Abstract
At present, amphipod crustaceans comprise 9,980 species, 1,664 genera, 444 subfamilies, and 221 families. Of these, 1,940 species (almost 20%) have been discovered within the last decade, including 18 fossil records for amphipods, which mostly occurred in Miocene amber and are probably all freshwater species. There have been more authors describing species since the 1950s and fewer species described per author since the 1860s, implying greater taxonomic effort and that it might be harder to find new amphipod species, respectively. There was no evidence of any change in papers per author or publication life-times of taxonomists over time that might have biased apparent effort. Using a nonhomogeneous renewal process model, we predicted that by the year 2100, 5,600 to 6,600 new amphipod species will be discovered. This indicates that about two-thirds of amphipods remain to be discovered which is twice the proportion than for species overall. Amphipods thus rank amongst the least well described taxa. To increase the prospect of discovering new amphipod species, studying undersampled areas and benthic microhabitats are recommended.
Keywords: Amphipoda, Crustacea, Taxonomy, Biodiversity
Introduction
Research into the progress of species discovery is of great interest to determine how many species exist on Earth (Costello, 2016). Recent studies on species discovery indicate that about two-thirds of species on Earth have already been described, and show that increasing numbers of people have been describing species new to science (e.g., Appeltans et al., 2012; Costello, May & Stork, 2013; Costello & Chaudhary, 2017). Thus, the present rate of species discovery is being sustained by an increasing number of taxonomists (both professional and amateur), and the relative number of species described per author has been decreasing for decades (e.g., Appeltans et al., 2012; Costello, May & Stork, 2013). These findings indicate that species discovery is flourishing and contradict the view that species discovery and description is facing a crisis (Wheeler, 2004; Wilson, 2004). Regardless of debates about how many species remain to be discovered, there is consensus that the new extinction crisis makes species description urgent to understand life on Earth and use resources sustainably (Costello, May & Stork, 2013; Wheeler et al., 2012).
Coleman (2015) found that the annual rate of species description has increased in recent decades. The most productive taxonomists with numbers of species described, the age distribution of taxonomists, annual rates of new taxa during the careers of four taxonomists, and the most productive taxonomists with working status (having permanent positions or temporary contracts) were also presented. However, trends in subgroups across environments and lifestyles were not studied and these may indicate where new species are most likely to be discovered. In this work, we reviewed trends in species description across all, benthic, pelagic, marine, freshwater, and subterranean amphipods. We used a statistical model to predict the number of amphipod species remaining to be described and analyzed taxonomic effort indicators such as the proportion of “oncer” authors (only ever described one species), the number of single and multiple authorship species, and authors’ publication lifetime and productivity. Although taxonomic effort has been increasing, the proportion of species remaining to be discovered is double that for all species on Earth. Amphipods are thus amongst the least well-known taxa globally.
Methods
Data source
Our analysis was based on the world list of amphipod crustaceans in the World Register of Marine Species (WoRMS) (Horton et al., 2016) as recorded on 3rd November 2016. We presented the results of the analysis for all species as well as for marine, freshwater, subterranean, benthic and pelagic species. We classified species as benthic and pelagic based on literature (Barnard, 1969; Bousfield, 1987; Brusca, 1967; Brusca, 1973; Lowry & Myers, 2009; Pillai, 1957; Streets, 1878; Vinogradov, 1988; Vinogradov, 1990). Only species names with accepted status and those that had been checked by the taxonomic editor were analyzed to avoid the over-estimation of the actual number of known species. Species described by Chevreux (1919–1920) were counted as species described in 1919.
Taxonomic effort
The number of authors is an indicator of taxonomic effort which may influence the rate of description. However, it could be biased by changing authorship practices whereby species are increasingly described by two or more authors. Thus, only the surname of the first author was considered to minimize estimates of effort. Dissimilar names or spelling variants (e.g., Kröyer and Krøyer) for the same author were corrected and counted as the same author. It was assumed that the number of authors with the same surnames at the same time were negligible and random over time (Costello, Wilson & Houlding, 2012). As the changes in description effort reflect the changes in taxonomic citation practices, we also analysed trends in the number of multiple and single authorships.
The trends in taxonomic effort might also be biased if there was a change in the relative productivity of individuals over time. Thus, we counted the proportion of “oncer” authors (described only one species) and calculated Pearson’s skewness coefficient on the number of species named by different authors. Working on another taxon might distract a taxonomist’s attention from describing amphipods. Thus, we analysed the number of non-amphipod species described by the most prolific authors to observe if taxonomists are now more specialized than they were in the 18th century. We also presented linear regressions between the number of non-amphipod species described, the number of amphipods species described, and the first year of an author’s publication. Non-amphipod species description data were gained through the advanced search feature on WoRMS and only non-amphipod species with accepted status were included. As WoRMS is primarily marine species it is possible that authors also described some non-marine species we have not accounted for.
An author’s publication lifetime was calculated as the number of years between their first and most recent species described. If authors’ publication lifetimes have been decreasing over time this may suggest a decrease in the number of amphipod specialists. Accordingly, we provided the linear regression on the publication lifetime against species per year for all authors either including or excluding Dybowsky (who described 97 species in one year).
We counted the number of species described per number of authors in a year. In the early years of discovery, it is easy for authors to find and name new species. After this period, it becomes more difficult and the proportion of species described per number of authors decline. Following Costello, Wilson & Houlding (2012); and Costello, May & Stork (2013), we used Muggeo’s (2003, 2008) method to determine the breakpoint between the increasing and decreasing number of species described per author in the time series.
