Abstract
Background:
The structured breast implant uses different technology than saline or silicone gel implants, making it a third type of implant. The U.S. Food and Drug Administration and Health Canada granted approval in November of 2014. This implant is filled with saline but has an internal structure consisting of a series of nested shells that support the upper pole when upright and control fluid movement. It combines certain key features and benefits of saline and silicone gel implants. As with saline, the filler is only saline, which women like for peace of mind in case of rupture/deflation. As with silicone gel, it has a natural feel, but without the risk of silent rupture and U.S. Food and Drug Administration–recommended magnetic resonance imaging scans—women can simply look in the mirror and know their implants are intact.
Methods:
This U.S. trial enrolled 502 women: 399 primary augmentations and 103 replacements of existing augmentation implants. Investigators were 45 American Board of Plastic Surgery–certified plastic surgeons at 35 sites. Of the 502 women enrolled, 438 (87.3 percent) completed 6-year follow-up visits, a higher percentage than other Core breast implant trials.
Results:
At 6 years, patient satisfaction was 89.7 percent for primary and 91.6 percent for replacement augmentations; surgeon satisfaction was 92.6 percent for primary and 94.0 percent for replacement augmentation. Kaplan-Meier adverse event rates were as follows: Baker grade III and IV capsular contracture, 5.7 percent for primary and 11.5 percent for replacement augmentation; and rupture/deflation, 1.8 percent for primary and 4.7 percent for replacement augmentation.
Conclusion:
Six-year results from 438 women show that the structured breast implant has high patient and surgeon satisfaction, a low rate of capsular contracture, and a low rate of rupture/deflation.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, IV.

The IDEAL IMPLANT (structured breast implant) was approved by the U.S. Food and Drug Administration and Health Canada in November of 2014. Previously, women had the choice of two types of implants: saline (an unsupported silicone elastomer balloon filled with saline) or silicone gel (a silicone elastomer balloon filled with silicone gel). Each of these implant types has certain well-known advantages and disadvantages.
The saline implant does not have a natural feel because movement of the saline filler is uncontrolled, allowing rapid displacement with motion. Because the implant shell is unsupported, the upper pole collapses when upright and the shell tends to wrinkle (Fig. 1, above, left). However, the advantage of the saline implant is that the filler is saline, which gives women peace of mind. In case of a rupture (deflation), the implant gets smaller as the saline is harmlessly absorbed by the body; thus, a woman can simply look in the mirror to know whether her implants are intact or ruptured.
Fig. 1.

(Above, left) Mentor Moderate Plus 325 cc saline implant at minimum fill volume (total implant volume, 345 cc from 325-cc fill volume plus 20-cc empty implant volume). (Above, center) Allergan Moderate style 15 silicone gel implant (total implant volume, 339 cc). (Above, right) Allergan Inspira style SRF silicone gel implant (total implant volume, 365 cc). (Center, left) Allergan Inspira style SSF silicone gel implant (total implant volume, 365 cc). (Center, center) Allergan Inspira style SCF silicone gel implant (total implant volume, 365 cc). (Center, right) IDEAL IMPLANT 335-cc structured implant at minimum fill volume (total implant volume, 335 cc). (Below) A 335-cc structured IDEAL IMPLANT at maximum fill volume (total implant volume, 375 cc). Standardized oblique photographs were taken perpendicular to the surface of a curved form with a 10-inch diameter that simulates the convexity of the chest wall. The form was tilted 45 degrees up from the horizontal; a 2-cm lip at the bottom of the form kept the implant from sliding off and simulates support from the inferior capsule. (Photographs courtesy of Ideal Implant Incorporated.)
The silicone gel implant has a natural feel because the viscosity of the silicone gel filler mimics breast tissue. The cross-linked silicone gel supports the implant shell, so there is less upper pole collapse when upright and less wrinkling compared with the saline implant (Fig. 1, above, center). Increasing the silicone gel fill to 95 percent without increasing the cross-linking increases support for the implant shell; thus, there is even less upper pole collapse when upright (Fig. 1, above, right). Progressive increases in cross-linking at the same 95 percent fill (Fig. 1, center, left and center, center) further increase support for the implant shell, further reducing upper pole collapse when upright. The disadvantage of the silicone gel implant is that ruptures are silent (i.e., not clinically detectable), and occur at a relatively high rate (9.3 to 24.2 percent in 10-year Core studies1,2), which concerns many women. The U.S. Food and Drug Administration recommends a magnetic resonance imaging scan to detect rupture at 3 years after implantation and then every 2 years for life. Also, the U.S. Food and Drug Administration recommends removal of a ruptured silicone gel implant, which may entail additional time-consuming procedures such as capsulectomy for complete removal of the silicone gel. The IDEAL IMPLANT was designed to combine the peace of mind of the saline implant and the natural feel of the silicone gel implant, without the drawbacks that concern women most (i.e., unnatural feel of the saline implant and silent rupture of the silicone gel implant).
