Abstract
Background
The effectiveness of nonconsummatory reinforcers habituate, as their ability to maintain reinforced responding declines over repeated presentations. Preclinical research has shown that nicotine can delay habituation of reinforcer effectiveness, but this effect has not been directly demonstrated in humans.
Objective
In preliminary translational research, we assessed effects of nicotine from tobacco smoking (versus a no smoking control) on within-session patterns of responding for a brief visual reinforcer.
Methods
Using a within-subjects design, 32 adult dependent smokers participated in two experimental sessions, varying by smoking condition: no smoking following overnight abstinence (verified by CO≤10 ppm), or smoking of own cigarette without overnight abstinence. Adapted from preclinical studies, habituation of reinforcer effectiveness was assessed by determining the rate of decline in responding on a simple operant computer task for a visual reinforcer, available on a fixed ratio schedule.
Results
Reinforced responding and duration of responding were each significantly higher in the smoking vs no smoking condition. The within-session rate of responding declined significantly more slowly during the smoking versus no smoking condition, consistent with delayed habituation of reinforcer effectiveness. Follow-up analyses indicated that withdrawal relief did not influence the difference in responding between conditions, suggesting the patterns of responding reflected positive, but not negative, reinforcement.
Conclusions
These results are a preliminary demonstration in humans that smoked nicotine may attenuate habituation, thereby maintaining the effectiveness of a reinforcer over a longer period of access. Further research is needed to confirm habituation and rule out alternative causes of declines in within-session responding.
Keywords: Nicotine, Reinforcement, Habituation, Smoking
Introduction
Nicotine reinforcement may involve dynamic and complex processes that underlie smoking persistence. Self-administration studies in both animal models and humans have reliably shown that nicotine and nicotine-associated stimuli reinforce drug intake (Henningfield, Smith, Kleykamp, Fant, & Donny, 2016; Rupprecht et al., 2015). Beyond these primary and secondary reinforcing effects, nicotine can also enhance the reinforcing efficacy of non-drug stimuli unrelated to smoking (Caggiula, Donny, Palmatier, Liu, Chaudhri, & Sved, 2008; Chaudhri, Caggiula, Donny, Palmatier, Liu, & Sved, 2006; Donny et al., 2003). Preclinical research has shown that rats will increase responding for non-drug reinforcers (e.g., light onset) following nicotine administration, despite no contingent association between nicotine and those reinforcers (Donny et al., 2003; Palmatier, O’Brien, & Hall, 2012). The non-drug reinforcers used in this research are perhaps best described as nonconsummatory or “not biologically important” (Lloyd, Medina, Hawk, Fosco, & Richards, 2014b), in an effort to rule out changes in responding for consummatory reinforcers due to satiety (McSweeney, 2004). Clinical research designed to closely match procedures used in research with animal models recently has translated this nicotine effect on non-drug reinforcement to humans. In a series of controlled studies, operant responding for non-drug reinforcers (i.e., preferred music, preferred video clips) has consistently been shown to increase following acute nicotine administration compared to no nicotine (Perkins & Karelitz, 2013a, 2013b, 2014; Perkins, Karelitz, & Michael, 2015; for a review see Perkins, Karelitz, & Boldry, 2017).
Recent preclinical research suggests that nicotine may also prolong the duration of a reinforcer’s efficacy. The effectiveness of a nonconsummatory (i.e., non-satiating) reinforcer habituates, in that its ability to maintain operant responding declines with each subsequent presentation of the reinforcer (McSweeney & Murphy, 2009; Thompson, 2009). In rodent models, some stimulant drugs have been shown to delay habituation of reinforcer effectiveness (i.e., to maintain responding for that reinforcer a bit longer; Gancarz, et al., 2012; Lloyd, Hausknecht, & Richards, 2014). For example, rats receiving either nicotine or methamphetamine had more gradual within-session declines in responding for a visual reinforcer (i.e., turning on a cage light) compared to a sharp reduction in those receiving saline. These drug effects may be specific to nonconsummatory reinforcers. When receiving methamphetamine, rats showed delayed habituation of reinforcer effectiveness for a visual stimulus (turning on a light), but not for a consumable (i.e., water) reinforcer (Lloyd et al., 2014a). Taken together, these preclinical studies suggest that select stimulant drugs, including nicotine, may attenuate habituation of reinforcer effectiveness (Lloyd et al., 2014b; cf. Wright, Ren, Constantin, & Clarke, 2018).
