Abstract
Umbilical cord blood transplantation (UCBT) has a high early mortality rate primarily related to transplanted stem cell dose. To decrease early mortality and enhance engraftment, a portion of selected cord blood units (20-50%) was expanded with cytokines and the copper chelator tetraethylenepentamine (carlecortemcel-L) and transplanted with the un-manipulated fraction after myeloablative conditioning. The primary endpoint was 100 day survival which was compared to a contemporaneous double-unit cord blood transplant (DUCBT) group. We enrolled 101 patients at 25 sites; the DUCBT comparison (n = 295) was selected from international registries using study eligibility criteria. Baseline carlecortemcel-L study group unit nucleated cell (NC) and CD34+ cell dose/kg were 3·06 × 107 and 1·64 × 105. Median NC and CD34+ fold expansion were 400 and 77, with a mean total CD34/kg infused of 9.7 × 105/kg. The 100 day survival was 84.2% for the carlecortemcel-L study group vs 74.6% for the DUCBT group [odds ratio 0.50 (95% CI 0·26-0.95); p = 0.035]. Survival at day 180 was similar for the two groups; the major cause of death after day 100 was opportunistic infections. Faster median neutrophil (21 vs 28 days; p < 0.0001), and platelet (54 vs 105 days; p =0.008) engraftment was seen in the carlecortemcel-L study group; acute and chronic GVHD rates were similar. In this multi-national comparative study, transplanting expanded CD34+ stem cells from a portion of a single UCB unit, with the remaining un-manipulated fraction improved 100 day survival compared to DUCBT controls while facilitating myeloid and platelet engraftment. This trial was registered at www.clinicaltrials.gov as #NCT00469729.
Keywords: ex vivo, umbilical cord blood, tansplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT), potentially curative for patients with hematologic malignancies, is limited by the availability of suitably matched donors. Only 20-30% of patients have a human leukocyte antigen (HLA)-matched sibling and while HLA-matched unrelated donors (MUD) can now be identified for many, only 25% of patients from ethnic or racial minority groups have such donors.1–2 To address this unmet need, umbilical cord blood transplantation (UCBT) has been utilized as HLA matching criteria are not as stringent as other matched donor transplants.3–6 However, UCBT in adults is limited because the stem cell dose/kg is often only 10% of a typical transplant, frequently inadequate to achieve prompt hematopoietic reconstitution, causing high early morbidity and mortality.7 Increasing, related haploidentical HSCT are being used for patients without donors8–10, and while potentially curative for a number of hematologic malignancies, these transplants are associated with a high rate of graft rejection and relapse.
As infusing a higher number of HSC, identified as CD34+, leads to earlier engraftment for patients undergoing UCBT and thus potentially improving transplant outcome, several strategies are being explored to maximize infused CD34. The leading strategy to date has been to combine two UCBUs (double UCB, DUCBT)11–13; which is of advantage mainly in adults undergoing CBT if the cell dose in their single unit graft is < 3.5 107/kg, while in pediatric patients with appropriately sized (> 3.5 107/kg) single UCB unit there seems to be no need for 2 cord blood units (CBUs)14–15. Another alternative is to transplant expanded HSC grown ex vivo from an UCB unit, increasing the infused CD34+ cell dose and thus facilitating engraftment and potentially improving transplantation outcome16. One of the first such methodologies evaluated is the method utilized in the current clinical trial. Specifically, carlecortemcel-L (StemEx®) is a stem/progenitor cell-based therapy product composed of ex vivo expanded allogeneic UCB cells expanded with cytokines and the copper chelator TEPA. Units selected from FACT/Netcord approved cord blood banks (CBB) were suitable for the study only if cryopreserved in two fractions. The larger (or equal) fraction, containing at least 1×107 total nucleated cells (TNC)/kg body weight to be served as the un manipulated fraction. While, HSC from the smaller (or equal) fraction to be expanded, underwent CD133+ selection followed by incubation for 21 days with 4 cytokines cocktail (SCF, TPO, IL-6 and FLT3-L), in order to promote proliferation, and the copper chelator TEPA, which inhibits transiently stem cell differentiation during the expansion period yielding a 225% expansion of the CD34+ HSC 17,18. The expanded product, enriched in early progenitor cells was then transplanted together with the remaining un-manipulated fraction.
Based on pre-clinical expansion efficacy an adult Phase I/II study was undertaken.19 Ten patients with hematological malignancies were transplanted with carlecortemcel-L and unfractionated NC from the same unit after myeloablative conditioning with impressively only a 10% 100 day mortality rate, significantly less than the 40% seen in other UCBT trials of that era20–24, without severe acute GvHD (aGVHD). Based on this scientific and clinical background, a multicenter concurrent cohort-controlled transplant study was undertaken with the primary end point to reduce the high 100 day mortality associated with UCBT.
Patients and Methods
Design and Study Population
The study was designed as a 100 patient cohort-controlled study to evaluate the efficacy and safety of transplantation of carlecortemcel-L, UCB stem and progenitor cells expanded ex vivo from a portion of a single UCB unit along with the remaining unexpanded cells, in patients with hematologic malignancies undergoing UCBT following myeloablative therapy. Outcomes were compared to a contemporaneous pre-specified cohort of all patients between the years 2006-2010 who underwent myeloablative non-expanded DUCB transplants and whose data were reported to the Center for International Bone Marrow Transplant Research (CIBMTR), and Eurocord cord blood registries. The final comparison group consisted of all such patients who met the same eligibility criteria as for the carlecortemcel-L study group. The study was a multicenter trial that was conducted at 25 international sites in Europe, US and Israel.
