Abstract
A treosulfan (Treo)-based conditioning regimen prior to hematopoietic stem cell transplantation (HSCT) has been successfully used in treating hematological malignant and nonmalignant diseases. We report Treo pharmacokinetics (PK) in patients with thalassemia major undergoing HSCT (n = 87), receiving Treo at a dose of 14 g/m2/day. Median Treo AUC and clearance (CL) was 1,326 mg*h/L and 10.8 L/h/m2, respectively. There was wide interindividual variability in Treo AUC and CL (64 and 68%) which was not explained by any of the variables tested. None of the Treo PK parameters were significantly associated with graft rejection or toxicity; however, Treo CL <7.97 L/h/m2 was significantly associated with poor overall (hazard ratio (HR) 2.7, confidence interval (CI) (1.09–6.76), P = 0.032) and event-free survival (HR 2.4, CI (0.98–5.73), P = 0.055). Further studies in a larger cohort are warranted to identify the factors explaining the variation in Treo PK as well as to establish a therapeutic range of Treo for targeted dose adjustment to improve HSCT outcome.
Treosulfan (Treo), originally registered as Ovastat, is being successfully used as a part of conditioning regimen prior to hematopoietic stem cell transplantation (HSCT). HSCT unanimously made its way as the only curative treatment option that is currently available for patients with thalassemia major (TM). Improving outcome in high-risk patients with significant liver dysfunctions has been a challenge. Patients above 7 years and with significantly enlarged liver have been shown to have poor outcomes due to regimen-related toxicity, especially sinusoidal obstruction syndrome (SOS) or rejection of the graft with a busulfan (BU) / cyclophosphamide (CY) conditioning regimen.1 A regimen containing Treo, fludarabine (F-araA), and thiotepa has been used since 2009 at our center (Christian Medical College, Vellore, India) for high-risk TM patients with significantly improved transplant outcomes compared to the historical cohort of patients receiving a BU/CY-based myeloablative regimen.2 However, complications such as graft vs. host disease (GVHD), SOS, and mucositis are still of major concern.
Treo is a bifunctional alkylating agent, which is structurally similar to busulfan except for the introduction of two hydroxyl moieties. Unlike BU, Treo is the prodrug that is water-soluble and nonenzymatically converts into its monoepoxide and diepoxide active derivatives, (S,S)-EBDM and (S,S)-DEB, respectively. These metabolites are known to exhibit their cytotoxicity by DNA alkylation and double-strand breakage.3 Although no known drug-metabolizing enzymes or transporters are involved in the Treo biotransformation, the active epoxide derivatives are known to be detoxified with the help of epoxyhydrolases and glutathione S-transferase (GST) enzymes.4–6
Treo in combination with thiotepa and F-araA has been widely used prior to HSCT to treat a variety of hematological malignant and nonmalignant conditions.7–13 Despite a low toxicity and high antileukemic activity exhibited by this regimen, the nonrelapse mortality (NRM) and incidence of relapse was still high (20% and 24%, respectively).12,13 At the maximum tolerated dose and above prior to HSCT, there was severe myelosuppression, grade III and IV mucositis and diarrhea,14 sinusoidal obstruction syndrome (SOS), renal toxicity, heart failure, nausea, infections, and gastrointestinal toxicity.13,15–17 However, there is no report directly comparing the Treo systemic exposure to the HSCT outcome endpoints including toxicity or graft rejection/relapse. Hence, the therapeutic range of Treo to achieve better HSCT outcome has not been established so far. This is probably due to a limited number of pharmacokinetic (PK) studies of Treo conducted in the HSCT setting with a relatively small number of patients with a difference in interindividual variability (IIV).18–22 These studies included various disease conditions, the majority being hematological malignant disorders, dose of Treo, rate of infusion, and method of Treo analysis. Hence, the goal of the present study was to describe the PK of Treo in a uniform cohort of patients with TM undergoing HSCT, evaluate the factors influencing the IIV in PK, and the role of Treo PK on HSCT outcome.
Results
Patients
Eighty-seven patients with TM (median age 9 years; range 1.5–25 years) receiving a Thiotepa/F-araA/Treo-based conditioning regimen were included in this study. All were children except for two who were 19 and 25 years. The patient demographics are listed in Table 1. There were 55 males and 32 females. The majority of the patients (n = 67; 77%) had an HLA-matched sibling donor (MSD); 11 (13%) had MRD and 9 (10%) had MUD transplants.
Table 1. Patient demographics.
| Parameters | N= 87 (%) Median (range) |
|---|---|
| Age, yrs | 9.0 (1.5–25) |
| Body weight, kg | 23.2 (11.4–55.0) |
| BSA, m2 | 0.93 (0.48–1.56) |
| Liver size, cm | 4.0 (0–14) |
| Ferritin, ng | 3047 (536–13084) |
| Sex | |
| Male | N = 55 (63%) |
| Female | N = 32 (37%) |
| Splenectomy | |
| Yes | N = 10 (11.5%) |
| No | N = 77 (88.5%) |
| Liver fibrosis | |
| Yes | N = 81 (93%) |
| No | N = 06 (7%) |
| Risk | |
| Class III HR | N = 42 (48.3%) |
| Class III LR | N = 32 (36.8%) |
| Class II | N = 13 (14.9%) |
| Donor source | |
| MSD | N = 67 (77%) |
| MRD | N = 11 (13%) |
| MUD | N = 9 (10%) |
Class III HR, class III high risk; LR, low risk; MSD, matched sibling donor; MRD, matched related donor; MUD, matched unrelated donor.