We analysed whether families and genera which were described first had more species than those newly described by running a simple linear regression analysis between the ages of family against number of species in each family and the age of genus against number of species in each genus. The ratio of species to genera depend strongly on the number of species available and is expected to increase with discoveries overtime. Therefore, we depicted the trend in the number of genera described and the number of species per genus described. The Non-Homogenous Renewal Process (NHRP) was fitted to the description data to make predictions of numbers of amphipod species to be described by 2050 and 2100 by the following equation:
The parameters of the function were: N, the total number of species to be discovered; α, the year of the maximum rate of discovery; and β which describes the overall rate of discovery, with a larger β implying a faster rate (Costello & Wilson, 2011; Wilson & Costello, 2005).
Results
In total, 9,980 accepted names of marine, brackish, freshwater and terrestrial amphipods species were described from 1758 to 2016. There were 221 families, 86 subfamilies, and 1,674 genera. The number of non-accepted species names was 3,332 or 25% of all 13,312 names (Table 1). There were 18 fossil amphipod species discovered up to the year 2016, all of them attributed either to the family Gammaridae or Pontogammaridae, and at least 14 were from the freshwater environment (Table 2). The top-five species-rich genera were Niphargus, Gammarus, Ampelisca, Caprella, and Stygobromus (Table 3). Six amphipod families had only one species (monotypic) for more than 100 years (Table 4).
Table 1. Currently valid and non-valid amphipod families, genera, infraorders, species and subfamilies.
| Taxonomy status | Infraorder | Family | Subfamily | Genus | Species |
|---|---|---|---|---|---|
| Accepted | 8 | 221 | 86 | 1,674 | 9,980 |
| Non-accepted: | |||||
| -Alternate representation | 0 | 0 | 2 | 3 | 58 |
| -Interim unpublished | 0 | 0 | 0 | 0 | 2 |
| -Nomen dubium | 0 | 0 | 0 | 1 | 83 |
| -Nomen nudum | 0 | 0 | 0 | 1 | 25 |
| -Taxon inquirendum | 0 | 0 | 0 | 0 | 6 |
| -Temporary name | 0 | 1 | 0 | 5 | 25 |
| -Unaccepted | 3 | 20 | 8 | 210 | 3,133 |
| Total non-accepted | 3 | 21 | 10 | 220 | 3,332 |
| Total data | 11 | 242 | 96 | 1,894 | 13,312 |
Table 2. Documented fossil occurrences of 18 amphipod species.
| Scientific name | Authority | Year of description | Environment | Occurrence |
|---|---|---|---|---|
| Palaeogammarus sambiensis | Zaddach | 1864 | Freshwater | Eocene-Oligocene Baltic amber |
| Gammarus oeningensis | Heer | 1865 | Freshwater | Miocene amber |
| Hellenis saltatorius | Petunnikov | 1914 | Freshwater | Miocene amber |
| Gammarus alsaticus | Van Straelen | 1924 | Freshwater | Miocene amber |
| Praegmelina archangelskii | Derzhavin | 1927 | Freshwater | Miocene amber |
| Andrussovia bogacevi | Derzhavin | 1927 | Freshwater | Miocene amber |
| Andrussovia sokolovi | Derzhavin | 1927 | Freshwater | Miocene amber |
| Praegmelina andrussovi | Derzhavin | 1927 | Freshwater | Miocene amber |
| Palaeogammarus balticus | Lucks | 1928 | Freshwater | Eocene-Oligocene Baltic amber |
| Andrussovia vassolevitschi | Derzhavin | 1941 | Freshwater | Miocene amber |
| Gammarus praecyrius | Derzhavin | 1941 | Probably freshwater | Miocene amber |
| Gammarus retzi | Maikovsky | 1941 | Probably freshwater | Miocene amber |
| Palaeogammarus danicus | Just | 1974 | Freshwater | Eocene-Oligocene Baltic amber |
| Tethorchestia palaeorchestes | Bousfield & Poinar Jr. | 1995 | Terrestrial | |
| Niphargus groehni | Coleman & Myers | 2001 | Freshwater | Eocene-Oligocene Baltic amber |
| Palaeogammarus polonicus | Jażdżewski & Kulicka | 2002 | Freshwater | Eocene-Oligocene Baltic amber |
| Palaeogammarus debroyeri | Jażdżewski, Grabowski & Kupryjanowicz | 2014 | Freshwater | Eocene-Oligocene Baltic amber |
| Synurella aliciae | Jażdżewski, Grabowski & Kupryjanowicz | 2014 | Freshwater | Eocene-Oligocene Baltic amber |
Table 3. The 35 families with the most species, their number of genera and the year of description of the first and last species as per Horton et al. (2016).
