Abstract
Tomato is a wonder fruit fortified with health-promoting phytochemicals that are beneficial in preventing important chronic degenerative disorders. Tomato is a good source of phenolic compounds (phenolic acids and flavonoids), carotenoids (lycopene, α, and β carotene), vitamins (ascorbic acid and vitamin A) and glycoalkaloids (tomatine). Bioactive constituents present in tomato have antioxidant, anti-mutagenic, anti-proliferative, anti-inflammatory and anti-atherogenic activities. Health promoting bioactivities of tomatoes make them useful ingredient for the development of functional foods. Protective role of tomato (lycopene as a potent antioxidant) in humans against various degenerative diseases are known throughout the world. Intake of tomato is inversely related to the incidence of cancer, cardiovascular diseases, ageing and many other health problems. Bioavailability of phytoconstituents in tomato is generally not affected by routine cooking processes making it even more beneficial for human consumption. The present review provides collective information of phytochemicals in tomato along with discussing their bioactivities and possible health benefits.
Keywords: Tomato, Phenolics, Carotenoids, Bioactivities, Health benefits
Introduction
Human diet has been evolving since the dawn of civilization. Selection and consumption of food depend upon the availability of resources, climatic conditions, and socio-economic needs. Food not only provides the necessary calories and nutrition to the human body but is also a source of bioactive compounds that help us combating degenerative effects of toxins and preventing many health problems (Singh et al. 2016). Vegetables, fruits, and legume seeds provide carbohydrates, proteins, minerals, and vitamins to our body. Besides these, they are also a source of health-promoting biologically active compounds (Ezekiel et al. 2013; Singh et al. 2017a, b, c). Some food items provide many health benefits to our body along with basic nutrition and tomato is one such food present in the platter of people all over the world.
Tomato (Solanum lycopersicum L.) is a highly popular fruit crop grown and consumed by people across the globe. The worldwide production of tomato in 2014 was 170.75 Mt from 50,238.1 km2 areas under cultivation which was much higher as compared to that recorded in 2010 when the production was 151.89 Mt from 44,955.8 km2 areas under cultivation (FAOSTAT 2014). Tomato production is highest in China followed by India and USA and it is consumed more in Mediterranean countries. In India, the cultivation of tomato in 2014 was carried out on an area of 8820 km2 with a total production of 18.74 Mt while in 2010 the area was 6344 km2 and production was 12.43 Mt (FAOSTAT 2014). Cooked or raw tomato is consumed in the various parts of the world in different forms viz, curries, sauces, salads etc. Consumption of tomatoes exert positive effects on human health and is known for anti-inflammatory, anti-genotoxic, anti-mutagenic, anti-proliferative and chemopreventive activities (Rafi et al. 2007; Scolastici et al. 2007, 2008; Polívková et al. 2010; Feng et al. 2010).
Tomatoes are considered as part of healthy diet regime as they are low in fats and are without any harmful cholesterol. Nutrients like Vitamin A, ascorbic acid, potassium, and folate are present in significant concentrations in tomatoes. Non-nutritive phytochemicals like carotenoids (lycopene, phytoene, and β-carotene) and polyphenols (flavonoids, flavanones, and flavones) are also present in significant amount in tomato (Tan et al. 2010). The contents of nutrients present in raw, ripe and cooked tomatoes are given in Table 1. Phytochemicals are fortified with ripening and cooking of tomatoes. Carotene is present in high concentration in ripened red tomatoes and cooking fortifies the lycopene content in tomatoes. α-carotene is not present in the cooked tomatoes whereas it is present in significant amount in ripe red tomatoes. Lutein is also present in high concentration in ripe tomatoes while it is absent in unripe tomato. Epidemiological evidence has suggested the potential role of tomato phytochemicals in preventing blindness, respiratory disorders, cardiovascular diseases (CVD) and some forms of cancers (Agarwal and Rao 2000; Sesso et al. 2004; Tan et al. 2010). Also, the potential role of these phytochemicals has been observed in the prevention of mutations in DNA (Hazewindus et al. 2014). Tomato is a vegetable of great interest because of its high content of health benefiting compounds. The present review is an attempt to compile information about beneficial phytochemicals present in tomato, their bioactive potential and health benefits reported in various research findings.
