INTRODUCTION
Mother-to-child transmission (MTCT) of HIV remains a global health challenge despite being largely preventable. An estimated 150,000 children were infected with HIV in 2015, mostly through MTCT; most of these infections occurred in sub-Saharan Africa.[1] The Joint United Nations Programme on HIV/AIDS (UNAIDS) has renewed efforts to virtually eliminate MTCT, with a target of reducing the final rate to 5% or lower among breastfeeding populations, and 2% or lower among nonbreastfeeding populations.[2]
Effective interventions for the prevention of MTCT (PMTCT) are available, which, if implemented correctly, should achieve the UNAIDS goal of virtually eliminating pediatric HIV infection. The current WHO guidelines (called Option B+) recommend that all HIV-infected pregnant or breastfeeding women be initiated and maintained on antiretroviral therapy (ART) for life, regardless of clinical or immunologic status.[3] However, earlier WHO guidelines in 2010 recommended 2 different PMTCT regimens based on maternal CD4 cell count.[4] Both of these (called Option A and Option B) recommended lifelong ART for women with CD4 counts ≤350 cells/mm3. For women with higher CD4 cell counts, Option A included zidovudine from 14 weeks of pregnancy, single-dose nevirapine (sdNVP) during labor, and zidovudine/lamivudine through 1 week postpartum for women, plus daily nevirapine for infants until cessation of breastfeeding. Option B included ART for women during pregnancy and for the duration of breastfeeding, and nevirapine for infants for 6 weeks after birth. Data for HIV-free survival rates among HIV-exposed children at 18–24 months is limited for these options in a programmatic setting.
MTCT rate (i.e., the proportion of HIV-infected children born to HIV-infected mothers) and HIV-free survival rate (i.e., the proportion of HIV-exposed children who are alive and HIV uninfected at 18–24 months of age) are the key outcomes used to measure the effectiveness of PMTCT programs.[5] WHO recommends several methods, including modeling, facility-based surveys and cohort data, analysis of early infant diagnosis and child HIV testing data, and population-/community-based surveys, to measure MTCT and HIV-free survival rates at 18 months.[5] However, there are very few recent community-based evaluations. The PMTCT Effectiveness in Africa: Research and Linkages to Care (PEARL) study evaluated the effectiveness of PMTCT services at the community level in Cameroon, Côte d’Ivoire, South Africa, and Zambia, from 2007 to 2009. HIV-free survival at 24 months of age was 79.7% with the sdNVP regimen.[6] In 2009, a community survey in Rwanda estimated MTCT and HIV-free survival at 4% and 91.9%, respectively, among children aged 9–24 months who were on an AZT plus sdNVP regimen.[7]
Swaziland is a small country of 1.2 million people in southern Africa that has an estimated HIV prevalence of 28.8%[8] among adults and 41.1%[9] among pregnant women who attend antenatal care (ANC) clinics. Swaziland implemented the WHO Option A strategy for PMTCT from 2010 to 2014, at which time facilities transitioned to providing ART to all pregnant and postpartum women. No study, to our knowledge, has been done in Swaziland to determine the MTCT rate among children at the end of breastfeeding or to document their HIV-free survival rate. The primary objective of this study was to determine MTCT and HIV-free survival rates as estimates of the population-level effectiveness of a program based on Option A. This study will also serve as a baseline for Swaziland to assess the effectiveness of Option B+, which was adopted in 2015.
METHODS
Study design
We performed a cross-sectional study of children born to HIV-infected mothers between April and October 2013 to determine MTCT and HIV-free survival rates through a nationally representative community survey. The survey was conducted from April to June 2015.