| Rank | Family | Number of species | Number of genera | First species described (Year) | Last species described (Year) |
|---|---|---|---|---|---|
| 1 | Lysianassidae | 536 | 82 | 1830 | 2015 |
| 2 | Caprellidae | 425 | 90 | 1767 | 2016 |
| 3 | Gammaridae | 414 | 34 | 1758 | 2016 |
| 4 | Niphargidae | 377 | 10 | 1836 | 2016 |
| 5 | Phoxocephalidae | 369 | 79 | 1842 | 2015 |
| 6 | Maeridae | 367 | 47 | 1808 | 2016 |
| 7 | Talitridae | 316 | 72 | 1766 | 2016 |
| 8 | Ampeliscidae | 302 | 4 | 1840 | 2013 |
| 9 | Stenothoidae | 279 | 45 | 1815 | 2015 |
| 10 | Ischyroceridae | 264 | 43 | 1808 | 2014 |
| 12 | Aoridae | 250 | 25 | 1843 | 2016 |
| 11 | Oedicerotidae | 250 | 46 | 1842 | 2014 |
| 13 | Crangonyctidae | 226 | 7 | 1840 | 2016 |
| 14 | Photidae | 225 | 17 | 1828 | 2016 |
| 15 | Ampithoidae | 216 | 16 | 1816 | 2016 |
| 16 | Leucothoidae | 179 | 5 | 1789 | 2015 |
| 17 | Melitidae | 175 | 26 | 1804 | 2016 |
| 18 | Uristidae | 171 | 24 | 1774 | 2014 |
| 19 | Pontogeneiidae | 168 | 32 | 1838 | 2014 |
| 21 | Corophiidae | 158 | 26 | 1761 | 2016 |
| 20 | Hyalidae | 158 | 12 | 1830 | 2016 |
| 22 | Pleustidae | 141 | 33 | 1838 | 2009 |
| 23 | Dexaminidae | 125 | 12 | 1813 | 2016 |
| 24 | Acanthogammaridae | 122 | 34 | 1858 | 2002 |
| 25 | Liljeborgiidae | 119 | 2 | 1853 | 2012 |
| 26 | Eusiridae | 116 | 10 | 1780 | 2015 |
| 27 | Bogidiellidae | 113 | 37 | 1933 | 2016 |
| 28 | Eulimnogammaridae | 112 | 16 | 1858 | 2014 |
| 29 | Stegocephalidae | 109 | 27 | 1774 | 2012 |
| 30 | Synopiidae | 108 | 17 | 1853 | 2013 |
| 31 | Iphimediidae | 105 | 15 | 1843 | 2014 |
| 32 | Calliopiidae | 104 | 27 | 1830 | 2014 |
| 33 | Hadziidae | 91 | 27 | 1907 | 2016 |
| 34 | Amphilochidae | 90 | 15 | 1862 | 2016 |
| 35 | Podoceridae | 89 | 8 | 1814 | 2016 |
Table 4. The age of families consisting of one species (monotypic families).
| Family | Age (Year) | Species |
|---|---|---|
| Argissidae | 147 | Argissa hamatipes (Norman, 1869) |
| Baikalogammaridae | 142 | Baikalogammarus pullus (Dybowsky, 1874) |
| Dairellidae | 131 | Dairella californica (Bovallius, 1885) |
| Giniphargidae | 117 | Giniphargus pulchellus (Sayce, 1899) |
| Prolanceolidae | 109 | Prolanceola vibiliformis Woltereck, 1907 |
| Tryphanidae | 145 | Tryphana malmii Boeck, 1871 |
Progress in species descriptions
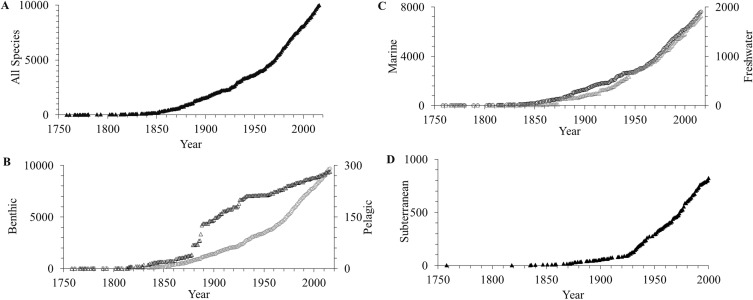
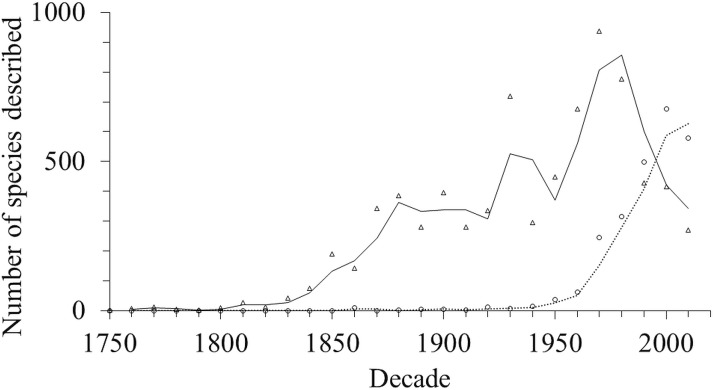
After an initial period of low discovery for 100 years, descriptions of new species accumulated steadily from the 1850’s to 1950’s, and have since maintained a linear rate of new species descriptions (Fig. 1A). A high proportion of pelagic amphipods were described in the late 19th century, but relatively few have been described compared to benthic species since then (Fig. 1B). Far more marine than freshwater species were described (Fig. 1C). The description of subterranean species was later than for marine and freshwater environments, with most species described since the late 18th century (Fig. 1D).
Figure 1. Accumulation curves of amphipod species described per year.
(A) all accepted, (B) benthic (gray circles) and pelagic (black triangles), (C) marine (gray circles) and freshwater (black triangles), and (D) subterranean up to the year 2016. Note scales vary.
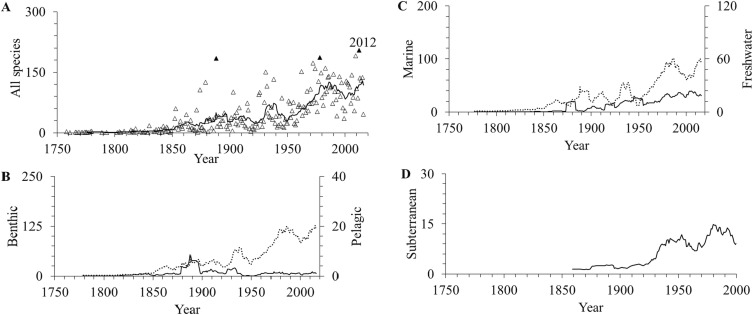
The greatest discovery of amphipods was in the 20th century, with the highest peak at 204 species in 2012 (Fig. 2A). A similar pattern also occurred for benthic, marine, freshwater and subterranean species (Figs. 2B–2D). However, the number of pelagic amphipods described had been declining since the 1890s (Fig. 2B). Overall, the average number of species described per year was 48 (95% confidence interval 41–54).