Returning to nicotine’s processes of reinforcement, habituation of reinforcer effectiveness may contribute to nicotine’s reinforcement enhancing effects. Following acute nicotine intake, there is rapid enhancement of reinforcement from non-drug associated stimuli over the bout of nicotine exposure (Caggiula et al., 2008; Donny et al., 2003; Perkins, Karelitz, & Boldry, 2017). Nicotine may also delay habituation of the reinforcer’s effectiveness which, in turn, may further sustain the nicotine-enhanced reinforcement (Lloyd et al., 2014b). Thus, nicotine may both initially increase and subsequently maintain the reinforcing efficacy of some non-drug associated stimuli.
The current study was intended as a first step in translating animal research assessing nicotine’s influence on habituation of reinforcer effectiveness to a human sample. To our knowledge, this is the first study to directly examine effects of any drug on habituation of reinforcer effectiveness in humans. We examined patterns of within-session responding on a simple operant computer task for a preferred visual reinforcer between two widely disparate smoking conditions, satiation vs. abstinence: 1) nicotine via cigarette smoke after smoking as usual vs 2) no smoking after overnight abstinence. We hypothesized slower rates of decline in within-session reinforced responding during the smoked nicotine (satiated smoking) condition compared to the no smoking (smoking abstinence) condition, as in the preclinical research. Secondary comparisons evaluated whether negative, rather than positive, reinforcing effects of nicotine could play a role.
Method
Participants
Participants (N=32; 15 men, 17 women) were those who smoked ≥10 cigarettes per day for at least one year and met DSM-V criteria for nicotine dependence (American Psychiatric Association, 2013). Mean (SD) sample characteristics were 15.2 (4.1) cigarettes per day, smoking at their current rate for 9.7 (8.1) years, and 34.1 (8.6) years old. The sample self-reported as 72% Caucasian, 25% African American or Black, and 3% more than one ethnicity. Sample characteristics did not vary between genders.
Measures
Operant Responding Task
Habituation of reinforcer effectiveness was measured using a simple computer task (“Apple Picker”; Norman & Jongerius, 1985). Participants used a keypad to move a cursor around a “field” on the monitor, pressing a button once the cursor highlighted a “tree” to check for an “apple”, representing a response. A fixed schedule of 10 responses (FR10 schedule) was required to earn each 7-second presentation of the reinforcer (i.e., picture of an attractive model; see Procedure), immediately displayed on the monitor when earned. Prior to engaging in the 15-min task, participants were instructed to work on the task only as long as they wanted to continue viewing the available picture. They were free to stop responding at any time, and general interest magazines were available to reduce likelihood of continued responding simply due to boredom. However, these magazines were purposefully “routine” in nature to not compete with the visual stimulus as an alternative reinforcer.
Procedure
Participants were initially screened over the phone for smoking and health histories and then scheduled for an introductory session to obtain informed consent and confirm eligibility. The two experimental sessions here, comparing the two most widely differing conditions of no smoking after overnight abstinence vs. smoking without overnight abstinence, were part of a larger project examining acute nicotine effects on responding for other reinforcers under a different procedure (progressive-ratio schedule) during trials that were separate from the habituation trials of this study. At the introductory session, participants were shown 80 pictures of attractive models and celebrities, from among those publicly available and collected from the Internet (40 men, 40 women, mixed ethnicity). They rated how much they liked seeing each picture on a 0-100 visual analog scale. Two pictures scoring ≥50 were used as reinforcers, one per session. Participants were then introduced to the Apple Picker task—without a reinforcer—to learn the task and become familiar with it.
