Abstract
Aim
To determine the relationship between plasminogen activator inhibitor-1 (PAI-1) activity rise during the first 24 hours of ST-elevation myocardial infarction (STEMI) treatment and death after 5 years.
Methods
From May 1, 2009 to March 23, 2010, 87 STEMI patients treated with primary percutaneous coronary intervention (PCI) at the Sestre Milosrdnice University Hospital Center were consecutively enrolled in prospective single-center cohort study. PAI-1 activity was determined on admission and 24 hours later. The primary end-point was death after 5 years. The predictive value of PAI-1 activity variables as biomarkers of death was assessed using receiver operating characteristic (ROC) curve, independent predictors of death were assessed using multivariate Cox regression, and covariates independently related to higher PAI-1 activity rise were assessed using linear regression.
Results
Two patients died during the hospital treatment and 11 during the follow-up. PAI-1 activity rise had the largest area under curve (0.748) for predicting death rate (optimal cut-off point 3.7 U/mL, sensitivity 53.8%, specificity 90.5%). Patients with PAI-1 activity rise higher than 3.7 U/mL had significantly higher mortality (P < 0.001). Kaplan-Meier survival curve diverged within the first year after STEMI. Independent predictors of death were PAI-1 rise and final TIMI flow. PAI-1 activity rise was independently related to heart failure, thrombus aspiration, and body weight.
Conclusion
PAI-1 activity rise higher than 3.7 U/mL is associated with higher 5-year death rate in STEMI patients treated with primary PCI.
Oxford Centre for Evidence-based Medicine level of evidence: 3.
Myocardial infarction (MI) is initiated by a coronary artery atherosclerotic plaque rupture or erosion, followed by a thrombotic event leading to a complete or partial occlusion (1). Thrombus stabilization and extension or spontaneous reperfusion depend on the interplay of endogenous prothrombotic and fibrinolytic factors (2). One of such factors is plasminogen activator inhibitor-1 (PAI-1). It is the central inhibitor of plasminogen activation, whose function is to deter endogenous fibrinolysis (3,4). PAI-1 is predominantly secreted by platelets and endothelial cells and is present in plasma in an active, inactive, and latent form (5). Its distinct circadian pattern, with the highest concentration and activity in the morning, has to be taken into account in studies using single-sample values (6).
PAI-1 role in acute MI has been studied only regarding short-term outcomes. Collet et al (7) reported the predictive value of PAI-1 antigen level rise (measured at patient presentation and after 24 hours to avoid the influence of circadian variations) and the final flow according to the Thrombolysis in Myocardial Infarction (TIMI) criteria for 30-day mortality of ST-elevation myocardial infarction (STEMI) patients. However, that study included both acute and subacute MI patients and had a penetration rate of urgent invasive strategies of 77.1%. Also, the majority of the deaths (9 of 11) occurred during hospital treatment. There are no available data on the predictive value of PAI-1 rise for the long-term outcome in STEMI patients treated with primary percutaneous coronary intervention (PCI). We hypothesized that PAI-1 activity rise in these patients was related to poorer long-term outcome. The aim of the study was to determine the relationship between PAI-1 activity rise and long-term outcome and to examine clinical, angiographic, and laboratory parameters related to higher rise in PAI-1 activity.
Patients and methods
Patients
One hundred consecutive acute STEMI patients treated with primary PCI within 12 hours from the symptoms onset at the Sestre Milosrdnice University Hospital Center from May 1, 2009 to March 23, 2010 were examined for eligibility to enter this prospective single-center cohort study. The only inclusion criterion was STEMI diagnosis, established if persistent ST-segment elevation ≥1 mm in at least 2 consecutive standard electrocardiogram (ECG) leads or persistent ST-segment elevation ≥2 mm in precordial ECG leads, or new onset complete left bundle branch block, was observed and accompanied by chest pain lasting more than 20 minutes. Inability or refusal to sign the informed consent was regarded as a non-inclusion criterion. Ethical approval was received from Sestre Milosrdnice Clinical Hospital Center Ethics Committee.
The exclusion criteria were as follows: not available or deceased at 24 hours (1 patient), chronic corticosteroid or immunosuppressive therapy (4 patients), no significant coronary artery disease on angiogram (2 patients), diagnosis of acute infective or inflammatory disease established within the first 24 hours (5 patients), active malignant disease (1 patient), and chronic hepatic failure requiring treatment (0 patients). A total of 87 patients entered the study. The number of participants was restricted by available financial resources, which were used for laboratory analyses.
Methods
Blood samples were collected in citrated tubes on admission (before PCI) in the catheterization laboratory and exactly 24 hours later in the Cardiac Intensive Care Unit. The samples were centrifuged for 10 minutes at 4000 rotations per minute, frozen at -20°C, and analyzed for PAI-1 activity after no more than 28 days (8).