Table 1.
Nutrients present in different types of tomatoes, red, ripe and raw
Source: Information derived from United States Department of Agriculture (USDA), Food Composition Databases (USDA 2016)
| Unit | Tomatoes, red, ripe, raw | Tomatoes, red, ripe, cooked | Tomatoes green, raw | |
|---|---|---|---|---|
| Nutrient | ||||
| Water | g | 94.52 | 94.34 | 93 |
| Energy | Kcal | 18 | 18 | 23 |
| Energy | kJ | 74 | 73 | 95 |
| Protein | g | 0.88 | 0.95 | 1.2 |
| Total lipid (fat) | g | 0.2 | 0.11 | 0.2 |
| Ash | g | 0.5 | 0.6 | 0.5 |
| Carbohydrate | g | 3.89 | 4.01 | 5.1 |
| Fiber, total dietary | g | 1.2 | 0.7 | 1.1 |
| Sugars, total | g | 2.63 | 2.49 | 4 |
| Glucose (dextrose) | g | 1.25 | 1.18 | – |
| Fructose | g | 1.37 | 1.31 | – |
| Calcium, Ca | mg | 10 | 11 | 13 |
| Iron, Fe | mg | 0.27 | 0.68 | 0.51 |
| Magnesium, Mg | mg | 11 | 9 | 10 |
| Phosphorus, P | mg | 24 | 28 | 28 |
| Potassium, K | mg | 237 | 218 | 204 |
| Sodium, Na | mg | 5 | 11 | 13 |
| Zinc, Zn | mg | 0.17 | 0.14 | 0.07 |
| Copper, Cu | mg | 0.059 | 0.075 | 0.09 |
| Manganese, Mn | mg | 0.114 | 0.105 | 0.1 |
| Selenium, Se | µg | 0 | 0.5 | 0.4 |
| Fluoride, F | µg | 2.3 | – | – |
| Vitamins | ||||
| Vitamin C, total ascorbic acid | mg | 13.7 | 22.8 | 23.4 |
| Thiamin | mg | 0.037 | 0.036 | 0.06 |
| Riboflavin | mg | 0.019 | 0.022 | 0.04 |
| Niacin | mg | 0.594 | 0.532 | 0.5 |
| Pantothenic acid | mg | 0.089 | 0.129 | 0.5 |
| Vitamin B-6 | mg | 0.08 | 0.079 | 0.081 |
| Folate, total | µg | 15 | 13 | 9 |
| Folic acid | µg | 0 | 0 | 0 |
| Folate, food | µg | 15 | 13 | 9 |
| Folate, DFE | µg | 15 | 13 | 9 |
| Choline, total | mg | 6.7 | 6.9 | 8.6 |
| Vitamin A, RAE | µg | 42 | 24 | 32 |
| Carotene, beta | µg | 449 | 293 | 346 |
| Carotene, alpha | µg | 101 | 0 | 78 |
| Vitamin A, IU | IU | 833 | 489 | 642 |
| Lycopene | µg | 2573 | 3041 | 0 |
| Lutein + zeaxanthin | µg | 123 | 94 | 0 |
| Vitamin E (alpha-tocopherol) | mg | 0.54 | 0.56 | 0.38 |
| Tocopherol, beta | mg | 0.01 | 0.01 | – |
| Tocopherol, gamma | mg | 0.12 | 0.21 | – |
| Tocopherol, delta | mg | 0 | 0.01 | – |
| Vitamin K (phylloquinone) | µg | 7.9 | 2.8 | 10.1 |
| Lipids | ||||
| Fatty acids, total saturated | g | 0.028 | 0.015 | 0.028 |
| Fatty acids, total monounsaturated | g | 0.031 | 0.016 | 0.03 |
| Fatty acids, total polyunsaturated | g | 0.083 | 0.044 | 0.081 |
| Phytosterols | mg | 7 | 9 | – |
| Amino acids | ||||
| Tryptophan | g | 0.006 | 0.008 | 0.009 |
| Threonine | g | 0.027 | 0.027 | 0.03 |
| Isoleucine | g | 0.018 | 0.026 | 0.029 |
| Leucine | g | 0.025 | 0.039 | 0.044 |
| Lysine | g | 0.027 | 0.039 | 0.044 |
| Methionine | g | 0.006 | 0.009 | 0.01 |
| Cystine | g | 0.009 | 0.014 | 0.016 |
| Phenylalanine | g | 0.027 | 0.028 | 0.031 |