The study population comprised adult and adolescent subjects (aged 12-55 years) with hematologic malignancies in remission or early relapse (≤ 10% blasts) who were eligible and in need of an allogeneic HSCT and without an HLA-matched or 1 antigen mismatched sibling donor. UCB units had to be matched at 4, 5 or 6/6 HLA class I (HLA-A and –B, low resolution) and II (HLA-DRB1, high resolution). The complete inclusion and exclusion criteria are shown in Supplementary Table S1 and were identical for the study group and the DUCBT controls. Unit selection was determined locally with units chosen primarily by match grade and nucleated cell dose/kg. Selected units were red cell depleted and volume reduced prior to cryopreservation and preserved in 2 portions of which the larger (or equal) portion contained an estimated minimum of pre-thaw nucleated cells of 1.0 × 107/kg. It was mandatory to have for each patient on the study a back-up stem cell source (either autologous graft or alternative cord blood) available. All patients received a myeloablative regimen specified by the protocol (eligibility and preparative regimens as per supplementary information). GVHD prophylaxis consisted of either cyclosporine or tacrolimus combined with mycophenolate mofetil (supplementary information).
Enrollment began October, 2007 and concluded February, 2012 at its accrual goal. The trial was designed by the sponsor of the trial, Gamida Cell, in concert with the investigators and approved by the US Food and Drug Administration (NCT00469729). The data were gathered by Gamida Cell and analyzed at the Biostatistics Unit of the Gertner Institute for Epidemiology. All authors reviewed the data and manuscript, affirm its completeness and accuracy and agree with submission of the manuscript for publications. The first draft was written by the first author.
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the local institutional review boards. Patients were required to provide written informed consent prior to enrollment.
Treatment Schema
After the determination of patient and UCB unit eligibility, the selected CBU was shipped to a central expansion GMP approved production site. The unit was separated into two fractions and the larger (or equal) portion was shipped cryopreserved to the clinical site, while the portion to be expanded remained at the expansion facility. The expansion process began on day -22 with the patients beginning their pre CBT myeloablative conditioning beginning on day -8 to -10 as shown (Figure 1) using one of five prescribed conditioning regimens (supplementary information).
Figure 1.
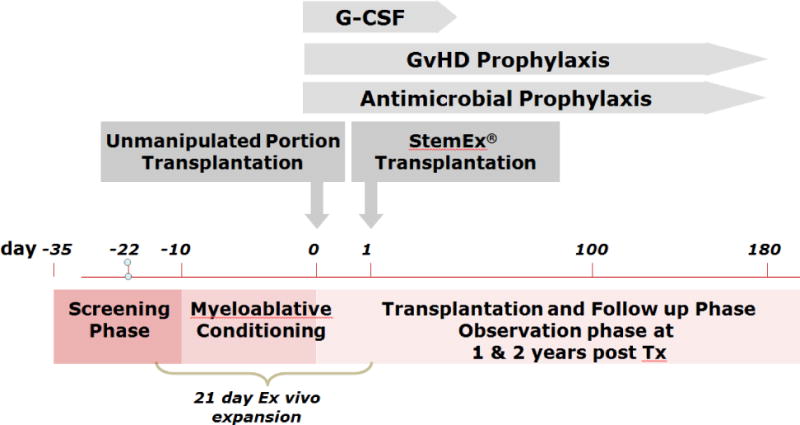
Study Schema
Production of the expanded cell product, carlecortemcel-L, consisted of the ex vivo culture of the smaller or equally sized CBU portion in the presence of the cytokines thrombopoietin, interleukin 6, flt-3L, and stem cell factor each at a concentration of 50 ng/ml, and 5 μM of the copper chelator TEPA (tetraethylenepentamine), for 21 days (Figure 2). On day of transplant patients received the unmanipulated, cryopreserved portion of the UCB unit and on that date the carlecortemcel-L expanded unit was prepared and transported to the transplant center by a courier with its infusion on day + 1.
Figure 2.
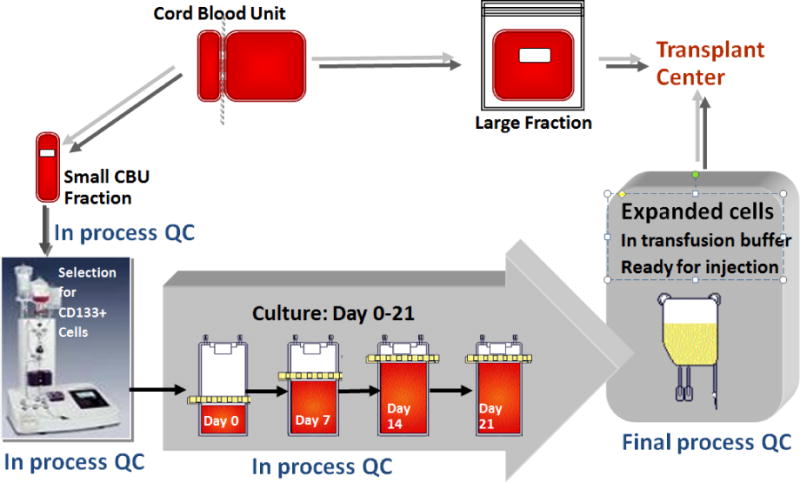
Expansion Schema
Post-transplant, patients received G-CSF(5 μg/kg/day)starting on day +5 until the absolute neutrophil count reached 2.5 × 109/kg. If aGVHD developed, treatment was per institutional protocols as was infection prophylaxis and therapy, and transfusion support.