Sampling
We used a multistage procedure to obtain a representative sample of HIV-infected mothers and HIV-exposed children from all 4 regions of the country. Constituencies (administrative districts) within each region were stratified as urban, rural high volume, or rural low volume. We randomly selected 1 constituency within each of the 3 strata for each region, for a total of 12 constituencies. Within each selected constituency, we randomly selected an enumeration area (EA) (similar to a census tract) from which recruitment began. Recruitment continued in the next-closest EA, according to a predetermined direction, until the approximated sample-size target was obtained. All eligible households were then surveyed. If the desired sample size for the constituency was not met, another EA was randomly selected until the desired sample size was achieved. The expected number of HIV-infected women who had given birth in each constituency was estimated in proportion to the constituency population size, and this was used to determine the sample size per constituency. In order to identify eligible households within the EAs, we engaged community health workers to identify households in which a mother had delivered a child between April 1 and October 30 in 2013, regardless of whether the child was currently alive. Study staff then visited each identified household to confirm whether a birth had occurred. If the mother or a primary caregiver was not available to complete the survey, a return appointment was made.
Measurements
Trained research assistants interviewed mothers primary caregivers using a structured questionnaire in SiSwati (the local language) that was uploaded to a portable electronic device. Maternal sociodemographic characteristics and clinical histories were collected. Information on PMTCT ART regimens and other clinical variables was obtained verbally and verified using data available on the mothers’ and children’s take-home ANC cards (when available).
If a mother was present in a household and did not have a verified HIV-seropositive status based on an ANC card, blood was drawn via a finger prick for rapid HIV testing (Alere Determine HIV-1/2, and Uni-Gold Recombigen® HIV). If the results of a rapid test were indeterminate or discrepant, we collected a dried blood spot (DBS) sample that was sent for DNA PCR (COBAS Ampliprep / Taqman HIV-1 QUAL Test System, ROCHE, New Jersey, USA) testing at the national reference laboratory in Mbabane, Swaziland. Children underwent HIV rapid antibody testing if their mother had a previously confirmed HIV-seropositive test or was found to be HIV seropositive during rapid testing, and in all cases in which the mother was not present in the household. We also collected DBS samples from all HIV-exposed children for DNA PCR testing at the national reference laboratory to confirm the rapid test results.
Data analysis
Data were analyzed using Stata Statistical Software (release 14, StataCorp LP, College Station, Texas, USA). We described the characteristics of the mothers using proportions for categorical variables and means with standard deviations (SD) for continuous variables. Our primary outcome of interest was the rate of HIV-free survival among HIV-exposed children, calculated as the number of children aged 18–24 months who were both alive and HIV uninfected, divided by the total number of children born to mothers confirmed to be HIV infected. We also calculated the MTCT rate as the proportion of HIV-exposed children who were confirmed to be HIV infected. We evaluated the relationship between maternal/child characteristics and MTCT of HIV, among children whose mothers were confirmed to be HIV infected. We used univariate and multivariate (multiple logistic regression) analysis to compute odds ratios (OR) and 95% confidence intervals (CI), with the rate of HIV-free survival among exposed infants as our primary outcome. Variables moderately associated (P<.1) with transmission in univariate analysis were included in the multivariate logistic regression. Children of mothers whose HIV status was unknown were excluded from the analysis. For women who had twin births, summaries of the variables were restricted to 1 of the twins. Women who were newly identified as HIV infected during the study were excluded from the analysis of variables for HIV care and treatment during pregnancy (eg, CD4-count testing and results, receipt of antiretroviral drugs for themselves and their infants).
Ethical considerations
The study was reviewed and approved by the Swaziland Scientific and Ethics Committee at the Ministry of Health, and by the institutional review board at George Washington University, Washington, DC, USA. Mothers and primary caregivers provided verbal informed consent, which was documented by the interviewer in study records.
