Abstract
Present study was conducted to evaluate the ability of Trichoderma viride as a source of cyclodextrin glucanotransferase that has shown transglycosylation activity in the presence of polyphenolic constituents extracted from Moringa oleifera leaves as its acceptor and wheat flour as its substrate to catalyze synthesis of polyphenolic glycosides as transglycosylation (transfer) reaction products. The enzymatic synthesized polyphenolic glycosides were then purified using octa-dodecyl-functionalized silica gel column chromatography prior to analysis using thin layer chromatography and high performance liquid chromatography and identified using nuclear magnetic resonance (NMR) spectroscopy. The high performance liquid chromatogram performed that the isolated transglycosylation products had retention times and concentration at 1.446 min (0.0017 mg/ml), 1.431 min (0.14 mg/ml), and 1.474 min (0.012 mg/ml), respectively, compared to the retention time of arbutin (1.474 min) that was applied as authentic standard for polyphenol glycoside. Moreover, observation using 1H NMR as well as 13C NMR showed that structures of the transglycosylation products were identified as gallic acid-4-O-β-glucopyranoside, ellagicacid-4-O-β-glucopyranoside, and catechin-4′-O-glucopyranoside, respectively.
Keywords: Trichoderma viride, Cyclodextrin glucanotransferase, Transglycosylation reaction, Moringa oleifera, Polyphenolic glycoside
Introduction
Polyphenolic compounds are utilized as an antipruritic or antiseptic in cosmetic products as a restrainer of melanogenesis, antioxidant, bacterial growth inhibitor, persuasive anti-mutagent, or antitoxic (Shimoda and Hamada 2010). Nevertheless, polyphenols are of restricted use since they are easily degraded in an aqueous solution following-on in rapid browning. It was reported that a number of physical properties of the polyphenols were enhanced through enzymatic transglycosylation reaction (Mathew et al. 2012). The absorption of polyphenolic compounds from the food is improved by conjugation with glucose (Paganga and Rice-Evans 1997). The amplified hydrophilicity, resulting from conjugation with glucose is supposed to make the absorption of phenolic compounds and its glycosides extra efficient as compare to its aglycone. Furthermore, polyphenols can be hydrolyzed in a digestive tract by glucosidases formed in human intestinal bacteria to liberate aglycone (Niu and Guo 2006).
Phenolic compounds exhibit instability due to oxidation, light and biochemical changes, which was indicated by occurrence of browning reaction. For that reason, while it is once oxidized then its properties and the use as biological compound will modify, decreased or even vanished. As compare to polyphenol glucosides that were enzymatically synthesized, they were able to demonstrated quite high stability toward the chemical changes (Aramsangtienchai et al. 2011). Enzymatic glycosylation of polyphenols into its glucosides has a number of advantages in contrast to chemical synthesis, which include low cost production, easiness on handling microbial strains and its enzyme for the purpose of synthesis of bioactive compounds. In contrast to conventional chemical synthesis that usually requires tedious protection/de-protection manipulations in order to achieve regio- and stereo-selectivity, enzymatic glycosylation usually provides perfect control of the anomeric configuration and high regio-selectivity without the need of any protecting groups (Crout and Vic 1998).
Enzymic transglycosylation permits insoluble and less stable bioactive compounds to be changed into the resultant soluble and more stable compounds during suitable single-step biological glucosylation (Shimoda et al. 2006). Additionally, glucosides of physiologically bioactive substances, i.e. vitamin glucosides, have been suggested to be functional anti-allergic agents (Satoh et al. 2001). Cyclodextrin glucanotransferase [CGTase, 1,4-α-D glucan 4-α-d-(1,4-glucano)-transferase, EC 2.4.1.19] catalyzes three types of reactions: (1) reaction with the nonreducing ends of starch chains to give a mixture of cyclomaltodextrins (CDs), linked α-(1 → 4) in a nonreducing cyclic structure, designated α-, β-, γ-CDs, 1, 2 respectively; (2) coupling reactions that occur when the CD rings are opened and the resulting MD is transferred to carbohydrate acceptors, such as d-glucose, maltose, sucrose, and so forth to water, which gives a very low degree of hydrolysis; and (3) disproportionation reactions, which occur between two MD molecules in which part of one is transferred to the nonreducing end of the other (Van der Veen et al. 2000; Yoon and Robyt 2002). Hence, it could be applied to catalyze a reversible conversion of polysaccharides and polyphenol compounds into polyphenol glycosides.
Through transglycosylation reaction, polyphenol glycosides as the main transfer product, was found to be higher resistant than that of polyphenol aglycone to light irradiation. CGTase is a starch degrading extracellular enzyme which relates to α-amylase family of glucoside hydrolases (van der Maarel et al. 2002). It is a distinctive enzyme which is able to produce cyclodextrins (CDs) via intra-molecular transglucosylation called cyclization and transferring glucose residues to an acceptor which has an OH-group. CGTase also has capability to degrade starch and CDs into simpler compounds (Kometani et al. 1996; Ibrahim et al. 2005). Through the cyclization process, CGTase is also capable of catalyzing coupling and disproportionation reactions and apparently it possesses weak hydrolytic activity (Alcalde et al. 2003).
CGTase is produced by various bacteria and used for transglycosylation of glycosides. These reactions have been carried out by traditional technique and take longer time durations to complete (Abelyan et al. 2004). Application of transglycosylation reaction for catalyzing glycosides are known well (Funayama et al. 1993; Kometani et al. 1994; Kitao and Sekine 1994; Kitao et al. 1995) however synthesis of polyphenolic glycosides by application of CGTase derived from T. viride in the present of polyphenolic compounds extracted from Moringa oleifera leaves have remained unexplored.