PAI-1 activity was analyzed using the commercially available Berichrom PAI on BCS XP analyzer (both from Siemens, Marburg, Germany) (9). After STEMI diagnosis was established, all patients received a routine acute-phase STEMI treatment, consisting of a loading dose of 300-mg aspirin and 600-mg clopidogrel. Patients with STEMI transferred from county hospitals in Karlovac (53 km) and Sisak (59 km) and patients presenting at the emergency department of Sestre Milosrdnice University Hospital Center and diagnosed with STEMI were immediately admitted to the catheterization laboratory. All PCIs were performed using transfemoral approach. The attending cardiologist decided on the dose and indication for the use of heparin, eptifibatide, thrombaspiration, type of intervention, and materials. The initial and final coronary flow were determined according to standard TIMI flow classification (10). No-reflow phenomenon was diagnosed if post-PCI residual culprit lesion stenosis was <50% and final TIMI flow was <3. After the procedure, patients were administered medications, including dual antiplatelet therapy, beta blockers, angiotensin converting enzyme (ACE) inhibitors, and statins according to indications and contraindications, by the attending cardiologist. Medical history, clinical parameters (occurrence of heart failure, cardiogenic shock, arrhythmias, and death), and laboratory findings were recorded prospectively. Creatine kinase (CK) peak level was determined as the highest value among the consecutive 6-hour findings; the analysis was stopped after the first value lower than the previous one was observed. Echocardiography was performed during the hospital stay. Primary end-point was defined as death after 5 years. The outcome data were obtained from the medical records and by a telephone interview (with family members for deceased patients) at a fixed time point of 5 years after the initial hospitalization.
Statistical analysis
Depending on the normality of distribution, tested by Kolmogorov-Smirnov test, descriptive data are presented as means and standard deviations (SD) or medians and interquartile ranges (IQR). Categorical variables are presented as counts and frequencies. The difference between PAI-1 activity values on admission and after 24 hours was tested using Wilcoxon signed-rank test. PAI-1 activity rise was defined as the value on admission subtracted from the value obtained 24 hours later. Categorical variables were analyzed with χ2, Fisher exact, or Fisher-Halton-Freeman exact test, continuous by Mann-Whitney U test, and correlations by point-biserial correlation. Receiver operating characteristic (ROC) curves were used to determine variable’s predictive value and optimal cut-off points (using Youden index). Binary logistic and linear regression models were used to determine independent contribution of an individual variable to PAI-1 activity variability. Survival was analyzed using Cox regression and log-rank test, and presented as Kaplan-Meier curves. Two-tailed significance tests were performed, and P < 0.05 was considered significant. Statistical analyses were performed using SPSS for Windows, version 25 (IBM SPSS, Armonk, NY, USA, licensed to the University of Zagreb, School of Medicine).
Results
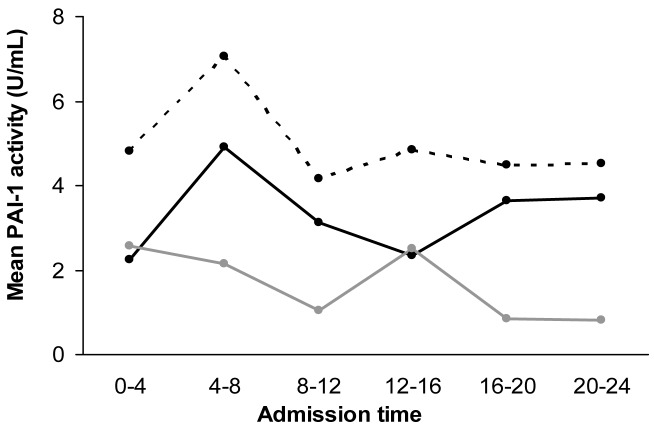
Study population was predominantly male, aged 61.1 ± 12.2 years, with a high prevalence of patients with hypertension and active smokers (Table 1). Low admission troponin T levels and high peak CK levels were observed, while all patients had initial TIMI flow lower than 3. High penetration of balloon angioplasty and frequent use of eptifibatide was observed, and 95.4% of interventions ended with stent implantation. Both PAI-1 measurements were obtained for all 87 patients. The highest mean PAI-1 activity levels in both samples were detected early in the morning (Figure 1); however, PAI-1 activity rise did not differ significantly among 4-hour time intervals of the day. PAI-1 activity measured after 24 hours was significantly higher than that on admission (4.71 ± 2.35 vs 3.25 ± 1.99 U/mL; P < 0.001, Wilcoxon signed-rank test). PAI-1 activity rise correlated with heart failure occurrence (rpb = 0.393, P < 0.001), thrombus aspiration (rpb = 0.344, P = 0.001), pulmonary edema (rpb = 0.267, P = 0.012), and final TIMI flow lower than 3 (rpb = 0.235, P = 0.029).
Table 1.