| Tyrosine | g | 0.014 | 0.018 | 0.021 |
| Valine | g | 0.018 | 0.027 | 0.031 |
| Arginine | g | 0.021 | 0.026 | 0.029 |
| Histidine | g | 0.014 | 0.016 | 0.018 |
| Alanine | g | 0.027 | 0.03 | 0.034 |
| Aspartic acid | g | 0.135 | 0.148 | 0.166 |
| Glutamic acid | g | 0.431 | 0.393 | 0.442 |
| Glycine | g | 0.019 | 0.026 | 0.03 |
| Proline | g | 0.015 | 0.02 | 0.023 |
| Serine | g | 0.026 | 0.028 | 0.032 |
| Flavonoids | ||||
| Naringenin | mg | 0.7 | 0 | – |
| Kaempferol | mg | 0.1 | 0 | – |
| Myricetin | mg | 0.1 | 0 | – |
| Quercetin | mg | 0.6 | 0.7 | – |
Historical background
Tomato is believed to have evolved from small green fruits present in the foothills of Andes (Peralta and Spooner 2007). In 700 AD, a species of yellow tomatoes of a size similar to present day tomato was cultivated in Central America. Domestication of tomatoes first started in Mexico. After colonization, the seeds of tomato were introduced to various parts of the world and in many places, monoculture was adopted (Peralta and Spooner 2007). After the independence of Mexico, by the development of transport system and some land reforms, the production of tomatoes increased (Saavedra et al. 2017). Documentary evidence suggests that in Europe during 1544 tomatoes were first used but were considered toxic at that time. Dietary inclusion of tomatoes in European cuisine was promoted during next two centuries. Later with the advent of the green revolution and use of better irrigation practices and agrochemicals, the production of tomatoes increased worldwide. Present day tomato has evolved largely with the increase in horticulture techniques. In the 1990s with the advancements in biotechnology and genetic modification techniques, the tomatoes having better color, taste, shelf life and nutrients were developed (Saavedra et al. 2017). Various techniques were adopted to inculcate desirable changes in appearance, size, and quality of the tomato fruit. A paradigm shift in crop improvement objectives for the enhancement of health benefits and disease resistance is observed in recent years (Tan et al. 2010).
Phytochemicals in tomato
Tomato is known as health stimulating fruit owing to the characteristic array of phytochemicals. Phenolics and carotenoids are the main bioactive compounds present in ripened tomatoes. The red color of a ripe tomato is because of a significant amount of lycopene (Martí et al. 2016; Perveen et al. 2015). The tomato fruit also contains β-carotene known for its provitamin A activity. The present section focuses on main phytochemicals (phenolics, carotenoids, vitamins, and glycoalkaloids) present in tomato. Chemical structures of important phytochemicals of tomato are given in Fig. 1.
Fig. 1.