Moringa oleifera has been the object of much research due to its multiple uses and some functional properties (Makkar and Becker 1997). It is rich in nutrients and, apart from a range of industrial and medicinal applications, is used to purify water for human consumption. The leaves of M. oleifera have been reported to be a valuable source of both macro- and micro-nutrients, rich source of β-carotene, protein, vitamin C, calcium, and potassium and act as a good source of natural antioxidants; and thus enhance the shelf-life of fat containing foods (Dillard and Bruce German 2000; Siddhuraju and Becker 2003).
To the best of our knowledge, enzymatic transglycosylation of glycoside by application of CGTase derived from T. viride has not been reported so far. In this study, we evaluated the enzymatic transglycosylation catalyzed by CGTase of fungal strain T. viride by application of wheat flour as donor substrate and polyphenol derived from M. oleifera leaves extract as its acceptor. The synthesized transglycosylation products were then purified and their chemical structures were elucidated through NMR spectroscopy techniques.
Materials and methods
Media for production of CGTase
Fungal T. viride was grown on a liquid medium, containing 30 g glucose, 0.5 g yeast extract, tryptone 1.0 g, 1.8 g NH4Cl, 2 g KH2PO4, 0.5 g MgSO4·7H2O, 0.1 g CaCl2·7H2O, 0.035 g FeSO4·7H2O, 0.007 g MnSO4·7H2O, 0.011 g ZnSO4·7H2O, 0.005 g CuSO4·7H2O, 0.002 g CoCl2·5H2O, 0.0013 g Na2MoO4·2H2O, 0.002 g H3BO4, 0.0005 g Al2(SO4)3, and 20 g of soluble starch dissolved in 1 l of phosphate buffer pH 6.0 (Hashmi et al. 2014). The culture of T. viride was incubated for 72–120 h, in a shaker incubator at 150 rpm and 40 °C. After inoculation then the medium was centrifuged at 8000×g for 20 min at 4 °C to obtain a supernatant and used as a source of crude CGTase (Hashmi et al. 2014).
Enzymatic assay for CGTase
The crude CGTase was assayed for cyclization activity performed by following the method as suggested by Shim et al. (2004). The procedure is based on the reduction in colour intensity due to inclusion complex formation of β-cyclodextrin (β-CD) with phenolphthalein (Alcalde et al. 1999). Soluble starch (2%, w/v) was used as substrate in 50 mM sodium acetate buffer pH 6.0. Approximately 0.1 ml of crude CGTase was added onto 1 ml of soluble starch solution and then incubated at 80 °C for 10 min. The amount of β-CD was determined by measuring the reduction in absorbance at 550 nm. One unit of cyclization activity was defined as the amount of enzyme producing 1 μmol of β-CD per minute.
Hydrolytic activity was determined by incubating 0.1 mL of CGTase with 0.3% (w/v) soluble starch in 50 mM sodium acetate buffer pH 6.0 for 20 min at 80 °C, and its absorbance was measured at 550 nm. The increase in reducing sugar content was calculated according to standard curve of glucose. One unit of hydrolytic activity was described as the amount of enzyme releasing 1 μmol of glucose per min under assay conditions.
Extraction of polyphenols
Extraction of polyphenol was done by following the method of Charoensin (2014) with a slight modification. The leaves of M. oleifera was purchased from local market at Kota Kinabalu, Sabah, Malaysia. The samples were dried in a hot air oven at 50 °C for 72 h and then ground into powder and stored at 4 °C prior to extraction M. oleifera powder (15 g) was extracted with 350 ml of methanol, and then the liquid extract was filtered through Whatman No. 1 filter paper. The residual solvent was removed by using rotary evaporator and all extracts were freeze dried to obtain the polyphenolic constituents.
Transglycosylation capacity
Approximately 0.5 ml of CGTase solution was added into 2.0 ml of reaction mixtures containing 0.1 g of wheat flour as substrates in sodium phosphate buffer (pH 6.0), and 0.04 g of polyphenols extracted from M. oleifera leaves as its acceptors and were incubated at 40 °C for 24 h. The enzyme reaction was stopped by boiling at 100 °C for 10 min. The reaction products were analyzed using TLC and developed using ethyl acetate, acetic acid and distilled water (3:1:1, v/v). The TLC plate was heated at 80–90 °C for 1 h prior to spray with a reagent of 20% H2SO4 in methanol, and then heated at 150 °C for 5–10 min. The spot of the reaction product that showed Rf value parallel to the spot of arbutin as the authentic standard for polyphenolic glycoside was referred as the spot of transfer product (Hashmi et al. 2014).
Isolation and purification of transfer product
A reaction mixture (200 ml) containing polyphenolic constituents of M. oleifera used as substrate-acceptor and wheat flour as substrate-donor were incubated with CGTase at 40 °C for 24 h as the same procedure mentioned above prior to extraction with diethyl ether to remove excess of polyphenols residue that might allegedly prefer to dissolve into solvent phase, where the constituents of glycosides as transfer product might allegedly be remained in water phase was concentrated and furthermore charged onto column chromatography containing octa-dodecyl-silica gel (ODS). The column chromatography was then eluted with gradient solvent of methanol in 1% formic acid (v/v). Fraction solutions resulted by flushing the column that is exhibited single spots on TLC plate within their RF values were parallel to the spot of arbutin were then collected and concentrated (Hashmi et al. 2014).
HPLC/UV–VIS analysis
The purified transfer products were also analyzed by using HPLC according to the method described by Mathew et al. (2012) with slight modification. The HPLC/UV–VIS system was comprised of Agilent HPLC system provided with a pump, an automatic injector, a UV–VIS detector and a degasser. Separations were carried out using Apollo C18 reversed-phase column at a room temperature. Acetonitrile (A) and 0.1% aqueous H3PO4 (B) was used as a mobile phase with a gradient elution of 24% (A) at 0–12 min, 24–50% (A) at 12–22 min, 50–24% (A) at 22–40 min and 24% (A) at 40–50 min. The separation was monitored through absorbance at 254 nm at flow rate of 0.5 ml/min.
