Abstract
In this work the influences of κ-carrageenan (CRG), konjac glucomannan (KGM) and inulin on lysozyme (Ly)’s structure, activity, and their complex phase behavior were investigated through spectroscopy and activity measurement in heated and unheated conditions. It was found that the impact on the structure and activity of Ly was determined by the interactions with polysaccharides. After heat treatment, KGM and CRG improved the stability of complex systems. However, inulin did not have significant impact. Heating process promoted to change the structure of Ly, and the intervention retard following the sequence of CRG > KGM > inulin. The worthwhile work indicated protein’s structure and activity could be regulated by the interaction with polysaccharide, which might provide theoretical basis for food preservation and processing in different temperature treatments. Besides, the bidirectional effects of polysaccharide on protein would be beneficial to rational selection of functional properties of polysaccharide/protein systems.
Keywords: Lysozyme, Inulin, κ-Carrageenan, Konjac glucomannan, Structure
Introduction
Proteins and polysaccharides, two kind of essential biopolymers, are widely used in food, cosmetics, pharmaceutical and many other fields (Maury et al. 2016; Bai et al. 2016). Many physical and biological properties in the development of products are dominated by their interactions (Sadahira et al. 2016; van de Velde et al. 2015). The properties of food, such as viscosity, stability, transparency and gel-forming ability, mainly depend on the interactions between polysaccharides and proteins. Meanwhile the functional properties of proteins could be affected in the presence of different polysaccharides (Derkach et al. 2015; Long et al. 2016). Besides, interaction between the two main biopolymers in food system provide the basis for food quality and food structure, which also give many opportunities and challenges for food research.
There has been extensive research about the relations between their interaction and functional properties. It has been confirmed that the protein’s thermal stability was observably different in various protein/polysaccharide systems. For example, chitosan could improve thermal stability of soybean separation protein, and carboxymethyl cellulose had the same ability for lactalbumin and milk globulin (Capitani et al. 2007; Huang et al. 2012). Meanwhile hyaluronic acid displayed opposite property in Ly’s thermal stability (van de Weert et al. 2004). Addition of polysaccharides commonly improves the functional properties of protein, including foaming and emulsifying ability and viscosity and gelling ability (Schmitt and Turgeon 2011). Some protein/polysaccharide systems, for example casein/xanthan gum and whey protein/carboxymethyl cellulose, especially after heat processing, displayed properties of the gel (Braga and Cunha 2004; Capitani et al. 2007).
However, the protein’s activity in protein/polysaccharide systems has not been systemically studied till now. Besides, the influence of polysaccharides on protein’s structure has no unified conclusion. Lysozyme (Ly), the main protein component of egg white fraction with molar mass of 14.3 kDa, has been received extensive attentions for biological function (Chang et al. 2015; Darwish et al. 2015). It is commonly used as a kind of additive for food preservation. In past decades, Ly commonly regarded as model protein or drugs, including research of protein conformation, enzymology and molecular space (Al Kayal et al. 2012). However, scarce reports cared about its structure and activity in presence of biopolymers.
Spectral features of protein is sensitive and easily captured. Therefore, UV/Vis absorption, fluorescence spectroscopy, CD spectra and other spectral technology are very practical approach to investigate the structural changes and complex formation. In our previous studies, we controllably fabricated nanogel by like charged xanthan gum and lysozyme, and further self-assembled into tunable superstructures with different sizes in control (Xu et al. 2014a, 2015). Through spectral analysis, we found κ-carrageenan (CRG) could spontaneously complex with Ly after gentle stirring for 30 min at 25 °C, which exerted impacts on spectrum of Ly (Xu et al. 2014b). The complex easily induced self-aggregation by intrinsic and extrinsic factors.
In this paper, CRG, konjac glucomannan (KGM) and inulin were selected as anionic and neutral polysaccharide with high and low molecular weight. The influences on structure and activity of Ly in the presence of different polysaccharides were investigated in heated and unheated conditions respectively through UV–Vis spectroscopy, circular dichroism, fluorescent spectroscopy and activity measurement. On the one hand, the study provide useful information for Ly’ application in food preservation with antimicrobial activity regulation. On the other hand, the worthwhile endeavor elucidated protein structure could be regulated by the interaction with polysaccharide, which provided a facile strategy to control activity and functions for food macromolecule.
Materials and methods
Materials and sample preparation
Lysozyme (Ly, Mw = 14.3 kDa) from chicken egg white was obtained from Sinopharm Chemical Reagent co., LTD. κ-carrageenan (CRG, 5.6 × 105Da) was purchased from Aladdin Chemistry Co. LTD. Konjac glucomannan (KGM, 1.4 × 106 Da) was provide by Hubei Konson Konjac Gum Co., LTD. Inulin (DP 10–12) was obtained from Tuofeng Co. LTD. Other chemicals were reagent grade.
