Abstract
Background
Currently, mismatch repair-deficient (dMMR) status is a promising candidate for targeted immune checkpoint inhibition therapy in colorectal cancer (CRC) patients, however, the potential immunological mechanism has not yet been well clarified and some other predictors need to be excavated as well.
Methods
We collected 330 CRC patients by the match of mismatch repair-proficient (167) and dMMR (163), explored the relationship between MMR status and some important immune molecules including MHC class I, CD3, CD4, CD8, CD56, programmed death-1 and programmed death ligand-1, and investigated the risk factors for dMMR status as well as low MHC class I expression. The Pearson Chi square test was used for analyzing the associations between clinicopathological and immune characteristics and MMR status, and two categories logistic regression model was used for univariate and multivariate analysis to predict the odds ratio of risk factors for dMMR status and low MHC class I expression.
Results
Multivariate logistic regression analysis showed that low MHC class I and CD4 expression and high CD8 expression were significant risk factors for dMMR status [odds ratio (OR) = 24.66, 2.94 and 2.97, respectively; all p < 0.05] and dMMR status was the only risk factor for low MHC class I expression (OR = 15.34; p < 0.001).
Conclusions
High CD8 and low MHC class I expression suggests the contradiction and complexity of immune microenvironment in dMMR CRC patients. Some other immunocytes such as CD56+ cells might also participate in the process of immune checkpoint inhibition, whereas needs further investigations.
Keywords: Colorectal cancer, Mismatch repair-deficient, Mismatch repair-proficient, MHC class I molecules, Programmed death-1
Background
In the past few years, immune checkpoint inhibitors including anti-programmed death-1 (PD-1), programmed death ligand-1 (PD-L1) and CTL-associated antigen-4 (CTLA-4) have yielded positive effect in a series of malignancies such as melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, and Hodgkin’s lymphoma [1–3]. Nevertheless, anti-PD-1 therapy has not shown encouraging outcomes in colorectal cancer (CRC) until 2015, when a phase II study showed that 40% of mismatch repair-deficient (dMMR) CRC patients achieved an objective response, while none of the patients with mismatch repair-proficient (pMMR) status responded to anti-PD-1 therapy [4].
Currently, dMMR CRC patients are suggested to be promising candidates for targeted immune checkpoint inhibition therapy. The most mentioned explanation for the enhanced activity of anti-PD-1 therapy in dMMR patients is the generation of neo-antigens, which then activate T cell response to tumor cells [4, 5]. Previous studies have also presented some other possible mechanisms such as changes in signaling pathways that lead to more cytokine and chemokine expression [6–8]. However, which immune cells and how they participate in the process remains unclear. Since approximately only 10–20% of all colorectal tumors (about 20% in stage II, 12% in stage III and 4% in stage IV) have microsatellite instability (MSI) or to say dMMR [9–12], it is not easy to obtain enough dMMR cases to investigate the concrete mechanisms. Besides, as only less than half dMMR CRC patients benefit from anti-PD-1 therapy, MMR status might not be the only positive predictor for anti-PD-1 therapy, some other predictive indexes should be explored to combine with MMR status in order to better predict the curative effect.
In this study, we enrolled 330 CRC patients by the match of pMMR (167) and dMMR (163) and explored the relationship between MMR status and other clinicopathologic characteristics, especially some important anti-tumor immune molecules such as MHC class I, CD3, CD4, CD8, CD56, PD-1 and PD-L1 to explain the better effect of anti-PD-1 therapy in dMMR group and explore some other possible efficacy predictors besides MMR status.
Methods
Patients and specimens
We collected surgical specimens from 330 patients who underwent primary surgery and diagnosed with stage I to IV CRC according to the seventh edition of the TNM Classification from March 2009 to December 2013 at Sun Yat-Sen University Cancer Center in Guangzhou, China. Clinicopathological characteristics are summarized in Table 1. The obtained surgical specimens were fixed in 10% formalin and embedded in paraffin for further use.
Table 1.