Endpoints
The primary objective end point was to assess the effectiveness of a UCB transplant consisting of both unmanipulated and carlecortemcel-L expanded cells in comparison with a DUCBT 2006-2010 contemporaneous control group from US and European registries as measured by 100-day overall survival. Both safety and futility assessments included all subjects who signed consent, and those transplanted with only the unmanipulated fraction of UCB cells were included in the carlecortemcel-L study group using intent to treat (ITT) analysis. However when the trial was first conceived in 2005, a different control group, specifically a single unit UCBT group was selected from Eurocord, the New York Blood Center registry and the CIBMTR groups from the years 1995-2005. This group formed the basis of the initial comparison to this current carlecortemcel-L study group, also a single unit UCBT control group, and used the same selection criteria as the current control group for matching with the patients actually entered on trial. However as in 2006 and beyond, e.g the time period of enrollment for this trial the field had evolved to using two units for transplantation, so a second and now contemporaneous control group of DUCBT was selected as the comparator group and used for this analysis.
Secondary efficacy end points were: time from infusion of UCB cells to death from any cause up to day 100, proportion of overall mortality at 180 days, time from infusion of UCB cells to death from any cause up to 180 days, proportion of subjects who developed aGvHD grades III-IV,25 proportion of subjects with neutrophil engraftment failure. Neutrophil engraftment was defiuned as the first date of three consecutive days in which the neutrophil count was ≥ 500/mm3, and platelet engraftment was defined as the first date of three consecutive days in which the platelet count was ≥ 20,00/mm3 without platelet transfusion support for the previous 7 days. Adverse events and acute toxicities of the expanded cell infusions were also assessed.26
The study also had several supportive efficacy endpoints assessing early engraftment and its relation to mortality, as well as exploratory endpoints assessing time to neutrophil and platelet engraftment and additional explorations of the relationships of graft characteristics to clinical outcomes.
Statistical Analysis
Survival estimates were calculated using the Kaplan-Meier estimator and confidence intervals were calculated by Greenwood’s method. For the efficacy analysis, a test for superiority of the outcome of the carlecortemcel-L study group over the control group was performed. The test was one-sided at a significance level of 0.025. The primary analysis tested the log odds ratio of 100-day mortality for carlecortemcel-L study group versus the DUCBT control group.27,28 The null and alternative hypotheses were: H0 : β=0 versus H1 : β<0. The overall one-tailed 2.5% level test is equivalent to a two-tailed 5% significance level, except that there was no formal final test that the outcome for the carlecortemcel-L study group compares unfavorably to the DUCBT control group for the primary endpoint (protection against this event is provided by the safety and futility analyses). The two-sided p-value will be reported in this analysis.
The 100-day mortality outcome was compared with the DUCBT 2006-2010 control group via a logistic regression analysis. The model included: disease risk (early leukemia, intermediate leukemia (including MDS), advanced leukemia, lymphoma), age (<18 years, 18-39 years, ≥40 years), CMV status, gender, and treatment group, The covariates to be included in the model were determined in a pre-trial meta-analysis of the original historical single unit UCBT control group.7 Engraftment and acute GVHD rates were calculated as cumulative incidence rates considering death as the competing risk, and the times to event were compared using the Fine and Gray method.
Results
Patient Characteristics
A total of 126 subjects were screened for enrollment into this study (Figure 3). There were 25 screen failures. Thus, 101 subjects were enrolled into the study at the 25 international clinical sites (US 14, Spain 6, Israel 3, Italy 1, Hungary 1).
Figure 3.
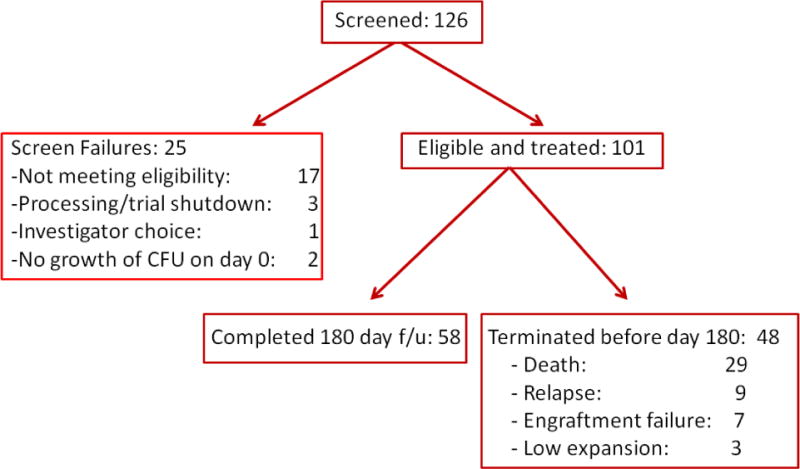
Consort Diagram
Key demographics and baseline characteristics are summarized for the carlecortemcel-L study group and DUCBT 2006-2010 control group are shown in Table 1. Compared to the DUCBT control group, the carlecortemcel-L study group had a lower proportion of patients aged <18 years (10.9% vs. 24.4%) and a higher proportion of patients aged ≥40 years (42.6% vs. 19.7%), as well as a lower proportion of patients with intermediate risk leukemia29 (33.7% vs. 54.2%) and a higher proportion of subjects with early leukemia29 (52.5% vs. 35.6%) and lymphoma (11.9% vs. 1.7%). These differences are adjusted for in the efficacy analyses when comparing the primary endpoint between the two groups.