All the polymer were prepared with the same concentration (1.0 mg/mL, pH 6.75, 10 mM). Ly solution and inulin solution were prepared by gentle magnetic stirring for 2 h, while for KGM solution was 6 h. CRG solution (1.0 mg/mL) was prepared at 70 °C for 30 min. The polysaccharide/Ly systems were prepared with different ratios (3:1, 2:1, 1:1 and 1:2) of polysaccharide to Ly. The blank of all the samples were formed using aqueous solution in instead of Ly.
Spectral analysis
UV/Vis absorption spectra of Ly was recorded in the absence and presence of polysaccharides on a UV spectrophotometer (Shimadzu UV-1700) at room temperature using quartz cells with 1 cm path-length. The intrinsic fluorescence spectra of Ly were obtained using a fluorescence spectrometer (RF-5301PC) at an excitation wavelength of 280 nm. Slit widths (5 nm), scan speed (240 nm/min) and excitation voltage (500 V) were kept throughout. The CD spectra were recorded on a J-1500 spectropolarimeter (JASCO, Tokyo, Japan) using a 1 mm path-length quartz cell at 25 °C. The conduct parameters were bandwidth 1 nm, step resolution 0.1 nm, scan speed and 100 nm/min and response time 1 s. Each spectrum was the average of three scans and PBS solution (pH 6.24, 66 mM) was used as a blank. The secondary structure retention was calculated by the ratio of the ellipticity intensities of Ly to that of the Ly/polymer at 222 nm.
Activity of Ly measurement
Activity of lysozyme was measured by the turbidimetric assay as describe before. Briefly, the decreased optical density of a turbid cell suspension (Micrococcus lysodeiticus) was monitored as a function of time by a UV spectrophotometer (Shimadzu UV-1700) at 450 nm in the absence and presence of Ly solutions or Ly/polymer solutions.
Phase transition induced heat treatment
The Ly/biopolymer systems with different weight ratios were incubated at different temperature ranges (20–80 °C) for 30 min. The corresponding systems were classified into the following four levels: transparent, translucent, turbid and phase separation.
Results and discussion
UV–Vis spectra of Ly in the absence and presence of polysaccharides
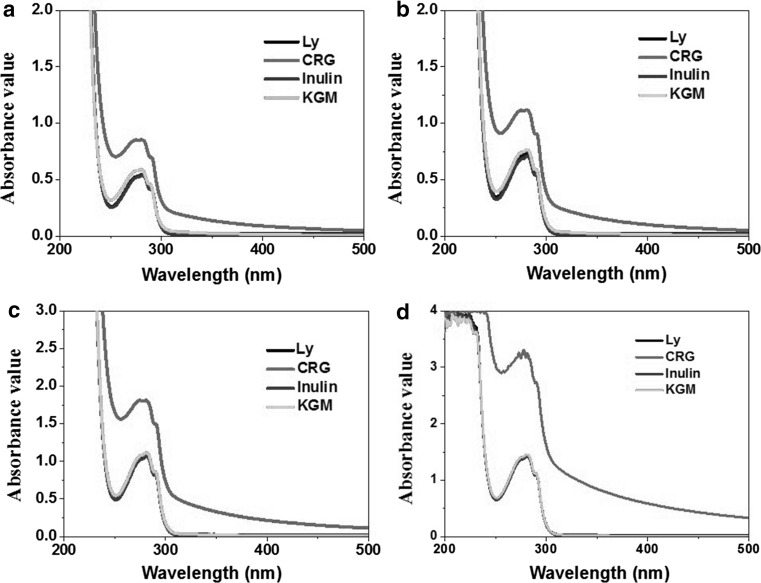
As shown in Fig. 1, resulted from characteristic polypeptide backbone structure, tryptophan and tyrosine, Ly displayed a strong absorption around 280 nm (Zhang et al. 2013). The characteristic peak at 257 nm was produced by phenylalanine. Obviously, the two characteristic peaks were greatly changed in the presence of different polysaccharides, reflecting the framework conformation of Ly when complex with polysaccharides. With gradual addition of polysaccharides, the absorption peak of Ly exhibited different phenomenon for physical interaction. KGM and inulin, two kinds of neutral polysaccharides with different molecular weight and viscosity, both did not show significant influence on the absorption spectra of Ly in any concentration. However, CRG clearly improved the absorption both in the visible light and ultraviolet region. The enhancement in the visible light may be induced by the Tindall effect for complex formation, while the change of ultraviolet absorption caused by changes in Ly’s structure by strong electrostatic interaction. The results were well agreed with our previous study (Xu et al. 2014a, b). The hyperchromic effect for complexation was strengthened as the concentration of Ly increased. Because CRG with strong negative charges could combine with much more Ly molecules. The intense electrostatic interactions resulted that Ly conformation changed, in which the backbone structure originally in a hydrophobic microenvironment exposed to a polar one.
Fig. 1.