RESULTS
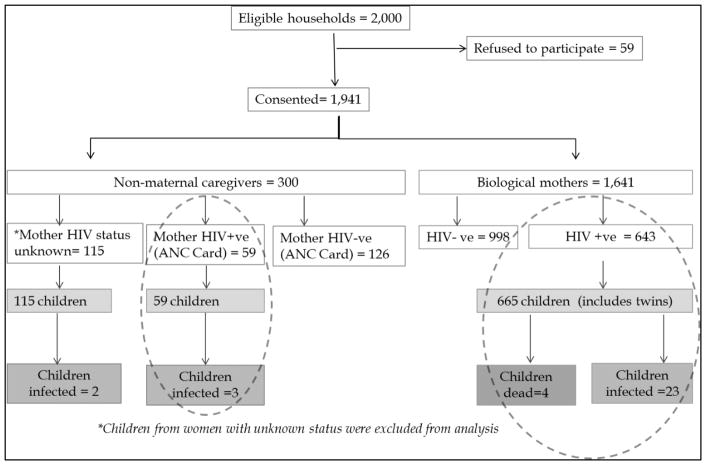
In total, there were 497 enumeration areas (EAs) in the 12 selected constituencies. Because of the small sizes of these EAs, 489 were visited in order to reach the desired sample size. We identified 2000 households that had a mother who had delivered a child between April and October in 2013; within these households, 1941 mothers and caregivers (97.1%) consented to participate in the study (Figure 1). A total of 59 refused, citing lack of time (n=10), lack of incentives for participation (n=2), discomfort with participating (n=8), not wanting to know their HIV status (n=4) or their child’s HIV status (n=3), lack of husband’s permission (n=6), and other reasons (n=26). The majority of study participants were the biological mothers of their children (84.5%, n=1641), and the rest were caregivers (n=300). Maternal HIV-positive status was observed in 643/1641 (39.2%) of the biological mothers. Caregivers reported that 144 biological mothers (48%) permanently lived elsewhere, 139 mothers (46.3%) lived elsewhere temporarily, 13 mothers (4.3%) had died, and the reason for mother’s absence was unknown in 4 households (1.3%). Caregivers reported (and confirmed with antenatal care [ANC] cards) 59 mothers as HIV infected and 126 mothers as HIV uninfected. However, caregivers did not know the HIV status of 115 mothers, and ANC cards were not available to review HIV testing information. A total of 724 children (including 22 sets of twins, none serodiscordant) were born to 702 HIV-infected women. Thirty-five (5.0%) of the 702 HIV-infected mothers were newly identified as HIV infected during study testing and therefore had not yet been initiated in HIV care. No children born to newly diagnosed mothers were HIV infected.
Figure 1.
Flow chart of study participants.
Table 1 presents the key characteristics of the HIV-infected mothers. The mean (±SD) age was 28.8 ± 5.8 years. Almost all mothers (99.7%) attended ANC at least once, and the mean (±SD) gestational age at first ANC visit was 15.9 ± 6.2 weeks. The mean (±SD) CD4 cell count at baseline was 493.7 ± 264.3 cellss/mm3. Most of the HIV-infected mothers (91.8%), excluding the 35 newly diagnosed women, received PMTCT drugs during pregnancy; 41.0% received AZT prophylaxis, 14.2% were on ART at the time of pregnancy, and 41.6% initiated ART during ANC. However, 22 women who received antiretroviral (ARV) drugs did not have a documented regimen. Most mothers (89.4%) delivered their child at a health facility; 13.5% reported that their child was hospitalized at some point after birth. At the time of the survey, 7.6% of mothers were still breastfeeding their child.
Table 1.
Characteristics of HIV-infected mothers and their children, Swaziland, 2015.