Clinicopathological and immune characteristics of CRC patients based on MMR status
| Characteristics | pMMR | dMMR | r | p value |
|---|---|---|---|---|
| Number of cases (n, %) | ||||
| Age at diagnosis (years) | 0.194 | < 0.001 | ||
| < 60 | 80 (47.9) | 110 (67.5) | ||
| ≥ 60 | 87 (52.1) | 53 (32.5) | ||
| Gender | 0.24 | |||
| Male | 96 (57.5) | 104 (63.8) | ||
| Female | 71 (42.5) | 59 (36.2) | ||
| Primary tumor site | 0.264 | < 0.001 | ||
| Left colon | 81 (48.5) | 41 (25.2) | ||
| Right colon | 47 (28.1) | 86 (52.8) | ||
| Rectum | 39 (23.4) | 36 (22.1) | ||
| Stage (7th AJCC) | 0.965 | |||
| I | 27 (16.2) | 26 (16.0) | ||
| II | 89 (53.3) | 85 (52.1) | ||
| III | 42 (25.1) | 41 (25.2) | ||
| IV | 9 (5.4) | 11 (6.7) | ||
| Tumor stage | 0.193 | 0.012 | ||
| T1 | 8 (4.8) | 5 (3.1) | ||
| T2 | 25 (15.0) | 24 (14.7) | ||
| T3 | 118 (70.7) | 95 (58.3) | ||
| T4a | 11 (6.6) | 27 (16.6) | ||
| T4b | 5 (3.0) | 12 (7.4) | ||
| Node stage | 0.819 | |||
| Negative | 119 (71.3) | 118 (72.4) | ||
| Positive | 48 (28.7) | 45 (27.6) | ||
| Negative lymph nodes | 0.179 | 0.001 | ||
| Low (< 14) | 102 (61.1) | 70 (42.9) | ||
| High (≥ 14) | 65 (38.9) | 93 (57.1) | ||
| Tumor histological grade | 0.212 | 0.001 | ||
| Well-differentiated | 2 (1.2) | 1 (0.6) | ||
| Moderately differentiated | 109 (65.3) | 77 (47.2) | ||
| Poorly differentiated | 27 (16.2) | 27 (16.6) | ||
| Mucinous | 29 (17.4) | 58 (35.6) | ||
| Nerve invasion | 0.12 | 0.029 | ||
| No | 142 (85.0) | 151 (92.6) | ||
| Yes | 25 (15.0) | 12 (7.4) | ||
| Vascular invasion | 0.123 | 0.025 | ||
| No | 143 (85.6) | 152 (93.3) | ||
| Yes | 24 (14.4) | 11 (6.7) | ||
| MHC class I expression | 0.454 | < 0.001 | ||
| Low | 12 (7.2) | 88 (54.0) | ||
| High | 155 (92.8) | 75 (46.0) | ||
| CD3 expression | 0.026 | 0.661 | ||
| Low | 66 (43.4) | 63 (46.0) | ||
| High | 86 (56.6) | 74 (54.0) | ||
| CD8 expression | 0.156 | 0.009 | ||
| Low | 83 (59.3) | 57 (43.5) | ||
| High | 57 (40.7) | 74 (56.5) | ||
| CD56 expression | 0.228 | < 0.001 | ||
| Low | 41 (24.7) | 75 (47.2) | ||
| High | 125 (75.3) | 84 (52.8) | ||
| CD4 expression | 0.2 | < 0.001 | ||
| Low | 49 (30.1) | 78 (50.0) | ||
| High | 114 (69.9) | 78 (50.0) | ||
| PD-1 expression | 0.043 | 0.483 | ||
| Low | 62 (46.3) | 55 (42.0) | ||
| High | 72 (53.7) | 76 (58.0) | ||
| PD-L1 expression | 0.133 | 0.015 | ||
| Negative | 77 (46.1) | 97 (59.5) | ||
| Positive | 90 (53.9) | 66 (40.5) | ||
r: contingency coefficient
Immunohistochemistry
To detect MMR proteins, mouse anti-MLH1, MSH2, MSH6 and PMS2 monoclonal antibodies (DAKO, Denmark) were used. Mouse monoclonal antibodies against pan MHC class I molecule (EMR8-5, Hokudo, Japan), CD3, CD4, CD8 and CD56 (DAKO, Denmark) as well as rabbit monoclonal antibodies against PD-1 (Abcam, UK) and PD-L1 (Cell Signaling Technology, USA) were used to test the expression of corresponding proteins. Tissue sections of 4 μm thickness were deparaffinized in xylene, washed and dehydrated with graded ethanol, and then dipped in methanol with 0.3% hydrogen peroxide for more than 15 min followed by antigen retrieval. The slides were then incubated with the primary antibody at 4 °C overnight. After been washed three times with phosphate-buffered saline (PBS), the slides were subsequently co-incubated with the biotin-linked secondary antibodies according to the manufacturer’s instructions. Slides were then counter-stained with haematoxylin for color reaction, washed and dehydrated in graded ethanol, ultimately mounted under a cover slip. Each slide was examined by three pathologists to obtain coincident immunohistochemical (IHC) results.