Procedures in both experimental sessions were identical, varying only by smoking condition (no smoking vs smoking; counterbalanced between subjects). For the no smoking condition, participants were required to abstain from all nicotine or tobacco products overnight (>12 hours). Expired-air CO was measured upon arrival (Vitalograph BreathCO)—levels ≤10 ppm confirmed overnight abstinence (Benowitz et. al, 2002). As part of the larger project, participants received a placebo patch at least three hours before the session and were administered a placebo nasal spray 20 mins before the current study procedures, both under single-blind conditions. Administration of placebo patch and placebo nasal spray maintained participant expectations of potentially receiving nicotine, similar to their expectations for nicotine intake during the smoking condition. For the smoking condition, participants were instructed to smoke normally (i.e., ad lib) before the session. Expired-air CO levels ≥10 ppm confirmed typical and recent smoke exposure upon arrival. To further ensure smoking satiation during the smoking condition, all took four puffs from their preferred brand of cigarette 20 mins prior to engaging in the Apple Picker task. Finally, the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes, 2007), with each item rated on a 0-100 VAS and averaged for a total score, was completed upon arrival to each session and again immediately before engaging in the operant responding task.
Data Preparation and Analysis Plan
All analyses were performed using SPSS 24.0 (IBM, Chicago, IL). The time of each operant response was recorded (in seconds since trial onset) and binned into ninety 10-second epochs. The number of responses per 10-second epoch was the main dependent variable. Previous studies have used a single composite measure to quantify the within-session habituation rate (Lloyd et al., 2014a; Lloyd et al., 2014b). However, this composite measure of habituation rate (expressed as the percent decline per epoch per minute) assumes a linear decline in within-session responding, despite evidence for non-linear patterns of within-session responding (McSweeney, 1992; McSweeney, 2004). Thus, using the number of responses per 10-second epoch allowed for the possibility of non-linear patterns of responding over time.
Preliminary analyses used paired-samples t-tests to assess differences between smoking conditions in subjective ratings of the reinforcers, expired-air CO levels, nicotine withdrawal, absolute number of operant responses, and duration responding. A 2 × 2 repeated measures analysis of variance was used to compare self-reported MNWS nicotine withdrawal, using within-subjects factors of smoking condition and time across the session (upon arrival versus just prior to the operant responding task).
The primary analysis used multi-level modeling (MLM; performed using the MIXED command in SPSS) to assess whether within-session rates of responding across epochs varied between smoking conditions. This analysis approach is typically used to examine data arranged in a hierarchical structure (Raudenbush & Bryk, 2001; Singer & Willett, 2003). For the current study, lower-level, within-session epochs (Level 1) were nested within higher-level smoking conditions (Level 2), with the cross-level interaction between Level 1 epoch and Level 2 smoking condition as the main outcome of interest. To facilitate interpretation of the intercept parameter, the epoch variable was adjusted (i.e., centered) to begin at 0 by subtracting 1 from each value (changing the range from 1-90 to 0-89) and entered into the model as a continuous predictor. The no smoking condition was coded as the control (0) and the smoking condition was coded as 1.
This analysis method required estimation of several models (Shek & Ma, 2011; Shek & Ma, 2014). First, a null model was estimated without predictors from either level to calculate the intraclass correlation coefficient (ICC). The ICC quantified the proportion of total variance in responding due to smoking condition. Next, models incorporating linear, cubic, and quadratic effects of epoch were estimated to determine whether the rate of change in responding was linear or curvilinar across epochs. Once the cubic rate of change was found to be the best fit, a model was estimated to examine the effect of smoking condition on responding across epochs. Lastly, an exploratory follow up model examined whether rates of responding were related to self-reported MNWS withdrawal scores collected immediately before participants worked on the operant responding task. All models were computed using maximum likelihood (ML) estimation (Shek & Ma, 2014).
Results
Preliminary comparisons
As expected for the smoking vs. no smoking conditions, respectively, means (±SE) measured upon arrival to the session were significantly higher on CO, 16.9±1.3 vs. 3.8±0.4, t(31) = 10.00, p < .001 and lower for withdrawal, 9.0±2.8 vs. 22.5±3.3, t(31) = 4.08, p < .001. Together, these confirm participant compliance with smoking instructions prior to sessions. There was a main effect of smoking condition on self-reported withdrawal, F(1,31) = 31.76, p < .001, . However, neither the main effect of time across the session nor the smoking condition by time interaction were significant, F(1,31)’s < 1, p’s > .36. In another within-subjects preliminary comparison, participants’ initial ratings of the reinforcers did not differ between sessions, with mean (±SE) ratings of 85.5±2.2 and 85.5±2.1, t(31) = 0.02, ns, verifying successful randomization of pictorial reinforcers between conditions.
