Introduction
The human microbiome is comprised of the bacteria, archaea, viruses, fungi and other microeukaryotes that live on and within the human host. Alterations in the microbiome are associated with adverse transplant outcomes including the expected infectious complications following allogeneic hematopoietic cell transplantation (allo-HCT) in addition to diseases that are not classically “microbe-associated”. For example, recent data suggest an association between certain microbial community structures and mortality, disease relapse, risk of infection, and graft-versus-host disease (GVHD)1,2,3. Most studies inspecting the role of the microbiome in HCT patient outcomes, though compelling, are limited in scope: in general, data have been generated in single-center studies or preclinical models. Here, we summarize many of the main findings of the past several decades of research on this topic, and propose areas of focus for future research that will facilitate investigating the microbiome ands its role in disease (Table 1).
Table 1.
Proposed next steps for future research investigating the microbiome-host relationship in HCT patients
| Primary Research Gaps and Strategies |
|---|
|
The earliest days of microbiota research: germ-free mice and patients
Pioneering studies conducted in the early 1970s demonstrated that mice undergoing allo-HCT in germ-free conditions experienced less GVHD and had improved survival (Table 2)4–16. Soon thereafter, this observation led to attempts to reproduce these conditions in patients undergoing HCT through the use of laminar-airflow isolation rooms, “sterile” diets, gut decontamination with oral non-absorbable antibiotics, and skin cleansing (Table 3)17–29. Between-study heterogeneity and the lack of reproducible data supporting efficacy for GVHD prevention have limited evidence-based guidance for clinical practice and prophylactic strategies. However, while many of these previous approaches have largely been abandoned, broadly-adopted modern-day recommendations to prevent infectious complications include antimicrobial prophylaxis, sterilized positive air pressure rooms, low-microbial diet, and use of barrier precautions (e.g. gloves, face masks, gowns)30. Recently, there has been a resurgence of microbiome research across many disciplines of medicine spurred by advances in high-throughput methodologies for characterizing the microbiome, which extend beyond bacterial culture techniques and virus-specific molecular approaches for detection. Similarly, there has been rapid growth in the microbiome research as it relates to HCT.
Table 2.
The early years of microbiota research in germ-free mice
| Model system and intervention | Findings | References |
|---|---|---|
| Germ-free mice vs. conventional mice | Significantly milder GVHD symptoms and longer survival after MHC*-disparate allo-HCT in germ-free mice | Connell, 19654 Jones, 19715 Van Bekkum, 19616 Van Bekkum,19777 |
| Intact GVT and reduced GVHD after MHC-disparate allo-HCT in germ-free mice | Pollard, 19739 Pollard, 197410 Truitt, 197411 Truitt, 197612 | |
| Germ-free mice vs. conventional mice vs. mice with consortium of colonization resistant intestinal microflora (anaerobes) |
|
Van Bekkum, 197413 |
| Antibiotic-treated mice vs. untreated mice | Significantly milder GVHD in xenogeneic rat-to-mouse HCT; GVHD histology present but less inflamed in antibiotic-treated mice compared to conventional mice | Heit, 19738 |
| Significantly milder GVHD symptoms and longer survival after MHC-disparate allo-HCT in antibiotic-treated mice | Van Bekkum, 196714 Heit, 197715 | |
| Selective antibiotic decontamination of Enterobacteriaceae vs. conventional mice | Mitigation of delayed-type GVHD by selective decontamination of Enterobacteriaceae was minor and dependent on mouse model | Veenendaal, 198816 |
MHC=Major histocompatibility
Table 3.
Clinical studies of protective isolation with gut decontamination in HCT patients
| Intervention | Control | Outcomes (Intervention v Control) |
References |
|---|---|---|---|
| Randomized trials | |||
| Protective isolation (LAF* isolation, skin cleansing, sterile diets) + oral antibiotic decontamination (n=45) | Oral antibiotic decontamination (n=44) |
|
Buckner, 197817 |
| LAF* isolation + oral antibiotic decontamination (n=36) | Conventional rooms +hand-washing and mask precautions (n=31) |
|
Navari, 198418 |
| LAF* isolation + oral non-absorbable antibiotics + prophylactic systemic antibiotics (n=54) | LAF* + oral nonabsorbable antibiotics (n=68) |
|
Petersen, 198619 |
| Conventional rooms + prophylactic systemic antibiotics (n=45) | Conventional rooms + prophylactic granulocyte infusions (n=67) |
|
Petersen, 198620 |
| LAF* + prophylactic systemic antibiotics (n=49) | Conventional rooms+ prophylactic systemic antibiotics (n=50) |
|
Petersen, 198721 |
| Conventional rooms + ciprofloxacin + metronidazole gut decontamination (n=68) | Conventional rooms + ciprofloxacin gut decontamination (n=66) |
|
Beelen, 199922 |
| Observational trials | |||
| LAF* isolation + oral antibiotic decontamination (n=39) | Conventional rooms + oral antibiotic decontamination (n=91) |
|
Storb, 198323 |
| LAF* isolation +oral and topical antibiotic decontamination (n=26) | Conventional rooms + barrier nursing +oral and topical antibiotic decontamination (n=22) |
|
Mahmoud, 198424 |
| Strict reverse isolation** + oral non-absorbable antibiotics, complete gut decontamination (n=26) | Barrier nursing + oral non-absorbable antibiotics, selective gut decontamination (n=15) |
|
Schmeiser, 198825 |
| LAF* + complete antibiotic oral decontamination (n=44) | LAF* + selective decontamination (n=21) |
|
Vossen, 199026 |
| Sustained growth suppression of anaerobic bacteria with oral nonabsorbable and systemic antibiotics (n=41) | Incomplete growth suppression of anaerobic bacteria despite oral nonabsorbable and systemic antibiotics (n=153) |
|
Beelen, 199227 |
| Protective isolation with either LAF* or HEPA filters (n=423 8) | Conventional isolation (n=827) |
|
Passweg, 199828 |
| Protective isolation + successful gut decontamination with oral nonabsorbable and systemic antibiotics (n=57) | Protective isolation - successful gut decontamination despite oral nonabsorbable and systemic antibiotic (n=55) |
|
Vossen, 201429 |
LAF= Laminar air flow
Reverse isolation was achieved in sterile plastic isolators
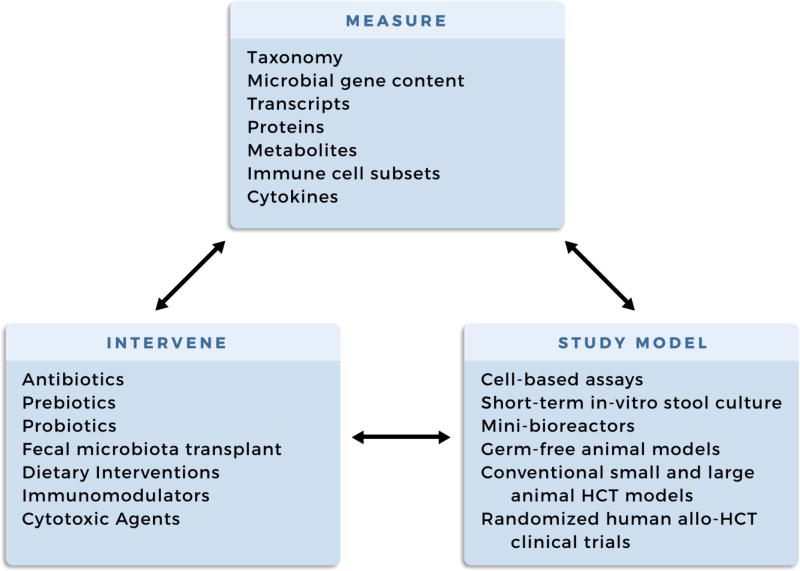
Section I: Methods to investigate the microbiome
With the advent of high-throughput molecular methods to study the microbiome, the field has grown significantly in the past decade. Commonly used methods include 16S ribosomal RNA gene sequencing for bacterial taxonomic classification, metabolomics, as well as shotgun metagenomic sequencing and subsequent taxonomic and functional classification of microbial genes; these methods have been reviewed in detail elsewhere (Table 4) 31,32–34. Each of these methods provide an orthogonal approach to study the microbiome from the perspective of answering important microbiota taxonomic and functional questions including “which microbes are there?”, “what do they make?” , “what genes do they contain?”, and “what is their relative and absolute abundance?”35. With the explosion of new molecular and bioinformatic approaches to study the microbiome, we anticipate an ever-growing toolkit to characterize potentially clinically relevant features of the microbiome such as antibiotic resistance, microbial virulence factors, and strain dynamics. Terms commonly used in microbiome studies and their definitions are listed in Table 532,36–38,39.
Table 4.
Sequencing technologies used in microbiome research
| Method | Definition | References |
|---|---|---|
| Metagenomics | The study of genes and non-coding genetic information in a mixed population of organisms in order to infer functional potential and taxonomic structure of the population and its individual organisms | Marchesi, 201532 |
| 16S ribosomal RNA/DNA sequencing | PCR amplification of bacterial RNA/DNA from the variable regions of the 16S ribosomal RNA gene for taxonomic profiling (the region selected is usually determined by the niche that is being investigated; e.g. V4 for stool, V1-3 for skin) | Weisburg, 199133 |
| Shotgun next-generation metagenomic sequencing | High-throughput DNA sequence generation and analysis from any organism or group of organisms via fragmentation, tagging, amplification, and massively parallel or deep sequencing. Allows for taxonomic identification in addition to generating information about gene presence, genetic bioregulation and potential metabolic pathways | Loman, 201234 |
| Metatranscriptomics | High-throughput RNA sequence generation and analysis from any organism or group of organisms via reverse transcription, tagging, amplification, and massively parallel or deep sequencing. Provides a snapshot of which genes are being transcribed | Marchesi, 201532 |
| Metabolomics | Characterization of the collection of metabolites produced by an organism or a single tissue. The term has been used to described characterization of the collection of metabolites produced by a collection of organisms (i.e. the microbiota), although some prefer the term “metabonomics” for that definition | Marchesi, 201532 |
| Metaproteomics | Characterization of all proteins within a clinical or environmental sample | Marchesi, 201532 |
Table 5.