| Characteristic | na | Percentage |
|---|---|---|
| Mothers (Na=702) | ||
|
| ||
| Age, mean (SD), years | 28.8 ± 5.8 | |
| Education (N=694) | ||
| None | 40 | 5.8 |
| Primary | 205 | 29.5 |
| Secondary | 246 | 35.4 |
| Postsecondary | 203 | 29.3 |
| Marital status (701) | ||
| Married | 300 | 42.8 |
| Cohabiting | 88 | 12.6 |
| Separated/divorced/widowed | 21 | 3.0 |
| Never married | 292 | 41.6 |
| Attended ANC (N=698) | 696 | 99.7 |
| Gestational age at first ANC visit, mean ± SD (weeks) | 15.9 ± 6.2 | |
| CD4 test performed during ANC (N=635)b | 582 | 91.7 |
| CD4 cell count, mean ± SD (cells/mm3)b | 493.7 ± 264.3 | |
| CD4 cell count (cells/mm3) (N=506)b | ||
| ≥350 | 365 | 72.3 |
| <350 | 141 | 27.7 |
| Took ARVs during pregnancy (N=646)b | 593 | 91.8 |
| ARV regimen received (N=571)b | ||
| AZT only | 234 | 41.0 |
| Initiated on ART during pregnancy | 238 | 41.7 |
| Already on ART | 81 | 14.2 |
| Other | 18 | 3.2 |
|
| ||
| Children (N=702a,c) | ||
|
| ||
| Delivered at health facility (N=700) | 626 | 89.4 |
| Given NVPb (N=654) | 617 | 94.3 |
| Hospitalized after discharge (N=689) | 93 | 13.5 |
| Ever breastfed (N=702) | 604 | 86.0 |
| Still breastfeeding at 18–24 months (N=603) | 46 | 7.6 |
| Duration of breastfeeding, mean ± SD (months) | 9.3 ± 4.6 | |
| Age breastfeeding stopped (N=548) | ||
| 0–6 months | 234 | 42.7 |
| 6–12 months | 246 | 44.9 |
| >12 months | 68 | 12.4 |
N/n vary due to small amounts of missing data.
Excludes 35 women who were newly identified as HIV infected during the study.
Includes only 1 pair of twins.
Table 2 summarizes the main outcomes of the study. Among the 724 known HIV-exposed children aged 18 to 24 months, 26 were HIV infected and 694 were HIV uninfected and alive. Four HIV-exposed children had died within 24 months of birth, all with an unknown HIV status. The overall HIV-free survival rate at 18–24 months of age was 95.9% (95% CI, 94.1–97.2). The estimated proportion of HIV-infected children among known HIV-exposed children was 3.6% (95% CI, 2.4–5.2). If we assume that all 4 children who died with unknown HIV status had been HIV infected, then the estimated proportion of HIV-infected children would be 4.1% (95% CI, 2.7–5.5).
Table 2.
Estimated HIV-free survival and MTCT rates at 18–24 months of age among 724 HIV-exposed children, Swaziland, 2015.
| HIV-free survival rate (alive and HIV negative) | MTCT rate among known HIV-exposed children | MTCT rate if all dead children were HIV infected | |||
|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) |
| 694/724 | 95.9% (94.1–97.2) | 26/720a | 3.6% (2.4–5.2) | 30/724 | 4.1% (2.7–5.5) |
Excludes 4 children who died (HIV status was unknown).
Table 3 shows the association between a child’s HIV infection or death and selected maternal and child characteristics. The risk for child HIV infection or death was significantly associated with the mother’s age and whether she received PMTCT drugs and delivered at a facility. Adjusting for facility delivery, infant nevirapine duration, and the mother’s PMTCT regimen, for every 10-year increase in the mother’s age, the odds of HIV transmission or child death were reduced by 58% (adjusted odds ratio [aOR]=0.42; 95% CI, 0.17–1.00). Adjusting for maternal age, facility delivery, and duration of infant nevirapine, the following characteristics were significantly protective against child HIV infection or death, compared with taking no PMTCT drugs: receipt of AZT only (aOR=0.15; 95% CI, 0.04–0.62), ART initiation during pregnancy (aOR=0.14; 95% CI, 0.03–0.58), and being ART experienced (aOR=0.08; 95% CI, 0.01–0.78). Delivering at a health facility significantly reduced the odds of HIV infection or death of a child (aOR=0.33; 95% CI, 0.12–0.92). A mother’s education level, marital status, gestational age at first ANC visit, and breastfeeding duration were not associated with child HIV infection or death.
Table 3.