Rates and duration of responding
Overall, there was significantly more reinforced responding during the smoking vs. no smoking condition, 185.2±33.2 vs. 132.8±18.2, respectively, t(31) = 2.22, p < .05. Similarly, participants responded significantly longer during the smoking vs. no smoking condition, 179.5±35.0 vs. 132.8±18.2 seconds, t(31) = 2.26, p < .05.
Figure 1 shows estimated mean responding across session epochs, estimated from the MLM analyses following procedures described in detail elsewhere (Ma & Shek, 2014). (Note that responding during the no smoking condition ended for all participants by Epoch 38, with the uptick in subsequent estimated responding presumed to be due to inclusion of a cubic term for epoch.) Table 1 displays the results from the final MLM model. Parameters for the fixed effects, γ00, γ10, γ20 and γ30, relate to responding only during the no smoking condition and are not of interest to the hypotheses for this study. There was no difference in responding during the first epoch between smoking conditions, γ01= 0.29, SE = 0.23, p = .21. Across smoking conditions, the response rate for those who responded more during the first epoch decreased more quickly than those who responded less during the first epoch, , SE = 0.04, p < .001. This is consistent with Rankin et al.’s (2009) fourth characteristic of habituation. As hypothesized, most importantly, the difference between conditions for the linear rate of change in responding over time was significant, γ11 = 0.03, SE = 0.01, p < .05, indicating that declines in responding were significantly slower during the smoking versus no smoking condition. Further, the quadratic rate of change was significantly different between smoking conditions, γ21 = − 0.0004, SE = 0.0001, p < .01, as the decline in responding was more gradual later in the session during the smoking condition relative to the no smoking condition.
Fig. 1.
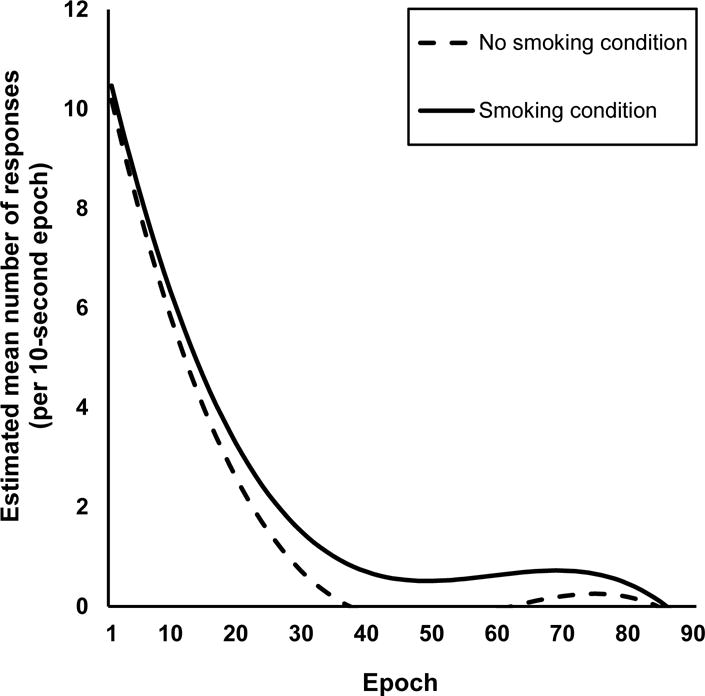
Line graph of estimated mean within-session responding, binned into 10-second epochs. Data were estimated using multi-level modeling. Fixed effects included in the model: intercept, epoch (linear term), epoch2 (quadratic term), epoch3 (cubic term), smoking condition, smoking condition × epoch, and smoking condition × epoch2. Random effects were included for the intercept and epoch (linear term). Solid black line represents responding during the smoking condition and the dashed line represents responding during the no smoking condition. Responding declined less sharply during the smoking (solid line) versus no smoking condition (dashed line), suggesting delayed habituation of the reinforcer’s efficacy due to acute nicotine.
Table 1.