Treo assay validation
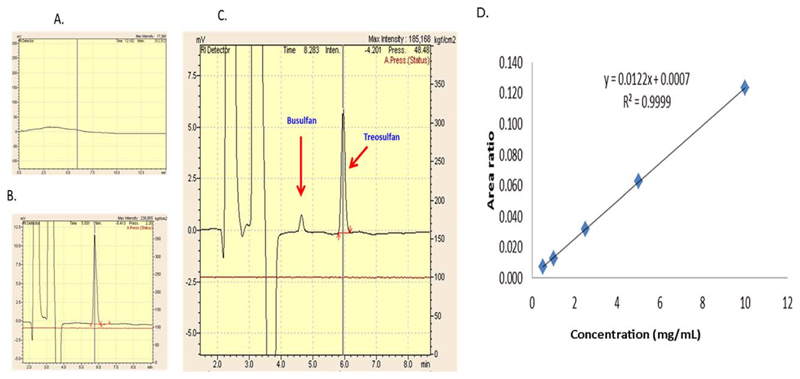
Treo assay was validated for its specificity, linearity, precision, accuracy, and recovery before it was used for measurement in patients’ plasma. There was no peak detected in unspiked blank plasma at the retention times of IS (4.6 min) and Treo (5.7 min) (Figure 1a–c). The method was linear for a concentration range of 0.5 to 10 mg/mL (Figure 1d). The interday assay precision as reported in %CV was <10% and intraday precision was <13%. The mean recovery of Treo from plasma was much higher in our assay, i.e., 63.7–81.8% as compared to a previously reported method,20 which was 40–48.1%. The lower limits of detection and quantification were 0.5 μg/mL and 1.0 μg/mL, respectively.
Figure 1. Treo assay in HPLC-coupled with RI detector.
(a) The blank plasma with no matrix effect and (b) the plasma spiked with standard Treo. (c) A patient’s sample showing Treo at 5.8 min and internal standard busulfan at 4.7 min. (d) The linearity and range from 0.5 to 10 mg/mL of Treo in human plasma and is depicted by the concentration vs. area ratio curve.
Population pharmacokinetics (PopPK) of Treo
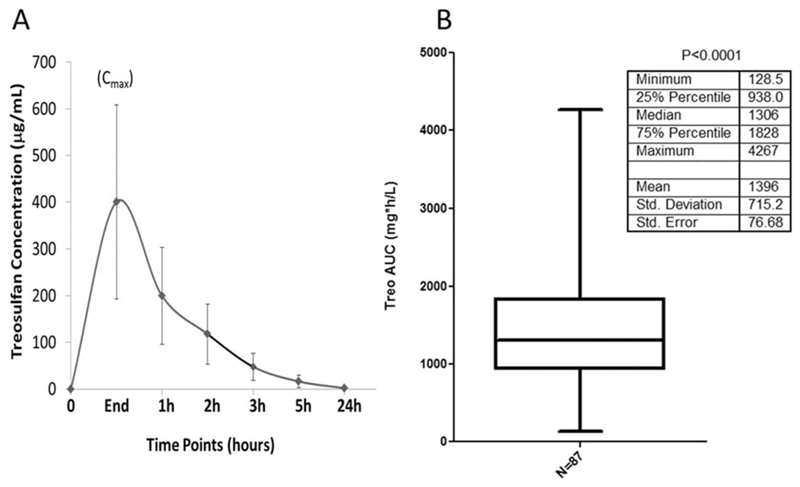
The PopPK model parameters comprising BSA normalized dose are shown in Table 2. Figure 2 shows the individual concentration timepoints for all the patients and IIV in Treo AUC. The median post-hoc estimated Treo clearance (CL) was 10.8 L/h/m2 (3.1–110.8 L/h/m2) and the median post-hoc estimated Treo AUC was 1,326 mg*h/L (126–4,484 mg*h/L). The PopPK estimated IIV in Treo Cl was 68% and post-hoc estimated IIV in AUC was 64% (CV%). None of the demographic or biochemical variables tested explained this wide IIV in Treo PK.
Table 2. Population pharmacokinetics of Treo as estimated using nonlinear mixed effects modeling.
| Parameter | Population estimate | RSE (%) |
|---|---|---|
| CL (L/h/m2) | 11.6 | 7.5 |
| V (L/m2) | 19.4 | 8.2 |
| Q (L/h/m2) | 2.14 | 17.5 |
| V2 (L/m2) | 2.01 | 29.5 |
| Residual (CV%) | 26.1 | 4.9 |
| –2 Log-likelihood | 4607.9 | |
| IIV | (CV%) | RSE (%) |
| CL | 67.8 | 8.1 |
| V | 66.5 | 9.7 |
| Q | 71.8 | 21.5 |
| V2 | 199.5 | 12.0 |
RSE, relative standard error; CL, clearance; V, volume of distribution; Q, intercompartmental clearance; CV%, coefficient of variation; IIV, interindividual variation.
Figure 2. Interindividual variation of Treo exposure in TM patients.
(a) The concentrations of all the patients at each timepoint represented as mean ± SD. (b) The distribution of AUC of all the patients. There was a significant interindividual variation (P < 0.0001) as analyzed by one-sample t-test.
Limited sampling model (LSM)
Using these data, we developed a three-point LSM including end of infusion, 2 h and 6 h postinfusion timepoints. This model was compared to the model with the full sampling timepoints (n = 7). The bias and error for CL and volume of distribution (V) were both reasonable (9% and –1%, respectively, for CL, and 10% and 8%, respectively, for V). Adding a 4th sampling timepoint 24 h after the end of the infusion did not significantly improve the bias and error of the parameter estimates.
HSCT outcome
The outcome endpoints for 87 patients are summarized in Table 3. Patients were followed up for a median of 31.77 months (0.37–55.33 months).