UV–Vis Spectra of Ly solutions (pH 6.75, 10 mM) in the absence and presence of polysaccharides with ratios of 3:1 (a), 2:1 (b), 1:1 (c) and 1:2 (d)
Secondary structural changes of Ly in the presence of polysaccharides
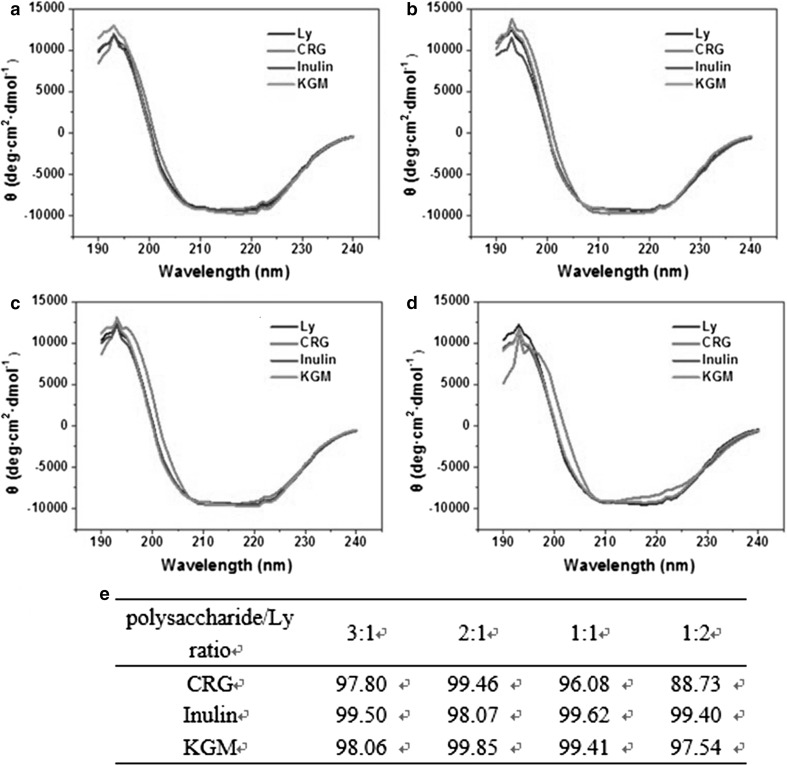
To further confirm whether KGM, CRG, inulin interacted with Ly, and the changes in the secondary structure after complex formation, the far-UV CD spectra of Ly was conducted in the absence and presence of polysaccharides. As showed in Fig. 2, Ly spectral features retained with some variation in ellipticity and all spectra displayed two characteristic negative peaks at 208 and 222 nm, which were the CD spectra characteristics of protein alpha helix. When the concentration of Ly was very low (polysaccharides/Ly 3:1), the ellipticity of polysaccharides/Ly systems nearly coincided with that of Ly, except for CRG which enhanced the ellipticity between 200 and 210 nm. As the concentration of Ly improved, the ellipticity of KGM/Ly and inulin/Ly coincided with that of Ly, illustrating that the two neutral polysaccharides did not complex with Ly and change its structure. Comparatively, CRG affected the structure of Ly as the concentration of Ly increased. When the ratio of polysaccharide/Ly was 2:1, 1:1 respectively, the ellipticity intensity between 200 and 210 nm was significantly higher than that of KGM/Ly, inulin/Ly and Ly systems. The phenomenon indicated the interaction between polysaccharide and Ly strengthened as the concentration of Ly increased. Especially at CRG/Ly ratio of 1:2, the differences of ellipticity were obvious as compared with other systems. Meanwhile further increase of the intensity at 208 and 222 nm illustrated the complexation of CRG and Ly was promoted by increasing Ly concentration resulting in changing its secondary structure.
Fig. 2.
CD spectra of Ly solutions (pH 6.75, 10 mM) in the absence and presence of polysaccharides with ratios of 3:1 (a), 2:1 (b), 1:1 (c) and 1:2 (d) and the secondary structure retention of lysozyme in presence of polysaccharide (%) (e)
The CD signal at 222 nm is characteristic and sensitive spectrum of α-helix of Ly, at which the intensity changes and reflects the variation of secondary structure (Hirst and Brooks 1994). Ghimire recorded the ratio of the intensities at 222 nm of Ly to that of the complex, using the structure retention to evaluate the effect of complexation on Ly structure (Ghimire et al. 2014). As shown in Fig. 2e, the structure retention of Ly in presence of KGM and inulin was nearly 100%, displaying the presence of KGM, while inulin exerted no significant effect on Ly structure. At low concentration of Ly, the structure retention was rarely affected and induced by CRG complexation. While the structural retention decreased 12% as the ratio of CRG/Ly was 1:2, indicating Ly structure changed. The results well coincided with prior data and previous work.