Figure 2. The number of amphipod species described per year.
(A) all species, (B) benthic (dotted line) and pelagic (solid line), (C) marine (dotted line) and freshwater (solid line), and (D) subterranean up to the year 2016. Solid triangles indicate top three years for species described. The lines are 10-year moving averages.
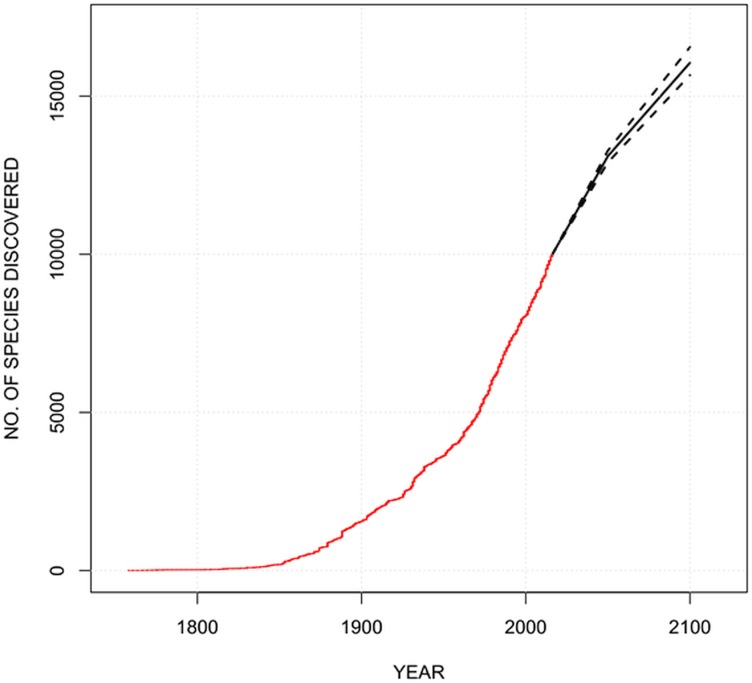
Using the nonhomogeneous renewal process model, with 95% probability, we predicted that by 2050 and 2100, there would be 2,900–3,300 and 5,600–6,600 more amphipod species to be described with the median prediction of 3,100 and 6,100, respectively (Fig. 3).
Figure 3. The cumulative number of amphipod species described over time (red line), with the predicted number of species based on the number of species described by 2050 and 2100.
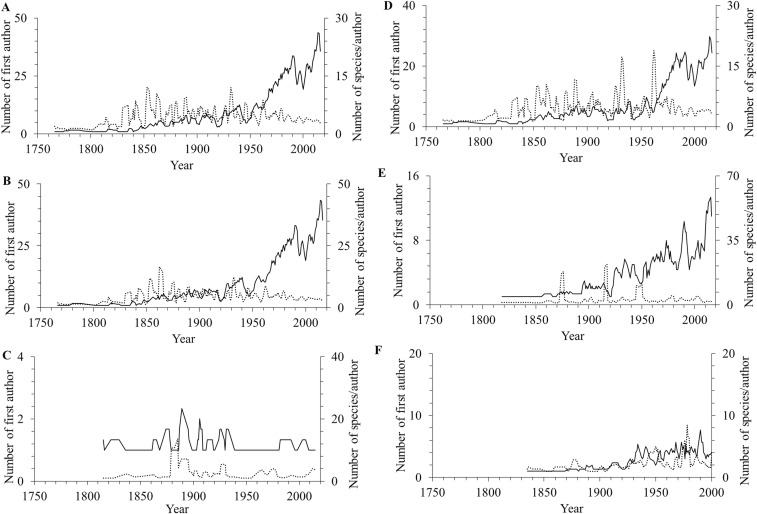
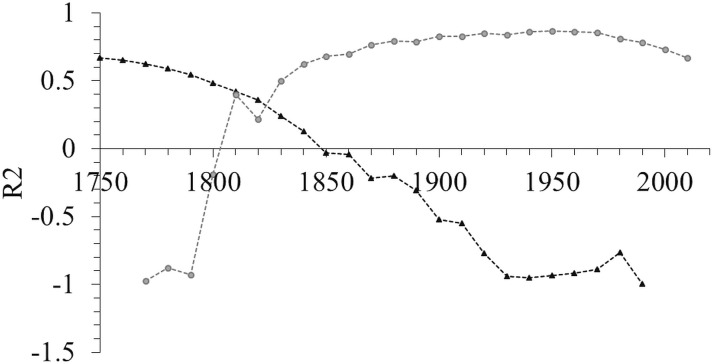
The number of authors describing species has increased in recent decades (Fig. 4A). The same increasing pattern was also found in benthic, marine, freshwater and subterranean species (Figs. 4D–4F), while there was no significant change in the number of authors describing pelagic species from the beginning of the 1950s (Fig. 4C). However, despite the increase in the number of authors describing species in recent decades, the number of species described per number of authors has decreased since the 1860s (Fig. 4A). The same downward trend occurred in the benthic, pelagic, marine, freshwater and subterranean species (Figs. 4B–4F). The break point in the time series for all species was in 1820 (Fig. 5). Even though there was a period with high productivity (a high number of species/author) between the 1830s and the 1970s, the overall trend since then has been a decreasing number of species per number of authors.
Figure 4. The number of first authors (solid line) and species/author (dotted line) of amphipod species described.
(A) all species, (B) benthic, (C) pelagic, (D) marine, (E) freshwater, and (F) subterranean per year up to the year 2016. The lines are three-year moving averages.