Definition of terms
| Term | Definition | References |
|---|---|---|
| Diversity | Measurement of the number of different types (taxa) of organisms and their abundance. Alpha-diversity and beta-diversity refer to diversity within and between samples, respectively | Lozupone, 201236 |
| Dysbiosis | Perturbation of the taxonomic structure and function of the microbiota from the healthy state. This can be associated with the development of disease | Petersen, 201437 |
| Germ-free mice | Mice raised in sterile conditions and free from colonization by all microorganisms. Also referred to as “axenic” mice | Giraud, 200838 |
| Fecal microbiota transplantation | The transfer of stool from a donor to a recipient via either endoscopy, nasogastric/duodenal tube, capsules, or enema for the purpose of altering the intestinal microbiota of the recipient and restoring health. Stool is obtained from healthy related or unrelated donors, and less commonly from the intended recipient | Borody, 201339 |
| Microbiota | The entirety of microorganisms (bacteria, archaea, viruses, fungi, and other eukaryotes) within a specific habitat | Marchesi, 201532 |
| Microbiome | Includes the biotic (microorganisms and their genomes) and abiotic (environmental) factors present within a particular habitat. This definition is modeled after the meaning of the term “biome”. Many in the field use the term microbiome to refer to the collection of genes and genomes within a particular habitat, although this definition is redundant with “metagenome” | Marchesi, 201532 |
A precedent has recently been set for the generation of multi-faceted data types (ranging from shotgun metagenomic sequencing to transcriptomes and epigenomes to metabolite profiling) that facilitate multi-dimensional and longitudinal characterization of both the host and the microbiome. Specifically, projects of the integrative Human Microbiome Project (iHMP), the US National Institutes of Health Common Fund’s second phase Human Microbiome Project, have collected longitudinal samples from three cohorts of individuals (comprising individuals with pregnancy and pre-term birth, type 2 diabetes, and inflammatory bowel disease)40,41. Given these advances in “multi-omic” data collection, we anticipate that the next decade of translational research in the microbiome field as it relates to HCT will extend far beyond simple characterization of community taxonomic structures within the microbiome. For example, advances in immunophenotyping and short-term, in vitro propagation of microbial mixtures will identify potential mechanistic relationships between microbes, microbial antigens, and host responses42,43.
While the advances in phenotyping and genotyping experiments may pave the way for the identification of biomarkers that may be clinically actionable, there are challenges and limitations to their effective, wide-scale application. For example, a specific challenge is the need for rapid turnaround of next-generation sequencing results to be clinically actionable; at present, this is not routinely available due to the need to batch samples to reduce the costs of sequencing. Thus, while next-generation sequencing and metabolomic approaches are the predominant technologies used in the research setting, some intriguing and potentially more easily deployed alternatives for microbiome measurement may be more translatable. Indeed, these technologies do exist and include approaches such as species- or bacterial-group-specific quantitative PCR33 and microarray approaches34,44. Despite the challenges to their clinical use, it is feasible for advancements in microbiota science currently based on next-generation sequencing to be optimized for real-time use both diagnostically and prognostically in the HCT setting.
Open Questions
How do we design and execute microbiome studies that permit simultaneous characterization of a) microbial characterization beyond taxonomy, b) both host impact on and host response to the microbiome, thus informing a deeper understanding of microbiota function?
What new technologies (such as single cell sequencing, T-cell receptor sequencing, long-read sequencing and advanced imaging) will emerge as new ways to measure the microbiome, its structure, function and interactions with the host; how should we apply these approaches in the HCT setting?
What strategies for multi-center collections of biospecimens and clinical data best support future integrative “multi-omic” approaches to illuminate host-microbe relationships as they pertain to HCT outcomes?
Section II: The Microbiome as Biomarker
Identifying biomarkers with high prognostic and predictive value is crucial for communicating risk to patients and selecting appropriate therapeutic strategies. Thus, it is no surprise that composition of the intestinal microbiota, which is affected by host genetic factors, immunological factors, diet, medications, lifestyle and environmental exposures, has been analyzed as a biomarker for important clinical outcomes after HCT. During transplantation, dramatic shifts in the composition of the intestinal flora are observed1. These shifts in species abundance and measures of diversity have been proposed as potential biological markers associated with patient outcomes after transplantation (Table 6)1,2,3,22,27,45,46,47,48,49,50 Biomarkers such as these may prove useful in the design of clinical trials to identify patients at risk of certain outcomes, as surrogate markers of clinical outcomes, or as early predictive markers of treatment response. If a causal relationship between the microbiota and transplant outcomes can be established, these relationships may inform the development of microbiota-based therapeutic interventions to improve transplant outcomes.
Table 6.
Clinical studies examining the microbiota as a biomarker for HCT outcomes*
| Microbiota Feature | Association | Sample size |
References |
|---|---|---|---|
| Sustained decontamination of gut anaerobes | Lower risk of GVHD | 194 | Beelen, 199227 |
| Decontamination of gut anaerobes | Lower risk of GVHD | 134 | Beelen, 199922 |
| Intestinal monodomination by Enterococcus and Proteobacteria | Higher risk of bacteremia and intestinal GVHD | 94 | Taur, 201245 |
| Intestinal monodomination, especially by Enterococcus | Higher risk of bacteremia and intestinal GVHD | 31 | Holler, 20143 |
| Decreased duodenal Paneth cell counts at GVHD | Higher GI GVHD severity, lower GVHD treatment response, and higher NRM** | 142 | Levine, 201346 |
| Low intestinal microbiota diversity | Lower OS, higher TRM | 80 | Taur, 20141 |
| Lower urinary 3-indoxyl sulfate | Higher intestinal microbiota dysbiosis, higher risk of GVHD | 31 | Holler, 20143 |
| Lower urinary 3-indoxyl sulfate | Higher intestinal microbiota dysbiosis, higher TRM, lower OS | 131 | Weber, 201547 |
| Higher fecal Blautia abundance | Lower GVHD-related mortality, higher OS | 115 | Jenq, 201548 |
| Higher abundance or presence of a cluster of bacteria including Eubacterium limosum in fecal microbiota | Lower risk of relapse or progression of disease, higher OS | 541 | Peled, 20172 |
| Higher gradient of positively to negatively correlated organisms at neutrophil recovery | Higher risk of severe acute GVHD | 66 | Golob, 201749 |
| Picobirnivirus presence | Severe GI GVHD | 44 | Legoff, 201750 |
All studies are observational except Beelen, 1999
NRM=non-relapse mortality
3-indoxyl sulfate as biomarker of intestinal microbiota health
An example of a recently proposed microbiome-derived biomarker in HCT is the small, aromatic tryptophan metabolite 3-indoxyl sulfate47,51. Indoxyl sulfate originates from the degradation of dietary protein-derived tryptophan to indole by the tryptophanase of commensal intestinal bacteria. After resorption of indole from the intestine, it is metabolized to indoxyl sulfate in the liver and finally excreted in the urine. Microbiota-derived indole and its derivatives are integral to the maintenance of human microbial communities through bacteriostatic effects on Gram-negative enteric bacteria, antifungal activities that provide colonization resistance to Candida albicans, as well as regulation of epithelial function and control of local inflammation through induction of anti-inflammatory cytokines52,53. Recent studies have demonstrated that indoxyl sulfate can serve as an important biomarker with lower urine levels being associated with significant and clinically relevant intestinal microbiota disruption in patients undergoing allo-HCT3,47,54. In the future, metabolites such as indoxyl sulfate may serve as a urine or serum marker for monitoring microbiota perturbations in patients being treated with antibiotics or in predicting the development of GVHD47.
Open Questions
Can the composition of the intestinal microbiota serve as a biomarker for clinical outcomes after HCT and can it be used to guide interventions?
Are there specific microbes that are causally associated with positive or negative outcomes of HCT, perhaps through their antigenic properties or through the action of generated metabolites? If so, might there be strain-specific differences in their capacity to induce inflammation or cytotoxic damage?
How can changes in the microbiome be assayed in real-time to allow for clinical decision-making?
Section III: Interactions Between the Microbiome and the Immune System
The microbiome and development of the immune system
Proper immune reconstitution is central to successful HCT. To better understand immune reconstitution in patients following HCT, it is helpful to turn to the well-studied and analogous process of immune development in neonates. The adaptive immune system and microbiota undergo a process of rapid change and development over the first three years of human life, and these two processes are intimately interconnected55. Developmental microbiota perturbations have been associated with short-term immune consequences early in life, and there is a strong suggestion that these early perturbations may have long-term deleterious effects on immune function as well56,57,58,59,60,61. As successful immune reconstitution is central to HCT efficacy in the short- and long-term, it is critical to understand exactly how the microbiota impacts that process.
Axenic or “germ-free” animal models have been an essential tool in defining the importance of microbes to immune development62,63,64. Studies in these systems have shown that the immune system of a germ-free neonate is under-developed65. Most notable are the changes observed in mucosal immunity, particularly in the intestine, with absence of gut-associated lymphoid tissue, including isolated lymphoid follicles, Peyer’s patches, and mesenteric lymph nodes66. While many similarities exist between the immunologic development process in infants and HCT recipients, some differences must also be considered. Notably, the adult intestinal microbiome is quite divergent from the infant microbiome, and individuals undergoing HCT have often received antimicrobial and other pharmacological agents that damage the microbiota composition. Thus, while the microbiota likely plays a role in immune reconstitution post-HCT, we must carefully consider both the similarities and differences to the process of infant immune development as we try to understand how the microbiota impacts both immune reconstitution and adverse immunological post-HCT outcomes such as GVHD.
The microbiome and its role in immune reconstitution post HCT
Impaired immune reconstitution after HCT is a significant cause of morbidity and mortality, and has been implicated in increased risk of infections, malignancy relapse, and development of secondary malignancies67,68,69,70,71,72,73,74,75. Given recent data on the interactions between the gut microbiome and transplant outcomes discussed above, as well as the immune system76,77,78, it is reasonable to hypothesize that the microbiome plays a direct role in post-transplant immune recovery. The study of post-transplant immune reconstitution now benefits from a variety of quantitative and qualitative assays, ranging from clinical parameters such as absolute lymphocyte counts (ALC), lymphocyte subsets (CD4+ and CD8+ T cells, NK cells, B cells) and antibody titers, to more complex functional assays and evaluations of T-cell and B-cell repertoire, to next-generation sequencing approaches to provide information on TCR diversity and specific clonotypes over time79. Currently, no integrated datasets comprised of simultaneous host and microbiome measurements are publicly available for analysis. Future prospective studies will need to integrate these areas of research to better define potential interactions between the immune system and the microbiome in HCT, as has been done in the iHMP for other diseases41,80.
Potential mechanisms of immune modulation by the microbiota that impact GVHD and GVT
The largest proportion of microorganisms in the body exists in the lower intestine, thus the intestine is believed to be the major interface between the microbiome and adaptive immune system. Intestinal homeostasis is a dynamic process that includes maintenance of bowel mucosa integrity and relies heavily on the interactions between immunologic function and the community of organisms that make up the gut microbiota. HCT leads to dysbiosis and disruption of intestinal homeostasis as a result of the conditioning regimen, use of broad-spectrum antibiotics, alterations in nutrition, and donor cell-derived immune reconstitution. There is clinical evidence for the regulatory effect of gut microbiota in the maintenance of intestinal homeostasis mediated primarily through regulatory T cells81,82,83. For example, emerging data suggest that alterations in the intestinal microbiota and metabolome are associated with the incidence and severity of acute GVHD (Tables 7a and 7b) 1,3,22,26,27,29,48,49,50,54,76,84,85,86,87,88,89,90,91,92,93,94,95,96. While the majority of the literature has focused on changes in intestinal microbiota diversity, others have focused on the role of particular organisms, such as Blautia spp. in protection from GVHD48. Reports of GVHD associated with blooms of eukaryotic viruses, such a picobirnaviruses, have also begun to emerge, suggesting a potential role for the human virome as, at the very least, a marker of this transplant complication50. In addition to increasing GVHD risk, disruption in intestinal homeostasis and dysbiosis is associated with increased treatment-related mortality (TRM), and decreased overall survival (OS) 2,3,49.