The secondary structure of Ly in the absence and presence of KGM, CRG and inulin was further calculated. The content of α-helix, β-sheet, β-turn and random were listed in Table 1. As the table showed, the secondary structural parameters of KGM/Ly, CRG/Ly, inulin/Ly with different ratios displayed some changes as compared with that of Ly. For KGM/Ly and CRG/Ly systems, the parametric variation range was small. The reason may be caused by error amplification during multiple data conversion. Comparatively, CRG exerted markedly influence. The content of α-helix and β-sheet increased as the concentration of Ly increased, while the content of β-turn declined and random remained stable. The results were well agreed with Fig. 2. In recent years, there is no regularity yet for the effects of polysaccharide on secondary structure of protein. There is no regularity yet. Qi and his co-worker confirmed that sugar beet pectin could complex with β-lactoglobulin and further changed its structure. Similar to our study, the content of α-helix and β-sheet in β-lactoglobulin increased in presence of sugar beet pectin (Qi et al. 2014). Meanwhile Souza investigated chitosan could improve α-helix content of β-lactoglobulin, but decreased its β-sheet content (Souza et al. 2011).
Table 1.
Secondary structure of Ly in the absence and presence of polysaccharide
| Ratio | Helix (%) | Beta (%) | Turn (%) | Random (%) | |
|---|---|---|---|---|---|
| Ly | 21.4 ± 0.98 | 45.9 ± 3.11 | 5.1 ± 1.90 | 27.6 ± 0.47 | |
| CRG + Ly | 3:1 | 23.57 ± 0.47 | 42.47 ± 1.97 | 8.57 ± 1.10 | 25.47 ± 0.50 |
| 2:1 | 25.1 ± 0.30 | 43.13 ± 1.13 | 7.3 ± 0.72 | 24.47 ± 0.47 | |
| 1:1 | 25.9 ± 0.14 | 39.5 ± 1.56 | 10.3 ± 0.99 | 24.25 ± 0.49 | |
| 1:2 | 29.15 ± 0.92 | 26.9 ± 2.97 | 19.25 ± 1.63 | 24.65 ± 0.49 | |
| Inulin + Ly | 3:1 | 20.6 ± 0.42 | 46.85 ± 1.16 | 4.05 ± 0.63 | 28.45 ± 0.17 |
| 2:1 | 22.55 ± 0.78 | 39.8 ± 1.98 | 7.75 ± 0.92 | 29.9 ± 0.28 | |
| 1:1 | 22.17 ± 0.21 | 43.1 ± 1.08 | 5.87 ± 0.55 | 28.9 ± 0.46 | |
| 1:2 | 22.3 ± 0.57 | 40.55 ± 2.19 | 7.6 ± 1.13 | 29.6 ± 0.57 | |
| 1:3 | 22.75 ± 0.78 | 41.25 ± 3.89 | 6.7 ± 2.12 | 29.3 ± 0.99 | |
| KGM + Ly | 3:1 | 20.15 ± 0.61 | 49.9 ± 2.07 | 2.2 ± 1.31 | 27.75 ± 0.38 |
| 2:1 | 19.3 ± 0.28 | 53.4 ± 0.28 | 0.25 ± 0.07 | 27 ± 0.57 | |
| 1:1 | 20.37 ± 0.06 | 49.2 ± 0.1 | 2.03 ± 0.21 | 28.37 ± 0.12 | |
| 1:2 | 21.63 ± 0.40 | 43.57 ± 1.45 | 6.1 ± 0.66 | 28.7 ± 0.6 | |
| 1:3 | 20.2 ± 0.1 | 49.5 ± 0.89 | 1.77 ± 0.75 | 28.5 ± 0.17 |
Activity of Ly in the absence and presence of polysaccharides
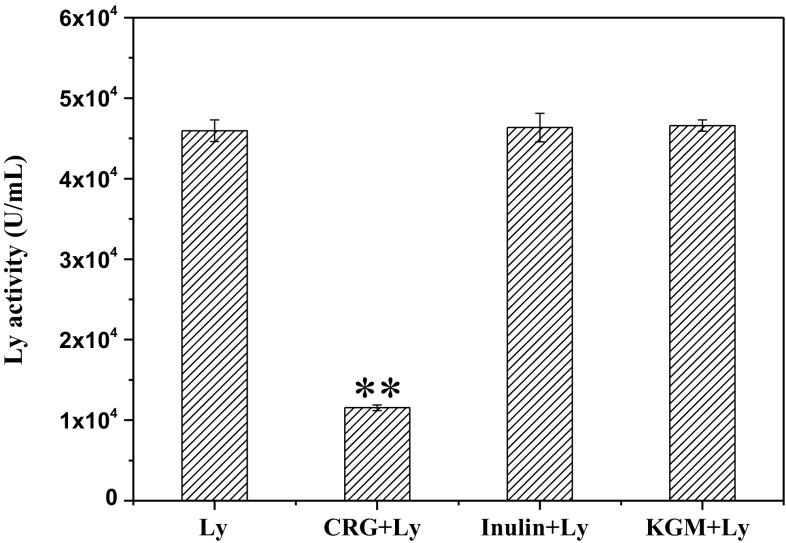
Ly, a kind of natural enzyme with sterilizing microorganism, has been widely applied in medical and food preservation for its satisfactory stability over a wide range of temperature and pH values. For example, Ly is commonly used in antimicrobial packaging for extending the shelf life of food. Benelhadj et al. found the lysozyme/Arthrospira platensis protein complexes possess favorable antimicrobial Properties for edible food packaging (Benelhadj et al. 2016). Due to its oval shape structure, there is a crack that created the active site for binding six carbohydrates, especially for the polysaccharides in Gram-positive bacteria cell walls (Shareghi et al. 2015). In prior studies it has confirmed that CRG, KGM and inulin have different effect on Ly structure. Here, we care about whether its enzyme activity will be affected in presence of different polysaccharides or not. As shown in Fig. 3, the enzyme activity of Ly in absence of polysaccharide was 45,950 ± 1343 U/mL, and its remained 46,600 ± 707 U/mL and 46,350 ± 1767 U/mL as coexist with KGM and inulin respectively. But the enzyme activity reduced to 11,550 ± 353 U/mL as combined with CRG. Obviously, KGM and inulin rarely changed the activity of Ly. While CRG produced significant differences to its enzyme activity and reduced to a quarter of the original enzyme activity. This phenomenon was well corresponded with spectral results in the presence of polysaccharides. The change in Ly activity might induce by strong electrostatic interaction between CRG and Ly, resulting in spatial structure changing.
