Abstract
The application of sugar-specific carbon isotope analysis by combining high performance liquid chromatography and isotope ratio mass spectrometry is described, for investigating the detection of added C4-plant sugars in coconut waters. Authenticity of coconut waters gains more importance since the product is considered a juice by the European Fruit Juice Association (AIJN), while it holds an increasing consumer preference as healthy, low-carb beverage. The detection potential was compared with the conventional total sugar carbon isotope analysis and it is demonstrated that the isotopic profile of individual sugars substantially improves the limit of detection of added C4-plant sugars in coconut water. The study includes 30 authentic coconut waters (extracted from coconuts in the lab), which provide the authentic carbon isotope range of pulp, total sugars, sucrose, glucose and fructose, and 24 commercial coconut waters (bottled) purchased from grocery stores. The market scan revealed that 38% of the tested samples contain undeclared added C4-sugars.
Keywords: Stable isotopes, Fruit juices, Food fraud, Fructose, Sucrose, Glucose
Introduction
Natural juices have been traditionally part of a healthy diet. For decades, the main focus was directed only to the word ‘juice’ which was strong enough by itself to guarantee the product’s nutritional value. Early scientific and experimental knowledge has brought up the importance of the presence of the notorious sugars, added to juices for improving the taste and as so the attractiveness of the product. This trend developed into actual campaigns and scientific case studies which proved the impact of sugar consumption to human body, correlating high consumption with a variety of diseases and pathogeneses (e.g. World Health Organization 2015). The commercial aspect of this trend was directly evident on products’ labeling and packaging concepts, introducing several terms like sugar-free, no sugar added, free from sugars, naturally sweet and others. Still, while these terms are used for adding value to a product, the undeclared addition of sugar remains a wide and common fraudulent practice in the juice industry. Coconut water, the clear liquid from inside the coconut, is rich in nutrients, has a palatable unobtrusive taste and a low viscosity. These properties made it a trend beverage on one hand but also convenient for adulteration with sugars on the other. Coconut water is made from Cocos nucifera (AIJN 6.27 Reference Guideline February 2017) and is considered by the Codex Alimentarius ‘coconut juice’ (AIJN Position Regarding Coconut water, April 2016). By taking this view that ‘coconut water’ is the juice of the coconut, products sold as ‘coconut water’ must comply with the requirements of the Fruit Juice Directive (Council Directive 2001/112/EC), which means that any product containing other ingredients such as sugar, food additives and flavourings can only be called ‘coconut water-based drink’ or ‘coconut juice-based drink’ and not just coconut water with ‘ingredients’ (AIJN Position Regarding Coconut water, April 2016).
Coconut water contains easily digestible carbohydrates in the form of sugar and electrolytes. Of course the positive effects of coconut water are forfeited when there is sugar added. In that case, the valuable product turns into a calorie bomb and the ‘100% natural’ beverages can not keep their promise. The problem is that also here, like in so many other products, the labels often are misleading and do not reflect correctly the real content of the beverages. According to a recently published article at The Grocer (2017), an investigation by FSA in the UK found undeclared added sugar in 60% of the tested coconut water samples. In Europe, the coconut water market is expected to grow at a CAGR (compound annual growth rate) of 25% during the period 2017-2021 (Research and Markets 2017).
Detection of added common sugars in juices is possible by stable isotope analysis, a fact reflected by the official standard methods of analysis related to sugar addition in juices (e.g. ENV12140, AOAC995.17, AOAC2004.01, AOAC992.09). However, when the amount of added sugar is getting low, the detection limit becomes challenging and the official methods face difficulties to detect variable added sugars in juices. Following this, several attempts have been made to use internal reference compounds in the juices on the basis of correlations between the δ13C values of individual sugars (e.g. González et al. 1999; Guyon et al. 2014), proteins (e.g. Jamin et al. 1998a), individual organic acids (e.g. Jamin et al. 1998b) and ethanol derived from the fermented sugars (e.g. Jamin et al. 2004). This multi-isotope approach can be efficiently used to detect sugars from plants following C3 or C4 metabolic pathways, using their different δ13C composition (e.g. O’Brien 2015).