Table 3. HSCT outcome parameters in TM patients.
| S. No | Parameter | N (%) |
|---|---|---|
| 1 | Engraftment | |
| Yes | 82 (97.6%) | |
| No | 02 (2.4%) | |
| NE | 03 | |
| 2 | Day28 chimerism | |
| Complete | 77 (92.5%) | |
| Mixed | 06 (7.5%) | |
| NE | 07 | |
| 3 | Rejection | |
| Yes | 05 (6.2%) | |
| No | 75 (93.8%) | |
| NE | 07 | |
| 4 | Mucositis | |
| Grade 1-2 | 17 (19.5%) | |
| Grade 3-4 | 17 (19.5%) | |
| Nil | 53 (61%) | |
| 5 | Pulmonary SOS | |
| Yes | 07 (8%) | |
| No | 80 (92%) | |
| 6 | Hepatic SOS | |
| Yes | 16 (18.4%) | |
| No | 71 (81.6%) | |
| 7 | OS status | |
| Alive | 68 (78.2%) | |
| Dead | 19 (21.8%) | |
| 8 | EFS status | |
| Event | 21 (24.1%) | |
| No event | 66 (75.9%) | |
| 9 | D+100 TRM | |
| Yes | 15 (17.2%) | |
| No | 72 (82.8%) |
NE, not evaluable; NA, not available; SOS, sinusoidal obstruction syndrome; aGVHD, acute graft versus host disease; cGVHD, chronic graft versus host disease; OS, overall survival; EFS, event-free survival; D+100 TRM, day 100 transplant related mortality.
Engraftment
Out of 87 patients, three patients died early of regimen-related toxicity and other transplantation-related complications. Hence, 84 patients were evaluable for engraftment, of which, 82 (97.6%) engrafted their grafts. The median day of engraftment was 16 days (range: 11–23 days).
Chimerism analysis
Posttransplant hematopoietic chimerism was evaluated in all patients who were alive beyond day +28 (n = 80). Six patients had mixed chimerism on day +28.
Rejection
Excluding the seven patients who died before day +28 post-HSCT, 80 were evaluable for rejection analysis. Five patients rejected their graft, out of which two had a primary graft failure.
Regimen-related toxicities
The incidence of regimen-related toxicities was documented in all 87 patients. Hepatic SOS was documented in 16 patients and seven had pulmonary SOS. Mucositis was mild to moderate (grade 1–2) in 17 patients and severe in 17 (grade 3–4) patients. Fifteen patients died before day +100 (D+100 transplant-related mortality (TRM)). Overall, 68 (78%) patients were alive at the last follow-up and the event-free survival (EFS) was 76%. The major cause of death was steroid refractory GVHD, SOS, multiorgan failure, sepsis, and infections.
Treo PK association with HSCT outcome
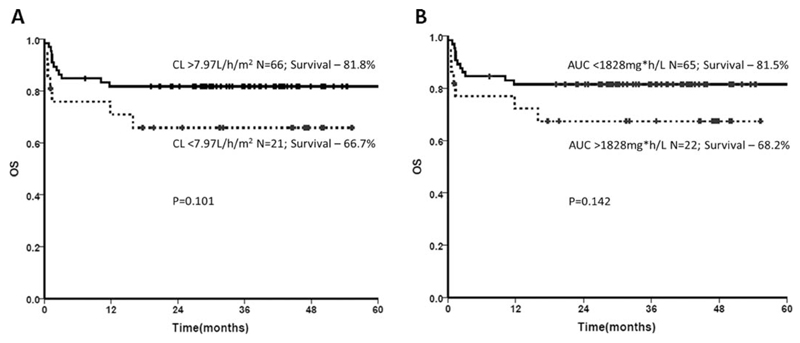
The influence of Treo PK on HSCT outcome parameters including rejection, regimen-related toxicities, OS, EFS, and TRM was evaluated. Since only two patients did not engraft, this parameter was not included for analysis. Despite a wide IIV, rejection, regimen-related toxicity, or TRM were not influenced by Treo PK (both as continuous variable and upon quartile analysis). Cox regression analysis showed a higher risk for low Treo CL (<7.97 L/h/m2) towards poor overall and EFS (hazard ratio (HR) 2.7, confidence interval (CI) (1.09–6.76), P = 0.03 & HR 2.4, CI (0.98–5.73), P = 0.055, respectively). Although not statistically significant, the Kaplan–Meier curve showed a high Treo Cl (>7.97L/h/m2) and low AUC (<1828mg*h/L) trending towards better OS (P = 0.101 and 0.142, respectively) (Figure 3).
Figure 3. Influence of Treo CL and AUC on OS.
(a) Cox-regression analysis on the Treo CL quartiles indicates patients with high clearance >7.97 L/h/m2 (75th percentile) tended to have a better OS (P = 0.101) as shown by the Kaplan–Meier curve. (b) Cox-regression analysis on the Treo AUC quartiles shows that patients with low AUC <1,828 mg*h/L (25th percentile) tended to have a better OS (P = 0.142).
Discussion
A Treo-based conditioning regimen for hematological malignant and nonmalignant diseases has proven to be beneficial in patients undergoing allogeneic HSCT,2,11,23,24 especially as an attractive alternative to a busulfan-based regimen. Although the dose-limiting toxicity and maximum tolerated dose of Treo have been documented,14,25–27 the PK and PD of Treo have not been extensively reported in the HSCT setting.
We established and validated a sensitive high-performance liquid chromatography (HPLC) Refractive Index (RI)-based method to measure the Treo concentrations in patients’ plasma. Although simple, the previously published method20 was difficult to reproduce in our system, either due to the nonavailability of the internal standard, barbital (use prohibited in India), or poor recovery of acetaminophen. Since busulfan is structurally similar to Treo, we used busulfan as an internal standard. Unlike what was reported previously,20 we found a constant, stable area and retention time for busulfan. Also, there was a 1.1 min difference in the retention time between Treo and busulfan. Since this method did not require any derivatization of busulfan to be analyzed in an RI detector (RID), it reduces the sample processing time and the total run time. This method has a 1 μg/mL quantitation limit and has a higher recovery rate (64–82%) than the previously published method20 (40.0–48.1%).
This is the first study evaluating the dose–exposure–response relationship of Treo in a large uniform cohort of patients with a nonmalignant condition such as TM receiving a thiotepa/F-araA/Treo-based conditioning regimen. Previous reports on Treo PK are in a small number of patients, and included a mixed cohort of adults and children with solid tumors, hematological malignant, and nonmalignant disorders.14,18–21,26,28,29 The dose and rate of infusion of Treo also was variable (8 g, 10 g, 12, and 14 g/m2 were administered at different rates from 30 min to 2 h infusion of entire dose). In our center, a fixed dose of Treo (14 g/m2) and a constant rate of infusion at 5 g/h is followed.