This implant is named a “structured” implant because of its internal structure, which supports the shell so there is less upper pole collapse when upright and less wrinkling compared to round saline and certain round silicone gel implants (Fig. 1, center, right). Increasing the fill volume in the outer lumen of the structured implant increases support for the shell, so there is even less upper pole collapse when upright (Fig. 1, below). Since its unique design and technology are different from saline and silicone gel implants, the structured implant is a third type of breast implant. Classifying breast implants on the basis of their saline or silicone gel filler material fails to identify differences in shell support that affect implant performance. A more informative classification is proposed here: “unsupported shell” (saline implant) or “supported shell” (silicone gel and structured implants).
The IDEAL IMPLANT is a round, smooth-surface, saline-filled implant with an internal structure. It has two lumens within two nested shells that are attached at the patch on the back. The inner lumen within the inner shell is filled through a valve in the patch with approximately two-thirds of the saline. The outer lumen within the outer shell, and surrounding the inner shell, is filled through a valve on the front with approximately one-third of the saline. Unattached and floating within the outer lumen is a baffle structure designed to restrict movement of the saline in the outer lumen. This internal structure is composed of one to three nested baffle shells that are perforated with slits so the saline is free to move through the slits, and around and between the shells. The number of baffle shells in an implant is proportionate to the size: 210- to 300-cc implants have one baffle shell, 335- to 555-cc implants have two baffle shells, and 595- to 675-cc implants have three baffle shells. A cutaway drawing (Fig. 2) of an IDEAL IMPLANT (335- to 555-cc) shows the inner shell, the outer shell, the baffle structure floating in the outer lumen composed of two baffle shells perforated with slits, the valve in the patch to fill the inner lumen, and the valve on the front to fill the outer lumen. The shape of this round implant was designed with the edge low, to contour to the convexity of the chest wall, and tapering from the dome to the edge so that the side of the implant does not bulge outward toward the arm (Fig. 3).
Fig. 2.
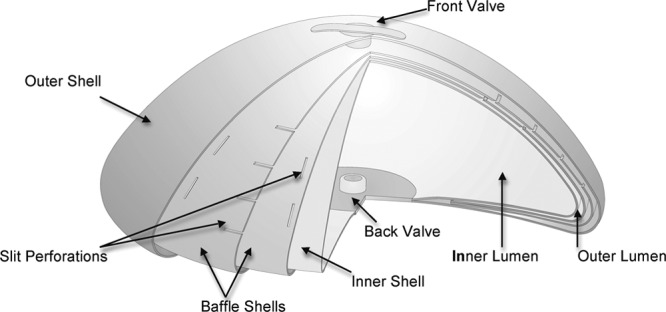
Cutaway of IDEAL implant (335- to 555-cc) to show internal structure. (Drawing courtesy of IDEAL IMPLANT Incorporated.)
Fig. 3.

IDEAL implant on a curved surface simulating the convexity of the chest wall. (Photograph courtesy of IDEAL IMPLANT Incorporated.)
Overfilling, a common practice for some surgeons using a saline implant, is not needed or recommended for the structured implant. For all 14 implant sizes, inner lumen fill volumes were engineered to be the same percentage of the inner shell mandrel volume, and are not adjustable. The outer lumen fill volumes are adjustable within a range that is proportionate to the implant size, from a range of 25 cc for the smallest size to a range of 80 cc for the largest size (Table 1). The minimum and maximum outer lumen fill volumes were engineered so that each is the same percentage of the outer shell mandrel volume. Because of this design, all implant sizes have the same shape and contour when at the minimum fill volume and when at the maximum fill volume.
Table 1.
Approximate Dimensions and Volumes*
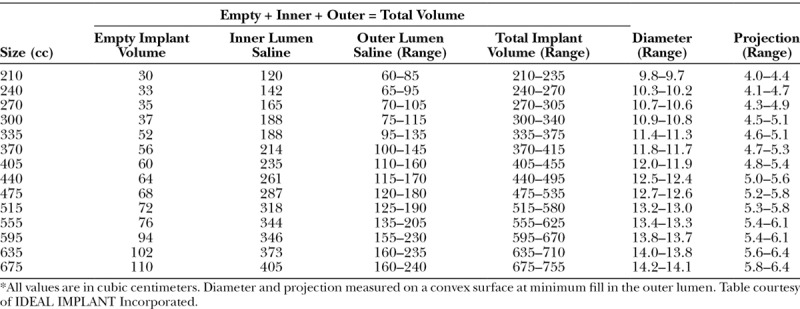
The diameter and projection of the implant were measured on a convex surface simulating the curve of the chest wall (Fig. 3 and Table 1), instead of on a flat surface as done by other manufacturers. This difference in measurement methods should be considered when comparing dimensions of the structured implant to other implants. For example, the diameter of a 210-cc implant at minimum fill volume measures 3 mm less on a curved surface than when measured on a flat surface. Similarly, the diameter of a 675-cc implant at minimum fill volume measures 7 mm less on a curved surface than when measured on a flat surface. The same adjustment applies to projection measurements: a 210-cc implant at minimum fill volume projects 3 mm more on a convex surface than when measured on a flat surface; and the projection of a 675-cc implant at minimum fill volume projects 7 mm more on a convex surface than when measured on a flat surface.