The AIJN 6.27 Reference Guideline (2017) for Coconut water, using this isotopic differentiation, recommends in its identity and authenticity criteria some threshold values of δ13C in ethanol (from fermented sugars) and whole juice, as measures of detection of added sugars deriving from C4-plants (e.g. cane sugar). Although these threshold values provide a handy tool for authentication purposes, the range of the authentic carbon isotope composition of the coconut’s sugars allows a wide tolerance of undetectable added C4-plant sugars, ending up to unsatisfactory detection limit of the method. Guyon et al. (2014) presented the utilization of carbon isotope ratios of individual sugars (fructose, glucose, sucrose) for authentication purposes of lemon juices by the application of high performance liquid chromatography (HPLC) coupled with elemental analyzer-isotope ratio mass spectrometry (EA-IRMS). The potential of this combination in food authenticity testing is still growing fast, considering that this technique can be applied in complex matrices without preparation or purification steps to obtain quick and reliable data.
This study describes the application of HPLC/EA-IRMS to individual coconut water sugars and presents for the first time in our knowledge a comprehensive dataset of carbon isotope ratios of total sugars, pulp, fructose, glucose and sucrose in coconut waters. At first, an evaluation of the traditional official method (ENV12140) for the detection of added sugars in juices was performed, together with the outcome of using the cut-off value as suggested by AIJN. Then, the isotope composition of the individual sugars was determined and the detection limit of this application was evaluated, aiming to test the hypothesis that this approach substantially improves the limit of detection of added sugars in coconut water.
Materials and methods
Chemicals
Acetone ≥ 99.9% GC Ultra Grade (Carl Roth KK40), Ca(OH)2 96% p.a. (Carl Roth 3529), H2SO4 96% p.a. ISO (Carl Roth 4623) were purchased from Lactan GmbH, Graz, Austria. Reference and carrier gases for the isotope ratio mass spectrometer (IRMS) were purchased from Messer Austria GmbH, Gumpoldskirchen, Austria.
Samples
The application stage consisted of the analysis of 30 authentic coconut waters (extracted from coconuts in the lab) and 24 commercial coconut waters (bottled) purchased from grocery stores, all declared as “pure”, “without added sugars” or “naturally sweet”. The spiking experiment stage involved one reservoir of coconut water (900 mL), extracted from 14 coconuts in the lab (origin Thailand, same batch). This reservoir was split into 6 sets of 3 aliquots each (total 18 aliquots of 50 mL). Each set represented one step of sugar addition (commercial cane sugar ID: 14453) (K with 0 g, L with 0.2 g, M with 0.4 g, N with 0.6 g, O with 1 g and P with 2 g).
Preparation of pulps and total sugars acc. ENV12140
All samples were treated according to the ENV12140 (following the procedure of Rossmann et al. 1997). Briefly, ca. 45 mL of coconut water were centrifuged and the supernatant was transferred to another vessel. In the case of some commercial coconut waters, the pulp content was too low and larger volume of the initial sample was used. The residue pulp was washed three times with ultra clean water and three times with acetone to remove remnant sugars, org. acids and lipids, and subsequently dried under vacuum, homogenized and stored until analysis by EA-IRMS (A, pulp). 2 g of Ca(OH)2 were added into the supernatant and the solution was stirred. After heating for 3 min at 90 °C in a water bath and centrifugation, the supernatant was transferred into a beaker and 0.1 mol/L H2SO4 was added until pH 5 was achieved. The solution was stored in the fridge overnight and then an aliquot of the supernatant was dried and stored until analysis by EA-IRMS (B, sugars).
High performance liquid chromatography (HPLC)
The HPLC system (Prominence, Shimadzu Corp., Kyoto, Japan) was used to chromatographically separate the fructose, glucose and sucrose fractions of each sample and collect them in order to be analyzed by EA-IRMS. About 5 mL of coconut water were centrifuged to remove particles, then filtered through a 0.2 µm filter. 10 µL of the filtered sample were injected on a Shodex SP0810 carbohydrate column (300 × 8 mm, Showa Denko, Japan), warmed at 80 °C. Flow rate was set at 0.5 mL/min (mobile phase: ultra clean water). A RID-10A differential refractometric detector (RI, Shimadzu Corp., Kyoto, Japan) was used for the identification of the individual sugars and their retention time. A FRC-10A Fraction Collector Module (Shimadzu Corp., Kyoto, Japan) was used to collect the fructose, glucose and sucrose fractions separately in PP tubes. Each sample ran twice so each individual sugar was collected in duplicate. The PP vials were dried under vacuum, then the residue sugar fraction was re-diluted with 200 µL ultra clean water, transferred in tin capsules, dried under vacuum and stored until analysis by EA-IRMS (F fructose, G glucose and S sucrose).