Structure identification by using NMR spectroscopy
The 1H NMR, 13C NMR, H–H COSY, C–H COSY, and HMBC spectra were measured using a Varian XL-400 spectrometer in DMSO solution (Shimoda and Hamada 2010).
Results and discussion
Enzymatic assay of CGTase
It is well known that CGTase catalyzes various kinds of chemical reactions including cyclization, where the enzyme acts on a linear polysaccharide chain to cleavage at two sides of fragments to form CDs. The activity of CGTase for cyclization was analyzed based on reduction in color intensity owing to the formation of inclusion complex of β-cyclodextrin with phenolphthalein. The cyclization activity of T. viride CGTase was 120 U/mg, while its hydrolytic activity on 0.3% starch solution as its substrate was recorded as 38 U/mg.
Transglycosylation activity assay of CGTase
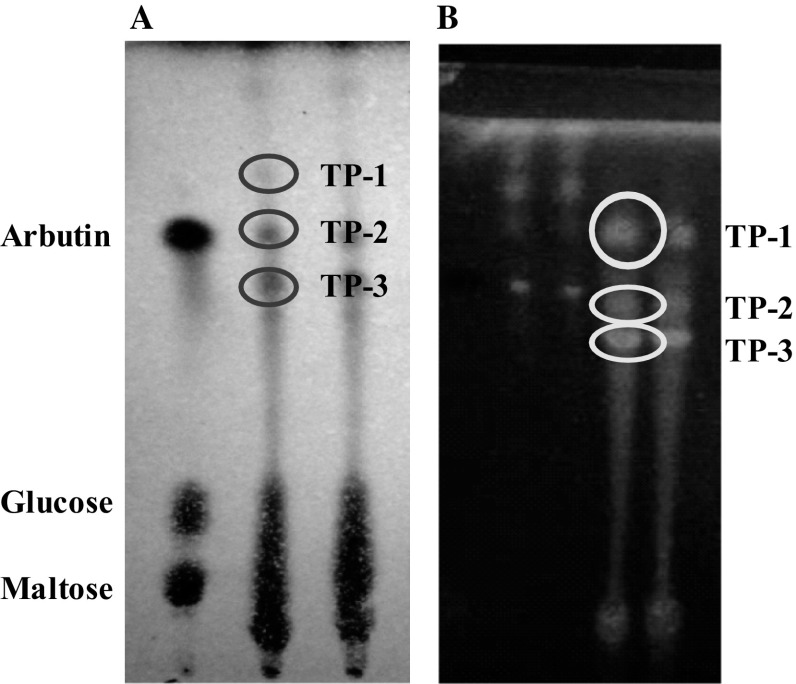
Reaction mixtures containing wheat flour as its substrates, polyphenol extracted from M. oleifera leaves as its acceptor and crude CGTase derived from T. viride were incubated to synthesize polyphenolic glycosides as transglycosylation (transfer) products. They were synthesized enzymatically via intermolecular transglycosylation reactions, where is a process to transfer glucose residues to any acceptor having OH- group. After conducting the transglycosylation reaction using T. viride CGTase with polyphenolic substituents derived from moringa leaves were applied as acceptors, we found three kinds of transfer products could be determined from the sample containing polyphenolic extract of moringa leaves through TLC analysis (Fig. 1). Arbutin (4-hydroxyphenyl-d-glucanopyranoside) was applied as an authentic standard component for determination of existing transfer product on the TLC chromatogram.
Fig. 1.
Chromatogram of transglycosylation products in the presence of polyphenolic extracts derived from moringa leaves after visualizing with chemical reagent (a) and observing under UV light irradiation (b). Arbutin, glucose, maltose used as reference for authentic standard solution and TP-1, TP-2, T-3 indicated to the synthesized transglycosylation products
Trichoderma viride CGTase showed capability to synthesize polyphenol glycosides through transglycosylation reaction in the presence of wheat flour as its substrates and polyphenols extracted from M. oleifera leaves as its acceptors. The spots of transfer products those were parallel corresponding to RF value of the spot of arbutin were considered as the enzymatic synthesized polyphenol glycosides.
The results exhibited that T. viride CGTase had shown high activity in transferring glucose units from its donor-substrate to the acceptor of polyphenols due to effect of strong interaction between OH-groups of donor-substrate and the OH-group of polyphenols-acceptor by synthesizing several polyphenol glycosides.
Separation and purification of polyphenol glycosides
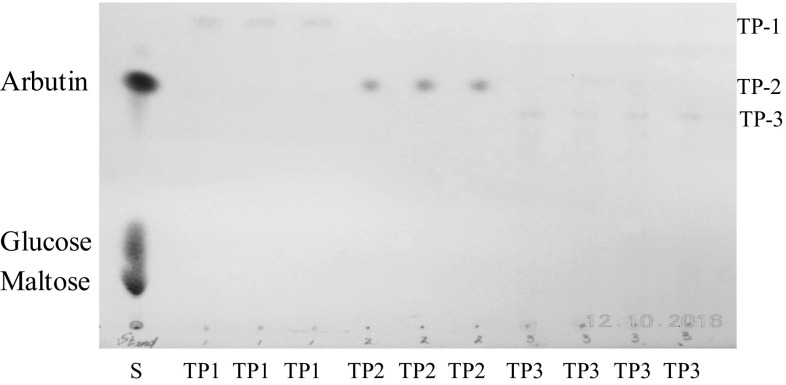
Synthesis of polyphenolic glycosides on a large scale was carried out by same procedure of synthesis on a small scale one. The result showed that the transfer products could be separated and isolated from other unwanted components after some fractionation processes were furthermore analyzed by using TLC. All the excessive polyphenolic residues and sugars were removed by continuous fractionation according to the gradient concentration of the solvent. After conducting ODS chromatography followed by HPLC purification, we obtained the following yields for transfer producs (TP-1, TP-2, and TP-3, respectively). All the synthesized polyphenolic glycosides referred as TP-1, TP-2, and TP-3 were recovered successfully by using solvent of methanol containing 1% (v/v) formic acid. The presence of transfer products could be determined on the TLC plate showing the Rf values of their spots were approximately parallel to the spot of arbutin (Fig. 2). Lane 2–4 exhibited TP-1 spots having Rf value higher than the spot of arbutin was eluted using gradient solvent methanol to formic acid 50:50 (v/v). Lane 5–7 exhibited TP-2 spots having Rf value parallel to the spot of arbutin. However, since the TP-2 spots were yet separated well to TP-1 spots, thus further purification was carried out using gradient solvent (60:40, v/v). Similarly, Lane 8–11 indicated the spots of TP-3 having the Rf value lower than the spot of arbutin, however since the TP-3 spots were yet separated well to TP-2 spots, thus further purification was carried out using gradient solvent (70:30, v/v).