Table 7.
| a. Studies of microbiota changes associated with GVHD in murine models | ||
|---|---|---|
| Intervention/comparison | Outcome (Intervention vs. control) | References |
| Reduced-intensity allogeneic vs. syngeneic HCT |
|
Heimesaat, 201084 |
| MHC-disparate and MHC-matched/minor antigen-mismatched allo-HCT +/− donor T-cells |
|
Eriguchi, 201285 |
| MHC-disparate allo-HCT +/− donor T-cells |
|
Jenq, 201276 |
| MHC-disparate allo-HCT using T-cells from specific pathogen-free vs. germ-free donor |
|
Tawara, 201386 |
| MHC-disparate allo-HCT +/− intestinal helminth infection |
|
Li, 201587 |
| MHC-matched/minor antigen-mismatched allo-HCT with anti-anaerobic antibiotics (imipenem-cilastin or piperacillin-tazobactam) vs. antibiotics lacking in anti-anaerobic activity (aztreonam) |
|
Shono, 201688 |
MHC-disparate vs. syngeneic allo-HCT
|
|
Mathewson, 201689 |
MHC-disparate allo-HCT + levofloxacin
|
|
Simms-Waldrip, 201790 |
| b. Microbiota changes associated with GVHD in clinical studies | |||
|---|---|---|---|
| Intervention or observational group |
Control | Outcomes (Intervention v Control) | References |
| Randomized trials | |||
| Ciprofloxacin with metronidazole prophylaxis (n=68) | Ciprofloxacin prophylaxis (n=66) |
|
Beelen, 199922 |
| Observational trials | |||
| Complete GI decontamination (n=40) | Selective GI decontamination (n=18) |
|
Vossen, 199026 |
| Sustained suppression of anaerobic intestinal flora (n=41) | Incomplete suppression of anaerobic intestinal flora (n=153) |
|
Beelen, 199227 |
| Acute GVHD (n=8) | No GVHD (n=10) |
|
Jenq, 201276 |
| Successful GI decontamination (n=57) | Unsuccessful GI decontamination (n=55) |
|
Vossen, 201429 |
| Lowest GI microbial diversity at engraftment (n=34) | Intermediate or high GI microbial diversity at engraftment (n=20 intermediate, n=26 high) |
|
Taur, 20141 |
| Colonized with Candida in the intestine (n=54) | Not colonized with Candida in the intestine (n=99) |
|
van der Velden, 201391 |
| Acute GI GVHD (n=8) | No GI GVHD (n=23) |
|
Holler, 20143 |
| Acute GVHD (n=5) | No GVHD (n=5) |
|
Biagi, 201592 |
| Lower microbial diversity (n=32), lower Blautia abundance (n=58) | Higher microbial diversity (n=32), higher Blautia abundance (n=57) |
|
Jenq, 201548 |
| Colonized with antibiotic resistant bacteria (ARB) pre-transplant (n=33) | Non-ARB colonized (n=74) |
|
Bilinksi, 201693 |
Treatment of febrile neutropenia with antibiotics effective against anaerobic bacteria:
|
Treatment with antibiotics less effective or ineffective against anaerobic bacteria:
|
|
Shono, 201688 |
| Acute GVHD (n=6) | No GVHD (n=9) |
|
Simms-Waldrip, 201790 |
| Pre-transplant antibiotic prophylaxis or treatment (n=239) | No pre-transplant antibiotics (n=261) |
|
Routy, 201794 |
| Early pre-transplant antibiotics (n=236) | Late post-transplant antibiotics (n=297) or no antibiotics (n=88) |
|
Weber, 201754 |
| Pre-conditioning low microbial diversity (n=18) | Pre-conditioning intermediate (n=48) and high diversity (n=41) |
|
Doki, 201795 |
| Severe acute GI GVHD (n=14) | Non-severe acute GI GVHD or no GVHD (n=52) |
|
Golob, 201749 |
| Acute GI GVHD (n=26) | No GI GVHD (n=18) |
|
Legoff 201750 |
| Acute GVHD (n=34) No GVHD (n=23) | HLA-matched sibling donors (n=22) |
|
Liu, 201796 |
Commensal bacteria can also play a role in tumor immunosurveillance. Although the precise mechanisms by which intestinal microbes can promote tumor immunity are unknown, one hypothesis invokes antigen mimicry, as microbial proteins can bear close resemblance to tumor associated antigens97. An alternative pathway might be through non-specific activation of innate immune cells and pathways. Consistent with the notion of the microbiome influencing anti-tumor immunity, it was recently shown that specific members of the intestinal microbiota are associated with a decreased risk of relapse after allo-HCT2. Achieving a comprehensive understanding of the mechanisms driving both mucosal and systemic immune modulation by the gut microbiota may facilitate the simultaneous mitigation of GVHD while maintaining or improving GVT effects.
Whether particular microbiota signatures correspond to a causal or contributing factor to the development of various disease phenotypes remains to be elucidated, as most clinical studies have established only associations, with rare exception22. Specifically, analyses of alterations in intestinal microbiota have focused on time-course compositional descriptions and correlations with clinical and biological outcomes, in particular acute GVHD. Future research will undoubtedly bring greater focus on both intestinal and extra-intestinal microbial alterations and their mechanistic impact on the development and severity of acute and chronic GVHD in addition to other transplant outcomes. The experimental data so far indicate that modification of the gut ecosystem to restore intestinal homeostasis may represent a novel approach to modulate complications of HCT. While attempts at altering therapeutically the established intestinal dysbiosis could potentially improve transplant outcomes, we are in the beginning phases of comprehending the full impact and the mechanistic role of microbiota in HCT, not only for GVHD outcomes but also for tumor relapse, infectious complications, and long-term outcomes after HCT.
Open Questions
Can we identify specific associations between the microbial taxa, antigens or metabolites and post-HCT immune recovery?
Is the pre-treatment microbiome (prior to any treatment for the underlying disorder) prognostic of immunologic and other outcomes post-transplant?
What is the role of oral and skin microbiomes as well as microbes from other organs on the incidence and severity of acute and chronic GVHD? Is there a specific set of intestinal and extraintestinal (e.g. ocular, skin, vaginal) microbial taxonomic structures over time that correlate with or are causally related to chronic GVHD and late effects of HCT?
How can we use interventions that modify the microbiome to improve post-HCT outcomes, specifically mediated by immune effects on GVHD and GVT?
Do specific T-cell responses against bacterial antigens affect donor and recipient T-cell repertoires and therefore play a role in HCT outcomes?
Do donor lymphocyte infusions, checkpoint inhibitors, CAR T-cell and other T-cell therapies impact the microbiome? If so, does the microbiome in any way mediate clinical outcomes following these therapies?
Section IV: The Microbiome and Its Role in Infection and Idiopathic Post-HCT Disorders
Infection is a major cause of non-relapse morbidity and mortality after HCT, second only to GVHD. Unfortunately, other than administering prophylactic antibiotics or antiviral agents to susceptible patients, the therapeutic approach against these infections is largely reactive. Thus, identifying modifiable host or microbiome features that can be manipulated to prevent infection is a very attractive and promising proposition. The gut microbiota plays a critical role in maintaining colonization resistance against intestinal pathogens, and the mechanisms that underlie this regulation are becoming increasingly well understood. The composition of intestinal microbiota is actively regulated by a number of internal and external factors, ranging from diet to antibiotics, to the elements of the adaptive and innate immune system. An important element of the innate immune system that shapes the microbiota is host-derived antimicrobial peptides (AMPs). Examples of such AMPs include Paneth cell-derived α-defensins and REG3α, which selectively eliminate non-commensals while preserving commensals, and thus serve as microbiome modulators98,99. Intestinal commensal bacteria can stimulate the gut epithelium to produce AMPs that kill pathogenic bacteria100 and fungi52. In GVHD, for example, Paneth cell loss is associated with both reduced secretion of α-defensins and intestinal dysbiosis85,101,102. Additionally, disruption of gut microbial communities by antibiotics can increase susceptibility to intestinal pathogens103,104. Microbiota disruption that leads to gut microbial monodominance (e.g. a microbiome dominated by Enterobacteriaceae or Enterococcus spp.) precedes and significantly increases the risk of bacteremia (with Enterobacteriaceae or Enterococcus spp.) in HCT patients45. In pre-clinical models, a one- to two-log fold reduction in bacterial105 or fungal52 gut colonization levels is sufficient to significantly decrease pathogen dissemination and mortality. Similarly, specific gut commensals can provide resistance to Clostridium difficile infection103. Thus, efforts targeted at protecting the commensal microbiome may protect against intestinal pathogens and infections.
The role of microbiome-host crosstalk and whether specific molecules or pathways in this crosstalk can be manipulated toward therapeutic benefit remains an active field of investigation. Oral administration of synthetic AMPs may restore gut ecology and shape the host immune system to decrease the risk of infection as well as reducing GVHD, while preserving the graft-versus-leukemia effect. As with nearly all antibacterial agents known to date, resistance to specific AMPs has been described106. An alternative strategy that leverages a larger spectrum of AMPs and thus protects against rapid acquisition of resistance might be stimulation of the intrinsic production of AMPs. Although augmenting innate cellular function or mucosal integrity is difficult, it may be possible in the future through modulation of gut microbiota or directly inducing gut mucosal immune effectors to tip the balance back towards gut homeostasis, restore colonization resistance, and reduce the risk of severe infections.
In addition to efforts focused on microbiome modulation to protect against bacterial infections, the importance of microbiota in the control of viral infections and host immune responses has been increasingly recognized. Studies specific to HCT patients or to viruses commonly encountered in HCT (i.e. CMV, EBV, adenovirus, and RSV) are still very limited. The interactions among microbiota, host immune response, and viral infections are complex and multi-directional: for example, the microbiome may influence viral-specific CD8 T cell memory107, which can modulate clinical symptoms, severity, and clearance of viral infections108, or in reverse, a viral infection may result in a change in the microbiome through host-immune responses and changes in cytokines, including interferon109. Pathogenic or nonpathogenic viruses within the respiratory tract110, skin, or gut111 may also interact with the bacterial microbiome in what has been termed “trans-kingdom interactions”112. Host immune responses to prophylactic vaccines or anti-viral drugs can also be influenced by the status of the microbiota, and this may impact future decision-making around routine decisions such as the schedule for immunizations post-HCT113.
A proportion of non-relapse related mortality in HCT patients results from non-GVHD and noninfectious complications for which clinicians are unable to ascribe a clear etiology. These so-called “idiopathic” disorders may be related to an underlying microbiome dysbiosis or a potential infectious trigger that sparks a self-perpetuating inflammatory cascade (i.e. a “hit and run” phenomenon)114,115. The application of next-generation sequencing methods and ultrasensitive molecular methods for both unbiased and candidate-base pathogen detection have illuminated several of these “mystery” cases114,116,. However, recurrent and abundant candidate pathogens have not yet been identified for highly morbid diseases such as the idiopathic pneumonia syndrome. While the evidence is still preliminary in most cases, it is proposed that the microbiome or novel opportunistic pathobionts may contribute to these disease phenotypes on occasion.