We observed 68% and 64% of IIV in Treo AUC and CL, respectively. Glowka et al.20 reported a 70% IIV in Treo exposure with three different doses (10, 12, and 14 g/m2/day) and in five children. In another study by Ten Brink et al.21 in 20 children with different indications for HSCT, the IIV was strikingly less (14.5%). Various PopPK models have been developed for drugs used in HSCT conditioning like BU,30–33 CY,34–37 F-araA,38–41 melphalan.42–45 So far, there is only one PopPK model of Treo including 12 children with a two-point LSM (4 h and 7 h postinfusion) which was validated in eight children.21 In this study we developed a three-point LSM including end of infusion, 2 h, and 6 h postinfusion. This model could best be used in the clinical setting if sample collection and analysis has to be done on the same day in the future.
The Treo PK was compared with previous studies in HSCT recipients receiving a Treo-based conditioning regimen (Table 4).). Beelen et al.26 first described the Treo PK in 18 adults (median age 44 years), undergoing a Treo/Cy-based regimen for hematological malignant diseases. The mean Treo AUC was 1,104 ± 173 mg/L*h. Glowka et al18 described the PK in seven children (median age 14 years) with various hematological malignant diseases and dose of Treo. Only one of these patients received 14 g/m2, while the others received 10 or 12 g/m2 Treo. The authors did not describe the combination of drugs used in the conditioning regimen along with Treo. However, they have demonstrated a linear increase in AUC with dose. The same group19 measured Treo and its metabolite (S,S, EBDM) in 16 children (median age 7.5 years) using an liquid chromatography / tandem mass spectrometry (LC-MS/MS)-based method. The mean AUC was 2,400 ± 1,267 mg/L*h in seven patients receiving 14 g/m2 per day for 3 days. This mean AUC was higher than what they have previously observed in one patient receiving 14 g/m2/day for 3 days (1,960 mg/L*h). However, the linear relationship of Treo dose vs. AUC was contradicted. One report8 has shown in 16 adults (median age 34 years) with AML/ALL/MDS, undergoing a Treo/F-araA-based regimen that the mean Treo AUC was not different between patients receiving either 12 or 14 g/m2 (1,365 ± 293 vs. 1,309 ± 262 mg/L*h). Another study46 in six pediatric oncology patients showed that Treo AUC was not significantly different in patients receiving either 12 or 14 g/m2 (1,486 ± 235 vs. 1,412 ± 215 mg/L*h). Of note, these authors used RP-HPLC coupled with a UV detector to measure the total Treo and its metabolites. Hence, the obtained Treo AUC is not just the parent prodrug but also the cumulative AUC of Treo and its active metabolite S,S-DEB. Previously, another group21 also described a similar RP-HPLC with a UV-based method to study the derivatized Treo in patients’ serum. The authors developed a PopPK model with 12 children (median age 6.9 years) receiving Treo along with F-araA ± ATG/Alemtuzumab/Thoracoabdominal radiation at a dose of 14 g/m2/day × 3 days. The mean AUC was 1,639 ± 237 mg/L*h.
Table 4. Comparison of Treo PK parameters with the existing literature.
| N | Median age (yrs) | Conditions | Method | Regimen | Treo dose, g/m2 | AUC, µgxh/mL (mean ± SD) | CL, L/h (mean ± SD) | Cmax, µg/mL (mean ± SD) | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 8 10 |
40 51 |
AML, ALL, CML, T-NHL, MDS | RP-HPLC + RID | Treo:D -6 to -4 Cy:D -3 to -2 |
12 × 3 14 × 3 |
898 ± 104 1104 ± 173 |
— | 260 ± 35 322 ± 47 |
— | 27 |
| 1 5 1 |
14 | AML, AML/ALL, HL, ALD, WAS, SAA | RP-HPLC + RID | Treo | 10 × 3 12 × 3 14 × 3 |
735 1309 ± 921 1960 |
23.1 13.9 ± 7.1 3.6 |
271 420 ± 250 632 |
Linear increase in AUC with dose | 16 |
| 4 12 |
34 34 |
AML, ALL, MDS | RP-HPLC + RID | Treo:D -6 to -4 F-araA:D -6 to -2 |
12 × 3 14 × 3 |
1365 ± 293 1309 ± 262 |
9.2 ± 2.1 11.1 ± 2.2 |
461 ± 102 409 ± 84 |
— | 8 |
| 12 8 |
6.9 5.4 |
Hemoglobinopathies, Hematological malignancies, Immune deficiency | RP-HPLC + UV | Treo+F-ara/ATG/Alem/TAI | 14 × 3 | 1639 ± 237 | 6.85 | — | Patients’ serum samples were used. Treo AUC is the total of Treo + metabolite. | 19 |
| 1 8 7 |
7.5 | NBL, ALL, ES, DBA, SCN, ALD, ALL, CML, AML, WAS | LC-MS/MS | Treo | 10 × 3 12 × 3 14 × 3 |
1560 1478 ± 552 2400 ± 1267 |
4.68 5.41 ± 0.60 4.07 ± 1.58 |
791 544 ± 272 857 ± 582 |
— | 17 |
| 3 3 |
1 4 |
Pediatric oncology | RP-HPLC + UV | Treo + F-araA | 12 × 3 14 × 3 |
1486 ± 235 1412 ± 215 |
2.7 ± 1.0 9.6 ± 4.3 |
560 ± 89 525 ± 88 |
AUC is the total of Treo + metabolite. No difference in AUC with increasing dose | 47 |
| 87 | 9 | Thalassemia | RP-HPLC + RID | Treo:D -6 to -4 F-araA:D -6 to -2 |
14 × 3 | 1396 ± 715 | 15.9 + 14.8 | 401 + 208 | - | Present study |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; NHL, Non-Hodgkin’s lymphoma; MDS, myelodysplastic syndrome, HL, Hodgkin’s lymphoma, ALD, adrenoleukodystrophy; WAS, Wiskott–Aldrich syndrome; SAA, severe aplastic anemia; ES, Ewing’s sarcoma; DBA, Diamond–Blackfan anemia; SCN, severe congenital neutropenia; RP-HPLC, reverse phase high performance liquid chromatography; RID-refractive index detector; UV, ultraviolet detector; LC, MS/MS-liquid chromatography tandem mass spectrometry; Treo, Treosulfan; Cy, cyclophosphamide; F-araA, fludarabine; AUC, area under the time vs. plasma concentration curve; CL, clearance; Cmax, maximum concentration.