The total implant volume comprises the empty implant volume plus the saline volume added to each lumen. If either lumen deflates, considerable implant volume remains to maintain much of the breast augmentation. Nevertheless, the deflation of either lumen is obvious to a patient by looking in the mirror, and replacement can be scheduled electively.
PATIENTS AND METHODS
Study Design
This study was a prospective, multicenter, clinical trial to document the safety and effectiveness of the IDEAL IMPLANT in two patient cohorts: bilateral primary breast augmentation and bilateral revision of existing saline or silicone gel augmentation implants. If a primary augmentation cohort patient had a study implant or implants replaced, she remained in the primary augmentation cohort. The protocol specified that patients were required to return for follow-up visits at 2 months; 6 months; and 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 years after implantation. Safety was assessed by means of the incidence and timing of all adverse events and subsequent breast operations reported by the investigator. Effectiveness was assessed by change in breast size (for augmentation patients), quality-of-life assessments by the patient, and outcome satisfaction assessments by the patient and surgeon.
The study was approved by a central investigational review board (RCRC IRB, now Salus IRB, Austin, Texas) because all investigators conducted study procedures in their offices or a site without an investigational review board, such as a free-standing surgical center. To encourage high rates of patient follow-up, a unique financial incentive plan was devised. Instead of making payments for each follow-up visit over 10 years, Ideal Implant Incorporated placed $3500 for each trial patient into an independent, irrevocable study participants’ trust fund. Patients who complete all of the required follow-up visits during the 10 years of the study will receive a lump sum payment from the trust fund. If a patient misses any required follow-up visit, she is discontinued from the study and loses her share of the trust fund, but her share remains in the trust fund to be divided among those who remain in the study through 10 years. Details of this incentive plan have been reported elsewhere.3 Investigators receive nominal financial incentives for completing case report forms and study administration.
Subjects
Women 18 years or older were eligible to enroll. They had to agree to comply with their surgeon’s postoperative instructions and follow-up visit requirements. Exclusion criteria were pregnancy or planning to become pregnant within 6 months of implantation, nursed within the previous 3 months, cancer or premalignant breast disease, an infection or abscess, a condition that could compromise healing, inadequate tissue cover or compromised vascularity, any condition that constituted an unwarranted surgical risk, or unrealistic expectations of the results.
Statistical Analysis
Data were collected on standardized case report forms before the procedure and at each required follow-up visit, other follow-up visits, and subsequent breast operations. The forms were sent to a data management center (NAMSA, Inc., Minneapolis, Minn.) for statistical analysis.
All adverse events reported by the investigator were included in the statistical analysis, except for capsular contracture (only Baker grade III or IV capsular contractures were included) and palpable wrinkling/scalloping (only moderate or severe were included), because mild and very mild occurrences were not considered clinically significant problems. Kaplan-Meier analyses of individual adverse events were performed on a per-subject basis for each cohort. To avoid the problem of competing risks, a subject who experienced one adverse event was still considered a candidate to experience any other potential adverse event.
Breast size was defined as the difference between chest circumference measurements at the inframammary fold and at the nipple line. Baseline measurements and 1-year measurements were collected to assess effectiveness for primary augmentation patients only.
The Breast Evaluation Questionnaire4 is a 55-item assessment specifically designed to evaluate breast satisfaction (both self-esteem and body image) among breast surgery patients, including three domains: (1) comfort not fully dressed, (2) comfort fully dressed, and (3) satisfaction with breast attributes. Scoring is a simple summation of the rating responses provided within a domain. Changes from baseline in all three domains are presented as mean, standard deviation, number of patients, and range, and are tested for significance using a paired t test. The Breast Evaluation Questionnaire is used to assess subjects’ satisfaction with their breasts before and after implant surgery at 1, 2, 4, 6, 8, and 10 years.
Both patients and investigators were asked at each required follow-up visit to assess their satisfaction with the outcome achieved in each breast on a five-point scale that ranges from definitely satisfied to definitely dissatisfied. To be conservative, a per-subject analysis was performed by taking the worst assessment between the two breasts as the score.
RESULTS
Subjects and Surgical Characteristics
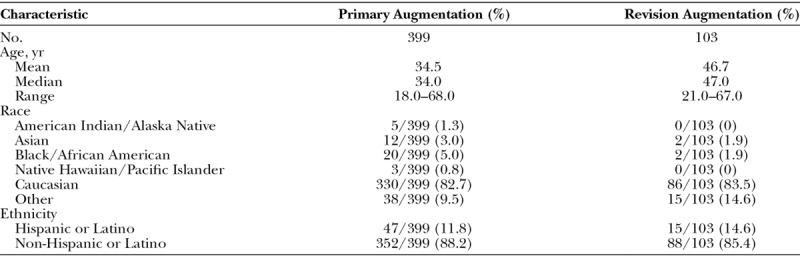
A total of 502 patients were enrolled by 45 American Board of Plastic Surgery–certified investigators at 35 investigational sites throughout the United States. Of these, 399 were in the primary augmentation cohort and 103 were in the revision augmentation cohort. The majority of women were Caucasian, and the mean age was 34.5 years for primary augmentation and 46.7 years for revision augmentation (Table 2).
Table 2.