Characteristics of patients with ST-elevation myocardial infarction (STEMI) for total study sample and according to 3.7 U/mL cut-off value of plasminogen activator inhibitor-1 (PAI-1) activity increase*
| Characteristic | No. of STEMI patients |
||||
|---|---|---|---|---|---|
| Total (n = 87) | PAI-1 rise >3.7 U/mL (n = 14) | PAI-1 rise ≤3.7 U/mL (n = 73) | P | ||
| Age (years; mean±SD) |
61.1 ± 12.2 |
66.2 ± 13.0 |
60.1 ± 11.9 |
0.104 |
|
| Female sex |
25 |
8 |
17 |
0.010 |
|
| Transferred patients |
33 |
7 |
26 |
0.310 |
|
| Hypertension |
58 |
8 |
50 |
0.409 |
|
| Diabetes |
14 |
1 |
13 |
0.451 |
|
| Smoking |
42 |
5 |
37 |
0.305 |
|
| Previous coronary artery disease |
11 |
2 |
9 |
1.000 |
|
| Chronic medications: | |||||
| aspirin |
13/86 |
4 |
9 |
0.213 |
|
| beta blockers |
12/83 |
3 |
9 |
0.417 |
|
| ACE inhibitors or ARB |
20/83 |
5 |
15 |
0.265 |
|
| statins |
6/86 |
0 |
6 |
0.583 |
|
| Pain-to-first medical contact (minutes; median, IQR) [n] |
122 (60-250) [86]† |
188 (57-300) [14] |
120 (61-247.5) [72] |
0.884 |
|
| First medical contact-to-balloon (minutes; median, IQR) [n] |
60 (48.75-85) [86]† |
60 (48-91.5) [14] |
59.5 (48.25-84.75) [72] |
0.893 |
|
| Occurrence of heart failure |
9 |
5 |
4 |
0.005 |
|
| Pulmonary edema |
1 |
1 |
0 |
0.161 |
|
| Cardiogenic shock |
3 |
2 |
1 |
0.066 |
|
| Ventricular fibrillation‡ |
11 |
4 |
7 |
0.072 |
|
| New onset atrial fibrillation |
4 |
0 |
4 |
1.000 |
|
| Anterior wall myocardial infarction§ |
34 |
9 |
25 |
0.035 |
|
| Infarction-related artery |
|||||
| Left anterior descending coronary artery |
36 |
10 |
26 |
0.048 |
|
| Circumflex coronary artery |
10 |
1 |
9 |
||
| Right coronary artery |
41 |
3 |
38 |
||
| Two- or three-vessel disease |
51 |
7 |
44 |
0.475 |
|
| In-stent thrombosis |
2 |
1 |
1 |
0.298 |
|
| Initial/final TIMI flow |
|||||
| 0 |
66/1 |
12/1 |
54/0 |
initial flow 0.882, final flow 0.033 |
|
| 1 |
12/1 |
1/1 |
11/0 |
||
| 2 |
9/10 |
1/2 |
8/8 |
||
| 3 |
0/75 |
0/10 |
0/65 |
||
| No-reflow phenomenon |
23 |
7 |
16 |
0.029 |
|
| Balloon angioplasty |
66 |
10 |
56 |
0.736 |
|
| Stent implantation |
83 |
13 |
70 |
0.511 |
|
| Use of drug-eluting stent (number of patients)‖ |
3/83 |
1 |
2 |
0.404 |
|
| Thrombaspiration |
12 |
5 |
7 |
0.009 |
|
| Eptifibatide |
63 |
10 |
53 |
1.000 |
|
| Medications within first 24 hours: | |||||
| beta blockers |
47 |
8 |
39 |
0.798 |
|
| ACE inhibitors or ARB |
38 |
4 |
34 |
0.252 |
|
| statins |
48 |
7 |
41 |
0.772 |
|
| Unfractionated heparin/kg (U/kg; median, IQR) [n] ¶ |
85.4 (71.2-100.0) [86]† |
94.2 (74.4-112.5) [14] |
84.7 (70.6-100.0) [72] |
0.165 |
|
| C-reactive protein (U/L; median, IQR) [n] |
2.9 (1.6-5.7) |
2.1 (0.9-3.5) [14] |
3.1 (1.6-6.8) [73] |
0.097 |
|
| Troponin T (μg/L; median, IQR) [n] |
0.083 (0.024-0.199) [64]† |
0.142 (0.036-0.243) [8] |
0.083 (0.022-0.163) [56] |
0.549 |
|
| Peak creatine kinase (U/L; median, IQR) [n] |
2639 (1480-4435) |
4354 (2560-7387) [14] |
2566 (1415-4075) [73] |
0.010 |
|
| Total cholesterol (mmol/L; mean±SD) [n] |
5.93 ± 1.29 [81]† |
6.09 ± 0.88 [13] |
5.90 ± 1.36 [68] |
0.463 |
|
| Low density lipoprotein (mmol/L; mean±SD) [n] |
3.91 ± 1.02 [81]† |
4.02 ± 0.59 [13] |
3.89 ± 1.09 [68] |
0.593 |
|
| High density lipoprotein (mmol/L; median, IQR) [n] |
1.10 (1.00-1.30) [81]† |
1.30 (1.15-1.50) [13] |
1.10 (0.92-1.30) [68] |
0.007 |
|
| Triglycerides (mmol/L; median, IQR) [n] |
1.80 (1.10-2.40) [81]† |
1.70 (0.95-2.20) [13] |
1.85 (1.20-2.47) [68] |
0.321 |
|
| Creatinine clearance <30 mL/kg |
8/86 |
3 |
5 |
0.118 |
|
| Left ventricular ejection fraction (%; median, IQR) [n] |
50 (45-56) [67]† |
51 (34-57) [10] |
50 (47-56) [57] |
0.441 |
|
| Weight (kg; mean±SD) [n] |
83.0 ± 14.9 [86]† |
74.1 ± 11.5 [14] |
84.7 ± 14.9 [72] |
0.007 |
|
| Body mass index (kg/m2; mean±SD) [n] |
27.7 ± 4.0 [86]† |
26.5 ± 3.7 [14] |
27.9 ± 4.0 [72] |
0.066 |
|
| Waist circumference (cm; mean±SD) [n] |
99.2 ± 13 [86]† |
95.4 ± 13.8 [14] |
99.9 ± 13.4 [72] |
0.109 |
|
| Hip circumference (cm; median, IQR) [n] |
98.5 (94-105) [86]† |
97.5 (91.5-100.7) [14] |
100.0 (95.0-107.5) [72] |
0.129 |
|
| Waist-to-hip (ratio; median, IQR) [n] | 0.990 (0.929-1.041) [86]† | 0.978 (0.926-1.027) [14] | 1.014 (0.930-1.043) [72] | 0.390 | |
*ACE – angiotensin converting enzyme; ARB – angiotensin receptor blocker; IQR – interquartile range; PAI-1 – plasminogen activator inhibitor-1; SD – standard deviation; TIMI – thrombolysis in myocardial infarction.