Fig. 2.
TLC of purified transglycosylation products eluted by ODS column chromatography. S, standard solution (arbutin, glucose, maltose). Lane 2–4 (TP1); lane 5–7 (TP-2); Lane 8–11 (TP3) referred to transglycosylation products
HPLC analysis of purified transfer products
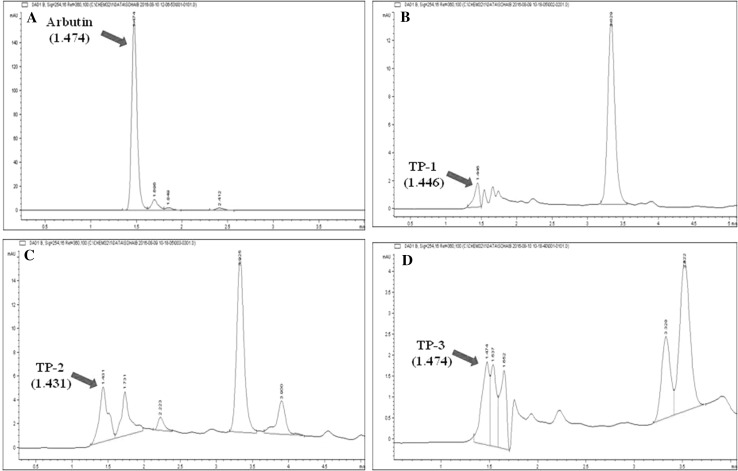
Approximately 20 μl of a fractioned solution containing expected polyphenol glycoside was directly applied onto HPLC under the optimum conditions as described earlier. The peaks performed on chromatogram were analyzed by comparing the retention time (RT) of arbutin to that of RT of the detected peaks of the respective fractioned solutions. The RT for arbutin as authentic standard was recorded at 1.474 min, whereas, the RT for TP-1, TP-2 and TP-3 were recorded at 1.446, 1.431 and 1.474 min, respectively (Fig. 3). According to the standard curve of arbutin prepared as authentic standard by plotting a graph regarding with the concentrations of arbutin against peak area obtained from the HPLC chromatogram, it showed that the concentration of these TP-1, TP-2 and TP-3 were 0.0017, 0.14 and 0.012 mg/ml, respectively.
Fig. 3.
High performance liquid chromatogram of partially purified product eluted with ODS column chromatography. Retention time of HPLC elution products were; a Arbutin (1.474 min); b TP-1 (1.446 min); c TP-2 (1.431 min); d TP-3 (1.474 min)
Structure determination of polyphenolic glycosides
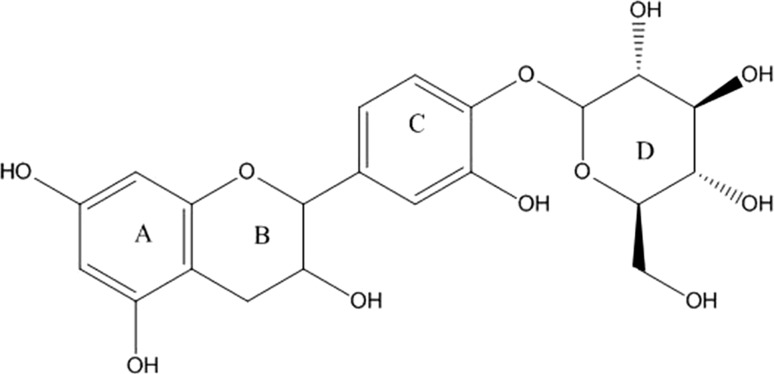
All the structures of these purified polyphenol glycoside constituents those were enzymatically synthesized by using T. viride CGTase in the present of wheat flour as its donor of glycosyl group and polyphenols were extracted from M. oleifera leaves as its acceptor were furthermore established on the basis of NMR spectroscopic techniques as referred as gallic acid-4-O-β-glucopyranoside (TP-1), ellagic acid-4-O-β-glucopyranoside (TP-2) and catechin-4′-O-β-glucopyranoside (TP-3) as shown in Table 1.
Table 1.