Demographic Data
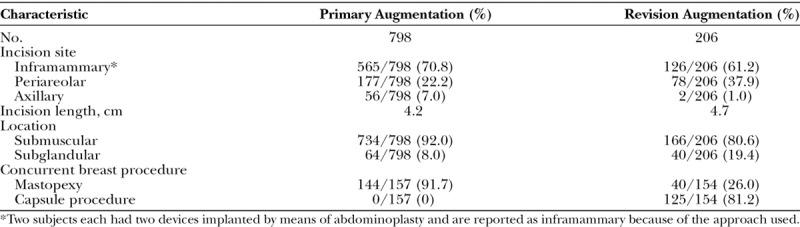
The majority of implants in both cohorts were placed in the submuscular position through an inframammary incision (Table 3). Concurrent breast procedures were performed in 157 of the 798 breasts undergoing primary augmentation (19.7 percent), with mastopexy being the most common. Concurrent breast procedures were performed in 154 of the 206 breasts undergoing revision augmentation (74.8 percent), with a capsule procedure being the most common.
Table 3.
Surgical Operative Data, per Implant
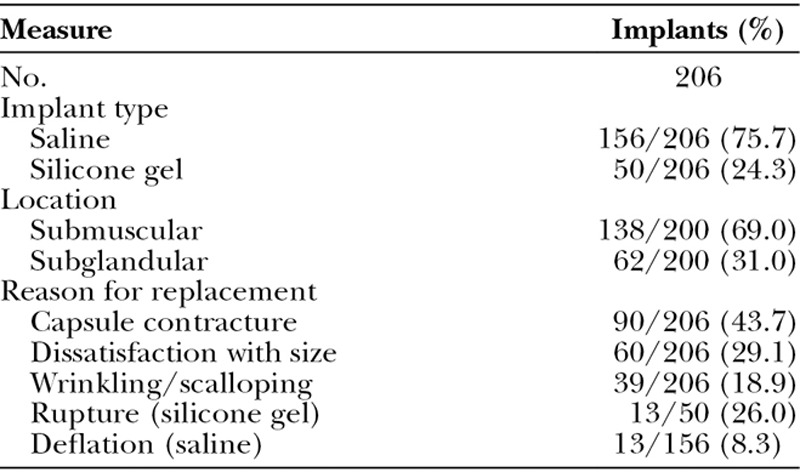
In the revision augmentation cohort (Table 4), 24.3 percent of the implants replaced were silicone gel and 75.7 percent were saline. The most common primary reason for revision augmentation was capsular contracture, seen in 43.7 percent of the breasts.
Table 4.
Initial Breast Implants in the Revision Augmentation Cohort
At the 6-year follow-up visit, excluding patients who voluntarily withdrew from the trial or had their study implants removed and replaced with another manufacturer’s implants, only 19 patients were lost to follow-up in the primary augmentation cohort, for a 93.8 percent follow-up rate, and only three patients were lost to follow-up in the revision augmentation cohort, for a 97.8 percent follow-up rate. As would be expected with the strong financial incentive provided by the participants’ trust fund, these percentages of follow-up are higher than in any prior U.S. Food and Drug Administration Core study of a breast implant.3
Safety
The safety analyses focus on the Kaplan-Meier risk rates of adverse events because this statistical analysis method takes into account the number of subjects followed over time and when a subject experiences the first occurrence of the event. Thus, these analyses provide an estimate of the true event rate at any given point in time because they consider only the subjects still at risk at the time of the event.
The key adverse events through 6 years in both cohorts are listed in Table 5. The most common adverse events in both cohorts were subsequent breast operations and implant removals. These are included with adverse events, because they are often performed attempting to correct adverse events. The most common local adverse events included palpable wrinkling/scalloping, dissatisfaction with implant size, capsular contracture, and dissatisfaction with the cosmetic result, which was a broad category that could include already reported adverse events (e.g., capsular contracture, deflation, wrinkling, size, scar, asymmetry).
Table 5.
Key Kaplan-Meier Risk Rates through 6 Years, per Patient
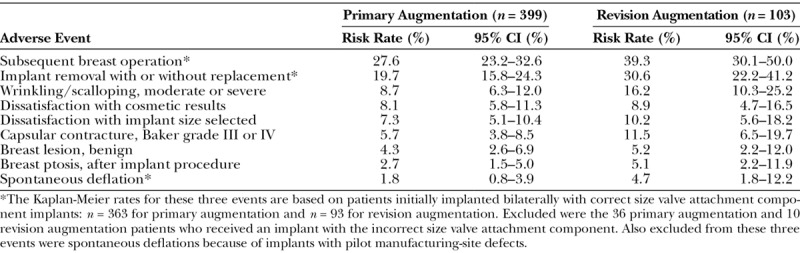
Macroscopic and microscopic analyses were performed on all study implants explanted for any reason. It is noteworthy that through follow-up of 7.5 to 8.5 years, no implant deflation was caused by a crease fold of the shell. The Kaplan-Meier deflation rate through follow-up of 6 years in Table 5 includes all reported deflations that were attributable to instrument damage and alleged leaks that could not be reproduced or could not be analyzed because the implant was damaged, and it excludes deflations attributable to implants with pilot manufacturing-site defects, such as valve damage during assembly, inadequate shell-patch bond, or inadequate valve-patch bond. These early manufacturing defects of trial implants at the pilot manufacturing site were addressed before U.S. Food and Drug Administration approval, with improved process controls and inspections5 at the commercial manufacturing site.