†Patients with incomplete data were omitted.
‡In 9 patients before and in 2 patients (within 6 hours) after percutaneous coronary intervention.
§According to electrocardiogram.
‖Out of all patients to whom stents were implanted.
¶During percutaneous coronary intervention.
Figure 1.
Mean plasminogen activator inhibitor-1 (PAI-1) activity levels on admission and 24 hours later according to the time of day when the samples were obtained. Full black line – PAI-1 activity on admission; dotted black line – PAI-1 activity after 24 hours; full gray line – PAI-1 activity rise.
PAI-1 rise was then expressed as a binary variable with the cut-off point of 3.7 U/mL, as determined by ROC analysis of long-term survival. Variables related to PAI-1 activity rise higher than 3.7 U/mL were heart failure, anterior wall myocardial infarction, left anterior descending artery as an infarction-related artery, use of thrombus aspiration, no-reflow phenomenon, worse final TIMI flow, and female sex among categorical variables (Table 1), and higher levels of peak CK, high-density lipoprotein, and lower body weight among continuous variables. The two PAI-1 activity rise groups did not differ significantly in therapy use (dose of heparin and use of eptifibatide during PCI, use of beta blockers, ACE inhibitors, and statins within first 24 hours).
Stepwise binary logistic regression included 7 variables significant in the univariate analysis (high-density lipoprotein was omitted due to a number of missing values, and TIMI flow and affected artery due to the lowest significance in the univariate analysis). It showed that independent predictors of PAI-1 rise higher than 3.7 U/mL were heart failure (exponentiation of b [eb] = 35.3; P = 0.004), thrombus aspiration (eb = 15.2; P = 0.008), female sex (eb = 5.0; P = 0.043), body weight (eb = 0.936; P < 0.001), and peak CK levels (eb = 1.0004; P = 0.013). Stepwise linear regression with PAI-1 rise expressed as a continuous variable showed that independent predictors of higher PAI-1 rise were heart failure (odds ratio [OR] 4.4, 95% confidence interval [CI] 1.8-4.8; P < 0.001), thrombus aspiration (OR 3.8, 95% CI 1.2-3.9; P < 0.001), and body weight (OR -2.2, 95% CI -0.06- to -0.01; P = 0.034).
Two patients (2.3%) died during hospital treatment, both due to acute complications of myocardial infarction. All discharged patients were available for 5-year follow-up. Eleven additional deaths (12.6%) occurred during follow-up due to the following causes: cardiac causes in 8 patients (3 sudden cardiac deaths, 4 recurrent myocardial infarctions, 1 terminal heart failure), shock following