The role of the microbiome in modifying the incidence and clinical outcomes of infection and idiopathic disorders in HCT patients is becoming increasingly recognized. To date, most research has focused on the bacterial contribution to these disorders, but increasingly, there is an appreciation of the contribution of viruses to these disorders and to the delicate balance of the microbiome. Both host and microbial factors participate in a complicated interplay to maintain homeostasis, and we are just now starting to understand the detailed elements in this complicated interaction. Little is known about the fungal contribution to both the healthy and diseased HCT microbiome, although we anticipate this will be an area of active and productive research in the future.
Open Questions
What is the composition of the human “virome” and “mycobiome” in HCT patients - and how do interactions between viruses, fungi, bacteria and the host impact HCT outcomes?
Do antimicrobial prophylaxis strategies adversely impact the microbiota and render HCT recipients susceptible to opportunistic infections beyond C. difficile?
Might microbiome-targeted therapeutics, aimed at protecting against loss of diversity in the microbiome, decrease the rate of infectious complications such as enteric Gram-negative and Gram-positive bacteremia believed to originate from the intestinal microbiome?
Which of the “idiopathic” complications of HCT are related to either infections or microbial dysbiosis? In cases where the offending organism acts through a “hit-and-run” type of mechanism, how might we identify these etiologies using existing technologies and sampling strategies?
Section V: Methods for Microbiota Modification
A clear rationale exists for targeting the microbiome with the eventual intention of both fine-tuning the immune system (balancing GVHD and GVT, for example) and decreasing the risk of downstream infectious complications of HCT. Several interventional studies are ongoing that will alter microbiota by means of diet and prebiotics, antibiotics, probiotics, microbial metabolites, and fecal microbial transplantation (Table 8). Below, we will discuss several clinical microbiome manipulation strategies and the implications of their use in future studies.
Table 8.
Clinical trials with microbiota-based interventions in HCT patients
| Name | Comparison | Outcomes | Study type | Clinical Trials.gov ID |
Status |
|---|---|---|---|---|---|
| Dietary Interventions | |||||
| Comparing Two Diets in Patients Undergoing HSCT or Remission Induction Chemo for Acute Leukemia and MDS | Standard hospital neutropenic diet vs. diet inclusive of fresh fruits and vegetables | Incidence of major infections | Randomized open-label | NCT03016130 | Recruiting |
| Gluten Free Diet in Preventing GVHD in Patients Undergoing HCT | Gluten-free diet | GVHD | Single-arm | NCT03102060 | Recruiting |
| Randomized, Prospective, Multicenter Study to Compare Enteral Nutrition to Parenteral Nutrition as Feeding Support in Patients With Hematologic Malignancies Undergoing Allo-HCT | Enteral vs. intravenous nutrition | Mortality | Randomized open-label | NCT01955772 | Recruiting |
| Donor Human Milk in Young Children Receiving Bone Marrow Transplantation | Enteral donor breastmilk vs. standard diet | Percentage of stool Lactobacillales | Randomized open-label | NCT02470104 | Active, not recruiting |
| Prebiotic and Probiotic Interventions | |||||
| Modification of the Intestinal Microbiome by Diet Intervention to Mitigate Acute GVHD | Bob’s Red Mill potato-starch-based prebiotic vs. standard diet | GVHD | Randomized open-label | NCT02763033 | Not yet open |
| Fructo-oligosaccharides in Treating Patients with Blood Cancer Undergoing HCT | FOS | Prebiotic tolerability | Single-arm | NCT02805075 | Recruitment completed |
| Lactobacillus rhamnosus GG in Reducing Incidence of GVHD in Patients Who Have Undergone HCT | Lactobacillus rhamnosus GG supplementation vs. control | GVHD | Randomized open-label | NCT02144701 | Active, not recruiting |
| Fecal Microbiota Transplantation | |||||
| Auto-FMT for Prophylaxis of CDI in Recipients of Allo-HCT | Auto-FMT vs. control | CDI | Randomized open-label | NCT02269150 | Recruiting |
| Fecal Transplant for Steroid-Resistant and Steroid-Dependent Gut Acute GVHD | Fecal microbiota transplant | Safety, GVHD | Single-arm | NCT03214289 | Recruiting |
| Antibiotic Interventions | |||||
| Choosing the Best Antibiotic to Protect Friendly Gut Bacteria During the Course of HCT | Piperacillin-tazobactam vs. Cefepime during febrile neutropenia | Change in Clostridial abundance | Randomized open-label | NCT03078010 | Recruiting |
| Gut Decontamination in Pediatric Allo-HCT | Oral Vancomycin-Polymixin B vs. standard of care | Intestinal microbiota diversity | Randomized open-label | NCT02641236 | Recruiting |
Antibiotics
Over the past 10 years, metagenomic and other culture-independent microbiota analyses have demonstrated the important role of the microbiome in health and disease117. Patients undergoing HCT represent a natural group for this line of research for the reasons that they are a) uniquely prone to perturbations in the normal microbiome as a result of toxicity from conditioning regimens, impaired diet, and antimicrobial exposure given for treatment and prophylaxis, b) their propensity for infectious and immune-mediated morbidity and mortality, c) their prolonged peri-transplant hospitalization that facilitates convenient sampling, and d) the availability of long-term outcomes that are universally gathered from transplant recipients. Early studies of prophylactic antibiotics in HCT demonstrated reductions in both infections and GVHD after suppression of the microbial flora26. Although the use of broad-spectrum antibiotics has led to dramatic improvement in infection-related TRM, antibiotics result in substantial microbiota disruption54. Importantly, the type of antibiotic therapy may determine the composition of intestinal microbiota and the extent of microbiome disruption. For example, antibiotics with anaerobic activity are associated with higher rates of GVHD-related mortality88. These new insights, which suggest an unfavorable impact of broad-spectrum antibiotics on intestinal microbiota and patient outcomes after HCT, raise the question of how we might preserve the protective effects of “healthy” commensal organisms without compromising treatment efficacy. In addition to the type of antibiotic, timing of treatment also appears to influence microbial diversity and may impact patient outcomes. Patients starting antibiotics before their day of transplantation showed significantly more microbiome disruption and had a higher TRM than those who began antibiotics on or following day 0 or who did not receive antibiotics54. Collectively, these studies support an argument for more selective use of broad-spectrum antibiotics along with early de-escalation strategies. Such strategies would preserve the microbiome but still ensure adequate prevention and treatment of bacterial infections. Further, the benefit of gut decontamination and prophylactic antibiotics should be examined through well-designed prospective trials. Definitive support for or against these practices will only come through the conduct of multicenter prospective trials designed to assess the short-term risk of bacterial infections during neutropenia with long-term endpoints (GVHD, immune reconstitution and microbial resistance) that may be affected by disruption of microbiome diversity.
Open Question(s)
How do we balance adequate prevention and treatment of bacterial infections with preservation of the microbiome in HCT recipients?
How can we incorporate microbiota stewardship practices in addition to antibiotic stewardship practices in our care of HCT recipients?
Diet, Prebiotics, Probiotics, and FMT
Ingested food contaminated by microbes has long been recognized as a potential source of bloodstream infection during chemotherapy-induced neutropenia with attendant gastrointestinal mucosal damage. The germ-free “sterile” diet was conceived in the 1960s as a way to reduce ingestion of potentially harmful microbes, but this was not palatable118. A “cooked-food” diet alternative, which eliminated raw foods with high bacterial counts, was shown in a randomized trial to have a similar effect on bacterial stool cultures as the germ-free diet, but it was also limited by patient dissatisfaction118. To expand and improve food palatability, Pizzo and colleagues cultured commercially available foods and identified low-microbial foods that were deemed suitable for a neutropenic diet119. The composition of neutropenic diets vary from center to center but, in general, consist of cooked and canned food products and exclude raw meat, fresh fruits, juices and vegetables, raw eggs and unpasteurized dairy products118,120. Despite limited evidence to support the merits of a neutropenic diet in HCT recipients as illustrated in Table 9121,122, dietary restriction of fresh fruits and vegetables continues to be standard practice for neutropenic patients in some centers, which likely has an impact on the amount of fiber that is consumed by HCT patients123. In the early post-transplant period, nutritional oral intake often declines to the point of necessitating nutritional supplementation. Retrospective comparisons of parenteral and enteral nutrition have suggested a benefit to the enteral route124,125. This may be due to enteral nutrition maintaining digestive function and the mucosal barrier, thus preventing bacterial translocation126. An ongoing trial is currently evaluating enteral vs. parenteral nutrition127.
Table 9.
Clinical trials investigating the efficacy of a neutropenic diet in HCT patients
| Intervention | Control | Outcomes (Intervention v Control) |
References |
|---|---|---|---|
| Randomized controlled, prospective trial | |||
| Unrestricted diet (n=21) | Neutropenic diet (n=25) |
|
Lassiter, 2015121 |
| Retrospective observational study | |||
| General hospital diet (n=363) | Neutropenic diet (n=363) |
|
Trifilio, 2012122 |
Specific elements of diet, called prebiotics, are particularly influential in the structure and function of the microbiota. The term “prebiotic” is traditionally applied to indigestible carbohydrates that are metabolized by gut bacteria to produce short chain fatty acids (SCFAs); recently the term is being redefined to refer to any substrate that is selectively utilized by host microorganisms and that confers a health benefit128. Apart from a single retrospective study, little is known about how prebiotics affect transplant outcomes such as GVHD129,130. However, studies in patients with inflammatory bowel disease treated with prebiotics such as inulin and fructo-oligosaccharides (inulin-type fructans or ITF) have demonstrated that these agents increase microbiota diversity and are associated with a corresponding decrease in disease markers and activity131,132,. Such studies provide a compelling rationale for studies of prebiotics in the HCT setting.
Probiotics are live microorganisms given to improve health and have long been used as part of traditional diets through the ingestion of fermented foods. Encapsulated preparations of one or more isolated live organisms have been used in attempts to treat a wide variety of gastrointestinal illness including infectious diarrhea or gastroenteritis133, and inflammatory bowel disease134,90. Some of these studies have shown evidence of efficacy, most likely mediated through direct antimicrobial effects, stimulation of immune responses that lead to up-regulation of anti-inflammatory cytokines and IgA, and promotion of intestinal barrier function135,136. To date, probiotics in the HCT setting have been limited to pre-clinical models and small pilot trials (Tables 10a and 10b)89,90,137–140. Of course, concern exists for the potential infectious complications associated with administration of live microbial organisms in high dose141. Indeed, case reports of bacteremia following ingestion of probiotics suggest the importance of exercising caution and judgment in the use of live bacterial therapies142. Further clinical studies are needed to fully determine the safety and efficacy of probiotics in patients undergoing HCT.