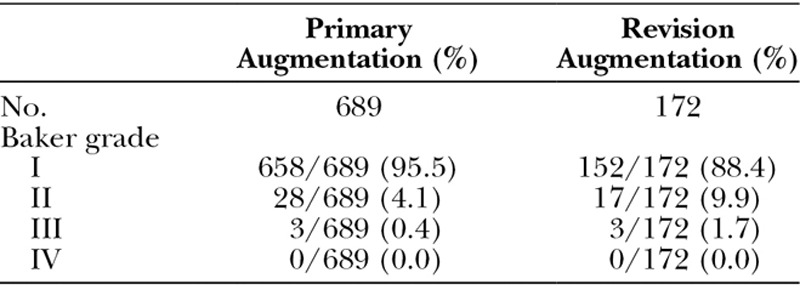
At each follow-up visit, patients were assessed on a five-point grading scale for palpable wrinkling/scalloping and by the Baker classification for capsular contracture. Tables 6 and 7 show the prevalence rates at the 6-year follow up visit, which are less than the Kaplan-Meier estimated adverse event rates presented in Table 5. This is because Kaplan-Meier rates incorporate the assessments at all prior follow-up visits, some of which could have been more severe. At the 6-year follow-up visit, only 1.7 percent of the implants in the primary augmentation cohort had palpable wrinkling/scalloping that was moderate or severe, and only 0.4 percent of the implants in the primary augmentation cohort had capsule contracture that was Baker grade III or IV. Wrinkling/scalloping may have been overreported in this trial because investigators were required to palpate for wrinkling/scalloping at each follow-up visit and assess the severity. This was not required in other breast implant trials.
Table 6.
Palpable Wrinkling/Scalloping Assessed at 6-Year Visit, per Implant
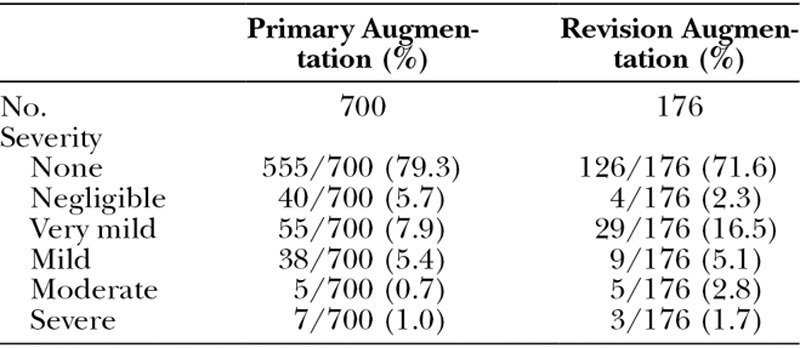
Table 7.
Capsular Contracture Assessed at 6-Year Visit, per Implant
Kaplan-Meier rates for subsequent breast operations in the primary and revision cohorts (27.6 and 39.3 percent) are comparable to those in Allergan (Allergan, Inc., Dublin, Ireland) (28.0 and 40.3 percent)6 and Mentor (Mentor Worldwide, Irvine, Calif.) (19.1 and 33.1 percent)7 silicone gel Core studies. The common primary reasons for subsequent breast operations are listed in Table 8. Through 6 years, there were 122 subsequent breast operations in 97 patients in the primary augmentation cohort and 61 subsequent breast operations in 36 patients in the revision augmentation cohort. As in other breast implant Core studies, one of the most common reasons for subsequent breast operations was dissatisfaction with implant size, which was the reason in 14.8 percent of primary augmentation patients and 13.1 percent of revision augmentation patients. Other key reasons were capsular contracture and wrinkling/scalloping. Spontaneous deflation was an uncommon reason for subsequent breast operations.
Table 8.