1H-NMR and 13C-NMR data of synthesized transglycosylation products
| C | TP-1 | TP-2 | TP-3 | |||
|---|---|---|---|---|---|---|
| δ1H, J (Hz) | δ13C | δ1H, J (Hz) | δ13C | δ1H, J (Hz) | δ13C | |
| 1 | 7.80 (s) | 126.4 | 6.84 (d, J = 1.93) | 130.2 | ||
| 2 | 7.03 (bs) | 123.6 | 140.6 | 108.6 | ||
| 3 | 138.9 | 132.7 | 144.8 | |||
| 4 | 144.9 | 146.2 | 7.24 (d, J = 8.43) | 147.96 | ||
| 5 | 138.9 | 101.6 | 116.9 | |||
| 6 | 7.03 (bs) | 123.6 | 120.2 | 7.01 (dd, J = 2.54, 8.69) | 119.9 | |
| 7 | 161.2 | |||||
| 1′ | 4.35 (d, 7.8) | 101.9 | 6.39 (s) | 111.4 | 4.60 (d, J = 1.93) | 82.4 |
| 2′ | 4.26 (dd, 8.3, 7.8) | 73.8 | 158.7 | 3.99 (m) | 67.7 | |
| 3′ | 3.26 (dd, 9.3, 8.3) | 78.4 | 160.9 | 2.52–2.94 (m) | 29.6 | |
| 4′ | 4.33 (dd 9.3, 9.3) | 71.1 | 140.4 | 5.84 (d, J = 2.06) | 156.4 | |
| 5′ | 3.37 (m) | 79.9 | 120.5 | 96.5 | ||
| 6′ | 108.5 | 157.5 | ||||
| 7′ | 161.2 | 5.93 (d, J = 2.06) | 95.9 | |||
| 1″ | 4.99 (d, 7.1) | 101.7 | 4.05 (m) | 100.7 | ||
| 2″ | 4.08 (m) | 74.9 | 3.48 (m) | 73.9 | ||
| 3″ | 4.30 (m) | 77.4 | 3.38 (m) | 80.4 | ||
| 4″ | 4.19 (m) | 70.1 | 4.13 (m) | 71.3 | ||
| 5″ | 4.06 (s) | 78.2 | 3.40 (s) | 78.9 | ||
| 6″α | 4.06 (dd, 11.3, 5.6) | 63.9 | 4.18 (m) | 63.2 | 3.68 (m) | 64.2 |
| 6″β | 4.37 (bd, 11.3) | 4.33 (m) | 3.89 (m) | |||
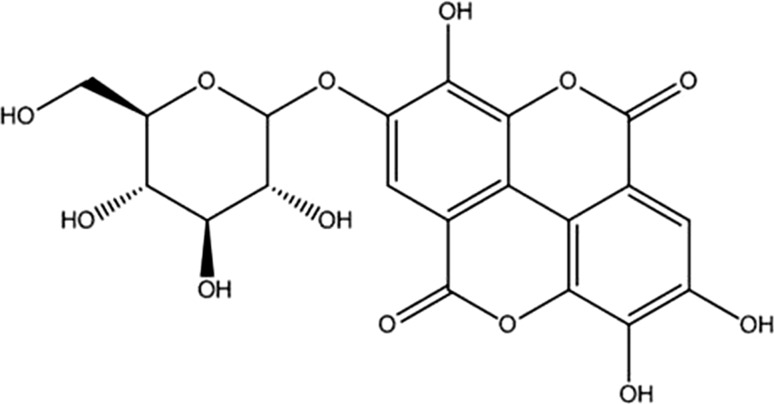
The 1HNMR and 13CNMR spectrum of compound TP-1 revealed the presence of glycosidic bond in gallic acid glucopyranoside. The broad singlet at δH 7.03 corresponds to the two protons of the aromatic ring. The 13CNMR showed one signal resonating at δc 114.96, which could be attributing to C-4. The shifting of this signal to the higher field confirms the glycosidic linkage at C-4. The presence of signal at δc 123.6 corresponds to C-2 and C-6 while the signal at 138.9 corresponds to C-3 and C-5 reveals that aromatic ring of TP-1 have plane of symmetry. More signals are provided in Table 1. Gallic acid-4-O-β-glucopyranoside: IRKBr (νmax/cm−1):1H-NMR (500 MHz; CD3OD-d4/ppm) δ: 7.03 (1H, bs, H-3), 7.03 (1H, bs, H-7), 4.35 (1H, d, J 7.8 Hz, H-1′), 4.26 (1H, dd, J 8.3, 7.8 Hz, H-2′), 3.26 (1H, dd, J 9.3, 8.3 Hz, H-3′), 4.33 (1H, dd, J 9.3, 9.3 Hz, H-4′), 3.37 (1H, m, H-5′), 4.06 (1H, dd, J 11.3, 5.6 Hz, H-6′α), 4.37 (1H, bd, J11.3 Hz, H-6′β).13C-NMR spectrum (125 MHz; DMSO-d6/ppm): 166.2 (C-1),123.6 (C-2),108.8 (C-3),144.96 (C-4), 138.9 (C-5), 144.9 (C-6), 108.8 (C-7), 101.9 (C-1′), 73.8 (C-2′), 78.4 (C-3′), 71.1 (C-4′), 79.9 (C-5′), 63.9 (C-6′) (Fig. 4).
Fig. 4.
Chemical structure of TP-1, gallic acid-4-O-β-d-glucopyranoside
The 1HNMR and 13CNMR spectrum of compound TP-2 revealed the presence of glycosidic bond in ellagic acid-4-O-β-glucopyranoside. The broad singlet at δH 7.03 corresponds to the two protons of the aromatic ring. The 13CNMR showed one signal resonating at δc 114.96, which could be attributing to C-4. The shifting of this signal to the higher field confirms the glycosidic linkage at C-4. The presence of signal at δc 123.6 corresponds to C-2 and C-6 while the signal at 138.9 corresponds to C-3 and C-5 reveals that aromatic ring of TP-2 have plane of symmetry. More signals are provided in Table 1. Ellagic acid-4-O-β-glucopyranoside: 1H NMR spectrum (500 MHz; DMSO-d6/ppm): δ 7.80 (1H, s, H-5), δ 6.39 (1H, s, H-5´), δ 6.05 (1H, m, H-1″), δ 4.08 (1H, m, H-2″), δ 4.30 (1H, m, H-3″), δ 4.19 (1H, m, H-4″), δ 4.06 (1H, m, H-5″), δ 4.18 (1H, m H-6″a), δ 4.33 (1H, m H-6″b). 13C NMR spectrum (125 MHz; DMSO-d6/ppm): δ 126.4 (C-1), 140.6 (C-2),132.7 (C-3), 140.2 (C-4), 101.6 (C-5), 120.2 (C-6), 161.2 (C-7), 111.4 (C-1′), 158.7 (C-2′), 160.9 (C-3′), 146.4 (C-4′),120.5 (C-5′), 108.5 (C-6′), 165.0 (C-7′), 101.7 (C-1″), 74.9 (C-2″), 77.4 (C-3″), 70.1 (C-4″), 78.2 (C-5″), 63.2 (C-6″) (Fig. 5).