Table 10.
| a. Probiotics and the microbiome in murine models of HCT | |||
|---|---|---|---|
| Treatment | Control | Outcomes | References |
| Lactobacillus rhamnosus GG | Ciprofloxacin |
|
Gerbitz, 2004137 |
| 17 butyrate-producing Clostridia spp. strains | Phosphate-buffered saline |
|
Mathewson, 201689 |
| Anti-inflammatory Clostridia spp. (AIC) | Phosphate-buffered saline |
|
Simms-Waldrip, 201790 |
| b. Probiotics and the microbiome in clinical studies of HCT patients | |||
|---|---|---|---|
| Trial design | Probiotic, dose | Outcomes | References |
| Observational | Self-reported yogurt intake, average of 150g/day (n=41) |
|
Tavil, 2012138 |
| Single-arm | Lactobacillus plantarum, 1 × 108 cfu/kg/day (n=30) |
|
Ladas, 2016139 |
| Randomized | Lactobacillus rhamnosus GG, 1 × 1010/day (probiotic group, n=20; control group, n=11) |
|
Gorshein, 2017140 |
Finally, fecal microbiota transplantation (FMT) is yet another intervention that could be employed to preserve or restore the GI microbiota in patients undergoing HCT. Pioneering physicians performed FMT in non-HCT patients with recurrent or refractory Clostridium difficile infections (CDI) and demonstrated efficacy in up to 90% of treated patients143,144. Literature on FMT in HCT patients is still scant; however, the limited data to date appears encouraging with a total of 25 reported HCT patients having undergone FMT without known complications (Table 11a–c)145–151,152. Highlighting the need for a cautious approach in HCT populations, case reports in non-HCT patients with CDI have documented infectious complications following FMT including norovirus infection and sepsis as a result of presumed bacterial translocation153,154. Taken together, while limited published experience suggests that FMT can be used in immunocompromised patients with CDI,155 prospective trials evaluating safety and efficacy in HCT recipients are needed.
Table 11.
| a. Fecal microbiota transplantation in auto- and allo-HCT patients with recurrent CDI | ||||||
|---|---|---|---|---|---|---|
| Patient population |
Total patients |
Route | Donor type | Outcomes | Adverse events |
References |
| 21-year-old female; allo-HCT | 1 | NG tube | Spouse | Resolution of CDI | None | Neemann, 2012145 |
| 60-year-old female; allo-HCT | 1 | Push enteroscopy | Anonymous, 2 donors | Resolution of CDI | None | de Castro, 2015146 |
| 64-year-old male; auto-HCT | 1 | Enema | Anonymous, 2 sequential FMTs from different donors | First FMT resulted in resolution of CDI. Recurrence in 6 months. Second FMT performed at 6 months resulted in resolution of CDI durable for at least 7 months | None | Mittal, 2015147 |
| Allo-HCT patients | 7 | NJ* tube or colonoscopy | Anonymous | Resolution of CDI in 6 patients; one patient recurred at day +156 post- FMT after receiving antibiotics. Repeat FMT with resolution of symptoms lasting >4 months | None | Webb, 2016148 |
| Auto- and allo-HCT patients | 8 | Oral capsules | Anonymous | No recurrence of disease in 7 patients; one had recurrence of CDI day +179 after FMT | None | Moss, 2017149 |
| b. Commercially available agents in Study Pharmacopeia | ||||||
|---|---|---|---|---|---|---|
| Patient population |
Total patients |
Route | Donor type | Outcomes | Adverse events |
References |
| Steroid-resistant GI GVHD | 4 | ND* tube | Related | Resolution of diarrhea in 3 patients, partial resolution in 1 patient | None | Kakihana, 2016150 |
| Steroid-refractory GI GVHD | 3 | Colonoscopy | Related or anonymous | Resolution of diarrhea in 2 patients, improvement in GI GVHD in 3rd patient | None | Spindelboeck, 2017151 |
| c. Fecal microbiota transplantation in allo-HCT patients with antibiotic-resistant bacteria (ARB) | ||||||
|---|---|---|---|---|---|---|
| Patient population |
Total patients |
Route | Donor type | Outcomes | Adverse events | References |
| Hematologic disorders + ARB* colonized | 20 (8 HCT) | ND* tube | Anonymous |
|
Bilinski, 2017152 | |
NJ: nasojejunal, ND: nasoduodenal,
ARB: antibiotic-resistant bacteria
Undeniably, the field of FMT is rapidly growing, yet several questions remain including what guidelines the field should adopt for identification of the best FMT donors and appropriate donor stool screening prior to FMT in immunocompromised patients. Autologous FMT donation may have an advantage of simple traceability of the preparation and control of the inoculum during donor procedures, as well as reducing the risk of potential transmission of diseases originating from the microbiota of an external donor. The opportunity to obtain a ‘pre-morbid/baseline’ stool may not always be feasible for autologous FMT and for this reason, several investigators are using 3rd-party FMT obtained from healthy donors156. Case reports have explored the role of FMT for the treatment of non-infectious complications. Finally, it is interesting to speculate about the role of the stem cell donor’s microbiota96,86, or the patient’s cohabitating family members157 from whom the microbiota may potentially reconstitute after transplant-induced dysbiosis.
Open Questions
What is the impact of neutropenic dietary restriction and use of parenteral nutrition on long-term outcomes post-HCT?
How might the screening protocol for FMT donors to HCT patients differ than standard screening protocols used for less immunocompromised patients?
Can targeting of specific microbes or microbial pathways result in modification of microbe-disease associations?
Is there a role for genetically-modified bacteria in the post-HCT setting? How might this tool be safely and effectively leveraged?
Are there novel bacterial natural products (small molecules, proteins, glycolipids, sugars) that can be investigated for salutary drug-like effects in the HCT patient population?
Might we create a defined microbial consortium as the next-generation microbial therapeutic to supplant FMT in the HCT recipient population?
How stable are microbial populations and microbial genomes over time? How does horizontal gene transfer affect the medium- and long-term safety and efficacy of potential novel bacterial therapies?
Section VI: Maximizing the Opportunity for Impact
Identifying associations between the microbiome and clinical phenotypes is critically important. With the advent of technologies such as metagenomic sequencing, metabolomics, and improved tools for studying human immunology, it is becoming increasingly affordable and feasible to perform longitudinal molecular characterization of patients following HCT. As we transition toward an increased reliance on human samples for the generation and querying of biological hypotheses, it is important that the same rigor used in carefully controlled in vitro or animal experiments be applied in the clinical setting. This is particularly important in light of the sometimes conflicting results seen between pre-clinical and clinical studies, possibly as a result of microbiota variability between animal strains or differences in practice between centers, but also potentially as a result of variation in sample management. Samples must be collected, stored and processed in a reproducible manner to avoid the unintended introduction of bias in the results158. This is of utmost importance when studying low biomass or low microbial burden samples, where the chance introduction of ambient microorganisms through handling or processing may confound the ability to draw robust, reproducible, and generalizable conclusions. Similar to standard sample collection and data generation practices, strict procedures for management of data generated through high-throughput techniques will ensure data quality and accuracy critical to reliable interpretation and analysis. For example, current efforts to understand the microbiome and its impact on transplant outcomes are often limited by incomplete information on antibiotic exposure and diet. The BMT community has a strong track record of collecting detailed clinical data regarding GVHD and immunosuppressive medication administration. This same rigor needs to be applied to infection reporting and antimicrobial exposure in order to draw meaningful conclusions regarding microbiome findings. To this end, the CIBMTR currently has a working group tasked with the development of reporting standards for infectious disease endpoints.
As clinical infectious data collection strategies improve, inclusion of patient-reported outcomes (PROs) should also be prioritized in microbiome-oriented studies. PROs provide greater accuracy of treatment-related symptoms than clinician report159, and given the role that the microbiome likely plays in mediating symptoms such as diarrhea, constipation, flatus and abdominal discomfort, inclusion of PROs will be critical in studying the impact of microbiome-modifying therapies. Specifically, validated instruments of physical function and health related quality of life are available and include patient-reported measures of gastrointestinal symptoms, with domains of severity, frequency, and interference related to these symptoms contained within the NCI PRO-CTCAE (http://healthcaredelivery.cancer.gov/pro-ctcae). The successful incorporation of PROs will require 1) the development of a scalable infrastructure for participating sites, 2) consensus choice of relevant measures160, 3) collection time points so that PROs can be appropriately linked to clinical data consistently across research studies and clinical practice, and 4) application of statistically sound approaches to handle missing data is of paramount importance.
It is expected that the collection of high quality high-resolution molecular, clinical and patient-reported symptom data will hasten the identification of potentially relevant and useful molecular biomarkers as well as associations between microbiome alterations or specific microbes and clinically relevant outcomes. We anticipate that “big-data” approaches, including clinical-informatics efforts that extract information from the electronic medical record will be a key part of this effort. As more and more data are collected, it will be increasingly important to leverage machine-learning approaches to data interpretation and analysis, as is already being done in the field of cancer genomics and beyond.
Perhaps most importantly, we must not lose sight of the importance of moving “beyond association” in study design. Strategies to model the hypothesized interactions between the microbiome and host, both in vitro and in vivo in models ranging from cell lines to small and large animal models, will allow for carefully controlled experiments to be carried out - something that is of course difficult or challenged by ethical considerations in patient studies. Lastly, whenever possible, it is critical that well-designed and thoughtfully targeted interventional studies of microbiome modification be performed in multi-center settings. This will provide the highest level of prospective data to help guide clinical practice, and the multi-center nature of these studies will ensure the highest level of generalizability. When these studies are done, adhering to rigorous standards of both biospecimen collection, clinical data collection, and PRO data collection will allow for the accurate measurement of the consequences of the tested interventions. This will shed light on potential mechanisms of action of these interventions, and will inform the next iteration of interventions (Figure 1).
Figure 1.
Structuring the design of future interventions aimed at establishing microbe-host disease causality
Open Questions
How can we facilitate more universal sample collection and support multi-institutional studies of the microbiome to improve the generalizability of findings?
How do we identify and implement standardized methods for sample and data collection that can be used for multicenter prospective clinical trials that study the microbiome in HCT patients?
How can we best collect information about infections and antimicrobial medication use in both the clinical trial setting and for registry purposes?
What are the critical clinical data that need to be collected for meaningful analysis of microbiome studies in HCT?
How can we hasten the design and execution of microbiome-targeted interventional clinical trials? What funding sources exist to support these critically important efforts, given the relative paucity of classical industry partners in this space?