Isotope ratio mass spectrometry (IRMS)
Stable carbon isotope ratios (13C/12C) were measured using a Horizon Isotope Ratio Mass Spectrometer (Nu Instruments Limited, Wrexham, UK), following total combustion in a Euro EA-CHNSO 2 Dual Elemental Analyzer (EuroEA3000, EuroVector Srl, Pavia, Italy). The values were denoted in delta (δ) in relation to the international V-PDB standard (Vienna-Pee Dee Belemnite) for δ13C, according to the following general equation:
where R is the 13C/12C ratio of the sample (s) and of the standard (st) (Coplen 2011). The δ13C values were calculated using 2-point scaled normalization against two in-house standards (acetanilide and sorghum flour), calibrated against international certified reference materials (IAEA-CH-6 and IAEA-600, International Atomic Energy Agency, Vienna, Austria). Quality control samples were analyzed frequently between the samples, using the principle of identical treatment. Analytical reproducibility is < 0.2‰ as determined from replicate measurements (according to the laboratory’s method validation report and quality assurance policy, EN ISO17025:2005).
Results and discussion
Carbon isotope composition of total sugars and pulp (acc. ENV12140)
The δ13C values of the analyzed authentic coconut waters pulp (A) and sugar (B) components (Table 1) were characteristic for C3-plants. The values were also in general agreement with the range suggested by the AIJN 6.27 Reference Guideline (2017) for Coconut water as well as with previously published values (Imaizumi et al. 2015). As a matter of data assessment, the total sugars δ13C values were in principle heavier, compared to the pulp values. The biggest δ13C difference observed between total sugars and pulp was 3‰ VPDB (pulp-total sugars warning limit). The heaviest value in total sugars was − 22.9‰, way below the absolute top limit for C3-plants (− 21‰, e.g. O’Leary 1988; Farquhar et al. 1989; O’Brien 2015).
Table 1.
Carbon isotope composition of authentic coconut water samples (extracted from coconuts, n = 30)
| Pulp (A) | Total sugars (B) | ΔA–B | Sucrose (S) | Glucose (G) | Fructose (F) | |
|---|---|---|---|---|---|---|
| Mean δ13C (‰ VPDB) | − 25.4 | − 24.6 | − 0.8 | − 24.2 | − 24.6 | − 23.3 |
| Median δ13C (‰ VPDB) | − 25.3 | − 24.7 | − 0.6 | − 23.9 | − 24.8 | − 23.3 |
| Max δ13C (‰ VPDB) | − 24.1 | − 22.9 | 0.5 | − 21.8 | − 22.1 | − 21.4 |
| Min δ13C (‰ VPDB) | − 27.7 | − 25.7 | − 3.0 | − 28.9 | − 27.2 | − 26.0 |
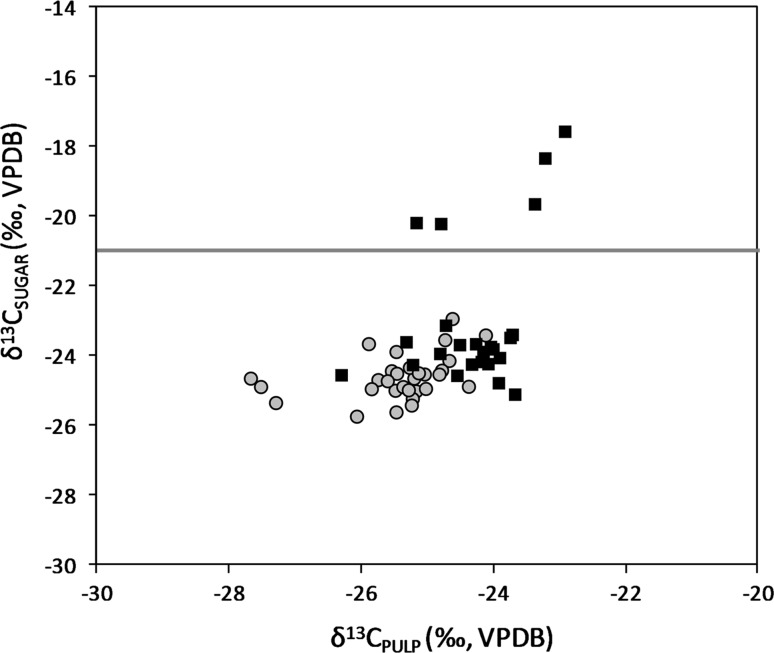
The δ13C values of the analyzed commercial bottled coconut waters pulp (A) component (Table 2) scattered in the typical range of C3-plants, while the sugar (B) component indicated 5 samples with δ13C values above − 21‰ (Fig. 1). Excluding these 5 samples, the heaviest δ13C value in total sugars was − 23.2‰ and the biggest difference between total sugars and pulp was 1.7‰. Significant difference between total sugars and pulp δ13C was detected for the 5 samples identified as adulterated (between 3.7 and 5.3‰).