Fig. 5.
Chemical structure of TP-2, ellagic acid-4-O-β-d-glucopyranoside
The 1H NMR spectrum of compound TP-3 revealed the presence of double doublet of H-6 at δH 7.01 of aromatic ring C where as two doublet at δH 6.84 and 7.24 having coupling constant 1.93 and 8.43 could be attributed to H-2 and H-5 respectively. The 13CNMR spectrum showed expected signals, which corresponds to all carbon present in the structure. The signal at δc 147.9 was at higher filed assigned to C-4 carbon atom of aromatic ring, confirming the glycosidic linkage. Another signal at δc 144.8 was attributed to C-3 as this carbon of aromatic ring C is attached to hydroxyl group. Further signals are included in Table 1. The 1H NMR spectrum of compound TP-3 revealed the presence of two characteristic singlets of H-5 at δH 7.80 and H-5′ at δH 6.39. The doublet at δH 4.99 having the coupling constant (J = 7.1 Hz) was assigned to H-1″ which confirms the presence of sugar moiety as β–d-glucose. The 13CNMR spectrum showed expected signals, which corresponds to all carbon present in the structure. The signal at δc 146.2 could be assigned to C-4. This higher field confirms the position of glycosidic linkage to the aglycone moiety. Further signals are included in Table 1. Catechin-4′-O-β-glucopyranoside:1H-NMR (500 MHz; CD3OD-d4/ppm) δ: 7.24 (1H,d,H-2), 6.84 (1H, d, H-5), 7.01 (1H, d, H-6), 4.60 (1H, d, H-1′), 3.99 (1H, dd, H-2′), 2.52-2.94 (2H, m, H-3′), 5.84 (1H, d, H-5′), 5.93 (1H, d, H-7′). δ 4.05 (1H, m, H-1″), δ 3.48 (1H, m, H-2″), δ 3.38 (1H, m, H-3″), δ 4.13 (1H, m, H-4″), δ 3.40 (1H, m, H-5″), δ 3.68 (1H, m H-6″a), δ 3.89 (1H, m H-6″b).13C NMR spectrum (125 MHz; DMSO-d6/ppm):130.2 (C-1),108.6 (C-2),144.8 (C-3),147.96 (C-4), 116.9 (C-5), 119.9 (C-6),82.4 (C-1′), 67.7 (C-2′), 29.6 (C-3′), 156.4 (C-4′), 96.5 (C-5′), 157.5 (C-6′), 95.9.0 (C-7′), 93.3(C-8′),100.7 (C-1″), 73.9 (C-2″), 80.4 (C-3″), 71.3 (C-4″), 78.9 (C-5″), 64.2 (C-6″) (Fig. 6).
Fig. 6.
Chemical structure of TP-3, catechin-4′-O-β-d-glucopyranoside
Recently, enzymatic transglycosylation has been employed in the modification of natural bioactive compounds, in an effort to improve their physicochemical qualities. Lee et al. (2004) reported that glycosylated ascorbic acids exhibited rather effective antioxidant properties, exerting a preventative effect against lipid oxidation, and also reported that they exhibited asynergistic effect superior to that observed for normal ascorbic acid. Gilly et al. (2003) also demonstrated that the glycosylation of resveratrol resulted in a strengthening of this compound’s preventative effect against enzymatic oxidation. Glycosylated naringin was 250 times more water soluble than naringin and10 times less bitter (Lee et al. 1999). Li et al. (2004) reported that the solubility of glycosylated puerarin was 14-168 times higher than that of puerarin. Glycosylated catechin was determined to be quite stable against ultraviolet (UV) radiation, although catechin could be degraded fairly readily (Kitao et al. 1993). Recently, a series of acceptor reactions have been conducted in a variety of studies, using various polyphenols with the glucan sucrose from L. mesenteroides. Yoon and Robyt (2002) synthesized two acarbose analogues using glucan sucrases from L. mesenteroides. Seo et al. (2005) synthesized a variety of salicin and phenol glycoside structures by carrying out of glucan sucrose acceptor reactions. That group determined that the salicin glycosides exerted an inhibitory effect against blood coagulation investigated the enzymatic synthesis of EGCG glycosides, in which a D-glucopyranosyl residue is attached to the 7-hydroxyl group of EGCG-G1 or to the 4¢-hydroxyl group of EGCG-G1¢, as well as both the 7- and 4¢-hydroxyl groups of EGCG, using the glucansucrase from L. mesenteroides (Moon et al. 2006).