The present study is the largest (n = 87) describing Treo PK in a uniform cohort of TM patients and a fixed dose of 14 g/m2. The mean AUCs were comparable to all studies except in one,26 where it is very low when compared to the present study and two others21,46 that measured the total Treo + metabolites. However, almost 1/4th of our patients (n = 20) are in the lowest quartile of Treo AUC, which is below the range described previously. This difference could be due to the small number of patients of varying underlying conditions included in the previous studies. Also, since the Treo dose is given based on body surface area (BSA), the adult patients receiving 14 g/m2/day will actually receive a cumulative Treo dose higher than those received by a child with the same 14 g/m2/day dose. Wide IIV in Treo PK was reported in various studies but none of these have described a possible reason for this variation. The PopPK model described in this study also fails to explain this variation.
Apart from the known factors influencing the HSCT outcome, we evaluated if Treo PK plays a role in the outcome. Since only two patients did not engraft and five rejected their grafts, we did not analyze these endpoints with Treo PK. Although 39% of patients had mucositis and at least 18% developed SOS, Treo PK was not significantly different between those who had toxicity and those who did not. Treo PK did not influence the early TRM or EFS either. In a post-hoc analysis, it was striking that lower Treo CL <7.97 L/h/m2 was significantly associated with poor overall and EFS (P = 0.032 and P = 0.055, respectively). We are continuing this study with increased sample size as a validation cohort to arrive at a therapeutic range for Treo to improve HSCT outcome, since the incidence of toxicities or rejection was lower in this cohort.
In summary, a wide IIV exists in Treo PK and the factors responsible for this variation still remain unknown. Our study suggests that lower Treo exposure is probably beneficial over a high exposure without the adverse effects of rejection or toxicity. Also, the plasma levels of Treo achieved at 12 g/m2 are comparable to 14 g/m2, as shown previously.8 Hence, instead of a maximum tolerated dose of Treo, a minimal beneficial dose should be identified in the HSCT setting with personalized dose–exposure monitoring which might reduce the cost of the procedure while improving the outcome. Also, the LSM we developed in this study is important, as it will allow us/others to efficiently conduct larger studies to better address questions related to PK variability and exposure outcome.
Methods
Patients
All patients diagnosed with TM (class III high risk and class II patients) undergoing HSCT with a thiotepa/F-araA/Treo-based conditioning regimen as previously reported7,10 between January 2012 and 2015 in the Department of Hematology, Christian Medical College, Vellore, India, were included in this analysis. Written informed consent was obtained from the patients/parents. This study was approved by the Institutional Review Board. Patients receiving any other combinational conditioning regimen, other comorbidity, or who did not consent to participate were excluded from the study.
Conditioning regimen
All patients received F-araA 40 mg/m2/day × 4 days over a 1-h infusion from day −5 to day −2 and Treo 14 g/m2/day × 3 days at the rate of 5 g/h from day −5 to day −3 prior to HSCT. Thiotepa at a dose of 8 mg/kg/day was administered on day −6. Cyclosporine (2.5 mg/kg/dose, b.d.) and methotrexate was given as GVHD prophylaxis. Lucarelli class III patients were further risk stratified based on the Vellore classification.47 HLA identical/matched sibling/related donors (MSD/MRD) or HLA-matched unrelated donors (MUD) were used for transplantation with GSF mobilized peripheral blood stem cells (PBSC) as the source (Table 1).
Reagents and chemicals
Standard Treo for the PK analysis was a kind gift from Medac (Hamburg, Germany). Busulfan, K2HPO4.3H20, K2EDTA.2H2O were purchased from Sigma Aldrich (St. Louis, MO); acetonitrile was purchased from Merck (Mumbai, India) and citric acid from Amersham Life Sciences (Cleveland, OH). Amicon Ultra 0.5 mL centrifuge filters (10 kDa cutoff) were purchased from Merck Millipore (Ireland). Standards for the Treo assay were prepared in drug-free blank plasma (obtained from the Christian Medical College hospital blood bank).
Sample collection and processing for pharmacokinetic analysis
Peripheral blood (5 mL) was collected in ice-cold sodium heparin tubes before the start (0 h), end of infusion, 1, 2, 3, 5, 7, and 24 h after the end of Treo infusion. The blood was immediately adjusted to a pH of 5.5 by adding 50 μL of 1M citric acid/mL of blood. This was done to avoid the artificial ex vivo degradation of Treo. The sample was centrifuged at 3,500 rpm for 10 min to obtain plasma and stored at −80°C until further analysis.
Measurement of plasma Treo in HPLC-RID
Measurement of plasma Treo levels were carried out as per the method reported previously,20 with some modifications. A Shimadzu LC-20AD UFLC coupled with a Refractive Index detector (RID-10AD) system controller with an autosampler (SIL 20AC HT) and column oven (CTO-20AC) (Shimadzu, Kyoto, Japan) was used. The modified method was validated in-house for accuracy, inter- and intraday precision, linearity and range, limit of detection, and quantification (LOD, LOQ) and recovery (Supplemental Methods). Busulfan was used as the internal standard. Phosphate buffer and acetonitrile were used as mobile phase and the separation was performed on a Synchronis C18 column (250 mm × 5 μ × 4.6 μ ID, ThermoFisher Scientific, Waltham, MA). Mobile phase, standard, and internal standard were prepared fresh before use (Supplemental Methods) for preparing the standard curve. Briefly, increasing concentrations of Treo and 15 μL of 1 mg/mL BU was spiked in drug-free plasma and mixed thoroughly. The contents were transferred to Amicon ultrafiltration tubes and centrifuged at 14,000g × 15 min. The clear filtrate (50 μL) was injected onto the column and maintained at 30°C through the autosampler. Treo was detected using the RID managed by LC solution software (Shimadzu). The patients’ plasma was also processed similarly and deionized water was spiked instead of standard Treo. The concentration is expressed in mg/L.