Table 2.
Carbon isotope composition of commercial coconut water samples (bottled, n = 24)
| Pulp (A) | Total sugars (B) | ΔA–B | Sucrose (S) | Glucose (G) | Fructose (F) | |
|---|---|---|---|---|---|---|
| Mean δ13C (‰ VPDB) | − 24.3 | − 23.0 | − 1.2 | − 21.0 | − 23.8 | − 22.3 |
| Median δ13C (‰ VPDB) | − 24.1 | − 23.8 | − 0.4 | − 22.2 | − 24.1 | − 22.7 |
| Max δ13C (‰ VPDB) | − 22.9 | − 17.6 | 1.5 | − 14.6 | − 20.1 | − 18.0 |
| Min δ13C (‰ VPDB) | − 26.3 | − 25.1 | − 5.3 | − 25.5 | − 24.7 | − 24.9 |
Fig. 1.
Carbon isotope composition of pulp and total sugars in authentic coconut waters (grey circles) and in commercial bottled coconut waters (black squares). The horizontal solid red line indicates the upper limit for C3-plants (− 21‰ VPDB). Five commercial coconut waters identified as adulterated with C4-plant sugars (color figure online)
Carbon isotope composition of the individual sugars (HPLC/EA-IRMS)
The concentration of the three major sugars detected in coconut waters (sucrose, glucose and fructose) was highly variable, depending on e.g. the ripening level of the nut. Irrespective of this variability in concentration, the carbon isotope composition of each sugar in authentic coconut waters fell within the expected range for C3-plants (Table 1). No correlation could be found (0.10 < r < 0.47, p(α) = 0.05, n = 30) between the isotope values and the percentile peak area of each sugar (relative concentration). Sucrose (S) and fructose (F) were slightly 13C-enriched in comparison to the total sugars δ13C values, with maxima at − 21.8 and − 21.4‰ VPDB respectively. The biggest δ13C difference observed between sucrose and pulp was 4.7‰ VPDB (pulp-sucrose warning limit), between glucose and pulp was 3.3‰ VPDB (pulp-glucose warning limit) and between fructose and pulp was 5.7‰ VPDB (pulp-fructose warning limit).
In the case of commercial bottled coconut waters (Table 2), the results provided enhanced detection of added C4-plant sugar. In particular, 8 samples presented sucrose δ13C values above − 21‰ VPDB (Fig. 2a, including the 5 samples identified as adulterated by their total sugars δ13C), 1 sample had glucose δ13C value above − 21‰ VPDB (Fig. 2b, the sample belongs to the group of 5 samples identified as adulterated by their total sugars δ13C) and 2 samples had fructose δ13C values above − 21‰ VPDB (Fig. 2c, 1 belongs to the group of 5 and the other not).