surgical treatment for intestinal ileus in 1 patient, and unknown cause in 2 patients. All deaths in patients with PAI-1 activity rise higher than 3.7 U/mL were of cardiovascular origin. Higher death rate was found in patients older than 65 (χ2 test, P = 0.034), women (χ2 test, P = 0.030), patients with heart failure (Fisher exact test, P = 0.026), worse final TIMI flow (Fisher-Halton-Freeman test, P = 0.001), lower left ventricular ejection fraction (LVEF) (Mann-Whitney U test, P = 0.014), and lower BMI (Mann-Whitney U test, P = 0.024).
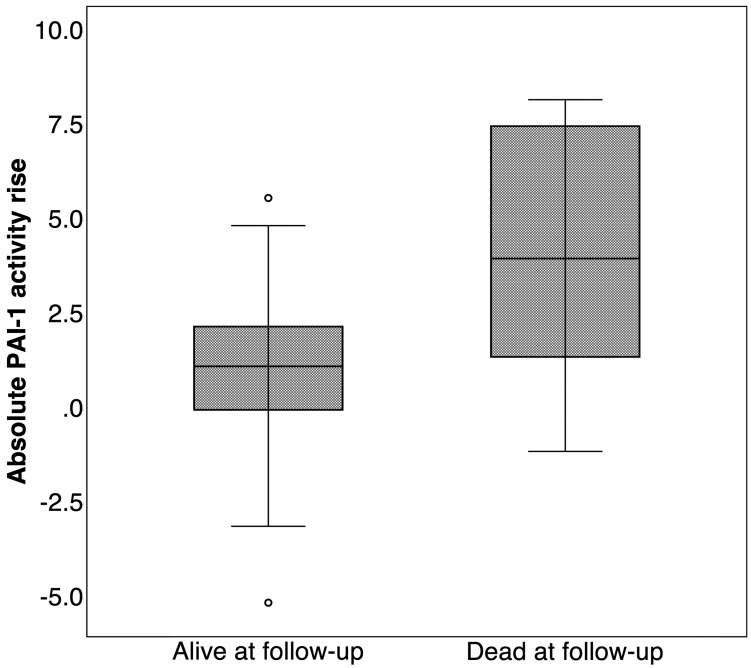
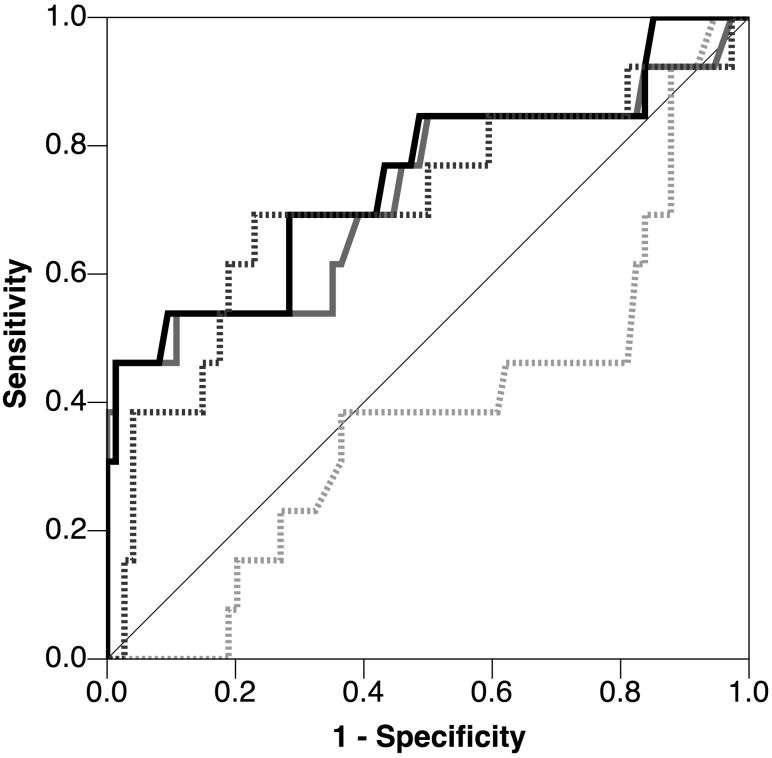
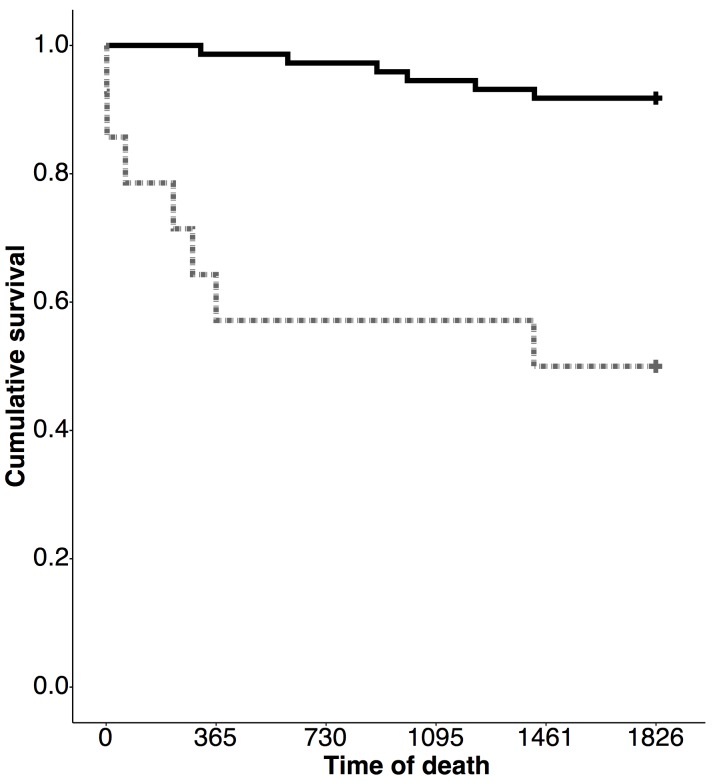
PAI-1 activity rise was higher in patients deceased during follow-up (Figure 2), which was also observed for patients deceased during hospital treatment, as well as after discharge (Table 2). In ROC analyses (Figure 3), significance was reached for PAI-1 activity in the second sample and PAI-1 activity rise, with the largest area under the curve for PAI-1 activity rise (area under the curve 0.748, 95% CI 0.579-0.917) and the optimal cut-off point of 3.7 U/mL. Kaplan-Meier survival curves showed poorer 5-year outcome in patients with PAI-1 activity rise higher than 3.7 U/mL (χ2 = 22.7 and P < 0.001, Figure 4).
Figure 2.
Plasminogen activator inhibitor-1 (PAI-1) activity rise according to patient status on follow-up (alive or dead). Boxes represent 25th and 75th percentile ranges, horizontal line represent medians, whiskers represent 1.5 times interquartile range, and circles represent outliers (patients with values higher than 1.5 times inter-quartile range).
Table 2.
Number of patients stratified by plasminogen activator inhibitor-1 (PAI-1) activity rise higher or lower than 3.7 U/mL and PAI-1 activity rise percentiles according to patient status at follow-up (alive or dead)
| No. of patients (% with PAI-1 rise) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PAI-1 rise | total death rate |
death during hospital stay |
death during follow-up |
|||||||
| alive | dead | P | alive | dead | P | alive | dead | P | ||
| >3.7 U/mL* |
no |
67 (92.0) |
6 (8.0) |
<0.001 |
73 (100.0) |
0 (0) |
0.024† |
67 (92.0) |
6 (8.0) |
0.001 |
| yes |
7 (50.0) |
7 (50.0) |
12 (86.0) |
2 (14.0) |
7 (58.0) |
5 (42.0) |
||||
| Percentile‡ | 25th |
0.00 |
1.40 |
<0.001 | 0.10 |
4.90 |
0.026 | 0.00 |
1.20 |
0.001 |
| 50th |
1.15 |
4.00 |
1.30 |
6.20§ |
1.15 |
2.19 |
||||
| 75th | 2.20 | 7.50 | 2.40 | 7.50 | 2.20 | 7.80 | ||||
*Cut-off values determined by receiver operating characteristic curve analysis regarding mortality. χ2 test was used if not stated otherwise.