Fig. 3.
Activity of Ly solutions (pH 6.24, 66 mM) in the absence and presence of polysaccharides. ** mean highly significant difference (p < 0.01)
Thermal stability of Ly/polysaccharides system
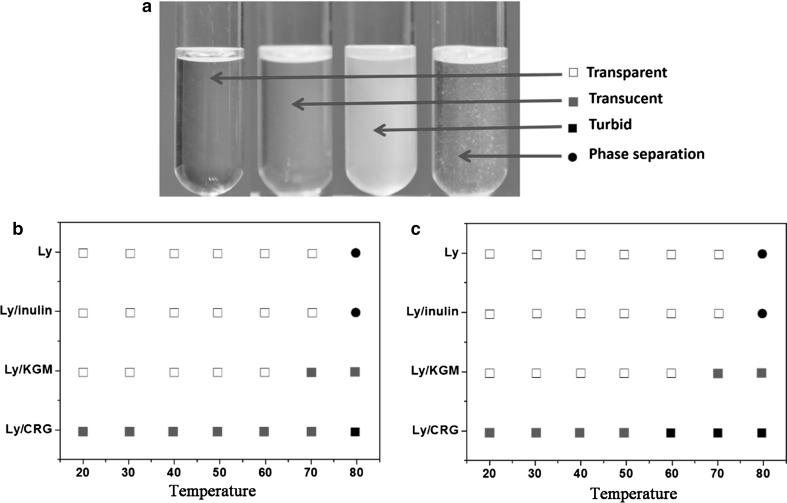
As shown in Fig. 4, polysaccharide/Ly systems displayed different phase behaviors when suffered from heat-treatment (20–80 °C) for 30 min and exhibited a temperature-dependent manner. Ly solutions were transparent at temperature under thermal denaturation temperature (70 °C), showing a very good heat resistance (Wong et al. 2014). However, denaturation, self-aggregation and phase separation occurred when temperature higher than 70 °C. The phase behavior of inulin/Ly system was identical with that of Ly indicating coexistence of neutral polysaccharide with low molecular weight that did not affect phase behavior of Ly solution. However, KGM addition could retard the process of phase separation. Even at temperature higher than 70 °C, KGM/Ly presented translucent and exhibited no phase separation occurred. This phenomenon may be resulted from the high viscosity endowed by KGM which effectively prevented Ly space conformation completely damage and self-aggregation. CRG/Ly complex was formed spontaneously. Temperature elevation promoted the interpolymeric complexes formation and increased the turbidity of CRG/Ly system. But finally no obvious phase separation appeared. Self-aggregation was promoted by higher Ly content, but CRG could suppress the phase transition for high viscosity. The phenomenon was well consistent with our previous studies (Xu et al. 2015).
Fig. 4.