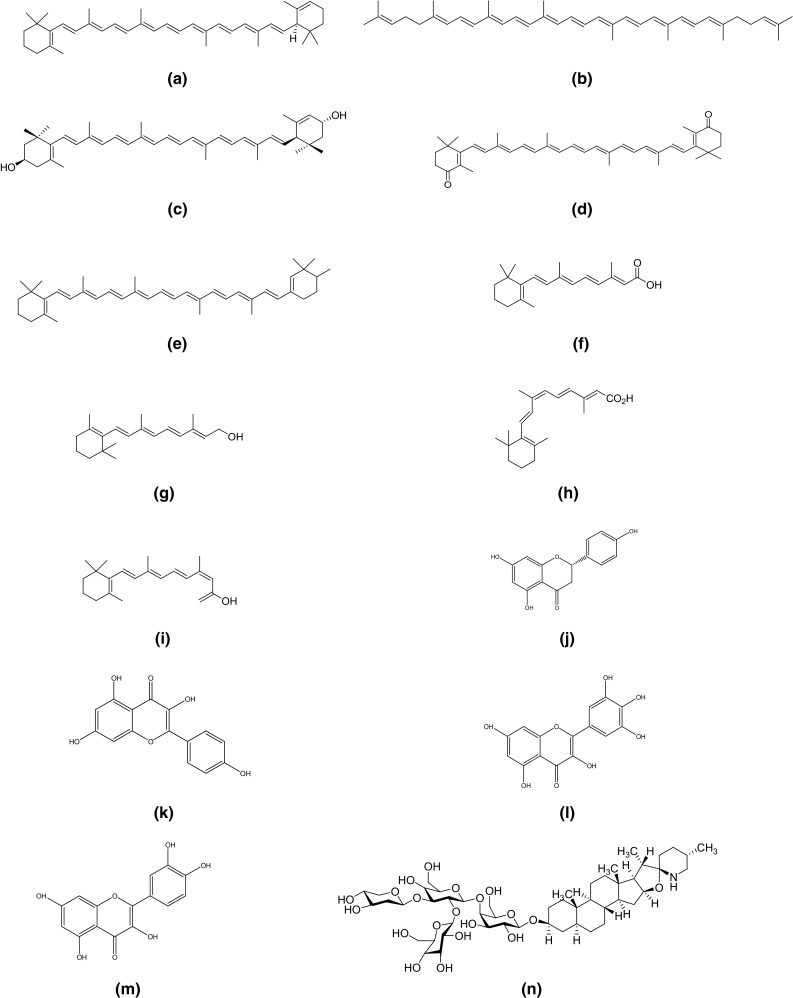
Chemical structures of some bioactive compounds in tomato; a α-carotene; b lycopene; c lutein; d canthaxanthin; e β-carotene; f all-trans-retinoic acid; g all-trans-retinol; h 9-cis-retinoic acid; i 13-cis-retinoic acid; j naringenin; k kampferol; l myricetin; m quercetin; n α-tomatine
Phenolic compounds
Phenolic compounds are the class of plant secondary metabolites that possess one or more hydroxyl groups attached to a benzene ring. Structurally, they vary from simple phenolics to complex polymers (polyphenols) on the basis of number and position of hydroxyl groups attached and structural elements that link phenolic rings (Singh et al. 2017b). Polyphenols are known to reduce the oxidative stress and thus counteract various health issues, including CVD and cancer (Singh et al. 2018). The phenolic compounds reported in tomato are phenolic acids (caffeic, chlorogenic, sinapic, p-coumaric and ferulic acids) and flavonoids (quercetin, rutin, kaempferol, and naringenin). Flavonoid accumulation occurs during maturation in tomatoes with a decrease in chlorophyll content and ripening of peels. Quercetin and chlorogenic acid are the most abundant flavonoids in tomato (Sharma et al. 2017). Tomas et al. (2017) reported contents of chlorogenic acid, rutin (quercetin-3-O-rutinoside), naringenin chalcone and naringenin as 17.9, 24.8, 2.45 and 0.12 mg/100 g DW, respectively in fresh tomato fruit. The chalconaringenin content decreases during post-harvest stage (15 mg/100 g at harvest decreased to 0.41 mg/100 g after 3 weeks of storage) of tomatoes.
Martí et al. (2016) summarized the literature by giving ranges of different polyphenols in ripened tomato fruits and enlisted naringenin chalcone as the major polyphenol with concentration range of 0.9–18.2 mg/100 g FW followed by rutin (0.5–4.5 mg/100 g FW), quercetin (0.7–4.4 mg/100 g FW), chlorogenic acid (1.4–3.3 mg/100 g FW), caffeic acid (0.1–1.3 mg/100 g FW) and naringenin (0–1.3 mg/100 g FW). Total phenolic content (TPC) in tomato varies with cultivar and is greatly influenced by variation in solar UV radiation (Sharma et al. 2017). Kaur et al. (2013) analyzed TPC in different commercial and wild cultivars of tomato. The wild cultivars found to have highest TPC (141.98 mg/100 g FW) and quercetin content (56 µg/g FW).