Scoring of immunohistochemistry
Both intratumoral and interstitial areas were included for analyzing the presence of CD3, CD4+, CD8+ and CD56+ cells using a modified semi-quantitative scoring method: 0 = “none” (no stained cells), 1 = “focal” (< 10% stained cells), 2 = “moderate” (10–40% stained cells), and 3 = “severe” (> 40% stained cells) [13, 14]. Cases of scores ≤ 1 were divided into low-expression group and scores ≥ 2 were considered to be high-expression group. The expression of MHC class I on tumor cells was graded as previously described: score 1 (< 20% positive cells), score 2 (20–80% positive cells), and score 3 (> 80% positive cells). Populations of scores 1 and 2 were classified into low-expression group and score 3 was regarded as high-expression group [15, 16]. PD-1 expression on lymphocytes was evaluated using a semi-quantitative scoring system (0 = none, 1 = < 5% of lymphocytes, 2 = 5–50% of lymphocytes, 3 ≥ 50% of lymphocytes). Scores 0 and 1 were graded as low and scores 2 and 3 were graded as high [13, 17]. PD-L1 expression on the surface of tumor cells and tumor-infiltrating immune cells was divided into two groups: specimens with < 5% membranous expression indicated “negative”, while ≥ 5% was considered “positive” [1]. Tumors lacking any one of MLH1, MSH2, PMS2, or MSH6 expression were identified as dMMR, only if tumors that express of all of the four markers were considered pMMR.
Statistical analysis
Statistical analyses were conducted using SPSS 22.0 software (SPSS Inc, USA). The Pearson Chi square test was used to assess the association between categorical variables. Two categories logistic regression model was used for univariate and multivariate analysis to predict the odds ratio (OR) of individual factors for dMMR status and low MHC class I expression. A p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
We selected the patients by the match of pMMR (167) and dMMR (163), including 255 colon cancer (left: 122; right: 133) and 75 rectum cancer patients. The clinicopathological characteristics of the 330 cases are listed in Table 1. Median age at the time of diagnosis was 57 years range from 22 to 84, and 60.6% were male patients. Majority of the patients were in stage II (52.7%) and III (25.2%), especially in T3 (64.5%) and N0 stage (71.8%). Moderately differentiated adenocarcinoma accounted for 56.4%, while well-differentiated adenocarcinoma made up only 0.9% of all pathological types. A total of 37 (11.2%) and 35 (10.6%) patients appeared nerve and vascular invasion, respectively. As negative lymph node represented the immune status against cancer, we incorporated this index into the study, and found no obvious difference between low (< 14; 52.1%) and high (≥ 14; 47.9%) group, according to the median of negative lymph nodes.
Relationship between MMR status and clinicopathologic and immunological features
Aberrant protein expression in dMMR patients was listed in Table 2. Single negative expression rates of MLH1, MSH2, MSH6 and PMS2 were 6.7, 3.1, 21.5 and 5.5%, respectively. Concurrent negative expression of two, three and all the four MMR proteins were observed in 52.1, 8.0 and 3.1% dMMR patients, respectively. We investigated whether MMR status has relationship with other clinical features, especially immune molecules. Representative IHC stainings for immune molecules were displayed in Fig. 1. As shown in Table 1, the MMR status was significantly correlated with the expression of MHC class I and CD8, and dMMR group displayed much less MHC class I and higher CD8 expression than pMMR group (all p < 0.01). Besides, CD56+ cell, CD4+ cell and PD-L1 expression were also reduced in dMMR group (all p < 0.05). Other characteristics such as age, primary tumor site, tumor stage, histological grade, number of negative lymph nodes, nerve and vascular invasion were also significantly correlated with the status of MMR (all p < 0.05). Univariate logistic regression analysis confirmed the above findings, however, when the above meaningful factors entered into multivariate logistic regression model (Table 3), negative lymph nodes, tumor histological grade, nerve invasion, CD56 and PD-L1 expression were found not to be significant risk factors for dMMR status (all p > 0.05).
Table 2.
Aberrant protein expression in dMMR patients
| Aberrant protein expression | Number of cases (n, %) |
|---|---|
| MMR (1) | 60 (36.8) |
| MLH1 | 11 (6.7) |
| MSH2 | 5 (3.1) |
| MSH6 | 35 (21.5) |
| PMS2 | 9 (5.5) |
| MMR (2) | 85 (52.1) |
| MLH1 + MSH2 | 1 (0.6) |
| MLH1 + MSH6 | 2 (1.2) |
| MLH1 + PMS2 | 49 (30.1) |
| MSH2 + MSH6 | 30 (18.4) |
| MSH2 + PMS2 | 2 (1.2) |
| MSH6 + PMS2 | 1 (0.6) |
| MMR (3) | 13 (8.0) |
| MLH1 + MSH2 + PMS2 | 4 (2.5) |
| MLH1 + MSH6 + PMS2 | 6 (3.7) |
| MLH1 + MSH2 + MSH6 | 2 (1.2) |
| MSH2 + MSH6 + PMS2 | 1 (0.6) |
| MMR (4) | 5 (3.1) |
MMR (1): negative expression of only one MMR protein
MMR (2): concurrent negative expression of two MMR proteins
MMR (3): concurrent negative expression of three MMR proteins
MMR (4): concurrent negative expression of four MMR proteins
Fig. 1.