Phase diagram of Ly/polysaccharide systems (b 1:3, c 1:1) after heat-treatment for 30 min
Absorption spectrum of Ly after thermal treatment
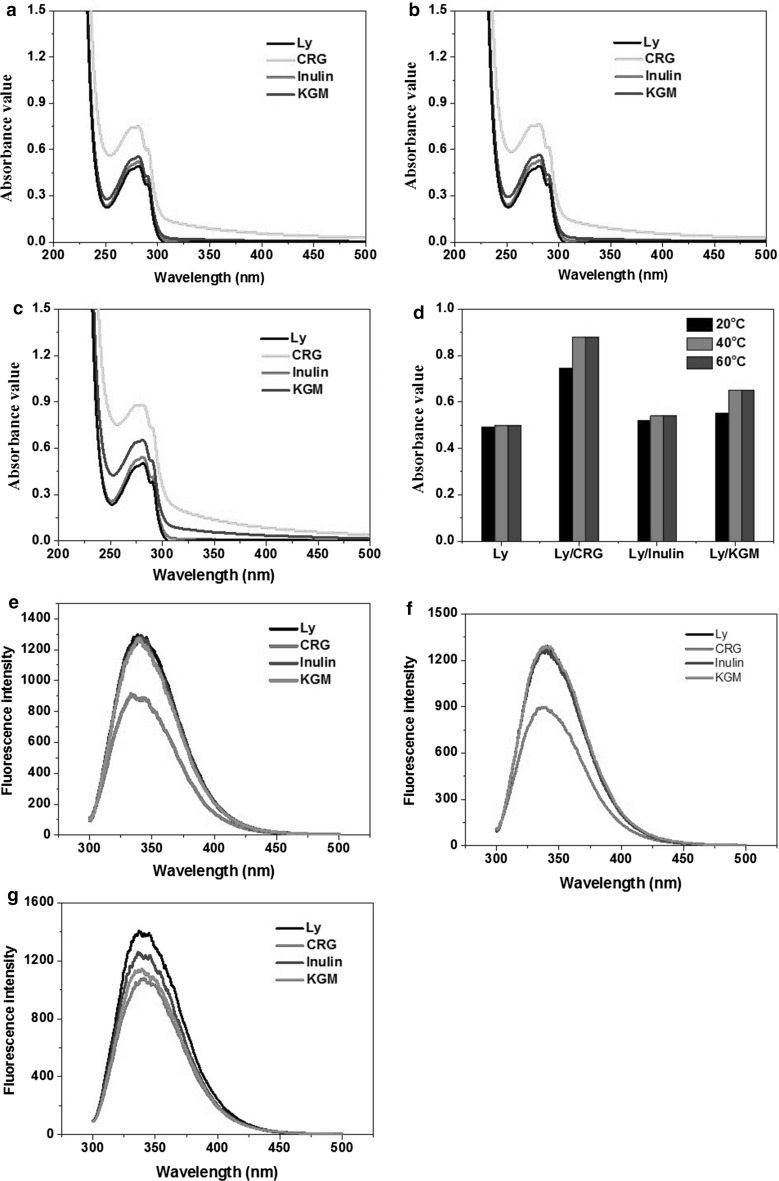
Figure 5 displayed UV–visible spectroscopy of Ly, CRG/Ly, KGM/Ly and inulin/Ly systems with different temperature disposing for 30 min. UV–visible spectroscopy of Ly solution nearly unaffected when disposed temperature was lower than 60 °C because the changes were reversible when temperature less than thermal denature temperature. While addition the three kinds of polysaccharides exerted different effects on the structure of Ly after thermal treatment. The obvious hyperchromic effects were induced by exposing amino acids in hydrophobic environment to a hydrophilic one. Inulin, a kind of neutral polysaccharide with small molecular weight and viscosity, has little impact on Ly structure. The spectroscopy of inulin/Ly has the most similarity with that of Ly, and the differences were amplified with temperature increasing. This phenomenon illustrated the reversible behavior of Ly structure after thermal treatment was hindered by hydrophobic interaction between inulin and Ly during heating and cooling process. With increase of molecular weight and viscosity, for example KGM, the hydrophobic interaction and hydrogen bonding between KGM and Ly may be strengthened. Comparatively, CRG has a greater ability to change Ly structure and prevent it back to the original state than that of KGM and inulin. The results displayed polysaccharides exerted bidirectional effects on structure of lysozyme. The most likely reason is strong electrostatic interactions between CRG and Ly, excepted for hydrophobic interaction and hydrogen bonding, to dominate Ly structure changing. The ultraviolet absorption at 280 nm contributed to tryptophan and tyrosine residues (Liang et al. 2013). From the amplitude of increase (Fig. 5d), we could arrange the sequence of the three kind of polysaccharide on changing Ly structure as: CRG > KGM > inulin.
Fig. 5.
UV–Vis (a–c), the absorbance (d) at 280 nm of Ly in presence of CRG, inulin and KGM with polysaccharide/Ly ratios of 3:1 at 20 °C (a), 40 °C (b) and 60 °C (c), and fluorescence spectra of Ly in presence of CRG, inulin and KGM with polysaccharide/Ly ratio of 3:1 at 20 °C (e), 40 °C (f) and 60 °C (g)
Intrinsic fluorescence of Ly after thermal treatment
Ly has three kinds of amino acid that contribute to intrinsic fluorescence, including tryptophane (Trp), tyrosine (Tyr), and phenylalanine (Phe), especially for Trp (Pang et al. 2012). As Fig. 5 showed, after thermal treatment of various polysaccharide/Ly solutions, the emission intensity dropped regularly with increasing temperature. The reduction of fluorescence intensity indicated that the polysaccharide as a quencher quenched the fluorescence of Ly, revealing that the interaction between the polysaccharide and protein occurred after thermal treatment. Inulin and KGM displayed no significant effect, and nearly coincided with intrinsic fluorescence of Ly when temperature was lower than 40 °C. The reduction fluorescence intensity by CRG showed no direct relation with thermal treatment. When the temperature was higher than 60 °C, all polysaccharide/Ly solutions with heat treatment exhibited fluorescence quenching phenomenon. But after 60 °C heating for 30 min, hydrophobic force and hydrogen bond that maintained Ly structure might partly destroyed. Trp exposed in a hydrophilic environment so that the fluorescence quenched. Through the reduction fluorescence intensity, the sequence of changing Ly structure was CRG > KGM > inulin, which was in agreement with the forward results of ultraviolet–visible spectroscopy.