Section VII: Future Directions
While the importance of the microbiome in immunological development, protection against infections, and patient symptoms has been investigated in the past decades, much remains to be understood on the importance and relevance of the contribution of the stability, the resilience and the redundancy of the microbial composition after transplantation. The field has come a long way from the earliest days of microbiome research, now nearly a half a century ago. There has been an explosion in the number of high-throughput tools for microbiome measurement and an increasing number of single institution biospecimen collections. These tools and resources have facilitated the testing of only a limited number of translationally important hypotheses. The taxonomic diversity of the microbiome has been shown to be a potential biomarker of HCT outcomes, ranging from overall survival to relapse. These early, single-institution findings are certainly compelling, and warrant further investigation. Interactions between the microbiome and immune system have been described for decades; deep immunophenotyping tools such as T-cell receptor sequencing and high-dimensional mass cytometry are now facilitating investigating the temporal relationships between the microbiome and immune system during immune reconstitution. Early data suggest a potential role for the microbiome in improving post-transplant immune reconstitution and helping to achieve the elusive goal of effective GVT without GVHD. A growing set of tools for microbiome manipulation using diet, prebiotics, probiotics and even FMT are being tested rigorously, both in preclinical models and in humans. It is anticipated that larger randomized multi-institutional studies of these approaches and their efficacy will be initiated. Beyond GVHD, the clear role of the microbiome in mediating risk of infection and perhaps idiopathic disorders also poses an exciting opportunity for investigation, and the potential to improve non-relapse, non-GVHD related morbidity and mortality. To maximize the impact of microbiome-focused investigation, there are many targets that represent low-hanging fruit: improving infection and antibiotic-related data collection, incorporating PROs, and the application of newer methods such as shotgun sequencing, metabolomics, metaproteomics, advanced microscopy and beyond, that will allow us to extend our investigational reach beyond the taxonomic realm. A more functional characterization of how these communities are structured, how they interact and what they do will undoubtedly inform progress in developing precision microbiome diagnostic and therapeutic strategies. Advances in technology have revealed many opportunities to better understand the mechanisms that underlie microbiome-host interactions. For example, recent studies have brought to the fore the role of metabolomes and host genes that are critical89, yet, there have been so far no corresponding studies on the RNA transcripts (metatranscriptomics) and proteins produced by the microbes in these processes in HCT patients. The abundance and transcript levels of genes encoding microbial resistance to antibiotics, drug metabolism and resistance to host mediated immune responses, for example, could shed light on better exploitation of the microbiome in HCT. Advances in bioengineering have resulted in the ability to generate microbes with specific, salutary effects. For example, oral administration of commensal bacteria genetically engineered to regulate endogenous or recombinant gene expression to alter their metabolic ability could hold great promise for restoring intestinal homeostasis and modulating host immune systems161. New technologies are rapidly being developed and applied; thus, in the coming years research will better define the role of microbiome on GVHD, GVT, infectious complications, and transplant outcomes. As this happens, we hope that carefully considered and planned investigations ranging from basic microbiology to immunology to large-scale, randomized-controlled interventional clinical trials will together result in improved outcomes for HCT and related patient populations. Simultaneously, we anticipate that such efforts will result in an improved understanding of the basic biological underpinnings of microbial bioregulation, microbiome community interactions, and human immunology. Vast opportunities exist for both scientific and translational advances in the realm of microbiome sciences. In order to capitalize quickly on these prospects for maximum impact, we propose ten areas of focus (Table 1) that may have the greatest promise for breakthrough discoveries regarding the dynamic and complex microbiota-host relationship in patients undergoing HCT.
Highlights.
Microbiome alterations are associated with infection, GVHD, and other adverse transplant outcomes
“Multi-omic” technologies may better identify clinically actionable microbiome biomarkers
Functional microbial characterization may improve diagnostic and therapeutic strategies
Knowledge gaps persist in chronic GVHD and extra-intestinal microbiome-host interactions
Multi-institutional studies and data collection expansion will maximize microbiome research impact
Acknowledgments
Funding
TAM is supported by the American Society of Blood and Marrow Transplantation New Investigator Award. ASB is supported by the Damon Runyon Cancer Research Foundation. The Blood and Marrow Transplant Clinical Trials Network is supported in part by grant #U10HL069294 and #U24HL138660 from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Other disclosures:
In the prior 2 years, MRMVDB has served as a consultant for Jazz Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, Flagship Ventures, Boehringer Ingelheim, Merck, and Evelo
LSK consults with Bristol Myers Squibb and Kymab, Ltd in addition to receiving research funding from these in addition to Regeneron Pharmaceuticals
MMM received honoraria and research support from MaaT Pharma
RRJ has consulted for Ziopharm Oncology
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflicts of interest to report: TMA, CH, PR, MLR, RS, TT, AA, SB, PC, WBC, EH, AH, AYK, PLM, JMM, RN, KR, BES, EJS, ADS, DW, JW, JRW, WAW, MAP, ASB
Seres Therapeutics Inc: JUP (licensing fees and research support), MRMVDB (research support), RRJ (advisory committee, holds patents with or receives royalties)
Contributor Information
Tessa Andermann, Division of Infectious Diseases, Department of Medicine, Stanford University, Stanford, CA, USA.
Jonathan Peled, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering, Cancer Center, New York, NY, USA; Department of Medicine, Weill Cornell Medical College, New York, NY, USA.
Christine Ho, Blood and Marrow Transplantation, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY, USA.
Pavan Reddy, Department of Medicine, University of Michigan Cancer Center, Ann Arbor, MI, USA.
Marcie Riches, Division of Hematology/Oncology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Rainer Storb, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Medicine, University of Washington School of Medicine, Seattle, WA, USA.
Takanori Teshima, Department of Hematology, Hokkaido University Faculty of Medicine, Sapporo, Japan.
Marcel van den Brink, Immunology Program, Memorial Sloan Kettering Center, New York, NY, USA; Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Amin Alousi, Multidiscipline GVHD Clinic and Research Program, Department of Stem Cell Transplant and Cellular Therapies, University of Texas, MD Anderson Cancer Center, Houston, TX, USA.
Sophia Balderman, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY, USA.
Patrizia Chiusolo, Hematology Department, Fondazione Policlinico Universitario A. Gemelli, Università Cattolica Sacro Cuore, Rome, Italy.
William Clark, Bone Marrow Transplant Program, Division of Hematology/Oncology and Palliative Care, Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, USA.
Ernst Holler, Department of Internal Medicine 3, University Medical Center, Regensburg, Germany.
Alan Howard, Center for International Blood and Marrow Transplant Research, Minneapolis, MN, USA.
Leslie Kean, Fred Hutchinson Cancer Research Center, Seattle WA; Department of Pediatrics, University of Washington School of Medicine, Seattle, WA; The Ben Towne Center for Childhood Cancer Research, Seattle Children’s Research Institute, Seattle, WA.
Andrew Koh, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Philip McCarthy, Blood and Marrow Transplantation, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY, USA.
John McCarty, Bone Marrow Transplantation Program, Virginia Commonwealth University Massey Cancer, Richmond, VA, USA.
Mohamad Mohty, Hôpital Saint-Antoine, AP-HP, Paris, France; Sorbonne Université, Paris, France; INSERM UMRs U938, Paris, France.
Ryotaro Nakamura, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope, Duarte, CA, USA.
Katy Rezvani, Section of Cellular Therapy, Good Manufacturing Practices Facility, Department of Stem Cell Transplant and Cellular Therapy, MD Anderson Cancer Center, Houston, TX, USA; Department of Medicine, MD Anderson Cancer Center, Houston, TX, USA.
Brahm Segal, Department of Medicine, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY, USA; Division of Infectious Diseases, Roswell Park Cancer Institute, Buffalo, NY, USA; Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY, USA.
Bronwen Shaw, Center for International Blood and Bone Marrow Transplant Research (CIBMTR), Medical College of Wisconsin, Milwaukee, WI, USA.
Elizabeth Shpall, Cell Therapy Laboratory and Cord Blood Bank; Department of Stem Cell Transplantation and Cellular Therapy, University of Texas, MD; Anderson Cancer Center, Houston, TX, USA.
Anthony Sung, Division of Hematologic Malignancies and Cellular Therapy, Duke University School of Medicine, Duke Cancer Institute, Durham, NC, USA.
Daniela Weber, Department of Internal Medicine 3, University Medical Center, Regensburg, Germany.
Jennifer Whangbo, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA, USA.
John Wingard, Department of Medicine, University of Florida Health Cancer Center, Gainesville, FL, USA; Bone Marrow Transplant Program, Division of Hematology/Oncology, University of Florida College of Medicine, FL, USA.
William Wood, University of North Carolina at Chapel Hill Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
Miguel-Angel Perales, Adult Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Department of Medicine, Weill Cornell Medical College, New York, NY, USA.
Robert Jenq, Departments of Genomic Medicine and Stem Cell Transplantation Cellular Therapy, Division of Cancer Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ami Bhatt, Division of Hematology, Department of Medicine, Department of Genetics, Stanford University, Stanford, CA, USA.