Key Reasons for Subsequent Breast Operations through 6 Years
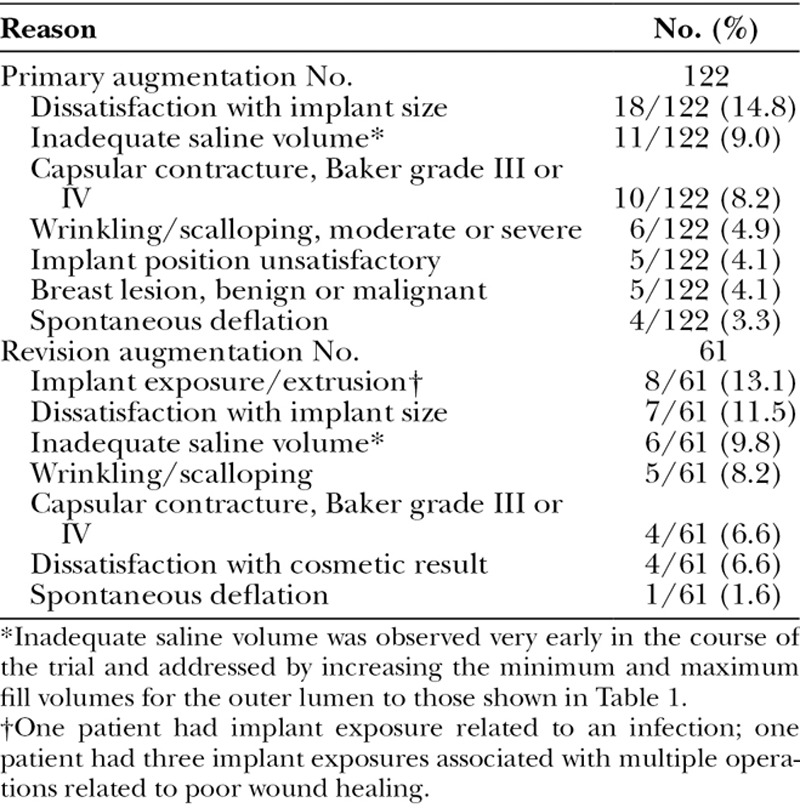
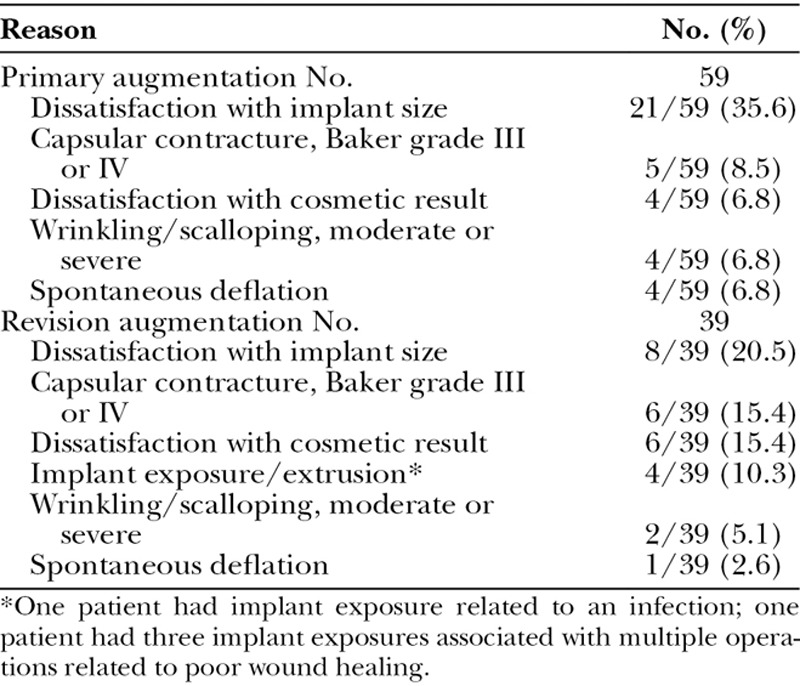
The common primary reasons for implant removal are listed in Table 9. There were 59 implants removed in the primary augmentation cohort and 39 implants removed in the revision augmentation cohort. The most common reason was dissatisfaction with implant size, prompting removal and replacement with the desired size. Other key reasons were capsular contracture and wrinkling/scalloping. Spontaneous deflation was an uncommon reason for implant removal.
Table 9.
Key Primary Reasons for Implant Removals through 6 Years
Effectiveness
Increase in breast size was analyzed for subjects in the primary augmentation cohort at 1 year. Patients experienced a mean increase of 2.5 inches in chest circumstance measurements (breast size), which represents an approximate increase of one to two bra cup sizes. This difference was statistically significant (p < 0.0001, t test).
Patients in the primary augmentation cohort and the revision augmentation cohort experienced significant increases from baseline in each domain of the Breast Evaluation Questionnaire at 1, 2, and 4 years. Patients continued to experience significantly significant increases from baseline in each domain of the Breast Evaluation Questionnaire at 6 years (p < 0.0001, t test).
Investigators and patients were satisfied with the outcome of the procedure at 6 years. Investigators were definitely or somewhat satisfied with patient outcomes in 315 of 340 in the primary augmentation cohort (92.6 percent) and 79 of 84 in the revision augmentation cohort (94.0 percent). Patients were definitely or somewhat satisfied with their outcomes in 305 of 340 in the primary augmentation cohort (89.7 percent) and in 77 of 84 in the revision augmentation cohort (91.6 percent).
DISCUSSION
The IDEAL IMPLANT has been studied in a clinical trial conducted by plastic surgeons in private practice, the typical users of breast implants. Patient follow-up at 6 years exceeded that in any other breast implant Core study, demonstrating a well-executed clinical trial conducted at 35 private practice sites in the United States.
The nature, frequency, and severity of the adverse events observed in the clinical trial through 6 years are consistent with the 2-year follow-up data reported previously.8 The most commonly reported adverse event was subsequent breast operation, and a common reason was dissatisfaction with implant size. The results from this clinical trial through 6 years show that the implant is safe and effective for primary and revision breast augmentation. This study will continue through 10-years of follow-up, providing additional clinical data.