Results of the final multilevel modeling analysis.
| Parameter | Value | Standard Error | |||
|---|---|---|---|---|---|
| Fixed Effects | |||||
| Composite Model | Intercept | γ00 | 10.18*** | 0.64 | |
| Epoch (linear term) | γ10 | − 0.57*** | 0.02 | ||
| Epoch2 (quadratic term) | γ20 | 0.01*** | 0.0004 | ||
| Epoch3 (cubic term) | γ30 | − 0.00006*** | 0.000003 | ||
| Smoking Condition | γ01 | 0.29 | 0.23 | ||
| Smoking Condition × Epoch | γ11 | 0.03* | 0.01 | ||
| Smoking Condition × Epoch2 | γ21 | − 0.0004** | 0.0001 | ||
| Variance Components | |||||
| Level 1: | Within-session |
|
8.57*** | 0.16 | |
| Level 2: | Intercept |
|
11.88*** | 3.02 | |
| Epoch (linear term) variance |
|
0.002*** | 0.001 | ||
| covar with intercept |
|
− 0.16*** | 0.04 | ||
p < .05,
p < .01,
p < .001
Follow-up analyses
Our prior research consistently showed that nicotine’s reinforcement enhancing effects were not secondary to withdrawal relief (which would suggest negative reinforcement) or to the initial efficacy of a reinforcer (Perkins & Karelitz, 2013a, 2013b). In the present study, we similarly examined whether withdrawal relief or magnitude of initial reinforcing efficacy related to nicotine’s delay of habituation. The first follow-up analysis tested whether the difference in rates of responding between smoking conditions may have been due to negative reinforcement (relief of withdrawal) rather than positive reinforcement (persistence of responding for pictorial reinforcer). When entered into the model as a continuous predictor, the effect of MNWS withdrawal (measured upon arrival to each session) was not significant (β = −0.02, SE = 0.04, p = .63), and other parameters in the model were unchanged. Another, secondary exploratory analysis tested for an effect of participants’ initial ratings of each reinforcer (i.e., whether patterns of within-session responding varied due to how participants rated the reinforcers). Participants’ ratings, entered as a continuous predictor, had no significant effect (β = 0.12, SE = 0.03, p = .57), and other parameters in the model were unchanged. Results of these exploratory analyses ruled out relief of withdrawal and individual differences in participants’ initial ratings of the reinforcers as influences on the rates of responding over time due to nicotine.
Discussion
The purpose of this study was to examine whether recent exposure to tobacco smoking versus no smoking following overnight abstinence would attenuate rate of decline in operant responding for a visual reinforcer (viewing an attractive photo), consistent with delay in the habituation of reinforcer effectiveness. To our knowledge, this is the first specific test of this concept in humans with acute administration of smoked nicotine, or any drug, and thus the first to translate the animal research examining nicotine’s influence on slowing habituation of reinforcer effectiveness to a human sample. Overall, total reinforced responding and the duration of responding were each significantly greater in the smoked nicotine condition compared to the no smoking condition, consistent with the notion of reinforcement enhancing effects of nicotine (Perkins et al., 2017). However, the most novel finding here emerged when examining the within-session patterns of responding, as the rates of responding declined less sharply during the smoking versus no smoking condition, suggesting delayed habituation of the reinforcer’s efficacy due to acute nicotine. Withdrawal relief from nicotine was not related to the patterns of within-session responding, ruling out the contribution of negative reinforcement. Overall, these results provide preliminary support for tobacco smoking’s ability to maintain the acute effectiveness of a reinforcer over a longer period of time, when compared to a no smoking control.
Clinical implications for the observed delay in habituation may be speculative, given the preliminary nature of the results. However, habituation to a reinforcing visual stimulus could partly help explain smoking persistence. Smokers commonly smoke when engaged in reinforcing leisure activities (Van Gucht, Van den Bergh, Beckers, Vansteenwagen, 2010) and most leisure activities U.S. adults typically engage in are tasks involving visual stimuli (e.g., watching TV, playing video games, using a computer, etc.; Bureau of Labor Statistics, U.S. Department of Labor, 2015). Taken together, smokers spend a significant part of their day engaging in activities involving visual rewards, so lapsing after trying to quit may be more likely at these times, perhaps partially due to more rapid habituation to visual rewards. In other words, extending the duration of reinforcing efficacy of visual rewards after a smoking lapse may attenuate the loss of enjoyment from those rewards due to maintaining abstinence, possibly promoting escalation of lapse to full relapse. Consistent with that possibility, earlier research has found that lapsing soon after initiating a quit attempt often happens at home when engaged in leisure activities that offer visual rewards, such as watching TV (Deiches, Baker, Lanza, & Piper, 2013).