References
- 1.Taur Y, Jenq RR, Perales M-A, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peled JU, Devlin SM, Staffas A, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(15):1650–1659. doi: 10.1200/JCO.2016.70.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2014;20(5):640–645. doi: 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell MS, Wilson R. THE TREATMENT OF X-IRRADIATED GERMFREE CFW AND C3H MICE WITH ISOLOGOUS AND HOMOLOGOUS BONE MARROW. Life Sci. 1965;4:721–729. doi: 10.1016/0024-3205(65)90011-1. [DOI] [PubMed] [Google Scholar]
- 5.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45(3):577–588. [PubMed] [Google Scholar]
- 6.van Bekkum D, Vos O. Treatment of secondary disease in radiation chimaeras. Int J Radiat Biol. 1961;3:173–181. doi: 10.1080/09553006114550181. [DOI] [PubMed] [Google Scholar]
- 7.van Bekkum DW, Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst. 1977;58(3):787–790. doi: 10.1093/jnci/58.3.787. [DOI] [PubMed] [Google Scholar]
- 8.Heit H, Wilson R, Fliedner TM, Kohne E. Mortality of secondary disease in antibiotic-treated mouse radiation chimeras. In: Heneghan JB, editor. Germ Free Research: Biological Effects and Gnotobiotic Environment. New York: Academic Press; 1973. pp. 477–485. [Google Scholar]
- 9.Pollard M, Truitt RL. Allogeneic bone marrow chimerism in germfree mice. 1. Prevention of spontaneous leukemia in AKR mice. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1973;144(2):659–665. doi: 10.3181/00379727-144-37657. [DOI] [PubMed] [Google Scholar]
- 10.Pollard M, Truitt RL. Allogeneic bone marrow chimerism in germ-free mice. II. Prevention of reticulum cell sarcomas in SJL-J mice. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1974;145(2):488–492. doi: 10.3181/00379727-145-37837. [DOI] [PubMed] [Google Scholar]
- 11.Truitt RL, Pollard M, Srivastava KK. Allogeneic bone marrow chimerism in germfree mice. 3. Therapy of leukemic AKR mice. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1974;146(1):153–158. doi: 10.3181/00379727-146-38061. [DOI] [PubMed] [Google Scholar]
- 12.Truitt RL, Pollard M. Allogeneic bone marrow chimerism in germ-free mice. IV. Therapy of “Hodgkin’s-like” reticulum cell sarcoma in SJL mice. Transplantation. 1976;21(1):12–16. doi: 10.1097/00007890-197601000-00003. [DOI] [PubMed] [Google Scholar]
- 13.van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974;52(2):401–404. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- 14.van Bekkum DW, de Vries MJ, van der Waay D. Lesions characteristic of secondary disease in germfree heterologous radiation chimeras. J Natl Cancer Inst. 1967;38(2):223–231. [PubMed] [Google Scholar]
- 15.Heit H, Heit W, Kohne E, Fliedner TM, Hughes P. Allogeneic bone marrow transplantation in conventional mice: I. Effect of antibiotic therapy on long term survival of allogeneic chimeras. Blut. 1977;35(2):143–153. doi: 10.1007/BF00996294. [DOI] [PubMed] [Google Scholar]
- 16.Veenendaal D, de Boer F, Van der Waaij D. Effect of selective decontamination of the digestive tract of donor and recipient on the occurrence of murine delayed-type graft-versus-host disease. Med Microbiol Immunol (Berl) 1988;177(3):133–144. doi: 10.1007/BF00232893. [DOI] [PubMed] [Google Scholar]
- 17.Buckner CD, Clift RA, Sanders JE, et al. Protective environment for marrow transplant recipients: a prospective study. Ann Intern Med. 1978;89(6):893–901. doi: 10.7326/0003-4819-89-6-893. [DOI] [PubMed] [Google Scholar]
- 18.Navari RM, Buckner CD, Clift RA, et al. Prophylaxis of infection in patients with aplastic anemia receiving allogeneic marrow transplants. Am J Med. 1984;76(4):564–572. doi: 10.1016/0002-9343(84)90274-2. [DOI] [PubMed] [Google Scholar]
- 19.Petersen FB, Buckner CD, Clift RA, et al. Laminar air flow isolation and decontamination: a prospective randomized study of the effects of prophylactic systemic antibiotics in bone marrow transplant patients. Infection. 1986;14(3):115–121. doi: 10.1007/BF01643474. [DOI] [PubMed] [Google Scholar]
- 20.Petersen FB, Buckner CD, Clift RA, et al. Prevention of nosocomial infections in marrow transplant patients: a prospective randomized comparison of systemic antibiotics versus granulocyte transfusions. Infect Control IC. 1986;7(12):586–592. doi: 10.1017/s0195941700065437. [DOI] [PubMed] [Google Scholar]
- 21.Petersen FB, Buckner CD, Clift RA, et al. Infectious complications in patients undergoing marrow transplantation: a prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Scand J Infect Dis. 1987;19(5):559–567. doi: 10.3109/00365548709032423. [DOI] [PubMed] [Google Scholar]
- 22.Beelen DW, Elmaagacli A, Müller K-D, Hirche H, Schaefer UW. Influence of Intestinal Bacterial Decontamination Using Metronidazole and Ciprofloxacin or Ciprofloxacin Alone on the Development of Acute Graft-Versus-Host Disease After Marrow Transplantation in Patients With Hematologic Malignancies: Final Results and Long-Term Follow-Up of an Open-Label Prospective Randomized Trial. Blood. 1999;93(10):3267–3275. [PubMed] [Google Scholar]
- 23.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308(6):302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud HK, Schaefer UW, Schüning F, et al. Laminar air flow versus barrier nursing in marrow transplant recipients. Blut. 1984;49(5):375–381. doi: 10.1007/BF00319885. [DOI] [PubMed] [Google Scholar]
- 25.Schmeiser T, Kurrle E, Arnold R, Krieger D, Heit W, Heimpel H. Antimicrobial prophylaxis in neutropenic patients after bone marrow transplantation. Infection. 1988;16(1):19–24. doi: 10.1007/BF01646924. [DOI] [PubMed] [Google Scholar]
- 26.Vossen JM, Heidt PJ, van den Berg H, Gerritsen EJ, Hermans J, Dooren LJ. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1990;9(1):14–23. doi: 10.1007/BF01969527. [DOI] [PubMed] [Google Scholar]
- 27.Beelen DW, Haralambie E, Brandt H, et al. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood. 1992;80(10):2668–2676. [PubMed] [Google Scholar]
- 28.Passweg JR, Rowlings PA, Atkinson KA, et al. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 1998;21(12):1231–1238. doi: 10.1038/sj.bmt.1701238. [DOI] [PubMed] [Google Scholar]
- 29.Vossen JM, Guiot HFL, Lankester AC, et al. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PloS One. 2014;9(9):e105706. doi: 10.1371/journal.pone.0105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzo VE, Bhatt AS. The human microbiome in hematopoiesis and hematologic disorders. Blood. 2015;126(3):311–318. doi: 10.1182/blood-2015-04-574392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loman NJ, Constantinidou C, Chan JZM, et al. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol. 2012;10(9):599–606. doi: 10.1038/nrmicro2850. [DOI] [PubMed] [Google Scholar]
- 35.Stämmler F, Gläsner J, Hiergeist A, et al. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome. 2016;4(1) doi: 10.1186/s40168-016-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16(7):1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giraud A. Axenic mice model. Methods Mol Biol Clifton NJ. 2008;415:321–336. doi: 10.1007/978-1-59745-570-1_19. [DOI] [PubMed] [Google Scholar]
- 39.Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15(8):337. doi: 10.1007/s11894-013-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Integrative Human Microbiome Project. Dynamic Analysis of Microbiome-Host Omics Profiles during Periods of Human Health and Disease. Cell Host Microbe. 2014;16(3):276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auchtung JM, Robinson CD, Britton RA. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs) Microbiome. 2015;3:42. doi: 10.1186/s40168-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci. 2016;113(1):E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajilić-Stojanović M, Heilig HGHJ, Molenaar D, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol. 2009;11(7):1736–1751. doi: 10.1111/j.1462-2920.2009.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55(7):905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine JE, Huber E, Hammer ST, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122(8):1505–1509. doi: 10.1182/blood-2013-02-485813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber D, Oefner PJ, Hiergeist A, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015;126(14):1723–1728. doi: 10.1182/blood-2015-04-638858. [DOI] [PubMed] [Google Scholar]
- 48.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2015;21(8):1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golob JL, Pergam SA, Srinivasan S, et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin Infect Dis. 2017 Aug; doi: 10.1093/cid/cix699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Legoff J, Resche-Rigon M, Bouque J, et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: new clues in enteric graft-versus-host disease. Nat Med. 2017;23(9):1080–1085. doi: 10.1038/nm.4380. [DOI] [PubMed] [Google Scholar]
- 51.Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci. 2010;72(1–2):1–11. [PMC free article] [PubMed] [Google Scholar]
- 52.Fan D, Coughlin LA, Neubauer MM, et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21(7):808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J-H, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34(4):426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 54.Weber D, Jenq RR, Peled JU, et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(5):845–852. doi: 10.1016/j.bbmt.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 56.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307) doi: 10.1126/scitranslmed.aab2271. 307ra152. [DOI] [PubMed] [Google Scholar]
- 57.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- 58.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- 60.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuo C-H, Kuo H-F, Huang C-H, Yang S-N, Lee M-S, Hung C-H. Early life exposure to antibiotics and the risk of childhood allergic diseases: an update from the perspective of the hygiene hypothesis. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2013;46(5):320–329. doi: 10.1016/j.jmii.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Ma D, Leulier F, Storelli G, Mitchell M. Studying host-microbiota mutualism in Drosophila: Harnessing the power of gnotobiotic flies. Biomed J. 2015;38(4):285. doi: 10.4103/2319-4170.158620. [DOI] [PubMed] [Google Scholar]
- 63.Milligan-Myhre K, Charette JR, Phennicie RT, et al. Methods in Cell Biology. Vol. 105. Elsevier; 2011. [Accessed September 12, 2017]. Study of Host-Microbe Interactions in Zebrafish; pp. 87–116. http://linkinghub.elsevier.com/retrieve/pii/B9780123813206000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hörmannsperger G, Schaubeck M, Haller D. Intestinal Microbiota in Animal Models of Inflammatory Diseases. ILAR J. 2015;56(2):179–191. doi: 10.1093/ilar/ilv019. [DOI] [PubMed] [Google Scholar]
- 65.Bauer H, Horowitz RE, Levenson SM, Popper H. The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- 66.Stěpánková R, Kovárů F, Kruml J. Lymphatic tissue of the intestinal tract of germfree and conventional rabbits. Folia Microbiol (Praha) 1980;25(6):491–495. doi: 10.1007/BF02897215. [DOI] [PubMed] [Google Scholar]
- 67.Baron F. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104(8):2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 68.Bartelink IH, Belitser SV, Knibbe CAJ, et al. Immune Reconstitution Kinetics as an Early Predictor for Mortality using Various Hematopoietic Stem Cell Sources in Children. Biol Blood Marrow Transplant. 2013;19(2):305–313. doi: 10.1016/j.bbmt.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Geddes M, Storek J. Immune reconstitution following hematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):329–348. doi: 10.1016/j.beha.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 71.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 × 106/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37(12):1119–1128. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 72.Novitzky N, Davison GM, Hale G, Waldmann H. Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome. Transplantation. 2002;74(11):1551–1559. doi: 10.1097/01.TP.0000031948.52799.C1. [DOI] [PubMed] [Google Scholar]
- 73.Porrata LF, Litzow MR, Inwards DJ, et al. Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33(3):291–298. doi: 10.1038/sj.bmt.1704355. [DOI] [PubMed] [Google Scholar]
- 74.Savani BN. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107(4):1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Storek J, Espino G, Dawson MA, Storer B, Flowers ME, Maloney DG. Low B-cell and monocyte counts on day 80 are associated with high infection rates between days 100 and 365 after allogeneic marrow transplantation. Blood. 2000;96(9):3290–3293. [PubMed] [Google Scholar]
- 76.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5):903–911. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 78.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Brink MRM, Velardi E, Perales M-A. Immune reconstitution following stem cell transplantation. Hematology. 2015;2015(1):215–219. doi: 10.1182/asheducation-2015.1.215. [DOI] [PubMed] [Google Scholar]