Conclusion
Effects of polysaccharide on the structure of Ly were investigated in the moderate and heating conditions. In the moderate condition whether interacting with Ly or not determined the change in structure and activity of Ly. CRG and Ly formed complex spontaneously. The strong electrostatic interaction changed the spectral characteristic of Ly, and reduced to a quarter of its original activity. While KGM and inulin did not exert significant impact. KGM, CRG and inulin exhibited different influences on phase behavior of Ly under thermal environment. KGM and CRG improved the stability of complex system so that it did not present apparent phase separation when suffered heat treatment. However, inulin did not have significant impact. Heating process promoted to change the structure of Ly. However, Ly’s structure could be prevented to destroy in the presence of polysaccharides. The intervention of polysaccharides on changing Ly’s structure was in the order of CRG > KGM > inulin. The endeavor provide theoretical basis for food preservation and processing in heat and unheat treatment. Rational selection of polysaccharide/protein system with partly preserve protein functional properties and enzyme activity will be beneficial to food storage and prolong its shelf life.
Acknowledgements
This work was financially supported by Natural Science Foundation of Henan province (Grant No. 162300410229), the National Natural Science Foundation of China (Grant No. 31701647), Youth sustentation Fund of Xinyang Normal University (Grant No. 16050), High level talent research foundation of Xinyang Normal University, and Nanhu Scholars Program for Young Scholars of XYNU. The authors greatly thank colleagues of Key Laboratory of Environment Correlative Dietology of Huazhong Agricultural University and Department of Nutrition and Food Hygiene of Wuhan University for offering many conveniences.
References
- Al Kayal T, Nappini S, Russo E, Berti D, Bucciantini M, Stefani M, Baglioni P. Lysozyme interaction with negatively charged lipid bilayers: protein aggregation and membrane fusion. Soft Matter. 2012;8:4524–4534. doi: 10.1039/c2sm06906g. [DOI] [Google Scholar]
- Bai L, Huan S, Gu J, McClements DJ. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: saponins, phospholipids, proteins, and polysaccharides. Food Hydrocolloid. 2016;61:703–711. doi: 10.1016/j.foodhyd.2016.06.035. [DOI] [Google Scholar]
- Benelhadj S, Fejji N, Degraeve P, Attia H, Ghorbel D, Gharsallaoui A. Properties of lysozyme/Arthrospira platensis (Spirulina) protein complexes for antimicrobial edible food packaging. Algal Res. 2016;15:43–49. doi: 10.1016/j.algal.2016.02.003. [DOI] [Google Scholar]
- Braga A, Cunha R. The effects of xanthan conformation and sucrose concentration on the rheological properties of acidified sodium caseinate–xanthan gels. Food Hydrocolloid. 2004;18:977–986. doi: 10.1016/j.foodhyd.2004.04.002. [DOI] [Google Scholar]
- Capitani C, Pérez OE, Pacheco B, Teresa M, Pilosof AM. Influence of complexing carboxymethylcellulose on the thermostability and gelation of α-lactalbumin and β-lactoglobulin. Food Hydrocolloid. 2007;21:1344–1354. doi: 10.1016/j.foodhyd.2006.10.022. [DOI] [Google Scholar]
- Chang CK, Chen WA, Sie CY, Lin SC, Lin TW, Lin TH, Hsu CC, Wang SSS. Investigating the effects of plasma pretreatment on the formation of ordered aggregates of lysozyme. Colloids Surf B. 2015;126:154–161. doi: 10.1016/j.colsurfb.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Darwish A, Robie T, Desch RJ, Thiel SW. Competitive adsorption of lysozyme and myoglobin on mesostructured cellular foam silica. Microporous Mesoporous Mater. 2015;210:101–109. doi: 10.1016/j.micromeso.2015.02.010. [DOI] [Google Scholar]
- Derkach S, Zhabyko I, Voron’ko N, Maklakova A, Dyakina T. Stability and the rheological properties of concentrated emulsions containing gelatin–κ-carrageenan polyelectrolyte complexes. Colloids Surf A. 2015;483:216–223. doi: 10.1016/j.colsurfa.2015.04.007. [DOI] [Google Scholar]
- Ghimire A, Kasi RM, Kumar CV. Proton-coupled protein binding: controlling lysozyme/Poly (acrylic acid) interactions with pH. J Phys Chem B. 2014;118:5026–5033. doi: 10.1021/jp500310w. [DOI] [PubMed] [Google Scholar]