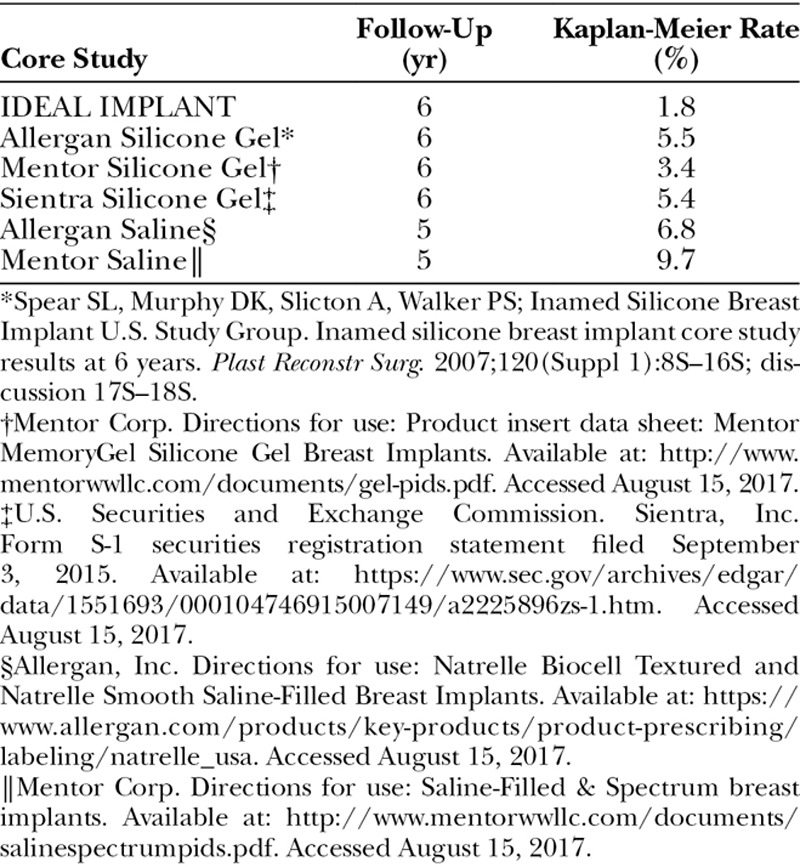
Although Core clinical trials follow similar protocols established by the U.S. Food and Drug Administration,9 there are differences in the patient populations and data collection methods. Nevertheless, it is useful to compare Core study data for two key adverse events, capsular contracture and implant failure (deflation/rupture). The IDEAL IMPLANT at 6 years had a substantially lower capsular contracture rate and lower failure rate than comparable round saline or silicone gel implants (Tables 10 and 11).6,7,10–12 The explanation for these favorable results is unknown but may be related to the unique technology of the structured implant compared with saline and silicone gel implants. Several theories to explain these results are offered here, but further studies are needed to evaluate each possibility.
Table 10.
Baker Grade III or IV Capsular Contracture in Primary Augmentation
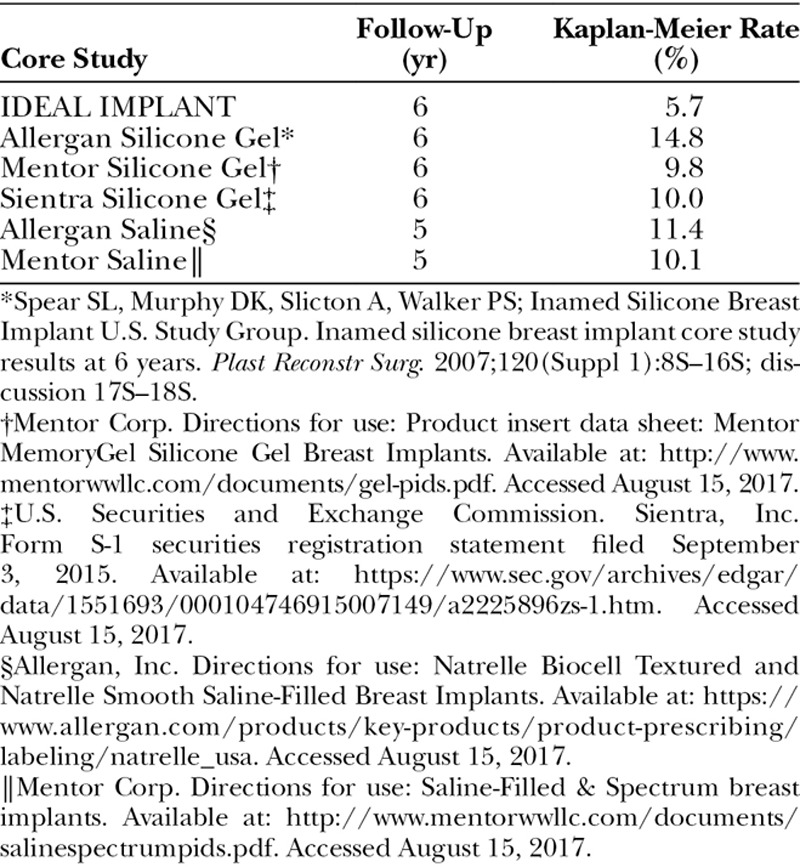
Table 11.