Figure 5. Change in the correlation coefficient of the number of all amphipod species described per author over 25 decades indicating the breakpoint was around 1820.
The grey dots indicate the correlation coefficients between the most recent decade and each previous decade. In contrast, the black triangles are the correlation coefficients between the first and each subsequent decade.
Authors as an indicator of taxonomic effort
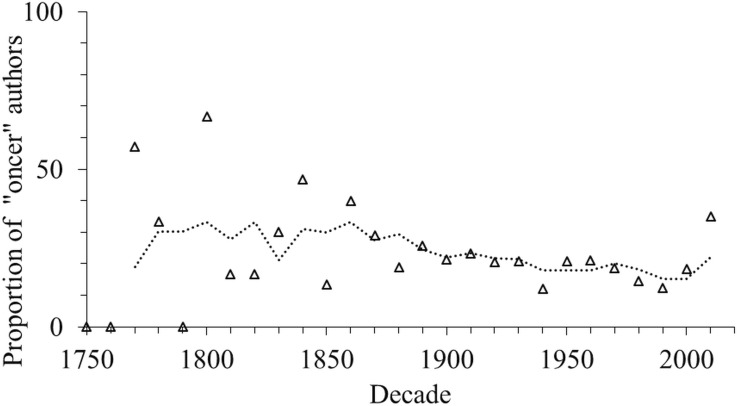
The trend in amphipod discovery above might be due to the increasing number of “oncers,” people who only describe one species. However, we found no trend in the proportion of oncers for more than one century and in the recent decade the highest proportion of “oncers” was only 35% (Fig. 6). Also, the skewness values were positive and were around 1.0 with an exception in the 1990s, which had high skewness (Fig. S1). Furthermore, it appears that “non-oncers” authors were responsible for almost all the new species descriptions (98%) since the 1850s, whereas “oncers” authors described only 2% of species.
Figure 6. The proportion of authors who described only one species (“oncers”) per decade, up to the year 2016.
The lines are three-year moving averages.
At the beginning of amphipod species descriptions in the 1750s, single authorship was the norm. Multiple authorship emerged more than 100 years later in the 1860s. Since the 1950’s the number of multiple authorships has been increasing (Fig. 7).
Figure 7. The number of amphipod species described with one (triangle) and multiple (circle) authors.
Among the 638 people involved in describing amphipod species from 1758 to 2016, 26 people described more than 90 species which we considered to be the most prolific authors (Table S1). We found that the average publication lifetime of all authors was nine years. However, there was no significant trend (R2 < 0.1, P > 0.05) in authors’ publication lifetimes over years for all authors, whether Dybowsky (who described 97 species in one year) was included or not (Fig. S2). Thus, there is no evidence of any change in author productivity since the 1850’s.
We found a significant trend (R2 < 0.5, P < 0.05) in the number of species between the “old” families or genera and the newly described families or genera (Fig. S3) reflecting a gradual accumulation of species over time through discoveries. Thus, families and genera which were described first had more species than those newly described. As observed in the number of species described, there was also an increase in the number of genera described and species to genus ratios in recent decades (Fig. S4).
There was no significant relationship (R2 = 0.0003, P > 0.05) between the number of amphipod and non-amphipod species described by the most prolific authors (Fig. S5A). However, there was a weak but significant relationship (R2 = 0.16, P < 0.05) in the number of non-amphipod species described with the first year of an author’s publication (Fig. S5B) indicating that recent authors are describing fewer non-amphipods.
Discussion
Species description rates
The number of species described for the entire fauna (benthic, marine, freshwater and subterranean species) increased in recent decades and the apparent decrease in the year 2016 may be explained by the time delay in entering new descriptions into WoRMS (Costello, Wilson & Houlding, 2012). As observed in the number of species described, there was also an increase in the number of genera described and species to genus ratios in recent decades. The decreased trend found in the number of pelagic species described for the past century is likely because pelagic species are more geographically widespread than benthic and thus are described earlier (Costello et al., 2015). The peak in the number of pelagic species described in 1879 was due to the contribution of a single author, Claus, who published three new genera and 29 new hyperiid amphipod species (Claus, 1879).
The nonhomogeneous renewal process model predicted that about one third of the described species would be described by 2050, and two-thirds more by 2100. These findings contrast with findings for all species on Earth and all marine species using the same model (Appeltans et al., 2012; Costello, Wilson & Houlding, 2012), and other assessments using proportions of undescribed species (Appeltans et al., 2012) and expert assessments (reviewed by Costello & Chaudhary, 2017). In these other cases, at least half and sometimes 80% of species are considered to have been described, and about two-thirds overall. Thus, amphipods appear to be only half as well described as other taxa.
The period of greatest discovery in the 20th century may have been influenced by the Global Taxonomy Initiative, Census of Marine Life (O’Dor et al., 2012) and PEET Program (Partnerships for Enhancing Expertise in Taxonomy) (Rodman & Cody, 2003). The peak in 2012 was due to the significant contribution of Ren (2012) in the second volume of Fauna Sinica which yielded 39 new species. Another contribution was from Lowry and coworkers who described 21 new species in the family Pachynidae, and established a new family Acidostomatidae and a new subfamily Conicostomatinae (Lowry & Stoddart, 2012; Stoddart & Lowry, 2012). Twenty-four new species of leucothoids from Japan (White & Reimer, 2012a; White & Reimer, 2012b; White & Reimer, 2012c) and six new species of Rhinoecetes from the South-eastern Australian Shelf were also contributing factors (Just, 2012). That families and genera which were described earlier had more species than those described later indicates that the major evolutionary lineages of amphipods have already been described. Thus, new species tend to be from already known families and genera.