Our findings are consistent with the previously discussed preclinical research, indicating that nicotine attenuates declines in operant responding attributed to habituation of reinforcer effectiveness (Gancarz, et. al, 2012; Lloyd et al., 2014a; Lloyd et al., 2014b). Although our results with humans need to be replicated, the consistency between preclinical and clinical paradigms lends further support to the translation of the behavioral effects of nicotine across species (O’Dell & Khroyan, 2009). This cross-species validation is important for demonstrating how findings from preclinical studies apply to human samples in clinical research.
Potential limitations of our study should be considered in designing research to replicate these results. The study design did not include experimental manipulations to confirm that the within-session declines in responding were due to habituation rather than another competing process (e.g., satiation, fatigue, etc.). However, satiation is an unlikely explanation for the observed within-session declines in responding when using a non-consummatory reinforcer (McSweeney, 2004). Also, fatigue seems unlikely given the modest response requirement for receipt of each reinforcer. Additional research using a dishabituating stimulus or a test of stimulus specificity (e.g., Kenzer, Ghezzi, Fuller, 2013) is needed to systematically confirm habituation and rule out alternate causes of within-session declines in responding.
While there was a cigarette smoking (non-abstinent) vs no smoking (abstinent) condition, there was no specifically matched placebo cigarette (i.e., denicotinized or very low nicotine cigarette) condition, to rule out simple smoking behavior per se. However, we have previously compared differences in reinforced responding due to sessions varying in nicotine smoking, denicotinized smoking, or no smoking conditions, finding effects due only to nicotine per se and not simple smoking behavior (Perkins, et al., 2013a, 2013b, 2014, 2017). Thus, simple smoking behavior would be unlikely to delay habituation relative to our no smoking control condition here, although this possibility remains to be empirically confirmed. Additionally, during the no smoking condition, participants received a placebo patch (at least three hours earlier) and administration of a placebo nasal spray (at least 20 minutes earlier) before the habituation trial of interest, as part of the larger project, again to equate expectations between sessions for receiving active nicotine. Although rather unlikely, it is conceivable that having received placebo patch and/or spray well before the habituation trial could have accelerated the decline in reinforced responding for the visual reinforcer beyond that during a simple no smoking condition. As such, follow up studies should include carefully matched comparison groups to allow for clearer causal relationships due to nicotine per se to be identified.
The current study was the first to directly test the effect of acute intake of any drug on habituation of reinforcer efficacy in humans. Our results provide preliminary support for the notion that, along with its other demonstrated reinforcing actions, nicotine from tobacco smoke maintains the reinforcing effectiveness of a preferred visual stimulus by delaying the rate of habituation to its reinforcing efficacy. Additional research utilizing manipulations to confirm habituation is needed to replicate our findings, test their generalizability with non-smoked nicotine administration and on duration of responding for other types of reinforcers, and more fully understand the mechanisms underlying nicotine’s role in this dynamic reinforcement process.
Acknowledgments
FUNDING
This research is based on a Master’s thesis conducted by JLK and presented at the 2017 meeting of the Society for Research on Nicotine and Tobacco in Florence, Italy. Research reported in this publication was supported by NIH Grants R01 DA035774 from NIDA (KAP) and T32 HL7560 (JLK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DECLARATION OF INTERESTS
None of the authors have any potential conflicts of interest to report.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Benowitz NL, Iii PJ, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics U.S. Department of Labor. Time spent in leisure activities in 2014 by gender, age, and educational attainment. The Economics Daily. 2015 Jun; https://www.bls.gov/opub/ted/2015/time-spent-in-leisure-activities-in-2014-by-gender-age-and-educational-attainment.htm.