- 80.Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129(6):729–739. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 82.Atarashi K, Tanoue T, Shima T, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 85.Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood. 2012;120(1):223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 86.Tawara I, Liu C, Tamaki H, et al. Influence of Donor Microbiota on the Severity of Experimental Graft-versus-Host-Disease. Biol Blood Marrow Transplant. 2013;19(1):164–168. doi: 10.1016/j.bbmt.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Chen H-L, Bannick N, et al. Intestinal Helminths Regulate Lethal Acute Graft-versus-Host Disease and Preserve the Graft-versus-Tumor Effect in Mice. J Immunol. 2015;194(3):1011–1020. doi: 10.4049/jimmunol.1303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339) doi: 10.1126/scitranslmed.aaf2311. 339ra71-ra339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simms-Waldrip TR, Sunkersett G, Coughlin LA, et al. Antibiotic-Induced Depletion of Antiinflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol Blood Marrow Transplant. 2017;23(5):820–829. doi: 10.1016/j.bbmt.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 91.van der Velden WJFM, Netea MG, de Haan AFJ, Huls GA, Donnelly JP, Blijlevens NMA. Role of the Mycobiome in Human Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2013;19(2):329–332. doi: 10.1016/j.bbmt.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Biagi E, Zama D, Nastasi C, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2015;50(7):992–998. doi: 10.1038/bmt.2015.16. [DOI] [PubMed] [Google Scholar]
- 93.Bilinski J, Robak K, Peric Z, et al. Impact of Gut Colonization by Antibiotic-Resistant Bacteria on the Outcomes of Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective, Single-Center Study. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016;22(6):1087–1093. doi: 10.1016/j.bbmt.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 94.Routy B, Letendre C, Enot D, et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology. 2017;6(1):e1258506. doi: 10.1080/2162402X.2016.1258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doki N, Suyama M, Sasajima S, et al. Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2017;96(9):1517–1523. doi: 10.1007/s00277-017-3069-8. [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Frank DN, Horch M, et al. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transplant. 2017 Oct; doi: 10.1038/bmt.2017.200. [DOI] [PubMed] [Google Scholar]
- 97.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and Anticancer Immunosurveillance. Cell. 2016;165(2):276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1(2):113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 99.Masuda K, Sakai N, Nakamura K, Yoshioka S, Ayabe T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J Innate Immun. 2011;3(3):315–326. doi: 10.1159/000322037. [DOI] [PubMed] [Google Scholar]
- 100.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eriguchi Y, Nakamura K, Hashimoto D, et al. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl Infect Dis Off J Transplant Soc. 2015;17(5):702–706. doi: 10.1111/tid.12423. [DOI] [PubMed] [Google Scholar]
- 102.Weber D, Frauenschläger K, Ghimire S, et al. The association between acute graft-versus-host disease and antimicrobial peptide expression in the gastrointestinal tract after allogeneic stem cell transplantation. In: Palaniyandi S, editor. PLOS ONE. 9. Vol. 12. 2017. p. e0185265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buffie CG, Jarchum I, Equinda M, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80(1):62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4(2):e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brandl K, Plitas G, Mihu CN, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bell G, Gouyon P-H. Arming the enemy: the evolution of resistance to self-proteins. Microbiol Read Engl. 2003;149(Pt 6):1367–1375. doi: 10.1099/mic.0.26265-0. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka K, Sawamura S, Satoh T, Kobayashi K, Noda S. Role of the indigenous microbiota in maintaining the virus-specific CD8 memory T cells in the lung of mice infected with murine cytomegalovirus. J Immunol Baltim Md 1950. 2007;178(8):5209–5216. doi: 10.4049/jimmunol.178.8.5209. [DOI] [PubMed] [Google Scholar]
- 108.de Steenhuijsen Piters WAA, Heinonen S, Hasrat R, et al. Nasopharyngeal Microbiota, Host Transcriptome and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am J Respir Crit Care Med. 2016 May; doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121(9):3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim ES, Zhou Y, Zhao G, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21(10):1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science. 2016;351(6270) doi: 10.1126/science.aad5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tarabichi Y, Li K, Hu S, et al. The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome. 2015;3:74. doi: 10.1186/s40168-015-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood. 2015;125(24):3789–3797. doi: 10.1182/blood-2014-12-617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrera AF, Soriano G, Bellizzi AM, et al. Cord Colitis Syndrome in Cord-Blood Stem-Cell Transplantation. N Engl J Med. 2011;365(9):815–824. doi: 10.1056/NEJMoa1104959. [DOI] [PubMed] [Google Scholar]
- 116.Bhatt AS, Freeman SS, Herrera AF, et al. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N Engl J Med. 2013;369(6):517–528. doi: 10.1056/NEJMoa1211115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124(10):4162–4165. doi: 10.1172/JCI78366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Preisler HD, Goldstein IM, Henderson ES. Gastrointestinal “sterilization” in the treatment of patients with acute leukemia. Cancer. 1970;26(5):1076–1081. doi: 10.1002/1097-0142(197011)26:5<1076::aid-cncr2820260516>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 119.Pizzo PA, Purvis DS, Waters C. Microbiological evaluation of food items. For patients undergoing gastrointestinal decontamination and protected isolation. J Am Diet Assoc. 1982;81(3):272–279. [PubMed] [Google Scholar]
- 120.Smith LH, Besser SG. Dietary restrictions for patients with neutropenia: a survey of institutional practices. Oncol Nurs Forum. 2000;27(3):515–520. [PubMed] [Google Scholar]
- 121.Lassiter M, Schneider SM. A pilot study comparing the neutropenic diet to a non-neutropenic diet in the allogeneic hematopoietic stem cell transplantation population. Clin J Oncol Nurs. 2015;19(3):273–278. doi: 10.1188/15.CJON.19-03AP. [DOI] [PubMed] [Google Scholar]
- 122.Trifilio S, Helenowski I, Giel M, et al. Questioning the role of a neutropenic diet following hematopoetic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18(9):1385–1390. doi: 10.1016/j.bbmt.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 123.Moody K, Charlson ME, Finlay J. The neutropenic diet: what’s the evidence? J Pediatr Hematol Oncol. 2002;24(9):717–721. doi: 10.1097/00043426-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 124.Guièze R, Lemal R, Cabrespine A, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2014;33(3):533–538. doi: 10.1016/j.clnu.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 125.Seguy D, Duhamel A, Rejeb MB, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94(3):287–294. doi: 10.1097/TP.0b013e3182558f60. [DOI] [PubMed] [Google Scholar]
- 126.Szeluga DJ, Stuart RK, Brookmeyer R, Utermohlen V, Santos GW. Nutritional support of bone marrow transplant recipients: a prospective, randomized clinical trial comparing total parenteral nutrition to an enteral feeding program. Cancer Res. 1987;47(12):3309–3316. [PubMed] [Google Scholar]
- 127.Lemal R, Cabrespine A, Pereira B, et al. Could enteral nutrition improve the outcome of patients with haematological malignancies undergoing allogeneic haematopoietic stem cell transplantation? A study protocol for a randomized controlled trial (the NEPHA study) Trials. 2015;16:136. doi: 10.1186/s13063-015-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 Jun; doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 129.Iyama S, Sato T, Tatsumi H, et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep Oncol. 2014;7(3):692–699. doi: 10.1159/000368714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andermann TM, Rezvani A, Bhatt AS. Microbiota Manipulation With Prebiotics and Probiotics in Patients Undergoing Stem Cell Transplantation. Curr Hematol Malig Rep. 2016 Jan; doi: 10.1007/s11899-016-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55(3):348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Casellas F, Borruel N, Torrejón A, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25(9):1061–1067. doi: 10.1111/j.1365-2036.2007.03288.x. [DOI] [PubMed] [Google Scholar]
- 133.Margreiter M, Ludl K, Phleps W, Kaehler ST. Therapeutic value of a Lactobacillus gasseri and Bifidobacterium longum fixed bacterium combination in acute diarrhea: a randomized, double-blind, controlled clinical trial. Int J Clin Pharmacol Ther. 2006;44(5):207–215. doi: 10.5414/cpp44207. [DOI] [PubMed] [Google Scholar]
- 134.Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vitetta L, Briskey D, Alford H, Hall S, Coulson S. Probiotics, prebiotics and the gastrointestinal tract in health and disease. Inflammopharmacology. 2014;22(3):135–154. doi: 10.1007/s10787-014-0201-4. [DOI] [PubMed] [Google Scholar]
- 136.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136(6):2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103(11):4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 138.Tavil B, Koksal E, Yalcin SS, Uckan D. Pretransplant nutritional habits and clinical outcome in children undergoing hematopoietic stem cell transplant. Exp Clin Transplant Off J Middle East Soc Organ Transplant. 2012;10(1):55–61. doi: 10.6002/ect.2011.0082. [DOI] [PubMed] [Google Scholar]
- 139.Ladas EJ, Bhatia M, Chen L, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51(2):262–266. doi: 10.1038/bmt.2015.275. [DOI] [PubMed] [Google Scholar]
- 140.Gorshein E, Wei C, Ambrosy S, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2017;31(5) doi: 10.1111/ctr.12947. [DOI] [PubMed] [Google Scholar]
- 141.Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38(1):62–69. doi: 10.1086/380455. [DOI] [PubMed] [Google Scholar]
- 142.Mehta A, Rangarajan S, Borate U. A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transplant. 2013;48(3):461–462. doi: 10.1038/bmt.2012.153. [DOI] [PubMed] [Google Scholar]
- 143.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 144.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 145.Neemann K, Eichele DD, Smith PW, Bociek R, Akhtari M, Freifeld A. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis Off J Transplant Soc. 2012;14(6):E161–E165. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 146.de Castro CG, Ganc AJ, Ganc RL, Petrolli MS, Hamerschlack N. Fecal microbiota transplant after hematopoietic SCT: report of a successful case. Bone Marrow Transplant. 2015;50(1):145. doi: 10.1038/bmt.2014.212. [DOI] [PubMed] [Google Scholar]
- 147.Mittal C, Miller N, Meighani A, Hart BR, John A, Ramesh M. Fecal microbiota transplant for recurrent Clostridium difficile infection after peripheral autologous stem cell transplant for diffuse large B-cell lymphoma. Bone Marrow Transplant. 2015;50(7):1010–1010. doi: 10.1038/bmt.2015.85. [DOI] [PubMed] [Google Scholar]
- 148.Webb BJ, Brunner A, Ford CD, Gazdik MA, Petersen FB, Hoda D. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis Off J Transplant Soc. 2016 May; doi: 10.1111/tid.12550. [DOI] [PubMed] [Google Scholar]
- 149.Moss EL, Falconer SB, Tkachenko E, et al. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PloS One. 2017;12(8):e0182585. doi: 10.1371/journal.pone.0182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spindelboeck W, Schulz E, Uhl B, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica. 2017;102(5):e210–e213. doi: 10.3324/haematol.2016.154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bilinski J, Grzesiowski P, Sorensen N, et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin Infect Dis. 2017;65(3):364–370. doi: 10.1093/cid/cix252. [DOI] [PubMed] [Google Scholar]
- 153.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8(3):252–253. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 154.Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol. 2013;108(8):1367. doi: 10.1038/ajg.2013.164. [DOI] [PubMed] [Google Scholar]
- 155.Di Bella S, Gouliouris T, Petrosillo N. Fecal microbiota transplantation (FMT) for Clostridium difficile infection: focus on immunocompromised patients. J Infect Chemother Off J Jpn Soc Chemother. 2015;21(4):230–237. doi: 10.1016/jjiac.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 156.Smith M, Kassam Z, Edelstein C, Burgess J, Alm E. OpenBiome remains open to serve the medical community. Nat Biotechnol. 2014;32(9):867–867. doi: 10.1038/nbt.3006. [DOI] [PubMed] [Google Scholar]
- 157.Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sinha R, Abu-Ali G, Vogtmann E, et al. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat Biotechnol. 2017 Oct; doi: 10.1038/nbt.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2016;24(8):3669–3676. doi: 10.1007/s00520-016-3297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaw BE, Lee SJ, Horowitz MM, Wood WA, Rizzo JD, Flynn KE. Can we agree on patient-reported outcome measures for assessing hematopoietic cell transplantation patients? A study from the CIBMTR and BMT CTN. Bone Marrow Transplant. 2016;51(9):1173–1179. doi: 10.1038/bmt.2016.113. [DOI] [PubMed] [Google Scholar]
- 161.Mimee M, Tucker AC, Voigt CA, Lu TK. Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst. 2016;2(3):214. doi: 10.1016/j.cels.2016.03.007. [DOI] [PubMed] [Google Scholar]