Characteristics of HIV-exposed infants and their mothers, and the association with HIV infection or death in the children, Swaziland, 2015.
| Characteristic | Na | HIV infected or dead Na (%) | mean ± SD | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|---|
| Mothers (N=702) | |||||
|
| |||||
| Age, for every 10 years increase | 697 | 29 | 26.3±4.3 | 0.38 (0.18–0.82) | 0.42 (0.17–1.00) |
| Education | |||||
| None | 40 | 2 (5.0) | Ref | ||
| Primary | 205 | 10 (4.9) | 0.97 (0.21–4.62) | ||
| Secondary | 246 | 7 (2.8) | 0.56 (0.11–2.78) | ||
| Postsecondary | 203 | 9 (4.4) | 0.88 (0.18–4.24) | ||
| Marital status | |||||
| Married | 300 | 11 (3.7) | Ref | ||
| Cohabitation | 88 | 6 (6.8) | 1.92 (0.69–5.36) | ||
| Separated/divorced/widowed | 21 | 0 (0) | n/a | ||
| Never married | 292 | 11 (3.8) | 1.03 (0.44–2.41) | ||
| Gestational age (weeks) at first ANC visit | 658 | 28 | 15.9±6.2 | 0.96 (0.89–1.03) | |
| CD4-count test done | |||||
| Yes | 582 | 18 (3.1) | 0.21 (0.08–0.53) | ||
| No | 53 | 7 (13.2) | |||
| CD4 cell count (cells/mm3) | |||||
| ≥350 | 365 | 7 (1.9) | Ref | ||
| <350 | 141 | 5 (3.5) | 1.88 (0.59–6.02) | ||
| Received PMTCT drugs during pregnancy | |||||
| 4 Yes | 593 | 17 (2.9) | 0.14 (0.06–0.34) | ||
| No | 53 | 9 (17.0) | Ref | ||
| PMTCT regimen/drug received | |||||
| None | 53 | 9 (17.0) | Ref | ||
| AZT only | 234 | 8 (3.4) | 0.17 (0.06–0.47) | 0.15 (0.04–0.62) | |
| Initiated ART during pregnancy | 238 | 6 (2.5) | 0.13 (0.04–0.37) | 0.14 (0.03–0.58) | |
| Already on ART | 81 | 1 (1.2) | 0.06 (0.01–0.50) | 0.08 (0.01–0.78) | |
|
| |||||
| Children (N=702b) | |||||
|
| |||||
| Facility delivery | |||||
| Yes | 626 | 20 (3.2) | 0.32 (0.13–0.77) | 0.33 (0.12–0.92) | |
| No | 74 | 7 (9.5) | Ref | ||
| Given NVP | |||||
| Yes | 617 | 22 (3.6) | 0.31 (0.10–0.94) | ||
| No | 37 | 4 (10.8) | Ref | ||
| Infant duration on NVP | |||||
| Never | 37 | 4 (10.8) | Ref | Ref | |
| 6 weeks | 266 | 10 (3.8) | 0.32 (0.10–1.08) | 2.36 (0.45–12.42) | |
| Throughout breastfeeding | 240 | 5 (2.1) | 0.18 (0.04–0.69) | 1.58 (0.24–10.51) | |
| Stopped during breastfeeding | 91 | 5 (5.5) | 0.48 (0.12–1.89) | 3.77 (0.68–20.95) | |
| Duration of breastfeeding | 19 | 10.3 ± 3.9 | 1.06 (0.96–1.17) | ||
| Age breastfeeding stopped | |||||
| No breastfeeding | 98 | 4 (4.1) | Ref | ||
| 0–6 months | 234 | 6 (2.6) | 0.62 (0.17–2.24) | ||
| 6–12 months | 246 | 11 (4.5) | 1.10 (0.34–3.54) | ||
| >12 months | 68 | 2 (2.9) | 0.71 (0.13–4.00) | ||
N/n vary due to small amounts of missing data.
Includes only 1 pair of twins.