Table 1.
Demographics and baseline characteristics (ITT analysis set)
| Number (%) subjects | |||
|---|---|---|---|
| Parameter | StemEx N=101 | DUCBT 2006–2010 N=295 | P value |
| Age | <.0001ℓ | ||
| <18 years | 11 (10.9%) | 72 (24.4%) | |
| 18–39 years | 47 (46.5%) | 165 (55.9%) | |
| ≥40 years | 43 (42.6%) | 58 (19.7%) | |
| Sex | 0.0903ℓ | ||
| Male | 47 (46.5%) | 166 (56.3%) | |
| Female | 54 (53.5%) | 129 (43.7%) | |
| Weight | 0.5113ℓ | ||
| ≤55 kg | 18 (17.8%) | 67 (22.8%) | |
| 56–75 kg | 45 (44.6%) | 116 (39.5%) | |
| >75 kg | 38 (37.6%) | 111 (37.8%) | |
| Disease | 0.0005£ | ||
| ALL | 30 (29.7%) | 127 (43.1%) | |
| AML | 43 (42.6%) | 125 (42.4%) | |
| CML | 8 (7.9%) | 18 (6.1%) | |
| MDS | 8 (7.9%) | 20 (6.8%) | |
| HD | 3 (3.0%) | 0 (–) | |
| NHL | 9 (8.9%) | 5 (1.7%) | |
| Disease risk | <.0001ℓ | ||
| Early leukemia | 53 (52.5%) | 105 (35.6%) | |
| Intermediate leukemia | 34 (33.7%) | 160 (54.2%) | |
| Advanced leukemia | 2 (2.0%) | 25 (8.5%) | |
| Lymphoma | 12 (11.9%) | 5 (1.7%) | |
| CMV status at baseline | 0.6ℓ | ||
| Negative | 34 (33.7%) | 103 (34.9%) | |
| Positive | 67 (66.3%) | 178 (60.3%) | |
| Missing | 0 (–) | 14 (4.8%) | |
By χ2 (chisq) test
By Fisher exact test
Expansion Data
The baseline UCB unit characteristics of the carlecortemcel-L study group and DUCBT control group are shown in Table 2. As expected the TNC and CD34/kg cell doses were higher for the DUCBT group. Considering the best matched of the 2 units in the DUCBT group, there was a higher proportion of better matched units for this group. There were 8 (8%) expansion failures in the carlecortemcel-L study group related to UCB unit quality. The reasons for the expansion failures were in 5, low or no growth of CFU-GM colony growth on day 0 of expansion (defined as < 10 CFU per 1000 plated cells), 2 had CD34 cell doses too low for expansion and one had an expansion less than 50 fold. Of these 8 patients, 4 went on to transplant with an alternative unit, 3 received the unmanipulated cells with a second unit and the one with a low expansion continued on trial. In the other 93 carlecortemcel-L study group patients the unmanipulated cells from the same unit were successfully transplanted within the stability limits of 18 hours, at a median time from production to infusion end of 11.55 hours (range 5.48–17).
Table 2.
Baseline CBU characteristics
| Characteristic | Carlecortemcel-L Group N=101 |
DUCBT 2006–2010 N=295 |
P value |
|---|---|---|---|
| N: Total NC dose ×107/kg | 101 | 265 | |
| Median dose1 (range) | 3.06 (Min 1.29 – Max 11.00) |
4.94 (Min 2.28 – Max 24.90) |
|
| N: Total CD34 ×105/kg | 101 | 248 | |
| Median (range) |
1.41 (Min 0.02 – Max 11739) |
2.07 (Min 0.39 – Max 9.45) |
|
| N: HLA match – minimal2 | 101 | 266 | |
| 0.9 | |||
| 4/6 2 | 70 (69.3%) | 182 (68.4%) | |
| 5/6 1 | 28 (27.7%) | 78 (29.3%) | |
| 6/6 0 | 3 (3.0%) | 6 (2.3%) | |
| N: HLA match – maximal3 | 101 | 266 | |
| 0.0001 | |||
| 4/6 2 | 70 (69.3%) | 131 (49.3%) | |
| 5/6 1 | 28 (27.7%) | 109 (41.0%) | |
| 6/6 0 | 3 (3.0%) | 26 (9.8%) |
Cell dose for the single unit used, or the sum of the cell doses for the two units used.
Minimal DUCBT HLA match: of the two UCBUs transplanted, the match of the lesser matched unit is counted, e.g., if one UCBU is 4/6 matched to the patient, and the other is 5/6 matched to the patient, then 4/6 is counted.
Maximal DUCBT HLA match: f the two UCBUs transplanted the match of the better matched UCBU is counted, e.g., if one unit is 4/6 matched to the patient, and the other is 5/6 matched to the patient, then 5/6 is counted.
The expansion data are shown in Table 3. Median expansion of total nucleated cells (TNC) was 401.5 fold (0-764). Median expansion of CD34+ cells was 90 fold (6-398) with an expansion overall of CD34 of from 0.33 to 9.26 × 105/kg. The median ratio of the infused CD34+ cells to the theoretical CD34+ cells (i.e., the CD34+ cell dose that the patient would have received from the entire UCBU without expansion) was 14 (1.6-93.1). Viability of the carlecortemcel-L expanded cells was excellent at 96% (81-100%).
Table 3.