†Fisher exact test.
‡Mann-Whitney U test.
§Median for only two cases.
Figure 3.
Receiver operating characteristic curve for plasminogen activator inhibitor-1 (PAI-1) activity (value on admission [dotted gray] and after 24 hours [full gray], absolute [full black] and percent rise [dotted black]) in relation to 5-year death rate.
Figure 4.
Kaplan-Meier survival curves according to plasminogen activator inhibitor-1 (PAI-1) rise (higher [gray line] or lower than 3.7 U/mL [black line]). Censored designated with cross.
Univariate Cox regression showed that PAI-1 activity rise higher than 3.7 U/mL was related to death at follow-up (hazard ratio 8.92, 95% CI 2.99-26.69, P < 0.001). Multivariate analysis using enter- and forward-stepwise approach included age, sex, heart failure occurrence, no-reflow phenomenon, thrombaspiration use, final TIMI flow (expressed as TIMI flow 3 or <3), and PAI-1 activity rise higher than 3.7 U/mL as covariates. In both approaches, the only independent predictors of death were final TIMI flow and PAI-1 activity rise higher than 3.7 U/mL (Table 3). After forcing LVEF into the model (using forward-stepwise approach), PAI-1 significance persisted despite 20 excluded patients due to missing LVEF values. PAI-1 and final TIMI flow remained significant after BMI, peak CK, multivessel disease detected on PCI, history of coronary artery disease, and diabetes were introduced into the model.
Table 3.
Multivariate Cox regression with status variable of occurrence of death at 5-year follow-up. Cox regression model was significant with χ2 = 38.47 and P < 0.001
| Hazard ratio | P | 95% confidence interval | |
|---|---|---|---|
| Final thrombolysis in myocardial infarction flow <3 |
6.60 |
0.009 |
1.60-27.14 |
| Plasminogen activator inhibitor-1 rise >3.7 U/mL |
5.55 |
0.018 |
1.34-22.98 |
| Occurrence of heart failure |
2.48 |
0.203 |
0.61-10.02 |
| No-reflow phenomenon |
2.18 |
0.327 |
0.46-10.36 |
| Female sex |
1.30 |
0.700 |
0.34-4.99 |
| Age |
1.01 |
0.658 |
0.95-1.08 |
| Thrombaspiration | 0.96 | 0.957 | 0.20-4.50 |
Discussion
We found that PAI-1 activity rise higher than 3.7 U/mL was associated with death during the 5-year follow-up in acute-phase STEMI patients treated with primary PCI. PAI-1 activity rise higher than 3.7 U/mL indicated poorer outcome, with Kaplan-Meier survival curves diverging within the first year after STEMI (suggesting the time frame in which PAI-1 activity rise exerts its impact), and the observed death rate of 50%. These results are in accordance with the study by Collet et al (7) on 30-day mortality of STEMI patients, in which the majority of deaths occurred during the hospital stay. However, the latter study included a heterogeneous patient population, a number of patients in the subacute MI phase, and had an incomplete penetration of invasive reperfusion strategy. Our study population comprised genuine STEMI patients with low initial TIMI flow levels, absence of initial TIMI 3 flow, substantial peak CK levels, and low initial troponin T levels in accordance with acute STEMI phase.
PAI-1 activity rise higher than 3.7 U/mL was associated with common high-risk features of STEMI, including heart failure, worse final TIMI flow, anterior myocardial wall infarction, left anterior descending artery as infarction-related artery, female sex, and higher peak CK levels. All these features except female sex are associated with a larger infarction size (11-13), which was estimated in our study by peak CK levels. Troponin T was used at recruitment only to rule in or rule out MI; therefore, consistent peak troponin T levels were not available for MI size estimation. Both heart failure and larger infarction size induce prominent inflammatory response in acute STEMI patients (14,15), while PAI-1 is one of the acute phase reactants (16). They might influence the outcomes separately, since PAI-1 activity rise was shown in multivariate Cox regression to be independently related to 5-year death rate. The only additional significant predictor of death in multivariate analysis was final TIMI flow, as was the case in the study by Collet et al (7). However, since no other predictors were significant, which might be the consequence of sample size, studies with larger sample size are needed to determine whether any additional variable is an independent predictor of death and whether PAI-1 is independent to these variables.
Higher plasma PAI-1 concentrations were detected in acute STEMI patients with fibrinolysis-resistant thrombi (17), while higher PAI-1 rise was detected in patients treated with streptokinase and persistent coronary artery occlusion (18). This indicates that PAI-1 rise influences the outcome similarly regardless of the reperfusion strategy. Enhanced PAI-1 activity may facilitate the persistence of microthrombi and a prothrombotic state in an early MI phase (19), which could worsen the short-term outcome in STEMI (7) and non-ST elevation acute coronary syndrome patients (20). On the other hand, PAI-1 plays a crucial role in myocardial fibrosis after MI, a fundamental component of unfavorable remodeling after STEMI and a possible pathway influencing the long-term outcome (21). PAI-1 deficiency protected against the induction of cardiac and perivascular fibrosis by a nitric oxide synthase inhibitor in an animal model (22). Yet, the complete PAI-1 absence in knockout murine models after artificially induced MI increased the incidence of myocardial rupture and death (23). Thus, both complete deficiency and high PAI-1 levels induced fibrosis in various organs, suggesting that the optimal therapeutic goal should be PAI-1 normalization (24). Accordingly, our results show that PAI-1 rise, rather than the initial PAI-1 activity, was related to a poorer long-term outcome. Hypothetically, PAI-1 rise could represent an individual phenotypic feature and could lead to a poorer outcome in subsequent cardiac or non-cardiac events after the patients survive the initial STEMI. Our data suggest that the influence of PAI-1 activity rise extends beyond the initial event. If future studies prove the causality of the observed association, PAI-1 antagonists use in MI treatment should be investigated. Currently, PAI-1 antagonists are still being developed, and no active substance is available for clinical use (25). Interestingly, in our study, concurrent medical therapy, including that with glycoprotein IIb/IIIa inhibitor (eptifibatide), did not influence PAI-1 activity rise. This is in accordance with previously published data showing deterred platelet aggregation and fibrin binding, but no difference in platelet PAI-1 secretion, after glycoprotein IIb/IIIa inhibition (26). Thus, even the most potent antiplatelet therapy did not affect the PAI-1 system.