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use. New York, NY: Springer; 2008. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184(3–4):353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Deiches JF, Baker TB, Lanza S, Piper ME. Early lapses in a cessation attempt: lapse contexts, cessation success, and predictors of early lapse. Nicotine & Tobacco Research. 2013;15(11):1883–1891. doi: 10.1093/ntr/ntt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Gancarz AM, Ashrafioun L, San George MA, Hausknecht KA, Hawk LW, Jr, Richards JB. Exploratory studies in sensory reinforcement in male rats: Effects of methamphetamine. Exp Clin Psychopharm. 2012;20(1):16–27. doi: 10.1037/a0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Smith TT, Kleykamp BA, Fant RV, Donny EC. Nicotine self-administration research: the legacy of Steven R. Goldberg and implications for regulation, health policy, and research. Psychopharmacology. 2016:1–20. doi: 10.1007/s00213-016-4441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Kenzer AL, Ghezzi PM, Fuller T. Stimulus specificity and dishabituation of operant responding in humans. Journal of the Experimental Analysis of Behavior. 2013;100(1):61–78. doi: 10.1002/jeab.29. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Hausknecht KA, Richards JB. Nicotine and methamphetamine disrupt habituation of sensory reinforcer effectiveness in male rats. Exp Clin Psychopharm. 2014a;22(2):166–175. doi: 10.1037/a0034741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. Habituation of reinforcer effectiveness. Front Integr Neurosci. 2014b;7:1–20. doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CM, Shek DT. How to plot growth curves based on SPSS output? Illustrations based on a study on adolescent development. International Journal on Disability and Human Development. 2014;13(2):183–190. doi: 10.1515/ijdhd-2014-0304. [DOI] [Google Scholar]
- McSweeney FK. Rate of reinforcement and session duration as determinants of within-session patterns of responding. Anim Learn Behav. 1992;20(2):160–169. doi: 10.3758/BF03200413. [DOI] [Google Scholar]
- McSweeney FK. Dynamic changes in reinforcer effectiveness: Satiation and habituation have different implications for theory and practice. Behav Analyst. 2004;27(2):171–188. doi: 10.1007/BF03393178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES. Sensitization and habituation regulate reinforcer effectiveness. Neurobiol Learn Mem. 2009;92(2):189–198. doi: 10.1016/j.nlm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Norman WD, Jongerius JL. Apple Picker: Computer software for studying human responding on concurrent and multiple schedules. Behav Res Methods Instrum Comput. 1985;17(2):222–225. doi: 10.3758/BF03214387. [DOI] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91(4):481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, O’Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology. 2011;219(4):1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013a;228(3):479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Influence of reinforcer magnitude and nicotine amount on smoking’s acute reinforcement enhancing effects. Drug Alcohol Depend. 2013b;133(1):167–171. doi: 10.1016/j.drugalcdep.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sensory reinforcement-enhancing effects of nicotine via smoking. Exp Clin Psychopharm. 2014;22(6):511–516. doi: 10.1037/a0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Boldry MC. Nicotine Acutely Enhances Reinforcement from Non-Drug Rewards in Humans. Front Psychiatry. 2017;8 doi: 10.3389/fpsyt.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Michael VC. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend. 2015;153:104–108. doi: 10.1016/j.drugalcdep.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. SAGE publications; 2001. [Google Scholar]
- Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. The Neuropharmacology of Nicotine Dependence. Springer; International Publishing: 2015. Behavioral mechanisms underlying nicotine reinforcement; pp. 19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek DT, Ma C. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. The Scientific World Journal. 2011;11:42–76. doi: 10.1100/tsw.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek DT, Ma CM. Application of SPSS linear mixed methods to adolescent development research: basic concepts and steps. International Journal on Disability and Human Development. 2014;13(2):169–182. doi: 10.1515/ijdhd-2014-0303. [DOI] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- Thompson RF. Habituation: a history. Neurobiol Learn Mem. 2009;92(2):127–134. doi: 10.1016/j.nlm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gucht D, Van den Bergh O, Beckers T, Vansteenwegen D. Smoking behavior in context: Where and when do people smoke? Journal of behavior therapy and experimental psychiatry. 2010;41(2):172–177. doi: 10.1016/j.jbtep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wright JM, Ren S, Constantin A, Clarke PB. Enhancement of a visual reinforcer by d-amphetamine and nicotine in adult rats: relation to habituation and food restriction. Psychopharmacology. 2018;235(3):803–814. doi: 10.1007/s00213-017-4796-1. [DOI] [PubMed] [Google Scholar]