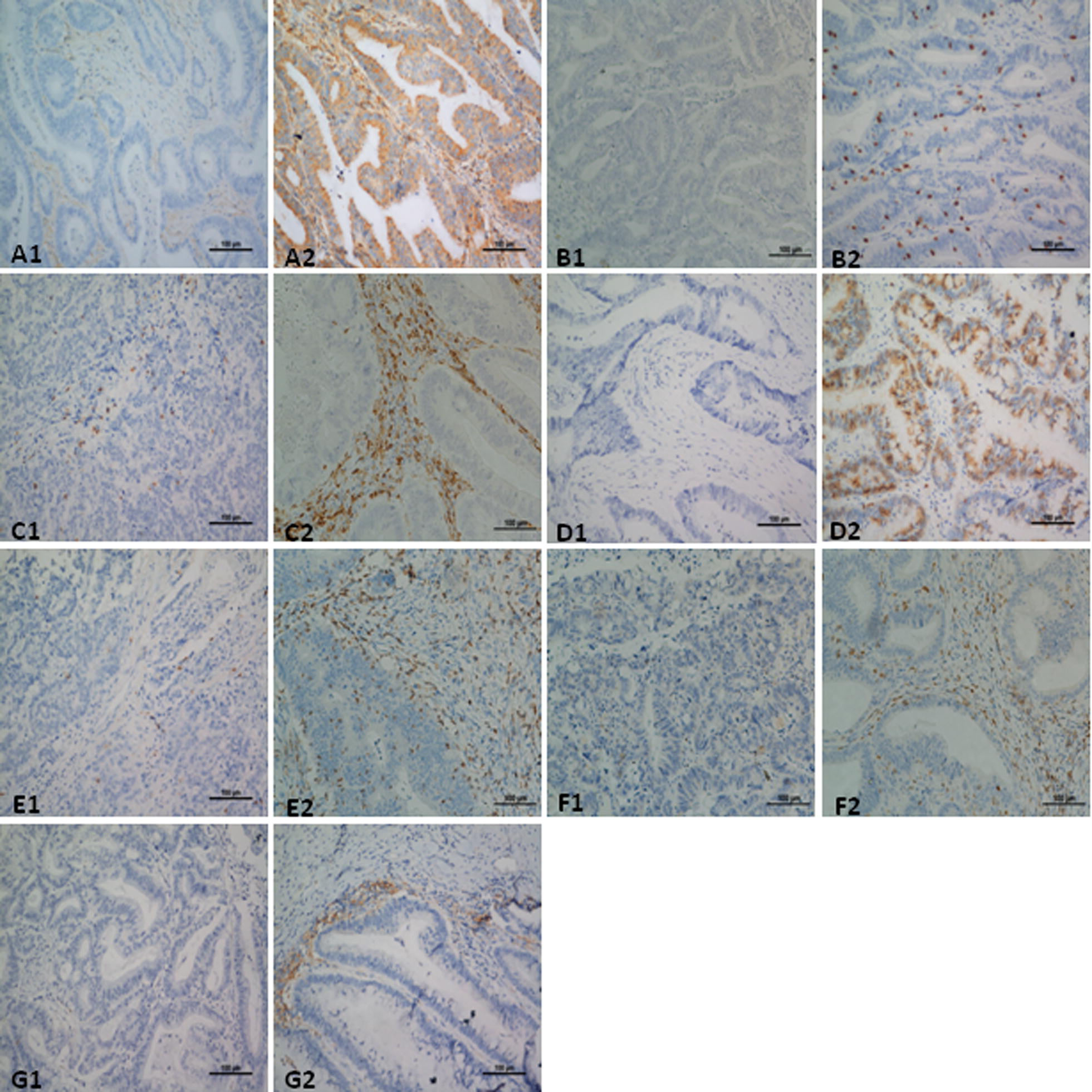
Representative IHC staining of immune molecules in CRC tissues. MHC class I expressed on tumor cells; CD3, CD4, CD8 and CD56 expressed in both intratumoral and interstitial areas; PD-1 expressed on lymphocytes; and PD-L1 expressed on tumor cells and tumor-infiltrating immune cells were evaluated. Low and high expression of MHC class I (A1 score 1, A2 score 3), CD3 (B1 score 1, B2 score 2), CD4 (C1 score 1, C2 score 3), CD8 (D1 score 0, D2 score 3), CD56 (E1 score 1, E2 score 3) and PD-1 (F1 score 0, F2 score 3); Negative and positive expression of PD-L1 (G1, G2). Images represent 200× microscopic fields used for analysis
Table 3.