PAI-1 activity values detected after 24 hours were significantly higher than admission values, which was not the case in the study by Collet et al (7), which analyzed PAI-1 antigen levels. Theoretically, PAI-1 activity reveals inhibitory capacity of PAI-1 system, while antigen levels cannot be used to assess PAI-1 protein status (active, inactive, or latent). This is why we believe that PAI-1 activity analyses are more appropriate for studying clinical entities with rapid changes in numerous biological systems, such as acute coronary syndromes.
At both measurement points, similar circadian rhythm was detected, with the highest values early in the morning. This pattern is described outside acute coronary syndrome (27) and is thought to be an evolutionary mechanism assuring higher blood clotting capability during physically most active part of the day. It is also responsible for peak MI occurrence in western societies (28). Our data suggest that intense events, such as acute STEMI, invasive treatment, and admission to the intensive care unit, do not affect the intrinsic circadian system in a short time period (24 hours), only consistently shift the PAI-1 time-activity curve upwards. Such circadian rhythm of PAI-1 variations in the acute phase of STEMI has not been previously described. Since single-sample PAI-1 activity determination cannot capture these variations in acute STEMI, we analyzed two samples 24 hours apart.
The major drawback of this study were the inadequate financial resources, which limited the sample size. However, the number of the included patients was not much smaller than in other PAI-1 studies in acute coronary syndromes (7,17,29,30). Post-hoc power analysis showed that χ2 and Mann-Whitney U tests for determining the difference in PAI-1 activities between dead and alive patients at 5-year follow-up had adequate power (1.000 for both tests at 5% significance level, G*Power 3.1.9.3, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) (31). Furthermore, the enrolment period had started before ticagrelor and drug-eluting stents for primary PCI became widely available in Croatia. Although this is a prospective study, due to its observational design the results may not be generalized to a larger population.
In conclusion, PAI-1 activity rise in acute STEMI patients treated with primary PCI was associated with high-risk features and poorer long-term survival. This finding could improve the accuracy of long-term prognosis in STEMI patients. Further studies are needed to clarify whether the determined relation is casual or causative. If the latter is true, additional efforts to produce and clinically test PAI-1 antagonists are required.
Acknowledgments
Funding All patients were participants in the scientific project funded by Ministry of Science and Technology (Metabolic Syndrome and Acute Myocardial Infarction, No. 108-1080230-0118).
Ethical approval received from Sestre Milosrdnice University Hospital Center Ethics Committee on February 16, 2006 (reference number EP#PRO-2-06).
Declaration of authorship MP, VNH, ZB, MM, IĆ, and VD conceived and designed the study; MP, VNH, ZB, KK, and IĆ acquired the data; MP, MM, KK, IĆ, and VD analyzed and interpreted the data; MP and VD drafted the manuscript; all authors critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Tanboga IH, Topcu S, Aksakal E, Kalkan K, Sevimli S, Acikel M. Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2014;20:716–22. doi: 10.1177/1076029613483169. [DOI] [PubMed] [Google Scholar]
- 2.Bainey KR, Fu Y, Granger CB, Hamm CW, Holmes DRJ, O’Neill WW, et al. Benefit of angiographic spontaneous reperfusion in STEMI: does it extend to diabetic patients? Heart. 2009;95:1331–6. doi: 10.1136/hrt.2008.160390. [DOI] [PubMed] [Google Scholar]
- 3.Binder BR, Christ G, Gruber F, Grubic N, Hufnagl P, Krebs M, et al. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol Sci. 2002;17:56–61. doi: 10.1152/nips.01369.2001. [DOI] [PubMed] [Google Scholar]
- 4.Gorlatova NV, Cale JM, Elokdah H, Li D, Fan K, Warnock M, et al. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J Biol Chem. 2007;282:9288–96. doi: 10.1074/jbc.M611642200. [DOI] [PubMed] [Google Scholar]
- 5.Aisina RB, Mukhametova LI. Structure and function of plasminogen/plasmin system. Russ J Bioorganic Chem. 2014;40:590–605. doi: 10.1134/S1068162014060028. [DOI] [Google Scholar]
- 6.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123:590–3. doi: 10.1182/blood-2013-07-517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collet JP, Montalescot G, Vicaut E, Ankri A, Walylo F, Lesty C, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108:391–4. doi: 10.1161/01.CIR.0000083471.33820.3C. [DOI] [PubMed] [Google Scholar]
- 8.Siemens healthcare diagnostics products GmbH. Berichrom PAI: Instruction manual. Marburg: 2008.