DISCUSSION
Our community household survey in Swaziland found that HIV-free survival at 18–24 months of age among children born to HIV-infected women was very high at 95.9%. The results of this 2015 survey measure the population-level effectiveness of the Swaziland PMTCT program that was implementing Option A of the WHO guidelines available at that time. Other community surveys have found considerably lower HIV-free survival rate estimates with implementation of less effective PMTCT regimens (sdNVP, AZT/sdNVP). In Zimbabwe, HIV-free survival at age 9–18 months was 90.5% before implementation of Option A.[10] In a survey of 4 African countries before implementation of Option A, HIV-free survival at 24 months of age among all HIV-exposed children was 79.7%. The country-level estimates were Cameroon, 72.6%; South Africa, 77.7%; Zambia, 83.1%; and Côte d’Ivoire, 84.4%.[6] These pre-Option A rates of HIV-free survival were lower than the estimates in our study, suggesting better effectiveness of Option A compared with previous PMTCT regimens.
HIV-free survival estimates in other country surveys after the Option A implementation were comparable with our estimates. In Rwanda, a similar community survey reported an HIV-free survival rate of 91.9% (95% CI, 90.4–93.3) among HIV-exposed children aged 9 to 24 months.[7] In a community-based survey in Zimbabwe in 2014 (post-Option A rollout), HIV-free survival was reported as 95.1%.[11] In South Africa, results in a prospective facility-based cohort study showed an HIV-free survival rate of 93.7% at 18 months[12], while in Nigeria, HIV-free survival was reported to be 86.2% at 18 months in a facility-based retrospective cohort study.[13]
HIV-free survival has been proposed as the gold standard for measurement of PMTCT program effectiveness.[14] Our study’s high estimate of HIV-free survival could be an indication of a strong PMTCT program in Swaziland. It could also be an overestimate, however, because there were very few child deaths captured, despite efforts to identify households that had had deliveries during the specified period and the child had died. This could be due to cultural difficulties in discussing child death, which might have limited our identification of such households. The Swaziland Multiple Indicator Cluster Survey (MICS) of 2014[15] found infant mortality at 50 per 1000 and mortality of children younger than 5 years at 67 per 1000. Given that 50% of HIV-infected children without treatment die by the age of 2 years, MTCT rates could be higher and HIV-free survival rates lower if our survey did not capture all child deaths in these communities.
Population-based surveys are one of the WHO-recommended methods for estimating MTCT of HIV at age 18 months or the end of breastfeeding.[5] Few studies have reported community-based prevalence estimates at 18–24 months. Our study found MTCT among known HIV-exposed children to be 3.6% at 18–24 months, which, as expected, is slightly higher than the 2.4% that was previously documented at 6–8 weeks [16] in Swaziland, due to postnatal HIV transmission during breastfeeding. Taha et al reported the cumulative risk for MTCT at age 24 months was 9.7% among breastfed children in Malawi who received short ARV prophylactic regimens and were uninfected at 1.5 months, with 87.4% of infections occurring after 6 months.[17] In South Africa, cohort data showed a cumulative MTCT rate of 4.3% at age 18 months.[12] In a Rwanda community survey, MTCT was reported to be 4.0% at 9–24 months, similar to our findings.[7] In Zimbabwe, MTCT of HIV was estimated at 4.8% in the post-Option A implementation period.[11] Using Spectrum models, MTCT in 2015 in Swaziland is estimated at 6.72% by the end of breastfeeding.[18] The difference between our findings and the Spectrum estimates may be due to differences in methodology (i.e., use of models vs observational study) and the possibility that our study had inaccurate ascertainment of child death, as discussed earlier. Assuming that all 4 children who had died were HIV infected, our MTCT rate would be 4.1%.