Population pharmacokinetics of Treo
A PopPK model was developed using nonlinear mixed effects modeling with Monolix (v. 4.3.3) using the Stochastic Approximation Expectation-Maximization (SAEM) method. A two-compartment PK model was used to describe the data. The PK parameters estimated clearance (CL: L/hr/m2), volume of distribution (V: L/m2), intercompartmental clearance (Q: L/hr/m2), and peripheral compartment volume (V2: L/m2). In addition, the individual post-hoc parameter values were used to estimate the area under the concentration curve (AUC mg*hr/L). The interindividual and interday variability of the parameters was assumed to be log-normally distributed. A proportional residual error model was used with assumed normal distribution of the residuals.
The relationships between the PK parameters and covariates were described using the following model: 𝚯Base*exp(β*covariate). A covariate was considered significant in the univariate analysis if the addition of the covariate to the model reduced the objective function value (OFV) at least 3.84 units (P < 0.05, based on the χ2 test for the difference in the −2 log-likelihood between two hierarchical models that differ by 1 degree of freedom). The covariates considered in this analysis were demographics (age, body weight, BSA, sex, liver size, liver fibrosis) and biochemical parameters (ferritin levels, liver enzymes, hemoglobin).
Limited sampling model
An LSM for Treo PK was developed with these data to reduce sample collection timepoints for future studies. Specifically, we generated datasets from our original population with subsets of three timepoints per individual chosen from the original times (end of infusion and 1, 2, 3, 5, 7, and 24 h after the infusion). We then estimated the individual post-hoc PK for each of the three timepoints per individual LSMs and compared the results to the individual post-hoc PK estimated using all seven timepoints per individual. The LSMs were ranked by their bias and error where bias was defined as: and error was defined as: where 𝚯i,full are the individual PK parameter estimates using all seven sample times and 𝚯i,LSM are the individual PK parameter estimates using the three-timepoint LSM.
Chimerism analysis
Whole-blood chimerism was evaluated by polymerase chain reaction (PCR) amplification of the short or variable number tandem repeats (STR/VNTR) markers followed by capillary electrophoresis (Genetic Analyzer ABI3130) as reported previously.48
HSCT outcome
The influence of Treo PK parameters (CL or AUC), biochemical, and demographic factors on HSCT outcome including rejection, regimen-related toxicity, TRM, overall and EFS were analyzed. Neutrophil engraftment and day +28 chimerism post-HSCT was documented as a part of the standard of care for these patients. Neutrophil engraftment was defined as the ANC ≥0.5 × 109/L on 3 consecutive days; complete chimerism was defined as ≥95% of donor pattern in the patient’s peripheral blood. Overall survival (OS) was defined as the percentage of patients who were alive at last follow-up and EFS was defined as the percentage of patients who were alive without rejection or graft failure at last follow-up. Early deaths before day +21 were excluded for engraftment analysis and patients who died before day +28 were excluded for rejection analysis. Deaths due to any cause directly or indirectly related to HSCT were included in the OS analysis.
Statistical analysis
IBM SPSS statistics 21.0 (Armonk, NY) and GraphPad PRISM5 software (San Diego, CA) were used for statistical analysis. Fisher’s exact test and Pearson’s chi-square test were used for studying the individual variables influencing HSCT outcome association. Age, body weight, BSA, liver size, ferritin levels, liver enzymes, Treo CL, and AUC were treated as continuous variables for the preliminary analysis. Age, sex, liver fibrosis, donor type, Treo CL, and AUC were taken as categorical and checked for the influence on outcome. We also did a quartile analysis on Treo CL and AUC on all the outcome parameters. Relative risk of variables on the HSCT outcome was performed by logistic regression. Log rank Cox regression was used for the survival analysis and the Kaplan–Meier curves were generated for OS and EFS. P < 0.05 was considered statistically significant.
Supplementary Material
Study Highlights.
What is the Current Knowledge on the Topic?
☑ There are limited reports on Treo pharmacokinetics in hematological disorders, especially in a nonmalignant condition like thalassemia major (TM). With the increasing use of treosulfan (Treo) in hematopoietic stem cell transplantation (HSCT) conditioning, it is mandatory to understand its kinetics and dynamics in the HSCT setting. Previously reported pilot studies established methods to measure Treo and was applied to very small number of patients with a range of hematological diseases and Treo dose.
What Question did this Study Address?
☑ The present study is the largest describing the population pharmacokinetics of Treo in a uniform cohort of patients receiving Treo at 14 g/m2/day × 3 days. Treo PK showed wide interindividual variability; however, none of the factors tested explained this variation.
What this Study adds to our Knowledge
☑ Treo CL was significantly associated with overall survival (OS) and event-free survival (EFS).
How this Might Change Clinical Pharmacology or Translational Science
☑ Further studies are warranted to establish a therapeutic window of Treo enabling targeted dose adjustment to improve HSCT outcome.
Acknowledgments
P.B. is supported by a senior fellowship program of Wellcome DBT India Alliance (IA/S/15/1/501842). This study is supported by grants from Department of Biotechnology, India BT/PR1387/MED/12/515/2011 and Wellcome DBT India Alliance (IA/S/15/1/501842) to P.B. We thank all the medical and nursing staff who managed the transplant patients. Technical assistance provided by Mr. Rajesh and Ms. Amirthavani is gratefully acknowledged.
Footnotes
Anu Korula: http://orcid.org/0000-0003-3207-3370
Conflict of Interest
The authors declare no competing interests for this work.
Author Contributions
E.M., P.B., J.C.P., V.M., and A.S. wrote the article; P.B. designed the research; E.M., P.B., J.C.P., E.S.E., A.K., F.N., A.A., A.V., B.G., V.M., and A.S. performed the research; E.M., P.B., and K.M.L. analyzed the data.