Carotenoids
Carotenoids are the major classes of bioactive compounds present in tomatoes. These plant pigments are produced by isoprenoid biosynthetic pathway with main roles as antioxidants and harvesting of light in plants (Singh et al. 2016). The main carotenoids present in tomatoes are lycopene, α-carotene, β-carotene, γ-carotene, δ-carotene, phytoene, phytofluene, neurosporene, and lutein. Martí et al. (2016) reviewed the literature and documented ranges of different carotenoids viz, lycopene (7.8–18.1 mg/100 g FW), α-carotene (0–0.002 mg/100 g FW), β-carotene (0.1–1.2 mg/100 g FW), γ-carotene (0.05–0.3 mg/100 g FW), δ-carotene (0–0.2 mg/100 g FW) and phytoene (1.0–2.9 mg/100 g FW) in tomatoes. Lycopene, phytoene, neurosporene, phytofluene, β-Carotene and lutein content in raw tomato is reported as 9.25, 1.86, 1.18, 0.80, 0.41 and 0.07 mg/100 g, respectively (Perveen et al. 2015). Lycopene is a major carotenoid responsible for characteristic red color and special antioxidant properties of tomatoes (Martí et al. 2016; Rafi et al. 2007). Tomatoes contain 8.8–42.0 µg/g of lycopene and provide nearly about 85% of total lycopene in the human diet (Rao and Rao 2007).
Vitamins
Tomato fruit is considered as the rich source of Vit C and also contains vitamin A, B and E. Studies have revealed that Vit C content at first increases with the maturation of fruit and then decreases as the fruit ripens (Watada et al. 1976). Organic farming increases the level of Vit C and this increase depends on the cultivar and site of cultivation (Martí et al. 2018). Vitamin A is present in the form of carotenoids. Vitamin B is present as thiamine, niacin, vitamin B6, and folates. Vitamin E is present in lesser quantities than other vitamins and is present as alpha and beta tocopherols. The vitamin content in tomato gets affected by the type of cultivar, time of harvest and ethylene supplementation (Watada et al. 1976).
Glycoalkaloids
Glycoalkaloids are a group of alkaloids in which sugar is attached. These compounds provide protection against the pathogenic attack as they are toxic (Friedman et al. 2009). The glycoalkaloids in tomato are present in the form of tomatine. Tomatine is a mixture of two glycoalkaloids; α-tomatine and dehydroxytomatine which are known to be present in both tomato leaves as well as fruits (Friedman 2013). In green fruit, the content of alpha-tomatine is high (500 mg/kg) as compared to ripe red fruit (5 mg/kg) (Friedman 2013). These compounds are known to provide protection against human pathogens like bacteria, viruses, and fungi. The high content of tomatine in green fruit makes it taste bitter and undesirable to eat. The content of glycoalkaloids is also affected by cultivar type and time of harvest.
Bioactivities
Tomato contains phytochemicals with anti-oxidative, anti-proliferative, anti-carcinogenic, anti-tumorigenic, anti-inflammatory, anti-mutagenic and anti-atherogenic properties. Tables 2 and 3 summarizes studies showing the bioactive potential of tomato and Fig. 2 demonstrates the bioactivities of tomato reported in various studies. Reactive oxygen species (ROS) induced oxidative stress is the chief cause of cancer and CVD. The ROS cause oxidative damage to crucial cellular biomolecules such as proteins, lipids, and nucleic acid. Antioxidative defense system provides protective effects against ROS. Antioxidants such as catalases, glutathione peroxidases, and superoxide dismutases are present within human cells whereas polyphenols, carotenoids, vitamin C and vitamin E can be obtained from food (Agarwal and Rao 2000). Lycopene is a potent natural antioxidant (with singlet-oxygen quenching potential higher than β-carotene and α-tocopherol) available in tomato with notable anti-cancerous and anti-atherogenic properties. Dietary lycopene enhances the level of lycopene in the body and elevates the overall antioxidant potential by trapping ROS, therefore reducing the oxidative damage to biomolecules (Agarwal and Rao 2000).