Our study also explored factors associated with child HIV infection and/or death. The factors associated with MTCT have been well documented in the literature.[19] Data on MTCT risk factors that were collected in our study as well as in previous studies include breastfeeding duration, CD4 count, use of ARVs, and maternal age. The factors identified in our study to be associated with increased, or markers of, child infection and/or death were young maternal age, not delivering at a health facility, and not receiving PMTCT drugs during pregnancy. In addition to strengthening uptake of PMTCT services for pregnant women, interventions to improve rates of HIV-free survival need to target younger women and to encourage facility delivery. Our study contributes to the body of evidence for factors associated with MTCT of HIV and HIV-free survival. Although mixed feeding and long duration of breastfeeding have been documented to be associated with MTCT,[19] our study did not show an association between duration of breastfeeding and child HIV infection and/or death. This could be due to the comprehensive use of nevirapine by the child during the breastfeeding period (94% of infants in our study received nevirapine prophylaxis), which has been shown to reduce MTCT.[20]
In addition to the possibility of missing children who had died, our study had other limitations. We did not collect any data on paternal characteristics, which can impact rates of HIV infection and HIV-free survival, as shown in the literature.[21] Our study also did not collect data on possible maternal and child migration from the study communities, which could have resulted in either over- or underestimating the HIV-free survival rates. Our study had 115 women with an unknown HIV status, and 2 HIV-positive children identified within this group; these women and children were excluded in our analysis, which could have resulted in over- or underestimating the rate of HIV-free survival. Another potential limitation of our study is that we did not use weighting to adjust for any potential differences in HIV-free survival in urban and rural constituencies. Also, potential differences in the number of HIV-infected women in rural and urban constituencies may not have been reflected in our study sample. Because we did not have the true population distribution numbers for HIV-infected women across rural and urban constituencies, we could not estimate the weights. We randomly selected constituencies within strata, and all households were approached; therefore, we expect that the distribution of HIV-infected women across rural and urban constituencies was maintained in the sample.
CONCLUSION
This is the first community survey conducted in Swaziland to assess the effectiveness of the PMTCT program that was implemented under Option A of the WHO guidelines. The target for PMTCT programs is to reduce final mother-to-child transmission of HIV to 5% or lower among breastfeeding populations, and 2% or lower among nonbreastfeeding populations. Findings from our study indicate that the Swaziland PMTCT program under Option A has been largely effective, with a high HIV-free survival rate of 95.9% and a low MTCT rate of 3.6% at age 18–24 months. Given the national rollout of Option B+ in late 2014, a similar household survey in 2 years is recommended to measure the effectiveness of these most recent guidelines. In any subsequent survey, efforts should be intensified to identify all households that have had a child death.
Acknowledgments
We would like to thank Samkelo Dladla- the Study Coordinator; Nelisiwe Masilela- the Field Coordinator; and Sandile Mchunu- the Data Management Officer for the great work in coordinating the study and ensuring timely completion. We would also like to thank all the study team (regional team leaders, data collectors, HIV testing counselors, and drivers) for ensuring timely and quality data collection. We also thank Epiphanie Nyirabahizi who conducted the initial and preliminary data analysis for the study. We also acknowledge all the mothers, caregivers and children who agreed to participate in this study.
We wish to acknowledge support from the University of California, San Francisco’s International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25MH064712.
Funding: This study was made possible through funding from PEPFAR through USAID under the Eliminating Paediatric AIDS in Swaziland (EPAS) Project Cooperative Agreement #674-A-00-11-00009-00.
Footnotes
Ethical considerations
The study was reviewed and approved by the Swaziland Scientific and Ethics Committee at the Ministry of Health, and the institutional review board of George Washington University, Washington, DC, USA. The mother or the primary caregiver provided verbal informed consent, which was documented by the interviewer in study records.
Competing interests
The authors declare that they have no competing interests.
Disclaimer: The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of USAID, PEPFAR or the US Government.
Authors’ contribution
CC designed study, wrote the protocol, oversaw study implementation, assisted in data analysis and wrote the manuscript. RM conducted data analysis. SM, MM, MMG, GM, GW and LG helped in designing study, developing the protocol, data analysis and writing the manuscript. KK helped in designing study, developing the protocol, supervising study implementation, data analysis and writing the manuscript. KL helped in writing of the manuscript. All authors read and approved the final manuscript.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed May 25, 2016];Fact sheet. 2015 http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed June 30, 2016];2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf. Published 2015.