References
- 1.Sabloff M, et al. HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood. 2011;117:1745–1750. doi: 10.1182/blood-2010-09-306829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews V, et al. Improved clinical outcomes of high risk β thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS One. 2013;8:e61637. doi: 10.1371/journal.pone.0061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feit PW, Rastrup-Andersen N, Matagne R. Studies on epoxide formation from (2S,3S)-threitol 1,4-bismethanesulfonate. The preparation and biological activity of (2S,3S)-1,2-epoxy-3,4-butanediol 4-methanesulfonate. J Med Chem. 1970;13:1173–1175. doi: 10.1021/jm00300a034. [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum LS, Keller DA. Metabolism and pharmacokinetics of 1,3-butadiene. Toxicology. 1996;113:14–16. [Google Scholar]
- 5.Bond JA, Himmelstein MW, Seaton M, Boogaard P, Medinsky MA. Metabolism of butadiene by mice, rats, and humans: a comparison of physiologically based toxicokinetic model predictions and experimental data. Toxicology. 1996;113:48–54. doi: 10.1016/0300-483x(96)03426-9. [DOI] [PubMed] [Google Scholar]
- 6.Jackson MA, Stack HF, Rice JM, Waters MD. A review of the genetic and related effects of 1,3-butadiene in rodents and humans. Mutat Res. 2000;463:181–213. doi: 10.1016/s1383-5742(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo ME, et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. Br J Haematol. 2008;143:548–551. doi: 10.1111/j.1365-2141.2008.07385.x. [DOI] [PubMed] [Google Scholar]
- 8.Nemecek ER, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:341–350. doi: 10.1016/j.bbmt.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casper J, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28:3344–3351. doi: 10.1200/JCO.2009.23.3429. [DOI] [PubMed] [Google Scholar]
- 10.Bernardo ME, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120:473–476. doi: 10.1182/blood-2012-04-423822. [DOI] [PubMed] [Google Scholar]
- 11.Burroughs LM, et al. Treosulfan-based conditioning and hematopoietic cell transplantation for nonmalignant diseases: a prospective multicenter trial. Biol Blood Marrow Transplant. 2014;20:1996–2003. doi: 10.1016/j.bbmt.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yerushalmi R, et al. Fludarabine and treosulfan compared with other reduced-intensity conditioning regimens for allogeneic stem cell transplantation in patients with lymphoid malignancies. Bone Marrow Transplant. 2015;50:1526–1535. doi: 10.1038/bmt.2015.174. [DOI] [PubMed] [Google Scholar]
- 13.Ruutu T, et al. Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica. 2011;96:1344–1350. doi: 10.3324/haematol.2011.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheulen ME, et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res. 2000;6:4209–4216. [PubMed] [Google Scholar]
- 15.Shimoni A, et al. Fludarabine and treosulfan: a novel modified myeloablative regimen for allogeneic hematopoietic stem-cell transplantation with effective antileukemia activity in patients with acute myeloid leukemia and myelodysplastic syndromes. Leuk Lymphoma. 2007;48:2352–2359. doi: 10.1080/10428190701671051. [DOI] [PubMed] [Google Scholar]
- 16.Greystoke B, et al. Treosulfan-containing regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. Br J Haematol. 2008;142:257–262. doi: 10.1111/j.1365-2141.2008.07064.x. [DOI] [PubMed] [Google Scholar]
- 17.Baronciani D, et al. Treosulfan/fludarabine as an allogeneic hematopoietic stem cell transplant conditioning regimen for high-risk patients. Am J Hematol. 2008;83:717–720. doi: 10.1002/ajh.21240. [DOI] [PubMed] [Google Scholar]
- 18.Glówka FK, Karazniewicz-lada M, Grund G, Wróbel T, Wachowiak J. Pharmacokinetics of high-dose i.v. treosulfan in children undergoing treosulfan-based preparative regimen for allogeneic haematopoietic SCT. Bone Marrow Transplant. 2008;42:S67–S70. doi: 10.1038/bmt.2008.287. [DOI] [PubMed] [Google Scholar]
- 19.Glówka F, et al. Pharmacokinetics of treosulfan and its active monoepoxide in pediatric patients after intravenous infusion of high-dose treosulfan prior to HSCT. Eur J Pharm Sci. 2015;68:87–93. doi: 10.1016/j.ejps.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Glówka FK, Lada MK, Grund G, Wachowiak J. Determination of treosulfan in plasma and urine by HPLC with refractometric detection; pharmacokinetic studies in children undergoing myeloablative treatment prior to haematopoietic stem cell transplantation. J Chromatogr B Anal Technol Biomed Life Sci. 2007;850:569–574. doi: 10.1016/j.jchromb.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Ten Brink MH, et al. Pharmacokinetics of treosulfan in pediatric patients undergoing hematopoietic stem cell transplantation. Ther Drug Monit. 2014;36:465–472. doi: 10.1097/FTD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 22.Slatter MA, et al. Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood. 2011;117:4367–4375. doi: 10.1182/blood-2010-10-312082. [DOI] [PubMed] [Google Scholar]
- 23.Gyurkocza B, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakellari I, et al. Survival advantage and comparable toxicity in reduced-toxicity treosulfan-based versus reduced-intensity busulfan-based conditioning regimen in myelodysplastic syndrome and acute myeloid leukemia patients after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:445–451. doi: 10.1016/j.bbmt.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Koenigsmann M, et al. High-dose treosulfan in patients with relapsed or refractory high-grade lymphoma receiving tandem autologous blood stem cell transplantation. Bone Marrow Transplant. 2004;34:477–483. doi: 10.1038/sj.bmt.1704626. [DOI] [PubMed] [Google Scholar]
- 26.Beelen DW, et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant. 2005;35:233–241. doi: 10.1038/sj.bmt.1704784. [DOI] [PubMed] [Google Scholar]