Summary of Carlecortemcel-L characteristics
| N=101 | |||
|---|---|---|---|
| Characteristic | N1 | Mean (SD) | Median (Range) |
| Volume of entire UCB unit, mL | 100 | 152.28 (157.67) | 101.0 (6.0–1139.0) |
| Number of viable cells, (×106) | 100 | 475 (451.50) | 322.80 (0.04–3140.00) |
| Before Expansion | |||
| Total cell dose ×107/kg | 101 | 3.33 (1.34) | 3.06 (1.29 – 11.00) |
| StemEx portion total cell dose | 101 | 0.94 (0.67) | 0.66 (0.26–3.37) |
| Unmanipulated portion total cell dose | 101 | 2.40 (0.99) | 2.20 (1.03–8.80) |
| Total CD34 × 105/kg | 101 | 117.98 (1168) | 1.41 (0.02–11739) |
| Unmanipulated portion CD34 | 101 | 94.22 (943.31) | 1.05 (0.02–9391) |
| StemEx portion CD34 | 101 | 23.75 (233.6) | 0.33(0–2348) |
| After Expansion | |||
| Total CD34 ×105/kg | 97 | 112.90 (952.6) | 10.28 (1.37–9396) |
| StemEx portion CD34 | 97 | 14.84 (17.52) | 9.26 (0.83–117.56) |
| Viability, % | 100 | 96 (2.46) | 96.00 (81.00–100.00) |
| Expansion of cells, fold | 100 | 406 (167.91) | 401.50 (0.00–764.00) |
| CD34+ cells, % | 97 | 19 (7.23) | 18.20 (6.80–43.40) |
| CD133+ cells, % | 97 | 13 (4.41) | 13.20 (3.70–23.90) |
| CD34+/CD38– cells, % | 97 | 4 (4.05) | 3.00 (0.00–15.50) |
| CD38+ cells, % | 97 | 67 (15.60) | 67.00 (28.70–94.70) |
| CXCR4+ cells, % | 97 | 46 (13.80) | 46.20 (7.90–87.60) |
| CFU per 1500 cells | 97 | 35 (20.57) | 30.0 (0.00–146.00) |
In one patient Carlecortemcel-L was not administered, due to expansion failure, and therefore the data for this batch are missing (the unmanipulated fraction of the UCBU was infused with an additional UCBU, therefore the patient remained on study)
Clinical Outcome
All 101 enrolled subjects were included in the ITT analysis set. Eight subjects had significant deviations from the treatment plan: there was a temporary halt to cell expansions at the processing site (3), no CFU growth of the unmanipulated cells (2), and in one each, relapse, positive donor specific antibodies to the unit, and investigator choice were the reasons for not moving forward. Four received their back-up unit, the two without CFU growth received a rescue transplant within 42 days, and two received the initial unfractionated portion only. Safety assays were outside specifications in 2 subjects who received their transplants without untoward toxicities. One was a sterility failure detected after infusion (no untoward effects noted), and the other involved an invalidated test result for the mycoplasma test due to yeast overgrowth in the culture.
Overall infusion toxicities of the unmanipulated cells on day 0 were mild and reversible consisting of hypertension, cough, urticaria, dyspepsia, oropharyngeal pain and throat irritation Infusional toxicity of expanded cells was minimal; cough was reported as an infusion toxicity in 3/101 patients and resolved with no change in the infusion rate. Severe adverse events after transplantation were seen in 42 patients, most commonly due to infections in 21 (20.8%), multiorgan failure in 7 (7%), and engraftment failure and sepsis each in 6 (6%).
The 100-day mortality rate in the ITT analysis set, the primary outcome for the study, was 15.8% (16/101 subjects: 95% CI: 8.7-22.9%) in the carlecortemcel-L study group compared to 25.4% (75/295 subjects: 95% CI: 20.4-30.4%) in the DUCBT control group. Thus the 100 day survival was 84.2% (95% CI: 77.1-91.3%) for the study vs 74.6% (95% CI: 69.6-79.6%) for the DUCBT groups (Figure 4; p=0.035, odds ratio 0.50 (95% CI: 0.26-0.95)). The observed treatment effect was particularly strong in subjects ≥40 years (88.4% vs. 58.6% survival) and CMV-positive subjects (86.6 % vs. 70.2%). The causes of death for the 16 patients before day 100 in the treatment arm were infections (non-interstitial pneumonitis) in 5, multi-organ failure and sinusoidal obstruction of the liver (venoocclusive disease) in 3 each, and one each due to GVHD, relapse, ARDS, and graft failure.
Figure 4.
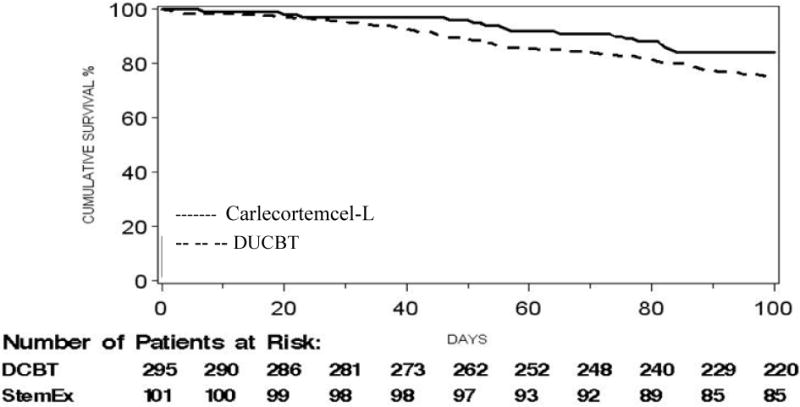
Overall survival – all cause mortality, for study (Carlecortemcel-L) (84.2%) and DUCBT (74.6%) treated patients: Odds ratio 0.50, 95% CI (0.26,0.95); p = 0.035
The 180-day mortality rate comparison for the carlecortemcel-Lstudy group vs DUCBT control group, a secondary endpoint analysis, was however not statistically significant (p=0.39), with an odds ratio of 0.79 (95% CI: 0.46, 1.35) and the comparison of disease-free survival rates up to 180 days, adjusted for the four prognostic factors that were used to adjust the mortality comparisons, had a hazard ratio of 0.81 (p = 0.29). The causes of death for the 17 patients that died between days 100-180 were infectious interstitial pneumonitis (7), and other infections (5) for a total infection death rate of 70.6%. Relapse and second malignancy were seen in 2 each and one patient had multi-organ failure.