Logistic regression analysis of risk factors for dMMR status
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age at diagnosis (≥ 60) | 0.44 (0.28–0.69) | < 0.001 | 0.48 (0.25–0.94) | 0.032 |
| Gender (female) | 0.77 (0.49–1.19) | 0.241 | ||
| Primary tumor site | < 0.001 | 0.001 | ||
| Left colon | 0.55 (0.30–0.99) | 0.045 | 0.43 (0.17–1.09) | 0.076 |
| Right colon | 1.98 (1.11–3.53) | 0.02 | 2.28 (0.86–6.07) | 0.098 |
| Rectum (reference) | – | – | – | – |
| Stage (7th AJCC) | 0.965 | |||
| I | 0.79 (0.28–2.21) | 0.651 | ||
| II | 0.78 (0.31–1.98) | 0.603 | ||
| III | 0.80 (0.30–2.13) | 0.653 | ||
| IV (reference) | – | – | ||
| Tumor stage | 0.007 | 0.004 | ||
| T1 | 0.26 (0.07–0.90) | 0.034 | 0.04 (0.01–0.33) | 0.002 |
| T2 | 0.39 (0.18–0.88) | 0.024 | 0.38 (0.11–1.37) | 0.139 |
| T3 | 0.33 (0.17–0.63) | 0.001 | 0.20 (0.07–0.59) | 0.004 |
| T4 (reference) | – | – | – | – |
| Node stage (positive) | 0.819 | |||
| Negative lymph nodes (high) | 2.09 (1.34–3.24) | 0.001 | 1.10 (0.52–2.30) | 0.810 |
| Tumor histological grade | 0.002 | 0.588 | ||
| Well-differentiated | 0.25 (0.02–2.87) | 0.266 | 7.91 (0.38–163.50) | 0.181 |
| Moderately differentiated | 0.35 (0.21–0.60) | < 0.001 | 1.12 (0.48–2.63) | 0.788 |
| Poorly differentiated | 0.50 (0.25–1.00) | 0.051 | 0.93 (0.30–2.89) | 0.896 |
| Mucinous (reference) | – | – | – | |
| Nerve invasion (no) | 2.22 (1.07–4.58) | 0.032 | 1.80 (0.59–5.45) | 0.299 |
| Vascular invasion (no) | 2.32 (1.10–4.91) | 0.028 | 3.66 (1.04–12.98) | 0.044 |
| MHC class I expression (low) | 15.16 (7.81–29.42) | < 0.001 | 24.66 (8.74–69.64) | < 0.001 |
| CD3 expression (high) | 0.90 (0.57–1.43) | 0.662 | ||
| CD4 expression (high) | 0.43 (0.27–0.68) | < 0.001 | 0.34 (0.14–0.83) | 0.017 |
| CD8 expression (high) | 1.89 (1.17–3.06) | 0.01 | 2.97 (1.30–6.78) | 0.01 |
| CD56 expression (high) | 0.37 (0.23–0.59) | < 0.001 | 0.92 (0.78–1.08) | 0.294 |
| PD-1 expression (high) | 1.19 (0.73–1.93) | 0.483 | ||
| PD-L1 expression (positive) | 0.58 (0.38–0.90) | 0.015 | 0.55 (0.27–1.13) | 0.103 |
OR: odds ratio; 95% CI: 95% confidence interval
Only the meaningful factors (p < 0.05) in univariate analysis were brought into the multivariate analysis
Risk factor analysis for low MHC class I expression
As dMMR status significantly correlated with low MHC class I expression, and MHC class I molecules play an important role in anti-tumor process, we next carried out the univariate and multivariate logistic regression analysis to explore the risk factors for low MHC class I expression. As shown in Table 4, age under 60, dMMR status, and low CD4 and CD56 expression were risk factors for low MHC class I expression in univariate model (all p < 0.05). Besides, tumor stage and tumor histological grade were also meaningful variables in the model (p < 0.05). However, when it came to multivariate logistic regression model, dMMR status was the only significant risk factor for low MHC class I expression (OR = 15.34, 95% confidence interval (CI) 7.24–32.50, p < 0.001).
Table 4.