Taxonomic effort
The increased number of people involved in describing species indicated increased taxonomic effort. Other studies have similarly reported the increasing trend in taxonomic effort (e.g., Costello, Wilson & Houlding, 2012; Costello, May & Stork, 2013; Costello, Houlding & Wilson, 2014; Costello et al., 2015; Essl et al., 2013; Irfanullah, 2013) which must have contributed to the sustained high number of species described (Appeltans et al., 2012). We found no change in skewness (of papers per author per year) over the past century, and that the percentage of “oncers” had not shown any trend in the past century and the largest proportion of “oncers” was only 35%. This ratio was lower than reported in previous research (42%–44%, Appeltans et al., 2012). Neither was there any significant trend in authors’ publication lifetimes or productivity of the 26 most prolific authors over time. By only using first authors we avoided any bias due to increasing multiple-authorships of species in recent decades. However, such co-authors are often first authors on other species, so our results will underestimate rather than overestimate the number of active authors of new species. Thus, there was no evidence of any change in author productivity that may affect description rates. The same results have also been reported for other taxa (Costello, Wilson & Houlding, 2012; Eschmeyer et al., 2010; Joppa, Roberts & Pimm, 2011; Pimm et al., 2010). Another measure of taxonomic productivity is the number of publications, and this is highly correlated with the number of authors per species (Edie, Smits & Jablonski, 2017).
As taxonomy develops, more time is needed to check synonyms and species relationships, to compare species characteristics with established species, and to produce more comprehensive publications (Frank & Curtis, 1979; Poulin & Presswell, 2016; Sangster & Luksenburg, 2015). However, these factors are likely compensated by the efficiency of the latest technology (Eschmeyer et al., 2010), the availability of reputable online database such as WoRMS to check the status of a species, the online availability of original descriptions from the Biodiversity Heritage Library, and greater ease of communication, transportation and improvement in sampling methods (Costello, Wilson & Houlding, 2012; Costello, May & Stork, 2013). For amphipods particularly, there is an extensive taxonomic character database (DELTA) and new techniques on amphipod descriptions initiated by Coleman (2003), Coleman (2006), Coleman (2009) and Coleman, Lowry & Macfarlane (2010), that provide more efficient ways of digital drawing for publication. We found that working on other taxa did not affect the number of amphipod species described. Also, we found that recent authors are having fewer non-amphipods described; thus there is no evidence of authors being distracted from working on amphipods.
Conclusions
Our results support previous studies on species description rates in finding increasing numbers of authors, a decreasing rate of species being described per authors, no changes in the relative productivity of authors over the past century, and that pelagic species are relatively better named than benthic. Most future new species are likely to be benthic and endemic, and from already known families and genera. However, we also find that two-thirds of amphipod species remain to be described, double the proportion of most other taxa, whether marine or terrestrial.
Supplemental Information
The correlation is less when excluding Dybowsky who published 97 species/year is excluded (r2 = 0.0003), for all authors.
The lines are five-year moving averages.
Acknowledgments
We thank Joko Pamungkas, Junita Duwi Purwandari, Danny McDougall, Chhaya Chaudhary, Irawan Asaad, Han-Yang Lin, Dinusha Jayathilake and Qianshuo Zhao for helpful discussion and tips regarding analyses.
Funding Statement
This work was supported by the New Zealand ASEAN Scholarships (NZAS) for the scholarship awarded to Tri Arfianti to study for a Ph.D. at the University of Auckland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Mark J. Costello is an Academic Editor for PeerJ.
Author Contributions
Tri Arfianti and Simon Wilson conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Mark John Costello conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Arfianti, Tri; Wilson, Simon; Costello, Mark (2018): WoRMS_Amphipoda_RawData.xlsx. figshare. Dataset. https://doi.org/10.17608/k6.auckland.6213965.v1.
References
- Appeltans et al. (2012).Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Błażewicz-Paszkowycz M. The magnitude of global marine species diversity. Current Biology. 2012;2223:2189–2202. doi: 10.1016/j.cub.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Barnard (1969).Barnard JL. The families and genera of marine gammaridean Amphipoda. Smithsonian Institution Press; Washington D.C.: 1969. [Google Scholar]
- Bousfield (1987).Bousfield EL. Amphipod parasites of fishes of Canada. Canadian Bulletin of Fisheries and Aquatic Sciences. 1987;217:1–37. [Google Scholar]
- Brusca (1967).Brusca GJ. The ecology of pelagic Amphipoda I species accounts vertical zonation and migration of Amphipoda from the waters off southern California. Pacific Science. 1967;21:382–393. [Google Scholar]
- Brusca (1973).Brusca GJ. Pelagic Amphipoda from the waters near Oahu Hawaii excluding the family Scinidae. Pacific Science. 1973;27:8–27. [Google Scholar]