We performed an exploratory analysis of survival at 100 days based on the use of TBI vs non-TBI-based regimens. In the DUCBT group: 15/57 (26%) receiving no TBI had died by 100 days compared to 60/238 (25%) in those receiving TBI; however for the carlecortemcel-L group. 9/76 (12%) receiving no TBI had died by 100 days compared to 7/25 (28%) in those receiving TBI(p = .066). The TBI group were more likely however to have intermediate/high risk acute leukemia (42 vs 33%).
Engraftment Outcome
Neutrophil engraftment failure occurred in 8 (cumulative incidence 8.1%) of carlecortemcel-L study group subjects and in 43 (14.5%) of DUCBT controls [p = .086, odds ratio 0.50 (95% CI 0.23-1.10)]. The median time to neutrophil engraftment, was 21 days (95% CI: 18.4-23.5) and 28 days (95% CI: 27.6-31.2), in the study and control groups respectively (Figure 5A) and the difference was statistically significant (p <0.0001), [hazard ratio of 1.79 (95% CI: 1.35-2.36)]. Median time to platelet engraftment, was 54 days (95% CI: 43.3-61.9) and 105 days (95% CI: 55.1-144.3), respectively (Figure 5B), and the difference was statistically significantly shorter in the carlecortemcel-L study group (p=0.008l), [hazard ratio 1.48 (95% CI: 1.11-1.96)]. The percentage of patients who did not engraft platelets by day 60 was also lower in the carlecortemcel-L study group at 34.4 vs 44.7% (p = 0.075).
Figure 5.
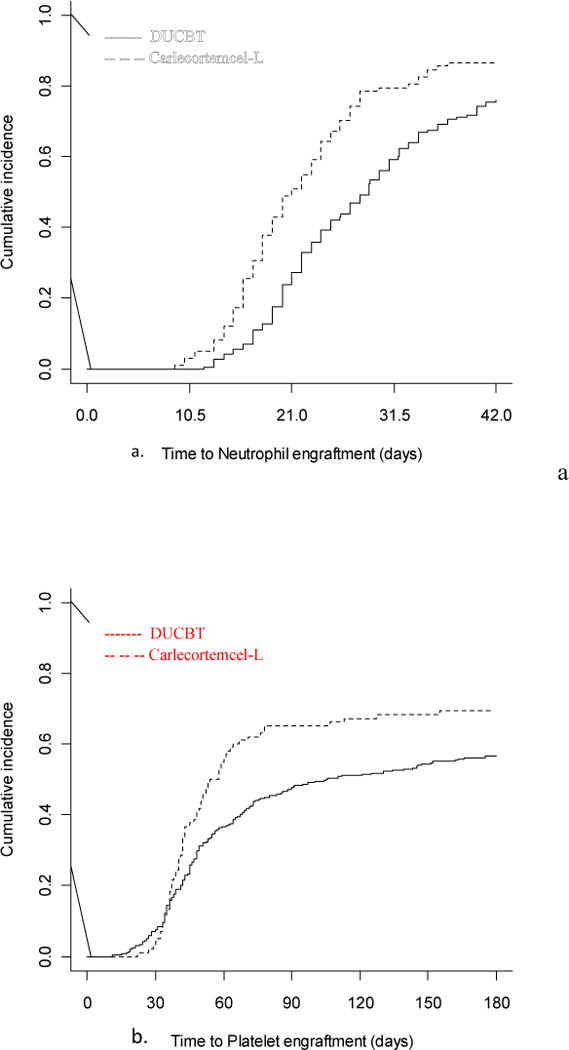
Cumulative incidence of a.) neutrophil and b.)platelet engraftment for the study (Carlecortemcel-L) and DUCBT patients
We constructed a model for the carlecortemcel-L study group that assessed the association of combined early engraftment (neutrophil before day 20 and platelet before day 60) with mortality up to 100 days. The hazard ratio of 0.17 (95% CI 0.05-0.54) indicated an association of reduced mortality (p=0.0028). The correlation was stronger for neutrophil engraftment only; those who engrafted neutrophils before day 20 had a 100 day survival rate of 94% vs 78% for those engrafting later.
Graft versus host disease
The cumulative incidence of aGVHD grade III-IV was similar in the carlecortemcel-L study group (19.4%) compared to the DUCBT control (16.9%; p=0.11), with a hazard ratio of 1.87 (95% CI: 0.87, 4.02). For grade II-IV aGVHD the cumulative incidence was lower in the carlecortemcel-L group (31.6 vs 46.8%). In the carlecortemcel-L study group, acute GVHD was the cause of death in only 2 (2%) patients due to GI and combined GI/hepatic involvement respectively. The cumulative incidence of chronic GVHD was similar as well (18.4%) vs (16.0%; p=0.73), with a hazard ratio of 1.12 (95% CI: 0.59, 2.13).