Implant Deflation or Rupture in Primary Augmentation
The low capsular contracture rate may be because the multiple shell layers of the implant offer significantly greater resistance to compression than single-shell implants; thus, it may have greater resistance to the compressive forces of capsular contracture. Another possible explanation may be that this greater resistance to compression reduces repeated stretching of the implant during activities of daily living, thereby reducing microtrauma to the capsule that could stimulate contracture. Another possibility is that the low capsular contracture rate may result from the unique geometry of the implant, which has a favorable effect on the scar capsule. Because the implant surface is smooth, the low capsular contracture rate cannot be attributed to surface texturing.
The low deflation rate may be explained by the absence of crease folds of the shell. This could be attributable to the underlying baffle shell layers supporting the outer shell, which prevents it from folding onto itself. Another reason for the absence of crease folds could be related to the shell manufacturing process, which uses a robot to dip mandrels while spinning on the longitudinal axis. This manufacturing technology results in shells that are more consistent and uniform in thickness compared with those made by dipping mandrels by hand.
Now, women and plastic surgeons can choose from three different breast implant technologies: the saline implant with an unsupported shell, the silicone gel implant with a supported shell, or the structured implant with a supported shell. The cross-linked silicone gel and the internal structure of the IDEAL IMPLANT both support the shell to minimize collapse and wrinkling. Because the filler of the implant is only saline, women can look in the mirror and know their implants are intact, giving them peace of mind. In addition to a natural feel, the structured IDEAL IMPLANT has demonstrated clinical advantages over silicone gel implants through 6 years, such as a lower capsular contracture rate and lower rupture rate in primary augmentation.
Footnotes
This trial is registered under the name “Core Study of the Safety and Effectiveness of IDEAL IMPLANT® Saline-filled Breast Implants,” ClinicalTrials.gov identification number NCT00858052 (https://clinicaltrials.gov/ct2/show/NCT00858052).
Presented in part at Plastic Surgery The Meeting 2017, Annual Meeting of the American Society of Plastic Surgeons, in Orlando, Florida, October 6 through 10, 2017.
Disclosure: Ideal Implant Incorporated designed and funded the study. The authors received research support for conducting this study. Gregg Anigian is an Ideal Implant Incorporated stockholder.
REFERENCES
- 1.Allergan, Inc. Directions for use: Natrelle Silicone-Filled breast implants and Natrelle Inspira breast implants. Available at: https://allergan-web-cdn-prod.azureedge.net/actavis/actavis/media/allergan-pdf-documents/labeling/natrelleus/siliconeimplants/1034rev11.pdf. Accessed August 15, 2017.
- 2.Government of Canada. Summary basis of decision: Mentor MemoryGel Silicone Gel-Filled breast implants: Health Canada. Available at: https://hpr-rps.hres.ca/reg-content/summary-basis-decision-medical-device-detailone.php?lang=en&linkid=sbd00412. Accessed August 15, 2017.
- 3.Mueller MA, Nichter LS, Hamas RS.Novel approach for maximizing follow-up in cosmetic surgery clinical trials: The Ideal Implant Core Trial experience. Plast Reconstr Surg. 2017;140:706–713.. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RC, Cunningham B, Tafesse E, Lenderking WR.Validation of the breast evaluation questionnaire for use with breast surgery patients. Plast Reconstr Surg. 2006;118:597–602.. [DOI] [PubMed] [Google Scholar]
- 5.Ideal Implant. Summary of safety and effectiveness data (SSED). Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf12/p120011b.pdf. Accessed August 15, 2017.
- 6.Spear SL, Murphy DK, Slicton A, Walker PSInamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(Suppl 1):8S–16S; discussion 17S18S.. [DOI] [PubMed] [Google Scholar]
- 7.Mentor Corp. Directions for use: Product insert data sheet: Mentor MemoryGel silicone gel breast implants. Available at: http://www.mentorwwllc.com/documents/gel-pids.pdf. Accessed August 15, 2017.
- 8.Nichter LS, Hamas RS.Two-year outcomes with a novel, double-lumen, saline-filled breast implant. Aesthet Surg J. 2012;32:861–867.. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Guidance for industry and FDA staff: Saline, silicone gel, and alternative breast implants. Available at: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm071233.pdf. Accessed August 15, 2017.
- 10.U.S. Securities and Exchange Commission. Sientra, Inc. Form S-1 securities registration statement filed September 3, 2015. Available at: https://www.sec.gov/archives/edgar/data/1551693/000104746915007149/a2225896zs-1.htm. Accessed August 15, 2017.
- 11.Allergan, Inc. Directions for use: Natrelle Biocell Textured and Natrelle Smooth Saline-Filled Breast Implants. Available at: https://www.allergan.com/products/key-products/product-prescribing/labeling/natrelle_usa. Accessed August 15, 2017.
- 12.Mentor Corp. Directions for use: Saline-Filled & Spectrum breast implants. Available at: http://www.mentorwwllc.com/documents/salinespectrumpids.pdf. Accessed August 15, 2017.