- Chevreux (1919–1920).Chevreux E. Note préliminaire sur les amphipodes recueillis par les expéditions du Travailleur et du Talisman 1880–1883. Bulletin du Muséum national d’Histoire naturelle Paris. 1919–1920;257:574–580. [Google Scholar]
- Claus (1879).Claus C. Die Gattungen und Arten der Platysceliden in Systematischer Übersicht. Arbeiten aus dem Zoologischen Institut der Universität zu Wien. 1879;2:147–198. [Google Scholar]
- Coleman (2003).Coleman CO. “Digital inking”: how to make perfect line drawings on computers. Organisms Diversity & Evolution. 2003;34:303–304. doi: 10.1078/1439-6092-00081. [DOI] [Google Scholar]
- Coleman (2006).Coleman CO. Substituting time-consuming pencil drawings in arthropod taxonomy using stacks of digital photographs. Zootaxa. 2006;13601:61–68. [Google Scholar]
- Coleman (2009).Coleman CO. Drawing setae the digital way. Mitteilungen aus dem Museum für Naturkunde in Berlin Zoologische Reihe. 2009;2:305–310. doi: 10.1002/zoos.200900008. [DOI] [Google Scholar]
- Coleman (2015).Coleman CO. Taxonomy in times of the taxonomic impediment—examples from the community of experts on amphipod crustaceans. Journal of Crustacean Biology. 2015;356:729–740. doi: 10.1163/1937240X-00002381. [DOI] [Google Scholar]
- Coleman, Lowry & Macfarlane (2010).Coleman CO, Lowry JK, Macfarlane T. DELTA for beginners. An introduction into the taxonomy software package DELTA. ZooKeys. 2010;45:1–75. doi: 10.3897/zookeys.45.263. [DOI] [Google Scholar]
- Costello (2016).Costello MJ. Parasite rates of discovery, global species richness and host specificity. Integrative and Comparative Biology. 2016;56(4):588–599. doi: 10.1093/icb/icw084. [DOI] [PubMed] [Google Scholar]
- Costello & Chaudhary (2017).Costello MJ, Chaudhary C. Marine biodiversity biogeography deep-sea gradients and conservation. Current Biology. 2017;2711:R511–R527. doi: 10.1016/j.cub.2017.04.060. [DOI] [PubMed] [Google Scholar]
- Costello, Houlding & Wilson (2014).Costello MJ, Houlding B, Wilson S. As in other taxa relatively fewer beetles are being described by an increasing number of authors: response to Löbl and Leschen. Systematic Entomology. 2014;393:395–399. doi: 10.1111/syen.12068. [DOI] [Google Scholar]
- Costello et al. (2015).Costello MJ, Lane M, Wilson S, Houlding B. Factors influencing when species are first named and estimating global species richness. Global Ecology and Conservation. 2015;4:243–254. doi: 10.1016/j.gecco.2015.07.001. [DOI] [Google Scholar]
- Costello, May & Stork (2013).Costello MJ, May RM, Stork NE. Can we name earth’s species before they go extinct? Science. 2013;339:413–416. doi: 10.1126/science.1230318. [DOI] [PubMed] [Google Scholar]
- Costello & Wilson (2011).Costello MJ, Wilson SP. Predicting the number of known and unknown species in European seas using rates of description. Global Ecology and Biogeography. 2011;202:319–330. doi: 10.1111/j.1466-8238.2010.00603.x. [DOI] [Google Scholar]
- Costello, Wilson & Houlding (2012).Costello MJ, Wilson S, Houlding B. Predicting total global species richness using rates of species description and estimates of taxonomic effort. Systematic Biology. 2012;615:871–883. doi: 10.1093/sysbio/syr080. [DOI] [PubMed] [Google Scholar]
- Edie, Smits & Jablonski (2017).Edie SM, Smits PD, Jablonski D. Probabilistic models of species discovery and biodiversity comparisons. Proceedings of the National Academy of Sciences of the United States of America. 2017;11414:3666–3671. doi: 10.1073/pnas.1616355114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschmeyer et al. (2010).Eschmeyer WN, Fricke R, Fong JD, Polack DA. Marine fish diversity: history of knowledge and discovery Pisces. Zootaxa. 2010;25251:19–50. [Google Scholar]
- Essl et al. (2013).Essl F, Rabitsch W, Dullinger S, Moser D, Milasowszky N. How well do we know species richness in a well-known continent? Temporal patterns of endemic and widespread species descriptions in the European fauna. Global Ecology and Biogeography. 2013;221:29–39. doi: 10.1111/j.1466-8238.2012.00787.x. [DOI] [Google Scholar]
- Frank & Curtis (1979).Frank JH, Curtis GA. Trend lines and the number of species of Staphylinidae. The Coleopterists’ Bulletin. 1979;33:133–149. [Google Scholar]
- Horton et al. (2016).Horton T, Lowry J, De Broyer C, Bellan-Santini D, Coleman CO, Corbari L, Daneliya M, Dauvin JC, Fišer C, Gasca R, Grabowski M, Guerra-García JM, Hendrycks E, Hughes L, Jaume D, Jazdzewski K, Kim YH, King R, Krapp-Schickel T, LeCroy S, Lörz AN, Mamos T, Senna AR, Serejo C, Sket B, Souza-Filho JF, Tandberg AH, Thomas J, Thurston M, Vader W, Väinölä R, Vonk R, White K, Zeidler W. World Amphipoda database. Amphipoda. 2016. http://www.marinespecies.org/aphia.php?p=taxdetails&id=1135. [03 November 2016]. http://www.marinespecies.org/aphia.php?p=taxdetails&id=1135
- Irfanullah (2013).Irfanullah HM. Plant taxonomic research in Bangladesh 1972–2012: a critical review. Bangladesh Journal of Plant Taxonomy. 2013;20(2):267–279. doi: 10.3329/bjpt.v20i2.17404. [DOI] [Google Scholar]
- Joppa, Roberts & Pimm (2011).Joppa LN, Roberts DL, Pimm SL. How many species of flowering plants are there? Proceedings of the Royal Society of London B: Biological Sciences. 2011;278(1705):554–559. doi: 10.1098/rspb.2010.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just (2012).Just J. Siphonoecetini Just 1983 Crustacea Amphipoda Ischyroceridae 10: Belkginoecetes gen nov Tropicoecetes gen nov and Rhinoecetes Just 1983 from north-eastern and northern Australian shallow water with eight new species. Zootaxa. 2012;35281:1–28. [Google Scholar]