This paper describes the isolation and identification of three novel transglycosylation products in the presence of polyphenolic substituents derived from M. oleifera. They were as gallic acid-4-O-β-glucopyranoside consisting of glucose and gallic acid, ellagic acid-4-O-β-glucopyranoside, consisting of glucose and ellagic acid and catechin-4′-O-glucopyranoside, consisting of glucose and catechin, respectively according to the results of 13C-NMR sand lH-NMR spectroscopy. In our previous paper, we reported that this glycoside was synthesized in the presence of commercial polyphenols such as hydroquinone, pyrocathecol and resorcinol through transglycosylation of CGTase derived from Xanthomonas campestris (Hashmi et al. 2014; Sulistyo et al. 2014). The transglycosylation products in the presence of various polyphenolic acceptors had also been observed on TLC. Considering different color of the transfer product spots were detected on the TLC plates under UV irradiation the result shown that the crude CGTase derived from T. viride used in this study exhibited transglycosylation capacity to synthesize different polyphenolic glycosides as novel transglycosylation products as indicated to the Rx value and the color of spots of the transfer products on TLC compared to that of authentic arbutin, however the Rx values of the transfer products were slightly shifted up and down of the spot of arbutin. According to the analysis using 13C-NMR and 1H-NMR spectroscopy, the results suggested that the respective structures of transfer products that were synthesized by the transglycosylation reaction catalyzed by T. viride CGTase in the present of starch as its substrate and extract of moringa leaves as its acceptor could be identified as gallic acid-4-O-β-glucopyranoside (TP-1), ellagic acid-4-O-β-glucopyranoside (TP-2) and catechin-4′-O-glucopyranoside (TP-3). These chemical structures were corresponded to composition of M. oleifera leaves extract where it contains approximately 10–11% gallic acid, 5–6% ellagic acid and 2–3% catechin.
Conclusion
This work reports on synthesis and stabilize polyphenolic constituent that was extracted from M. oleifera leaves as source of bioactive compounds by application of transglycosylation reaction CGTase derived from fungal culture of T. viride. The enzymatically synthesized polyphenol glycoside were qualitatively determined using TLC and quantitatively estimated using HPLC and furthermore determined as transfer products as referred to arbutin as authentic standard for commercial polyphenol glucopyranoside. The transglycosylation products were furthermore identified and recognized as novel structures of glycosides through 1HNMR and 13CNMR spectroscopy as gallic acid-4-O-β-d-glucopyranoside (GAGP), ellagic acid-4-O-β-d-glucopyranoside (EAGP) and catechin-4′-O-β-d-glucopyranoside (CGP), respectively.
Acknowledgements
This work was financially supported by UMS Research Grant 2015, under the Universiti Malaysia Sabah. We are also thankful to Institute for Tropical Biology And Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia for NMR Spectroscopy analysis services.
References
- Abelyan VA, Balayan AM, Ghochikyan VT, Markosyan AA. Transglycosylation of stevioside by cyclodextrin glucanotransferases of various groups of microorganisms. Appl Biochem Microbiol. 2004;40:129–134. doi: 10.1023/B:ABIM.0000018914.08571.50. [DOI] [Google Scholar]
- Alcalde M, Plo FJ, Andersen C, Martin MT, Pedersen S, Ballesteros A. Chemical modification of lysine side chains of cyclodextrin glycosyltransferase to α-amylase specificity. FEBS Lett. 1999;445:333–337. doi: 10.1016/S0014-5793(99)00134-9. [DOI] [PubMed] [Google Scholar]
- Alcalde M, Plou FJ, Boada MP, Arellano HG, Valdes I, Mendez E, Ballesteros A. Chemical modification of carboxylic residues in a cyclodextrin glucanotransferase and its implication in the hydrolysis/transglycosylation ratio of the α-amylase family. J Mol Catal. 2003;26:57–67. doi: 10.1016/S1381-1177(03)00166-8. [DOI] [Google Scholar]
- Aramsangtienchai P, Chavasiri W, Ito K, Pongsawasdi P. Synthesis of epicatechin glucosides by a β-cyclodextrin glycosyltransferase. J Mol Catal B Enzym. 2011;73(1):27–34. doi: 10.1016/j.molcatb.2011.07.013. [DOI] [Google Scholar]
- Charoensin S. Antioxidant and anticancer activities of Moringa oleifera leaves. J Med Plant Res. 2014;8(7):318–325. doi: 10.5897/JMPR2013.5353. [DOI] [Google Scholar]
- Crout DH, Vic G. Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr Opin Chem Biol. 1998;2:98–111. doi: 10.1016/S1367-5931(98)80041-0. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, Bruce German J. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80(12):1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Funayama M, Nishino T, Hirota A, Murao S, Takenisi S, Nakano H. Enzymatic synthesis of (+) catechin-α-glucoside and its effect on tyrosinase activity. Biosci Biotechnol Biochem. 1993;57(10):1666–1669. doi: 10.1271/bbb.57.1666. [DOI] [Google Scholar]
- Gilly RS, Oded S, Itzhak B, Zohar K. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem J. 2003;374:157–163. doi: 10.1042/bj20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi MI, Nazir S, Sulistyo J. Antimelanogenesis activity of polyphenol glycoside synthesized by transglycosylation reaction of CGTase from Xanthomonas Campestris. IJRAFS. 2014;2(8):2311–2476. [Google Scholar]