Fig. 2.
a Carbon isotope composition of pulp and sucrose in authentic coconut waters (grey circles) and in commercial bottled coconut waters (squares). The horizontal solid red line indicates the upper limit for C3-plants (− 21‰ VPDB). The blank (white) squares represent the samples identified as adulterated only by the sucrose carbon isotope analysis (and not by the total sugars). In total, eight commercial coconut waters identified as adulterated with C4-plant sucrose. b Carbon isotope composition of pulp and glucose in authentic coconut waters (grey circles) and in commercial bottled coconut waters (squares). The horizontal solid red line indicates the upper limit for C3-plants (− 21‰ VPDB). One commercial coconut water identified as adulterated with C4-plant glucose. c Carbon isotope composition of pulp and fructose in authentic coconut waters (grey circles) and in commercial bottled coconut waters (squares). The horizontal solid red line indicates the upper limit for C3-plants (− 21‰ VPDB). The blank (white) square represents the sample identified as adulterated only by the fructose carbon isotope analysis (and not by the total sugars). In total, two commercial coconut waters identified as adulterated with C4-plant fructose (color figure online)
Detection of added sugar
The carbon isotope composition of individual sugars in coconut waters provided improved detection of added C4-plant sugar, in comparison to the total sugars δ13C. Using the upper theoretical cut-off value for C3-plants (− 21‰ VPDB), the number of identified as adulterated commercial coconut waters with C4-plant sugars rose from 21% (δ13C of total sugars) to 38% (δ13C of individual sugars) of the tested commercial samples. The δ13C value of the ethanol, included in the AIJN 6.27 Reference Guideline for coconut water (2017), produced by fermentation of the total sugars in coconut water is expected to have the same potential as the δ13C of the total sugars. The root cause of this improvement is obviously the targeted investigation of the augmented isotopic effect of any C4-plant sugar to its respective saccharide.
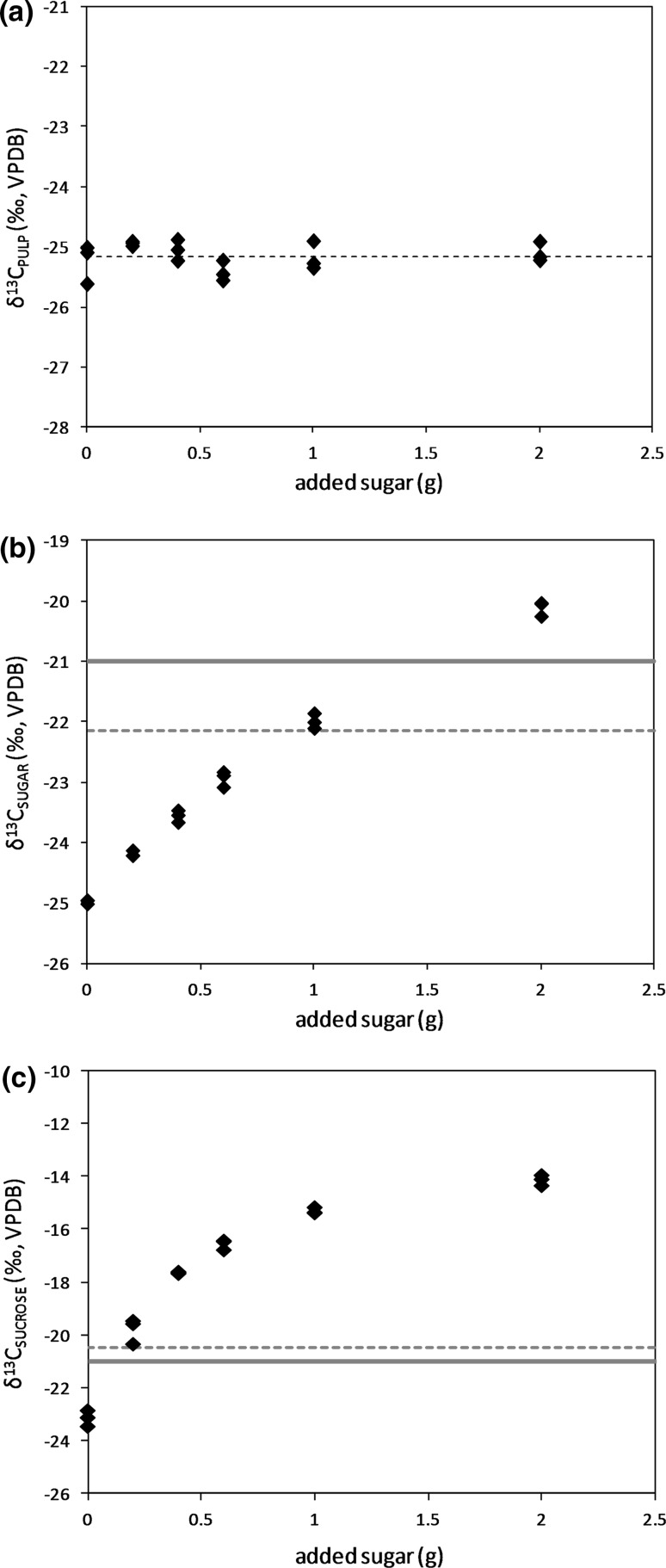
Apart from the detection potential of the sugar-specific carbon isotope analysis, the limit of detection is also expected to improve substantially. In order to estimate this, an adulteration spiking experiment was performed, following steps of sugar (sucrose, δ13C = − 12.2‰ VPDB) addition in pure coconut water. The samples (description in “Samples” section) were analyzed following both the carbon isotope composition of total sugars (acc. ENV12140) and pulp and the carbon isotope composition of the individual sugars (in this case, sucrose). The results showed that the carbon isotope composition of the pulp was, as expected, not affected by the addition of C4-plant sugar in the sample (Fig. 3a). The δ13C values of the total sugars