- Lowry & Myers (2009).Lowry JK, Myers AA. Benthic Amphipoda crustacea: peracarida of the great barrier reef. Zootaxa. 2009;2260(16):1–930. [Google Scholar]
- Lowry & Stoddart (2012).Lowry JK, Stoddart HE. Australian and South African conicostomatine amphipods Amphipoda: Lysianassoidea: Lysianassidae: Conicostomatinae subfam nov. Zootaxa. 2012;32481:43–65. [Google Scholar]
- Muggeo (2003).Muggeo VM. Estimating regression models with unknown break-points. Statistics in Medicine. 2003;2219:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- Muggeo (2008).Muggeo VM. Segmented: an R package to fit regression models with broken-line relationships. R News. 2008;81:20–25. [Google Scholar]
- O’Dor et al. (2012).O’Dor R, Boustany AM, Chittenden CM, Costello MJ, Moustahfid H, Payne J, Steinke D, Stokesbury MJW, Vanden Berghe E. A census of fishes and everything they eat: how the census of marine life advanced fisheries science. Fisheries. 2012;379:398–409. [Google Scholar]
- Pillai (1957).Pillai NK. Pelagic crustacea of Travancore: 3 Amphipoda. Bulletin of the Central Research Institute Uni Travancore. 1957;5:29–68. [Google Scholar]
- Pimm et al. (2010).Pimm SL, Jenkins CN, Joppa LN, Roberts DL, Russell GJ. How many endangered species remain to be discovered in Brazil. Natureza & Conservação. 2010;81:71–77. doi: 10.4322/natcon.00801011. [DOI] [Google Scholar]
- Poulin & Presswell (2016).Poulin R, Presswell B. Taxonomic quality of species descriptions varies over time and with the number of authors but unevenly among parasitic taxa. Systematic Biology. 2016;656:1107–1116. doi: 10.1093/sysbio/syw053. [DOI] [PubMed] [Google Scholar]
- Ren (2012).Ren XQ. Fauna Sinica. Invertebrata vol. 43, Crustacea Amphipoda Gammaridea (2) Science Press; Beijing: 2012. [Google Scholar]
- Rodman & Cody (2003).Rodman JE, Cody JH. The taxonomic impediment overcome: NSF’s partnerships for enhancing expertise in taxonomy PEET as a model. Systematic Biology. 2003;523:428–435. doi: 10.1080/10635150390197055. [DOI] [PubMed] [Google Scholar]
- Sangster & Luksenburg (2015).Sangster G, Luksenburg JA. Declining rates of species described per taxonomist: slowdown of progress or a side-effect of improved quality in taxonomy? Systematic Biology. 2015;641:144–151. doi: 10.1093/sysbio/syu069. [DOI] [PubMed] [Google Scholar]
- Stoddart & Lowry (2012).Stoddart HE, Lowry JK. Revision of the lysianassoid genera Acidostoma and Shackletonia Crustacea: Amphipoda: Acidostomatidae fam nov. Zootaxa. 2012;33071:1–34. [Google Scholar]
- Streets (1878).Streets TH. Pelagic Amphipoda. Proceedings of the Academy of Natural Sciences of Philadelphia. 1878;30:276–290. [Google Scholar]
- Vinogradov (1988).Vinogradov GM. Life forms of pelagic amphipods. Zoologichesky Zhurnal. 1988;6712:1765–1775. [Google Scholar]
- Vinogradov (1990).Vinogradov GM. Pelagic amphipods Amphipoda Crustacea from the south-eastern Pacific. Akademija Nauk SSSR Trudy Instituta Okeanologii. 1990;124:27–104. [Google Scholar]
- Wheeler (2004).Wheeler QD. Taxonomic triage and the poverty of phylogeny. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2004;359:571–583. doi: 10.1098/rstb.2003.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler et al. (2012).Wheeler QD, Knapp S, Stevenson DW, Stevenson J, Blum SD, Boom BM, Donoghue MJ. Mapping the biosphere: exploring species to understand the origin organization and sustainability of biodiversity. Systematics and Biodiversity. 2012;101:1–20. doi: 10.1080/14772000.2012.665095. [DOI] [Google Scholar]
- White & Reimer (2012a).White KN, Reimer JD. Commensal Leucothoidae Crustacea Amphipoda of the Ryukyu Archipelago Japan Part I: ascidian-dwellers. ZooKeys. 2012a;163:13–55. doi: 10.3897/zookeys.163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White & Reimer (2012b).White KN, Reimer JD. Commensal Leucothoidae Crustacea Amphipoda of the Ryukyu Archipelago Japan Part II: sponge-dwellers. ZooKeys. 2012b;166:1–58. doi: 10.3897/zookeys.166.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White & Reimer (2012c).White KN, Reimer JD. Commensal Leucothoidae Crustacea Amphipoda of the Ryukyu Archipelago Japan Part III: coral rubble-dwellers. ZooKeys. 2012c;173:11–50. doi: 10.3897/zookeys.173.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson (2004).Wilson EO. Taxonomy as a fundamental discipline. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2004;359(1444):739–739. doi: 10.1098/rstb.2003.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson & Costello (2005).Wilson SP, Costello MJ. Predicting future discoveries of European marine species by using a non-homogeneous renewal process. Journal of the Royal Statistical Society: Series C Applied Statistics. 2005;545:897–918. doi: 10.1111/j.1467-9876.2005.00513.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The correlation is less when excluding Dybowsky who published 97 species/year is excluded (r2 = 0.0003), for all authors.
The lines are five-year moving averages.
Data Availability Statement
The following information was supplied regarding data availability:
Arfianti, Tri; Wilson, Simon; Costello, Mark (2018): WoRMS_Amphipoda_RawData.xlsx. figshare. Dataset. https://doi.org/10.17608/k6.auckland.6213965.v1.