- Hirst JD, Brooks CL. Helicity, circular dichroism and molecular dynamics of proteins. J Mol Biol. 1994;243:173–178. doi: 10.1006/jmbi.1994.1644. [DOI] [PubMed] [Google Scholar]
- Huang GQ, Sun YT, Xiao JX, Yang J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012;135:534–539. doi: 10.1016/j.foodchem.2012.04.140. [DOI] [PubMed] [Google Scholar]
- Liang M, Liu R, Qi W, Su R, Yu Y, Wang L, He Z. Interaction between lysozyme and procyanidin: multilevel structural nature and effect of carbohydrates. Food Chem. 2013;138:1596–1603. doi: 10.1016/j.foodchem.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Long Z, Zhao M, Sun-Waterhouse D, Lin Q, Zhao Q. Effects of sterilization conditions and milk protein composition on the rheological and whipping properties of whipping cream. Food Hydrocolloid. 2016;52:11–18. doi: 10.1016/j.foodhyd.2015.06.015. [DOI] [Google Scholar]
- Maury C, Sarni-Manchado P, Poinsaut P, Cheynier V, Moutounet M. Influence of polysaccharides and glycerol on proanthocyanidin precipitation by protein fining agents. Food Hydrocolloid. 2016;60:598–605. doi: 10.1016/j.foodhyd.2016.04.034. [DOI] [Google Scholar]
- Pang B, Bi S, Wang Y, Yan L, Wang T. Investigation on the interactions of silymarin to bovine serum albumin and lysozyme by fluorescence and absorbance. J Lumin. 2012;132:895–900. doi: 10.1016/j.jlumin.2011.11.021. [DOI] [Google Scholar]
- Qi PX, Wickham ED, Garcia RA. Structural and thermal stability of β-lactoglobulin as a result of interacting with sugar beet pectin. J Agric Food Chem. 2014;62:7567–7576. doi: 10.1021/jf502699g. [DOI] [PubMed] [Google Scholar]
- Sadahira MS, Rodrigues MI, Akhtar M, Murray BS, Netto FM. Effect of egg white protein-pectin electrostatic interactions in a high sugar content system on foaming and foam rheological properties. Food Hydrocolloid. 2016;58:1–10. doi: 10.1016/j.foodhyd.2016.02.007. [DOI] [Google Scholar]
- Schmitt C, Turgeon SL. Protein/polysaccharide complexes and coacervates in food systems. Adv Colloid Interface. 2011;167:63–70. doi: 10.1016/j.cis.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Shareghi B, Farhadian S, Zamani N, Salavati-Niasari M, Moshtaghi H, Gholamrezaei S. Investigation the activity and stability of lysozyme on presence of magnetic nanoparticles. J Ind Eng Chem. 2015;21:862–867. doi: 10.1016/j.jiec.2014.04.024. [DOI] [Google Scholar]
- Souza HK, Gonçalves MdP, Gómez J. Effect of chitosan degradation on its interaction with β-lactoglobulin. Biomacromolecule. 2011;12:1015–1023. doi: 10.1021/bm101356g. [DOI] [PubMed] [Google Scholar]
- van de Velde F, de Hoog EH, Oosterveld A, Tromp RH. Protein-polysaccharide interactions to alter texture. Annu Rev Food Sci T. 2015;6:371–388. doi: 10.1146/annurev-food-022814-015558. [DOI] [PubMed] [Google Scholar]
- van de Weert M, Andersen MB, Frokjaer S. Complex coacervation of lysozyme and heparin: complex characterization and protein stability. Pharm Res. 2004;21:2354–2359. doi: 10.1007/s11095-004-7689-z. [DOI] [PubMed] [Google Scholar]
- Wong YH, Lim CH, Kadir HA, Tayyab S. Towards increasing chemical and thermal stability of lysozyme with a simulated honey sugar cocktail. RSC Adv. 2014;4:53891–53898. doi: 10.1039/C4RA09606A. [DOI] [Google Scholar]
- Xu W, Jin W, Hu Y, Liu S, Li B. Tunable self-assembly of nanogels into superstructures with controlled organization. RSC Adv. 2014;4:35268–35271. doi: 10.1039/C4RA06744D. [DOI] [Google Scholar]
- Xu W, Jin W, Zhang C, Li Z, Lin L, Huang Q, Ye S, Li B. Curcumin loaded and protective system based on complex of κ-carrageenan and lysozyme. Food Res Int. 2014;59:61–66. doi: 10.1016/j.foodres.2014.01.059. [DOI] [Google Scholar]
- Xu W, Jin W, Li Z, Liang H, Wang Y, Shah BR, Li Y, Li B. Synthesis and characterization of nanoparticles based on negatively charged xanthan gum and lysozyme. Food Res Int. 2015;71:83–90. doi: 10.1016/j.foodres.2015.02.007. [DOI] [Google Scholar]
- Zhang H, Hao F, Liu R. Interactions of lead (II) acetate with the enzyme lysozyme: a spectroscopic investigation. J Lumin. 2013;142:144–149. doi: 10.1016/j.jlumin.2013.03.061. [DOI] [Google Scholar]