Logistic regression analysis of risk factors for low MHC class I expression
| Factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age at diagnosis (≥ 60) | 0.60 (0.37–0.98) | 0.042 | 0.99 (0.54–1.85) | 0.998 |
| Gender (female) | 0.72 (0.44–1.17) | 0.187 | ||
| Primary tumor site | 0.136 | |||
| Left colon | 0.79 (0.41–1.50) | 0.464 | ||
| Right colon | 1.36 (0.74–2.50) | 0.323 | ||
| Rectum (reference) | – | – | ||
| Stage (7th AJCC) | 0.245 | |||
| I | 0.47 (0.17–1.35) | 0.161 | ||
| II | 0.38 (0.15–0.97) | 0.044 | ||
| III | 0.43 (0.16–1.17) | 0.097 | ||
| IV (reference) | – | – | ||
| Tumor stage | 0.005 | 0.122 | ||
| T1 | 0.89 (0.27–2.99) | 0.849 | 6.78 (0.97–47.42) | 0.054 |
| T2 | 0.37 (0.16–0.86) | 0.02 | 0.91 (0.22–3.72) | 0.897 |
| T3 | 0.35 (0.19–0.65) | 0.001 | 0.71 (0.33–1.52) | 0.377 |
| T4 (reference) | – | – | – | – |
| Node stage (positive) | 0.92 (0.54–1.56) | 0.753 | ||
| Negative lymph nodes (high) | 1.42 (0.89–2.28) | 0.143 | ||
| Tumor histological grade | 0.004 | 0.117 | ||
| Well-differentiated | 0.00 | 0.999 | 0.00 | 0.999 |
| Moderately differentiated | 0.38 (0.22–0.65) | < 0.001 | 0.47 (0.23–0.94) | 0.034 |
| Poorly differentiated | 0.76 (0.38–1.52) | 0.436 | 0.99 (0.42–2.38) | 0.989 |
| Mucinous (reference) | – | – | – | – |
| Nerve invasion (no) | 1.66 (0.73–3.77) | 0.227 | ||
| Vascular invasion (no) | 1.10 (0.51–2.38) | 0.814 | ||
| MMR status (dMMR) | 15.16 (7.81–29.42) | < 0.001 | 15.34 (7.24–32.50) | < 0.001 |
| CD3 expression (high) | 0.91 (0.54–1.52) | 0.714 | ||
| CD4 expression (high) | 0.57 (0.35–0.93) | 0.025 | 1.05 (0.57–1.96) | 0.871 |
| CD8 expression (high) | 1.42 (0.83–2.42) | 0.198 | ||
| CD56 expression (high) | 0.49 (0.30–0.79) | 0.004 | 0.92 (0.50–1.70) | 0.795 |
| PD-1 expression (high) | 0.95 (0.56–1.61) | 0.855 | ||
| PD-L1 expression (positive) | 0.70 (0.43–1.12) | 0.133 | ||
OR: odds ratio; 95% CI: 95% confidence interval
Only the meaningful factors (p < 0.05) in univariate analysis were brought into the multivariate analysis
Discussion
Anti-PD-1 therapy has shown good therapeutic effect in dMMR rather than pMMR CRC patients; however, the underlying mechanism remains not very clear. Several studies have shown that T cell activation by large proportion of mutant neoantigens in dMMR cancers make them sensitive to immune checkpoint blockade, and that immune score based on CD3+ and CD8+ T cell counts varied between dMMR and pMMR goups and had positive prognostic significance in CRC patients [18, 19]. These researches have revealed some immunological mechanisms in the process and the immunological characteristic differences between the two groups. We designed this retrospective study to provide some possible explanations in addition to T cell participation, as well as offer some other potential indexes in combination with MMR status for the efficacy prediction of immune checkpoint inhibition therapy in CRC patients.
Previous studies have shown that loss of MHC class I expression is very common in up to 60% of dMMR CRC patients [20, 21], in accordance with these researches, our result also revealed that about 54% (88/163) of dMMR patients had low expression rate of MHC class I molecules.
As known to all, MHC class I molecule is a crucial media expressed on the surface of cancer cells for the recognition by CD8+ cytotoxic lymphocytes (CTL). The MMR deficiency provoked enhanced immune response might be weaken by the immune evasion mediated by loss of MHC class I [22]. How could this phenomenon be in agreement with the anti-PD-1 clinical data that only dMMR patients can benefit? Former researches provided some possible explanations: First, antigen-presenting cells such as dendritic cells may cross-present MHC class I-restricted tumor related antigens to CTL and trigger the process of tumors killing. The mechanism has been confirmed in a mouse model [23]. Second, based on the findings that CD4+ T cells in MSI tumor environment express higher levels of PD-1 compared with microsatellite stable (MSS) tumors and CD4+ T cells could recognize mutated antigens in melanoma, they propose CD4+ T cells might play an important role in the anti-PD-1 therapy process [24, 25]. Furthermore, MHC class II-restricted T cell may also play an important role in the process, which has been verified in a pre-clinical CRC model [26]. In addition, cellular stress caused by high level DNA damage in dMMR tumors might be sensed by and activate innate immune cells [4].