Discussion
This is the first ever reported multicenter trial exploring the use of ex vivo expanded CB cells to decrease the high day 100 mortality rate seen with single or DUCBT8, 20–24. It was based on the previous data3 that the infusion of a significantly higher number of HSC measured as CD34+ cells/kg would lead to faster engraftment of both neutrophils and platelets as compared to the current UCBT standard, DUCBT and lead to a higher 100 day survival. In fact, despite expanding only a fraction of the UCB unit, patients in the carlecortemcel-L study group were transplanted with an average 14 fold total higher number of CD34 as compared to what they would have received from an unmanipulated UCB unit, which led to a faster engraftment of neutrophils (21 vs 28 days) and platelets (54 vs 108 days) and an improved 100 day survival of 84 vs 75%, without an increase in either severe aGVHD or relapse. Further we were able to successfully manufacture expanded UCB cells at central processing centers, and had them delivered to 25 different centers internationally where they were infused in a timely fashion, with minimal toxicity. Survival at 100 days correlated with early engraftment of both neutrophils and platelets which correlated with expansion of CD34+ cells, the median of which was 90 fold using this novel copper chelator cytokine combination designed to expand only primitive HSC. The manufacturing and delivery of the carlecortemcel-L was conducted efficiently in 97/101 patients. Seven of the 8 batch failures were predicted based on the lack of growth in CFU assays done on the initially thawed cells, a known risk factor for fatal non-engraftment at the time the study was in progress31–32, and permitted the investigators to make alternative plans for their patients.
The control group represents a current standard of care for patients without a matched sibling or unrelated donor and the unit and patient selection criteria here were similar to typical DUCBT. In the design, rigorous methodologies were applied to minimize bias and allow for a meaningful demonstration of clinical benefit, while accounting for potential differences between the two groups. The comparator group was chosen to meet the same eligibility criteria of the patients in the trial, but imbalances were expected and indeed seen, e.g a lower proportion of patients aged < 18 (10.9 vs 24.4%), a higher proportion > 40 (42.6 vs 19.7%) and a higher proportion of patients with early leukemia (52.5 vs 35.6%). These imbalances were adjusted for using the regression model approach implemented in the mortality comparisons.
The major alternative to UCBT is haploidential transplantation. In companion trials run by the Bone Marrow Transplant Clinical Trial Network, adults undergoing either a reduced intensity DUCBT or haploidentical transplant had an identical survival at 2 years albeit with differing causes of death.33 This led to the development of a Phase III comparison trial, now underway. However the limitations of DUCBT, specifically the slow engraftment rate especially for platelets and late opportunistic infections and the high relapse/graft failure rate seen in haploidentical transplants especially for those with refractory myeloid and lymphoid leukemias34 are not being addressed in this comparison trial. It would seem that with a lower relapse rate, the major limitations of UCBT, perhaps could be addressed easier with advances in cell manipulation technologies like the strategy employed here. Thus UCB expansion trials, building on the results here, may ultimately increase the transplant cure rate of patients with advanced hematologic malignancies.
While we demonstrated an improved 100 day survival, deaths were still seen after day 100 primarily related to opportunistic infections, and not relapse and appear to be correlate with the expected deficiencies in cellular immunity. This perhaps was related to the smaller number of infused non-manipulated immune cells, the discarding the immune cells in the cell fraction to be expanded, which could have been up to 50% of the total immune cell infusion normally infused with a single UCBT, and the fact that the pre-clinical expansion studies did not demonstrate an expansion of immune cells.18 Strategies are being developed to address this late immune deficiency. The stem cell differentiation blocking strategy used in this study with a copper chelator is being tested with alternative agents including an aryl hydrocarbon receptor antagonist,35,36 and nicotinomide29 or by culturing cells over beads that activate Notch signaling.37 Co-culturing UCB cells with third party mesenchymal stem cells as well as methods to improve homing of stem cells to the bone marrow are also being investigated and appear promising.38 In all instances engraftment is accelerated to the point that neutrophil engraftment appears more rapid than any other form of transplantation. However, these techniques, evaluated in a double cord configuration have traditionally not led to long term engraftment of the expanded cells, although strategies where the CD34 negative fraction, containing the T-cell fraction, is re-cryopreserved and infused with the expanded fraction potentially may aid the long term engraftment of the expanded UCB cells, including immune cells. 29,36
Based on recent registry data suggesting that TBI-based preparative regiments might be associated with an inferior leukemia-free survival (LFS)39, we did a retrospective ad hoc analysis of 100 day survival of the study group based on the preparative regimens used particularly since the thiotepa, busulfan and fludarabine regimen was utilized in 55% of the patients transplanted in this study and was associated with an improved LFS as compared to TBI-based regimens in this registry analysis. We found a trend toward inferior outcome using TBI; however, only 25% of the study group received TBI and this group tended to have a higher incidence of intermediate/high risk acute leukemia. Neertheless the 12% 100 day mortality for the non-TBI group deserves further study.
This initial multi-national allogeneic transplantation trial comparing DUCBT to a single unit UCBT containing both expanded and unexpanded cord blood cells demonstrated both a faster engraftment and a reduced 100 day mortality for the study group. While not a randomized prospective clinical trial, we believe that this strategy holds the promise of further enhancing the transplant options for patients without a related or unrelated living donor. Complications from delayed immune reconstitution after UCBT remain a challenge, but ongoing efforts to enhance immune cell engraftment hold the promise of improving long term outcome by both decreasing opportunistic infections and preventing relapse.