- 27.Sender V, et al. Preclinical analysis of treosulfan in combination with total body irradiation as conditioning regimen prior to bone marrow transplantation in rats. Immunopharmacol Immunotoxicol. 2009;31:595–600. doi: 10.3109/08923970902865683. [DOI] [PubMed] [Google Scholar]
- 28.Hilger RA, et al. Pharmacokinetics of treosulfan in a myeloablative combination with cyclophosphamide prior to allogeneic hematopoietic stem cell transplantation. Int J Clin Pharmacol Ther. 2004;42:654–655. doi: 10.5414/cpp42654. [DOI] [PubMed] [Google Scholar]
- 29.Glówka FK, Romañski M, Tezyk A, Zaba C, Wróbel T. HPLC method for determination of biologically active epoxy-transformers of treosulfan in human plasma: pharmacokinetic application. J Pharm Biomed Anal. 2012;62:105–113. doi: 10.1016/j.jpba.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Salinger DH, et al. Development of a population pharmacokinetics-based sampling schedule to target daily intravenous busulfan for outpatient clinic administration. J Clin Pharmacol. 2010;50:1292–1300. doi: 10.1177/0091270009357430. [DOI] [PubMed] [Google Scholar]
- 31.Gaziev JC, et al. Novel pharmacokinetic behavior of intravenous busulfan in children with thalassemia undergoing hematopoietic stem cell transplantation: a prospective evaluation of pharmacokinetic and pharmacodynamic profile with therapeutic drug monitoring. Blood. 2010;115:4597–4604. doi: 10.1182/blood-2010-01-265405. [DOI] [PubMed] [Google Scholar]
- 32.Paci A, et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children: the results of a population pharmacokinetic study from a large pediatric cohort undergoing hematopoietic stem-cell transplantation. Ther Drug Monit. 2012;34:198–208. doi: 10.1097/FTD.0b013e31824c2f60. [DOI] [PubMed] [Google Scholar]
- 33.Long-Boyle JR, et al. Population pharmacokinetics of busulfan in pediatric and young adult patients undergoing hematopoietic cell transplant: a model-based dosing algorithm for personalized therapy and implementation into routine clinical use. Ther Drug Monit. 2015;37:236–245. doi: 10.1097/FTD.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Jonge ME, Huitema ADR, van Dam SM, Rodenhuis S, Beijnen JH. Population pharmacokinetics of cyclophosphamide and its metabolites 4-hydroxycyclophosphamide, 2-dechloroethylcyclophosphamide, and phosphoramide mustard in a high-dose combination with thiotepa and carboplatin. Ther Drug Monit. 2005;27:756–765. doi: 10.1097/01.ftd.0000177224.19294.92. [DOI] [PubMed] [Google Scholar]
- 35.Balasubramanian P, et al. Population pharmacokinetics of cyclophosphamide in patients with thalassemia major undergoing HSCT. Bone Marrow Transplant. 2012;47:1178–1185. doi: 10.1038/bmt.2011.254. [DOI] [PubMed] [Google Scholar]
- 36.Kim I-W, et al. Population pharmacokinetics analysis of cyclophosphamide with genetic effects in patients undergoing hematopoietic stem cell transplantation. Eur J Clin Pharmacol. 2013;69:1543–1551. doi: 10.1007/s00228-013-1507-7. [DOI] [PubMed] [Google Scholar]
- 37.Salinger DH, et al. Real-time dose adjustment of cyclophosphamide in a preparative regimen for hematopoietic cell transplant: a Bayesian pharmacokinetic approach. Clin Cancer Res. 2006;12:4888–4898. doi: 10.1158/1078-0432.CCR-05-2079. [DOI] [PubMed] [Google Scholar]
- 38.Salinger DH, et al. A limited sampling schedule to estimate individual pharmacokinetic parameters of fludarabine in hematopoietic cell transplant patients. Clin Cancer Res. 2009;15:5280–5287. doi: 10.1158/1078-0432.CCR-09-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCune JS, et al. Association of fludarabine pharmacokinetic/dynamic biomarkers with donor chimerism in nonmyeloablative HCT recipients. Cancer Chemother Pharmacol. 2015;76:85–96. doi: 10.1007/s00280-015-2768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCune JS, et al. Population pharmacokinetic/dynamic model of lymphosuppression after fludarabine administration. Cancer Chemother Pharmacol. 2015;75:67–75. doi: 10.1007/s00280-014-2618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanghavi K, et al. Personalized fludarabine dosing to reduce nonrelapse mortality in hematopoietic stem-cell transplant recipients receiving reduced intensity conditioning. Transl Res. 2016;175:103–115. doi: 10.1016/j.trsl.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kühne A, et al. Population pharmacokinetics of melphalan and glutathione S-transferase polymorphisms in relation to side effects. Clin Pharmacol Ther. 2008;83:749–757. doi: 10.1038/sj.clpt.6100336. [DOI] [PubMed] [Google Scholar]
- 43.Mougenot P, et al. Population pharmacokinetics of melphalan, infused over a 24-hour period, in patients with advanced malignancies. Cancer Chemother Pharmacol. 2004;53:503–512. doi: 10.1007/s00280-003-0761-2. [DOI] [PubMed] [Google Scholar]
- 44.Nath CE, Shaw PJ, Montgomery K, Earl JW. Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients. Br J Clin Pharmacol. 2007;64:151–164. doi: 10.1111/j.1365-2125.2007.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nath CE, et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol. 2010;69:484–497. doi: 10.1111/j.1365-2125.2010.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koyyalamudi SR, et al. Development and validation of a high pressure liquid chromatography-UV method for the determination of treosulfan and its epoxy metabolites in human plasma and its application in pharmacokinetic studies. J Chromatogr Sci. 2016;54:326–333. doi: 10.1093/chromsci/bmv145. [DOI] [PubMed] [Google Scholar]
- 47.Mathews V, et al. A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with β thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:889–894. doi: 10.1016/j.bbmt.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Sellathamby S, et al. Developing an algorithm of informative markers for evaluation of chimerism after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2006;37:751–755. doi: 10.1038/sj.bmt.1705317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.