In our studies, we found that dMMR status was associated with low CD4 expression and high CD8 expression, and there was no significant correlation between MMR status and CD56. In addition, dMMR status was the only risk factor for low MHC class I expression. Previous research showed that tumors from dMMR CRC patients contained a greater density of CD8+ T cells and higher expression of PD-L1 than did tumors from pMMR patients in stage IV [4]. The inconsistency of PD-L1 expression between our studies is probably because our data were from stage I–IV especially stage II, and PD-L1 expression both on the surface of tumor cells and tumor-infiltrating immune cells was counted. As only 40% of dMMR CRC patients achieved an objective response to anti-PD-1 therapy, we infer that only the CRC cells of high MHC class I expression could be recognized and killed by CD8+ T cells, because previous studies have shown that about 60% of dMMR CRC patients lack of MHC class I expression. Our data also showed that approximate 46% of cases (75/163) in dMMR group displayed high MHC class I expression, which is close to the 40% response rate.
CD56 was used as a biomarker for NK cells existing in the colorectal tumor microenvironment in majority of IHC studies [27, 28], so we assessed for the presence of NK cell infiltration in CRC tissues using the expression of CD56. Based on our findings, and the conception that loss or alteration of MHC class I molecule is the essential condition for NK cell activation [29–31], we could speculate that CD56+ NK cell might also play a role in the process of immune checkpoint inhibition therapy. However, current result is insufficient to prove the above two hypotheses, and subsequent researches should be carried out to further confirm our assumptions. Some other mechanisms should also be taken into account, for example, subgroup of CD4+ cell and MHC class II should be detected to evaluate the role of helper T cells and MHC class II molecule in the process. Moreover, it would be even better to compare the immune molecules, especially MHC class I molecules before and after anti-PD-1 therapy. If MHC class I molecule were up-regulated, the doubts would be well resolved.
MMR status has relationship with the expression of CD4, CD8 and MHC class I molecule, however, the potential mechanism remains unclear. There might be correlations between them in transcriptional or post transcriptional levels, nevertheless lack of evidence. KRAS mutation and BRAF mutation are two important mutations that affect immunological and clinical outcomes of CRC patients. BRAF V600E mutations are overrepresented in dMMR tumors and can be used to rule out Lynch syndrome, while KRAS mutations (in codons 12 or 13) are inversely correlated with dMMR status [32, 33]. Those pMMR CRC patients exhibiting a KRAS or BRAF mutation had the poorest prognosis [34]. The above two aspects were not involved in our study, which are defects of this research and need further investigations.
Previous studies have shown that CRC patients with dMMR status, higher MHC class I expression, and higher CD8+ T cell and NK cell infiltration had a better prognosis [35–38]. Our results suggested a loss of MHC class I expression in dMMR group, revealing the complexity of immune related prognostic factors. Multivariate analysis should be carried out to determine the independent prognostic factors. However, due to the limited samples and the fact that most of the patients were in early stage without relapse or death, we failed to make the corresponding research, which should be implemented in the future.
Now, MMR status is thought to be a gold standard for prediction of clinical benefit from immune checkpoint blockade in CRC patients [4]. However, some other predictors should also be explored to improve the predictive efficiency. Tumor-infiltrating lymphocyte (TIL) and PD-L1 have been explored, whereas were not significantly associated with overall survival (OS) or progression-free survival (PFS) [4]. On the basis of our data, MHC class I molecules as another predictor in combination with MMR status is strongly recommend, that’s to say dMMR status with high MHC class I expression might be the most appropriate candidate for immune checkpoint inhibition therapy.
Conclusions
Taken together, based on our study, high CD8 and low MHC class I expression suggests the contradiction and complexity of immune microenvironment in dMMR CRC patients. Some other immunocytes such as CD56+ cells might also participate in the process of immune checkpoint blockade, whereas need further investigations. Besides, dMMR status with high MHC class I expression might be the best candidate for immune checkpoint inhibition therapy.
Authors’ contributions
S-SL, Y-ZY, CJ, QQ, Q-KX, P-FK and L-PX designed and performed the research; X-PW, W-ZH, Y-MR and PC collected and analysed the data; S-SL, QY, LY, BZ and X-JX analyzed the results and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated and analysed during this study are included in this manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The research was carried out according to the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee of Sun Yat-sen University. Informed consent was not applicable in this retrospective study.
Funding
This work was supported in part by funding from: Natural Science Foundation of Guangdong Province (5A030313010 to Liang-Ping Xia), Science and Technology Program of Guangzhou, China (1563000305 to Liang-Ping Xia), National Natural Science Foundation of China (81272641 and 81572409 to Liang-Ping Xia) and Natural Science Foundation of Guangdong Province (2017A030310337 to Shou-Sheng Liu).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CRC
colorectal cancer
- dMMR
mismatch repair-deficient
- pMMR
mismatch repair-proficient
- MSI
microsatellite instability
- MSS
microsatellite stable
- PD-1
programmed death-1
- PD-L1
programmed death ligand-1
- CTLA-4
CTL-associated antigen-4
- CTL
cytotoxic lymphocyte
- TIL
tumor-infiltrating lymphocyte
- OS
overall survival
- PFS
progression-free survival
Footnotes
Shou-Sheng Liu and Yuan-Zhong Yang contributed equally to this work
Contributor Information
Shou-Sheng Liu, Email: liushsh@sysucc.org.cn.