Supplementary Material
Highlights.
Expanding a portion of a UCB unit in cytokines with tetraethylenepentamine led to a median 77 fold expansion of CD34+ cells
Compared to a contemporaneous double UCB cohort, transplantation of expanded and remaining unexpanded cells led to a lower 100 day mortality
Faster neutrophil and platelet engraftment was also seen also for the group transplanted with the expanded cells
Acknowledgments
This study was supported (in part) by research funding from Gamida-Cell, Jerusalem, Israel.
The authors wish to acknowledge the help and support of the data managers, and research nurses at each institution, as well as the physicians and nurses who cared for these patients. But most of all we wish to acknowledge the patients and their families for enrolling on this innovative trial, we will long remember their heroic efforts to overcome their hematologic malignancies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship statement: TP, DAS, OSK, DH, YCC, YC, EG, MMH, ME designed the study, PJS, PM, AN, GS performed the transplants, and collected data, PJS, TP, EL, NGO, JM, NH, EO, EG, DAS, EGC, OSK, DB, DH, KB-A, EF, LSF, LO, RB, VR, EG, MMH, ME, AN performed data analysis and interpretation, PJS wrote the manuscript with the assistance of all of the authors, and all authors approved the final manuscript
Conflict of Interest: TP, EL, NRG, JM, NH, EO, EG, DAS, EGC, OSK, DB, DH, KB-A, EF, YCC, were employed by Gamida-Cell, Jerusalem, Israel during the study/analysis/writing period of the study. AN is a medical consultant to Gamida-Cell, Jerusalem, Israel
References
- 1.Pidala J, Kim J, Schnell M, et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and –DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplant. 2013;48:346–50. doi: 10.1038/bmt.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US registry. New Engl J Med. 2014;371:339–48. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballen KK, Gluc
- 4.Kman E, Broixmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eapen M, Rubenstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukemia: a retrospective analysis. Lancet Oncol. 2010;11:653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen YC, Scaradavou A, Stevens CE, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone Marrow Transplant. 2011;46:70–6. doi: 10.1038/bmt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciceri F, Labopin M, Aversa F, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112:3574–81. doi: 10.1182/blood-2008-02-140095. this is very old almost 10 years ago should be replaced. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:337–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 11.McCurdy SR, Kanakry JA, Showell MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;127:3024–31. doi: 10.1182/blood-2015-01-623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–47. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 13.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for hematologic malignancies: conditioning regimens, double cord blood transplants and infectious complications. Br J Haematol. 2009;147:207–16. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 14.Rocha V, Labopin M, Mohty M, et al. Outcomes after double unit unrelated cord blood transplantation (UCBT) compared with single UCBT in adults with acute leukemia in remission: a Eurocord and ALWP collaboration study [abstract] Blood. 2010;116:910a. [Google Scholar]
- 15.Wagner JE, Jr, Eapen M, Carter S, et al. Blood and marrow transplant clinical trials network. One- unit versus two-unit cord-blood transplantation for hematologic cancers. New Engl J Med. 2014;371:1685–94. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127:3450–7. doi: 10.1182/blood-2016-01-694349. [DOI] [PubMed] [Google Scholar]
- 17.Baron F, Ruggeri A, Nagler A. Methods of ex vivo expansion of human cord blood cells: challenges, successes and clinical implications. Expert Rev Hematol. 2016;9:297–314. doi: 10.1586/17474086.2016.1128321. [DOI] [PubMed] [Google Scholar]
- 18.Peled T, Landau E, Prus E, Treves AJ, Nagler A, Fibach E. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood-derived CD34+ cells. Br J Haematol. 2002;116:655–61. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 19.Peled T, Landau E, Mandel J, Glukjman E, Goudsmid NR, Nagler A, Fibach E. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increased their engraftment potential in NOD/SCID mice. Exp Hematol. 2004;32:547–55. doi: 10.1016/j.exphem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–8. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–81. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipient’s of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–77. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizieri DAA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 24.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor hematopoietic stem-cell transplantation in adults with acute leukemia. Lancet Oncol. 2010;11:653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 26.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life-tables (with discussion) J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–80. [Google Scholar]
- 29.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multi lineage engraftment. J Clin Invest. 2014;124:3121–28. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armand P, Kim HT, Logan BR, et al. Validation and refrinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad VK, Mendizabal A, Parikh SH, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S. Kurtzberg Unrelated donor umbilical cord blood transplantation for inheritied metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page KM, Zhang L, Mendizbal A, Wease S, Carter S, Gentry T, Balber AE, Kurtzberg J. Total colony-forming units are a strong, independent predictor of neutrophil and platelet engraftment after unrelated umbilical cord blood transplantation: a single center analysis of 435 cord blood transplants. Biol Blood Marrow Transplant. 2011;17:1362–1374. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 35.Boitano AE, Wang J, Romero R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–48. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner JE, Jr, Brunstein CG, Boitano AE, et al. Phase I/II trial of StemRegenis-1 expanded umbilical cord blood hematopoietic stem cell supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–55. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delaney C, Heimfeld S, Brasheem-Stein C, Voorhies H, Manger R, Bernstain ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nature Medicine. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. New Engl J Med. 2012;367:2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggeri A, Sanz G, Bittencourt H, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimens, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014;28:779–786. doi: 10.1038/leu.2013.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