were enriched by each step of sucrose addition (Fig. 3b), however, only the maximum quantity of added sucrose (i.e. 2 g in 50 mL) was detected as clear adulteration, reaching δ13C above − 21‰ VPDB. Using the mean δ13C value of the pulp (− 25.2‰ VPDB) and the pulp-total sugars warning limit (3‰), the detection limit improved to the next sucrose addition step (1 g in 50 mL). In contrast, the δ13C values of the sucrose fraction were substantially affected by the added sucrose (Fig. 3c), already from the first addition step (0.2 g in 50 mL), which corresponds to less than 10% of added sugar. This threshold is in agreement with previous sugar-specific carbon isotope applications (e.g. González et al. 1999; Jamin et al. 1997).
Fig. 3.
a Carbon isotope composition of pulp versus the amount of added sugar (sucrose). Each addition (spiking) step consists of three aliquots. Black dashed-line corresponds to the mean value (− 25.2‰ VPDB, n = 18). b Carbon isotope composition of total sugars versus the amount of added sugar (sucrose). Each addition (spiking) step consists of three aliquots. The solid red line indicates the absolute limit for C3-plants (− 21‰ VPDB). The dashed-red line corresponds to the pulp-total sugars warning limit (3‰ from the mean δ13CPULP). c Carbon isotope composition of sucrose versus the amount of added sugar (sucrose). Each addition (spiking) step consists of three aliquots. The solid red line indicates the absolute limit for C3-plants (− 21‰ VPDB). The dashed-red line corresponds to the pulp-sucrose warning limit (4.7‰ from the mean δ13CPULP) (color figure online)
Conclusion
The detection of C4-plant sugar addition in coconut waters can be substantially improved by the more sensitive sugar-specific carbon isotope analysis. In this study, a market scan was also performed, showing that 9 out of 24 tested samples (38%) of the commercial bottled coconut waters contained added C4-sugar, against the claim of the product. As expected, a quantitative estimation of the added C4-plant sugar in a sample is difficult, as the saccharide concentration of the natural pure product is highly variable. Nevertheless, the adulteration of a product like coconut water which gains rapidly market share due to its characteristics is of critical importance, especially when the adulteration has a direct counter-effect on the original health claims (e.g. by containing added sugar). As so, improved detection analytical methods like sugar-specific carbon isotope analysis of coconut waters should be considered as strong tools in food authenticity testing.
Contributor Information
David Psomiadis, Phone: +43 5 9010 8900, Email: psomiadis@imprint-analytics.at.
Nikoleta Zisi, Email: zisi@imprint-analytics.at.
Claudia Koger, Email: koger@imprint-analytics.at.
Balazs Horvath, Email: horvath@imprint-analytics.at.
Bernd Bodiselitsch, Email: bodiselitsch@imprint-analytics.at.