- Ibrahim HM, Yusoff WMW, Hamid AA, Illias RM, Hassan O, Omar O. Optimization of medium for the production of β-cyclodextrin glucanotransferase using central composite design (CCD) Process Biochem. 2005;40:753–758. doi: 10.1016/j.procbio.2004.01.042. [DOI] [Google Scholar]
- Kometani T, Terada Y, Nishimura T, Nakae T, Takii H, Okada S. Acceptor specificity of cyclodextrin glucanotransferase from an alkalophilic Bacillus species and synthesis of glucosyl rhamnose. Biosci Biotechnol Biochem. 1996;60:1176–1178. doi: 10.1271/bbb.60.645. [DOI] [PubMed] [Google Scholar]
- Kometani T, Terada Y, Nishimura T, Takii H, Okada S. Purification and characterisation of cyclodextrin glucanotransferase form an alkalophilic Bacillus species and transglycosylation at alkaline pH. Biosci Biotech Biochem. 1994;58:517–520. doi: 10.1271/bbb.58.517. [DOI] [Google Scholar]
- Kitao S, Sekine H. α-D-Glucosyl transfer to phenolic compounds by sucrose phosporylase from Leuconostoc mesenteroides and production of α-arbutin. Biosci Biotech Biochem. 1994;58:38–42. doi: 10.1271/bbb.58.38. [DOI] [PubMed] [Google Scholar]
- Kitao S, Ariga T, Matsudo T, Sekine H. The Syntheses of Catechin-glucosides by Transglycosylation with Leuconostoc mesenteroides Sucrose Phosphorylase. Biosci Biotech Biochem. 1993;57:2010–2015. doi: 10.1271/bbb.57.2010. [DOI] [Google Scholar]
- Kitao S, Matsudo T, Saitoh M, Horiuchi T, Sekine H. Enzymatic synthesis of two stable (-)-epigallocatechin gallate-glucosides by sucrose phosporylase. Biosci Biotech Biochem. 1995;59:2167–2169. doi: 10.1271/bbb.59.2167. [DOI] [Google Scholar]
- Lee SJ, Kim JC, Kim MJ, Kitaoka M, Park CS, Lee SY, Ra MJ, Moon TW, Robyt JF, Park KH. Transglycosylation of naringin by Bacillus stearothermophilus maltogenic amylase to give glycosylated naringin. J Agric Food Chem. 1999;47:3669–3674. doi: 10.1021/jf990034u. [DOI] [PubMed] [Google Scholar]
- Lee SB, Nam KC, Lee SJ, Lee JH, Inoue K, Park KH. Antioxidant effects of glycosyl-ascorbic acids synthesized by maltogenic amylase to reduce lipid oxidation and volatiles production in cooked chicken meat. Biosci Biotechnol Biochem. 2004;68:36–43. doi: 10.1271/bbb.68.36. [DOI] [PubMed] [Google Scholar]
- Li D, Park SH, Shim JH, Lee HS, Tang SY, Park CS, Park KH. In vitro enzymatic modification of puerarin to puerarin glycosides by maltogenic amylase. Carbohydr Res. 2004;339:2789–2797. doi: 10.1016/j.carres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J Agric Sci. 1997;128:311–322. doi: 10.1017/S0021859697004292. [DOI] [Google Scholar]
- Mathew S, Hedström M, Adlercreutz P. Enzymatic synthesis of piceid glycosides by cyclodextrin glucanotransferase. Process Biochem. 2012;47(3):528–532. doi: 10.1016/j.procbio.2011.11.012. [DOI] [Google Scholar]
- Moon YH, Lee JH, Ahn JS, Nam SH, Oh DK, Park DH, Chung HJ, Kang S, Day DF, Kim D. Synthesis, structure analyses, and characterization of novel epigallo catechin gallate (EGCG) glycosides using the glucansucrase from Leuconostoc mesenteroides B-1299CB. J Agric Food Chem. 2006;54:1230–1237. doi: 10.1021/jf052359i. [DOI] [PubMed] [Google Scholar]
- Niu PQ, Guo CY. Investigative development of pharmacology of resveratrol. Herald Med. 2006;6(6):524–525. [Google Scholar]
- Paganga G, Rice-Evans CA. The identification of flavonoids as glycosides in human plasma. FEBS Lett. 1997;401:78–82. doi: 10.1016/S0014-5793(96)01442-1. [DOI] [PubMed] [Google Scholar]
- Satoh T, Miyataka H, Yamamoto K, Hirano T. Synthesis and physiological activity of novel tocopheryl glycosides. Chem Pharm Bull. 2001;49:948–953. doi: 10.1248/cpb.49.948. [DOI] [PubMed] [Google Scholar]
- Seo ES, Lee JH, Park JY, Kim D, Han HJ, Robyt JF. Enzymatic synthesis and anti-coagulant effect of salicin analogs by using the Leuconostoc mesenteroides glucansucrase acceptor reaction. J Biotechnol. 2005;117:31–38. doi: 10.1016/j.jbiotec.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Shim JH, Kim YW, Kim TJ, Chae HY, Park JH, Cha H, Kim JW, Kim YR, Schaefer T, Spendler T, Moon TW, Park KH. Improvement of cyclodextrin glucanotransferase as an antistaling enzyme by error-prone PCR. Protein Eng Des Sel. 2004;17:205–211. doi: 10.1093/protein/gzh035. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Hamada H. Enzymatic synthesis and anti-allergic activities of curcumin oligosaccharides. Biochem Insights. 2010;3:1–5. doi: 10.4137/BCI.S2768. [DOI] [Google Scholar]
- Shimoda K, Kondo Y, Abe K, Hamada H, Hamada H. Formation of water soluble vitamin derivatives from lipophilic vitamins by cultured plant cells. Tetrahedron Lett. 2006;47:2695–2698. doi: 10.1016/j.tetlet.2006.02.082. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drum stick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Sulistyo J, Handayani R, Rahayu RD. Assay for transglycosylation reaction of Xanthomonas campestris on carbohydrate sources. IJRAFS. 2014;2(6):1–7. [Google Scholar]
- Van der Maarel MJEC, van der Ween B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L. Properties and applications of starch-converting enzymes of the α-amylase family. J Biotechnol. 2002;94:137–155. doi: 10.1016/S0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- Van der Veen BA, van Alebeek GJWM, Uitdehaag JCM, Dijkstra BW, Dijkhuizen L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem. 2000;267:658–665. doi: 10.1046/j.1432-1327.2000.01031.x. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Robyt JF. Synthesis of acarbose analogues by transglycosylation reactions of Leuconostoc mesenteroidesB-512FMC and B-742CB dextransucrases. Carbohydr Res. 2002;337:2427–2435. doi: 10.1016/S0008-6215(02)00350-6. [DOI] [PubMed] [Google Scholar]