- 9.Stief TW, Lenz P, Becker U, Heimburger N. Determination of plasminogen activator inhibitor (PAI) capacity of human plasma in presence of oxidants: a novel principle. Thromb Res. 1988;50:559–73. doi: 10.1016/0049-3848(88)90204-6. [DOI] [PubMed] [Google Scholar]
- 10.The TIMI Study Group The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med. 1985;312:932–6. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 11.Seneviratna A, Lim GH, Devi A, Carvalho LP, Chua T, Koh TH, et al. Circadian dependence of infarct size and acute heart failure in ST elevation myocardial infarction. PLoS One. 2015;10:e0128526. doi: 10.1371/journal.pone.0128526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todt T, Maret E, Alfredsson J, Janzon M, Engvall J, Swahn E. Relationship between treatment delay and final infarct size in STEMI patients treated with abciximab and primary PCI. BMC Cardiovasc Disord. 2012;12:9. doi: 10.1186/1471-2261-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laster SB, O’Keefe JH, Gibbons RJ. Incidence and importance of thrombolysis in myocardial infarction grade 3 flow after primary percutaneous transluminal coronary angioplasty for acute myocardial infarction. Am J Cardiol. 1996;78:623–6. doi: 10.1016/S0002-9149(96)00382-7. [DOI] [PubMed] [Google Scholar]
- 14.Stumpf C, Sheriff A, Zimmermann S, Schaefauer L, Schlundt C, Raaz D, et al. C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch Med Sci. 2017;13:1086–93. doi: 10.5114/aoms.2017.69327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim DS, Ahn Y, Kim YH, Seon HJ, Park KH, Yoon HJ, et al. The relationship among N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein and infarct size in patients with acute ST-elevation myocardial infarction. Korean Circ J. 2015;45:285–93. doi: 10.4070/kcj.2015.45.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panahloo A, Mohamed-Ali V, Gray RP, Humphries SE, Yudkin JS. Plasminogen activator inhibitor-1 (PAI-1) activity post myocardial infarction: the role of acute phase reactants, insulin-like molecules and promoter (4G/5G) polymorphism in the PAI-1 gene. Atherosclerosis. 2003;168:297–304. doi: 10.1016/S0021-9150(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 17.Sambola A, Garcia Del Blanco B, Ruiz-Meana M, Francisco J, Barrabes JA, Figueras J, et al. Increased von Willebrand factor, P-selectin and fibrin content in occlusive thrombus resistant to lytic therapy. Thromb Haemost. 2016;115:1129–37. doi: 10.1160/TH15-12-0985. [DOI] [PubMed] [Google Scholar]
- 18.Paganelli F, Alessi MC, Morange P, Maixent JM, Levy S, Vague IJ. Relationship of plasminogen activator inhibitor-1 levels following thrombolytic therapy with rt-PA as compared to streptokinase and patency of infarct related coronary artery. Thromb Haemost. 1999;82:104–8. doi: 10.1055/s-0037-1614637. [DOI] [PubMed] [Google Scholar]
- 19.Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1979–89. doi: 10.1161/01.ATV.0000091250.53231.4D. [DOI] [PubMed] [Google Scholar]
- 20.Sinkovic A, Pogacar V. Risk stratification in patients with unstable angina and/or non-ST-elevation myocardial infarction by troponin T and plasminogen-activator-inhibitor-1 (PAI-1). Thromb Res. 2004;114:251–7. doi: 10.1016/j.thromres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–56. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–44. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- 23.Zaman AK, Fujii S, Schneider DJ, Taatjes DJ, Lijnen HR, Sobel BE. Deleterious effects of lack of cardiac PAI-1 after coronary occlusion in mice and their pathophysiologic determinants. Histochem Cell Biol. 2007;128:135–45. doi: 10.1007/s00418-007-0300-z. [DOI] [PubMed] [Google Scholar]
- 24.Flevaris P, Vaughan D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin Thromb Hemost. 2017;43:169–77. doi: 10.1055/s-0036-1586228. [DOI] [PubMed] [Google Scholar]
- 25.Gong L, Proulle V, Fang C, Hong Z, Lin Z, Liu M, et al. A specific plasminogen activator inhibitor-1 antagonist derived from inactivated urokinase. J Cell Mol Med. 2016;20:1851–60. doi: 10.1111/jcmm.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mousa SA, Bozarth J, Forsythe M, Tsao P, Pease L, Reilly TM. Role of platelet GpIIb/IIIa receptors in the modulation of platelet plasminogen activator inhibitors type-1 (PAI-1) release. Life Sci. 1994;54:1155–62. doi: 10.1016/0024-3205(94)00837-X. [DOI] [PubMed] [Google Scholar]
- 27.Young ME. PAI at breakfast (whether you like it or not). Blood. 2014;123:466–8. doi: 10.1182/blood-2013-11-538330. [DOI] [PubMed] [Google Scholar]
- 28.Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation. 1989;79:101–6. doi: 10.1161/01.CIR.79.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Demircan S, Yazici M, Hamiseyev C, Demircan G, Sultansuyu S, Yasar E, et al. The role of fibrinolytic system in no-reflow after stenting with and without predilation in patients with acute coronary syndromes: fibrinolysis and no-reflow after coronary stenting. J Thromb Thrombolysis. 2007;24:109–14. doi: 10.1007/s11239-007-0012-1. [DOI] [PubMed] [Google Scholar]
- 30.Van Dreden P, Rousseau A, Savoure A, Lenormand B, Fontaine S, Vasse M. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis. 2009;20:635–41. doi: 10.1097/MBC.0b013e32832e05dd. [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang A-G, Buchner AG. *Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]