References
- AIJN Provisional Reference Guideline for Coconut Water/Juice 6.27, February 2017. AIJN, Brussels, Belgium
- AOAC992.09 Sugar-beet-derived syrups in frozen concentrated orange juice δ18O measurements in water. Stable isotope ratio mass spectrometric method. AOAC International, Gaithersburg, USA
- AOAC995.17 Beet sugar in fruit juices. Site specific natural isotope fractionation-nuclear magnetic resonance (SNIF-NMR©) method. AOAC International, Gaithersburg, USA
- AOAC2004.01 Carbon stable isotope ratio of ethanol derived from fruit juices and maple syrups. AOAC International, Gaithersburg, USA
- Coplen TB. Guidelines and recommended terms for expression of stable isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom. 2011;25:2538–2560. doi: 10.1002/rcm.5129. [DOI] [PubMed] [Google Scholar]
- Council Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption, EC, Brussels, Belgium
- ENV12140 Fruit and vegetable juices—determination of the stable carbon isotope ratio (13C/12C) of sugars from fruits juices—method using isotope ratio mass spectrometry. European Committee for Standardization, Brussels, Belgium
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. doi: 10.1146/annurev.pp.40.060189.002443. [DOI] [Google Scholar]
- González J, Remaud G, Jamin E, Naulet N, Martin GG. Specific natural isotope profile studies by isotope ratio mass spectrometry (SNIP-IRMS): 13C/12C ratios of fructose, glucose, and sucrose for improved detection of sugar addition to pineapple juices and concentrates. J Agric Food Chem. 1999;47:2316–2321. doi: 10.1021/jf981093v. [DOI] [PubMed] [Google Scholar]
- Guyon F, Auberger P, Gaillard L, Loublanches C, Viateau M, Sabathie N, Salagoity M-H, Medina B. 13C/12C isotope ratios of organic acids, glucose and fructose determined by HPLC-co-IRMS for meon juices authenticity. Food Chem. 2014;146:36–40. doi: 10.1016/j.foodchem.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Imaizumi VM, Sartori MMP, Ducatti C, Venturini Filho WG (2015) Use of stable isotopes of carbon in coconut water tampering detection. Anais do Simpósio Latino Americano de Ciências de Alimentos 2
- Jamin E, Gonzales J, Remaud G, Naulet N, Martin G. Detection of exogenous sugars or organic acids addition in pineapple juices and concentrates by 13C IRMS analysis. J Agric Food Chem. 1997;45:3961–3967. doi: 10.1021/jf9701087. [DOI] [Google Scholar]
- Jamin E, Gonzales J, Bengoechea I, Kerneur G, Remaud G, Iriondo C, Martin G. Proteins as intermolecular isotope reference for detection of adulteration of fruit juices. J Agric Food Chem. 1998;46:5118–5123. doi: 10.1021/jf980664g. [DOI] [Google Scholar]
- Jamin E, Gonzalez J, Bengoechea I, Kerneur G, Remaud G, Naulet N, Martin G. Measurements of 13C/12C ratios of sugars, malic acid, and citric acid as authenticity probes of citrus juices and concentrates. J AOAC Int. 1998;81:604–609. [Google Scholar]
- Jamin E, Martin F, Martin G. Determination of the 13C/12C ratio of ethanol derived from fruit juices and maple syrup by isotope ratio mass spectrometry: collaborative study. J AOAC Int. 2004;87:621–631. [PubMed] [Google Scholar]
- O’Brien D. Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr. 2015;35:565–594. doi: 10.1146/annurev-nutr-071714-034511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary M. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. doi: 10.2307/1310735. [DOI] [Google Scholar]
- Research and Markets (2017) European coconut water market 2017–2021—growing demand for organic coconut water. Dublin, Ireland. https://www.researchandmarkets.com/research/plnbm9/coconut_water. Accessed 01 Mar 2018
- Rossmann A, Koziet J, Martin GJ, Dennis MJ. Determination of the carbon-13 content of sugars and pulp from fruit juices by isotope-ratio mass spectrometry (internal reference method). A European interlaboratory comparison. Anal Chim Acta. 1997;340:21–29. doi: 10.1016/S0003-2670(96)00538-7. [DOI] [Google Scholar]
- The Grocer . FSA probe finds widespread addition of undeclared sugar in coconut water, by Julia Glotz, 12 October 2017. Crawley: William Reed Business Media Ltd; 2017. [Google Scholar]
- World Health Organization (WHO) Sugars intake for adults and children. Geneva: Switzerland; 2015. [PubMed] [Google Scholar]