Yuan-Zhong Yang, Email: yangyuanzh@sysucc.org.cn.
Chang Jiang, Email: jiangchang@sysucc.org.cn.
Qi Quan, Email: quanqi@sysucc.org.cn.
Qian-Kun Xie, Email: xieqk@sysucc.org.cn.
Xiao-Pai Wang, Email: wangxiaopai86@163.com.
Wen-Zhuo He, Email: hewzh@sysucc.org.cn.
Yu-Ming Rong, Email: rongym@sysucc.org.cn.
Ping Chen, Email: chenping@sysucc.org.cn.
Qiong Yang, Email: yangqiong05@126.com.
Lin Yang, Email: yanglin@sysucc.org.cn.
Bei Zhang, Email: zhangbei@sysucc.org.cn.
Xiao-Jun Xia, Email: xiaxj@sysucc.org.cn.
Peng-Fei Kong, Phone: +86 02087343455, Email: kongpf@sysucc.org.cn.
Liang-Ping Xia, Phone: +86 02087343455, Email: xialp@sysucc.org.cn.
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28(8):411–419. doi: 10.1093/intimm/dxw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 7.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto H, Imai K, Perucho M. Gastrointestinal cancer of the microsatellite mutator phenotype pathway. J Gastroenterol. 2002;37(3):153–163. doi: 10.1007/s005350200015. [DOI] [PubMed] [Google Scholar]
- 9.Raut CP, Pawlik TM, Rodriguez-Bigas MA. Clinicopathologic features in colorectal cancer patients with microsatellite instability. Mutat Res. 2004;568(2):275–282. doi: 10.1016/j.mrfmmm.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352(18):1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 11.Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100(2):266–273. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3(5):502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del MVM, Perea-Garcia F, Sanchez-Palencia A, Ruiz-Cabello F, Gomez-Morales M, Miranda-Leon MT, et al. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget. 2016;7(44):71608–71619. doi: 10.18632/oncotarget.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kukita K, Tamura Y, Tanaka T, Kajiwara T, Kutomi G, Saito K, et al. Cancer-associated oxidase ERO1-alpha regulates the expression of MHC class I molecule via oxidative folding. J Immunol. 2015;194(10):4988–4996. doi: 10.4049/jimmunol.1303228. [DOI] [PubMed] [Google Scholar]
- 16.Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012;62(5):303–308. doi: 10.1111/j.1440-1827.2012.02789.x. [DOI] [PubMed] [Google Scholar]
- 17.Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirta EV, Seppala T, Friman M, Vayrynen J, Ahtiainen M, Kautiainen H, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3(3):203–213. doi: 10.1002/cjp2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dierssen JW, de Miranda NF, Ferrone S, van Puijenbroek M, Cornelisse CJ, Fleuren GJ, et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong RA, Boerma A, Boezen HM, Mourits MJ, Hollema H, Nijman HW. Loss of HLA class I and mismatch repair protein expression in sporadic endometrioid endometrial carcinomas. Int J Cancer. 2012;131(8):1828–1836. doi: 10.1002/ijc.27449. [DOI] [PubMed] [Google Scholar]
- 22.Kloor M, Michel S, von Knebel DM. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127(5):1001–1010. doi: 10.1002/ijc.25283. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35(3):323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 25.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desbois M, Rusakiewicz S, Locher C, Zitvogel L, Chaput N. Natural killer cells in non-hematopoietic malignancies. Front Immunol. 2012;3:395. doi: 10.3389/fimmu.2012.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3(8):e952197. doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gras NA, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon SR, Kim TD, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med. 2015;47:e141. doi: 10.1038/emm.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 33.Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49(3):151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 34.Phipps AI, Buchanan DD, Makar KW, Win AK, Baron JA, Lindor NM, et al. KRAS-mutation status in relation to colorectal cancer survival: the joint impact of correlated tumour markers. Br J Cancer. 2013;108(8):1757–1764. doi: 10.1038/bjc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang S, Na Y, Joung SY, Lee SI, Oh SC, Min BW. The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors. Medicine (Baltimore) 2018;97(9):e19. doi: 10.1097/MD.0000000000010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling A, Edin S, Wikberg ML, Oberg A, Palmqvist R. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110(10):2551–2559. doi: 10.1038/bjc.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, et al. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. 2011;128(11):2663–2672. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson JA, Al-Attar A, Watson NF, Scholefield JH, Ilyas M, Durrant LG. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut. 2010;59(7):926–933. doi: 10.1136/gut.2009.194472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analysed during this study are included in this manuscript.