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach second edition, 2016. Geneva, Switzerland: World Health Organization; [Accessed August 16, 2016]. http://www.who.int/hiv/pub/arv/arv-2016/en/. Published 2016. [Google Scholar]
- 4.World Health Organization. [Accessed May 20, 2016];Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. Published 2010. [PubMed]
- 5.World Health Organization. [Accessed May 20, 2016];A short guide on methods: measuring the impact of national PMTCT programmes towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. http://apps.who.int/iris/bitstream/10665/75478/1/9789241504362_eng.pdf. Published 2012.
- 6.Stringer JSA, Stinson K, Tih PM, et al. Measuring coverage in MNCH: population HIV-free survival among children under two years of age in four African countries. PLoS Med. 2013;10(5):e1001424. doi: 10.1371/journal.pmed.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruton H, Mugwaneza P, Shema N, et al. HIV-free survival among nine- to 24-month-old children born to HIV-positive mothers in the Rwandan national PMTCT programme: a community-based household survey. J Int AIDS Soc. 2012;15(1):4. doi: 10.1186/1758-2652-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed June 20, 2016];Joint United Nations Programme on HIV/AIDS (UNAIDS) http://aidsinfo.unaids.org/#. Published 2016.
- 9.Swaziland Ministry of Health. 2010 ANC Sentinel Surveillance. Mbabane: Ministry of Health; 2010. [Google Scholar]
- 10.Buzdugan R, McCoy SI, Watadzaushe C, et al. Evaluating the impact of Zimbabwe’s prevention of mother-to-child HIV transmission program: population-level estimates of HIV-free infant survival pre-Option A. PLoS One. 2015;0(8):e0134571. doi: 10.1371/journal.pone.0134571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzdugan R, Kang Dufour M-S, McCoy SI, et al. Option A improved HIV-free infant survival and mother to child HIV transmission at 9 18 months in Zimbabwe. AIDS. 2016;30:1655–1662. doi: 10.1097/QAD.0000000000001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goga A. Highest risk of mother-to-child transmission of HIV or death in the first 6 months postpartum: results from 18 month follow-up of an HIV-exposed cohort, South Africa. 21st International AIDS Conference; Durban. 2016; abstract TUAE0106. [Google Scholar]
- 13.Anígilájé EA, Dabit OJ, Olutola A, Ageda B, Aderibigbe SA. HIV-free survival according to the early infant-feeding practices; a retrospective study in an anti-retroviral therapy programme in Makurdi, Nigeria. BMC Infect Dis. 2015;15:132. doi: 10.1186/s12879-015-0871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86(1):57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Central Statistical Office. Swaziland Multiple Indicator Cluster Survey 2014, Key Findings. Mbabane, Swaziland: 2015. [Google Scholar]
- 16.Swaziland Ministry of Health. Evaluation of the Effectiveness of the National Prevention of Mother to Child Transmission of HIV (PMTCT) Programme at 6–8 Weeks Post-partum; Swaziland. Mbabane, Swaziland: 2012. [Google Scholar]
- 17.Taha TE, Hoover DR, Kumwenda NI, et al. Late postnatal transmission of HIV-1 and associated factors. J Infect Dis. 2007;196:10–14. doi: 10.1086/518511. [DOI] [PubMed] [Google Scholar]
- 18.Swaziland Ministry of Health. Swaziland HIV Estimates and Projections Report. Mbabane, Swaziland: 2015. [Google Scholar]
- 19.Newell ML. Prevention of mother-to-child transmission of HIV: challenges for the current decade. Bull World Health Organ. 2001;79(12):1138–1144. [PMC free article] [PubMed] [Google Scholar]
- 20.Omer SB. Six Week Extended Dose Nevirapine (SWEN) Study Team. Twelve-month follow-up of Six Week Extended Dose Nevirapine randomized controlled trials: differential impact of extended-dose nevirapine on mother-to-child transmission and infant death by maternal CD4 cell count. AIDS. 2011;25(6):767–776. doi: 10.1097/QAD.0b013e328344c12a. [DOI] [PubMed] [Google Scholar]
- 21.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]