Table 2.
Summary of literature on effect of tomato products/active constituents on different bioactivities in vitro
| S. no. | Name of bioactive compound (concentration) | Cell lines used | Effect on biomarkers | Bioactivity | References |
|---|---|---|---|---|---|
| 1. | Lycopene (0–6 µM) |
HL-60 leukemia cells | Dose dependent reduction in cell growth Cell cycle arrest at G0/G1 phase Synergistic antiproliferative effects with 1, 25-dihydroxyvitamin D3 (natural anticancer compound) |
Anti-proliferative | Amir et al. (1999) |
| 2. | Lycopene (0.75, 1.5 and 3.0 µM) |
MCF-7 | Inhibition of cell growth by interfering in cell cycle progression and in IGF-I receptor signaling | Anti-proliferative | Karas et al. (2000) |
| 3. | Lycopene (2–3 µM) |
MCF-7 ECC-1 |
Cell cycle arrest at G1 phase ↓ phosphorylation of retinoblastoma and related pocket proteins ↓ cdk 4 and cdk 2 activities, cyclin D1 and D3 levels and p21Cip1/waf1 abundance |
Anti-carcinogenic | Nahum et al. (2001) |
| 4. | Lycopene (10−9–10−5 M) |
LNCaP | Inhibition of growth in dose dependent manner | Anti-oxidant Anti-proliferative |
Kim et al. (2002) |
| 5. | Synthetic all-E-lycopene (0–5 µmol/l) |
PrEC | Inhibition of growth of nonneoplastic PrEC in dose dependent manner | Anti-proliferative | Obermüller-Jevic et al. (2003) |
| 6. | Lycopene (0.1, 1 and 5 µM) |
LNCaP | ↓ LNCaP viability through cell cycle arrest at G2/M phase and apoptosis | Anti-tumorigenic/anti-proliferative | Hwang and Bowen (2004) |
| 7. | Lycopene (0.1–50 µM) |
SK-Hep 1 | ↓ gelatinolytic activities of MMP-9 and MMP-2 Inhibited cell growth and cell adhesion to matrigel-coated substrate in a dose-dependent manner Reduction in invasion and migration of SK-Hep1 cells |
Anti-metastatic | Hwang and Lee (2006) |
| 8. | Lycopene (0.1–50 µM) |
Hep3B | Inhibition of cell growth in concentration dependent manner Cell arrest at G0/G1 and S phase |
Antitumorigenic | Park et al. (2005) |
| 9. | Lycopene (0.125–100 µM) |
MCF-7 | Lycopene induced GJIC functionality in dose-dependent manner and increased expression of connexin 43 | Anti-proliferative | Fornelli et al. (2007) |
| 10. | Lycopene (1–10 µM) |
SK-Hep-1 | Inhibition of MMP-9 levels Inhibition of binding abilities of Sp1 and NF-кB ↓ expression of IGF-1R and ROS |
Anti-invasion | Huang et al. (2007) |
| 11. | Lycopene (0.01–10 µM) (0–100 µM) (80–800 nM) |
LNCaP PC-3 |
Mitotic arrest ↓ cyclin dependent kinase 4, cyclin D and E Suppressed retinoblastoma phosphorylation ↓ expression and activation of IGF-I receptor, ↓ AKT activation and ↑ IGFBP2 expression ↑ apoptotic response in LNCaP cells |
Anti-proliferative | Ivanov et al. (2007) |
| 12. | Lycopene (20, 40 and 60 µM) |
PC-3 | ↓ cell proliferation and IGF-IR expression ↑ IGFBP-3 levels Induced apoptosis |
Anti-proliferative | Kanagaraj et al. (2007) |
| 13. | Lycopene (1.0–4.0 µM) |
EHEB K562 HuCC Raji cell line |
↓ proliferation capacity of K562, HuCC and lymphoma Raji cell lines in concentration dependent manner whereas in case of EHEB, this worked only at higher concentrations ↑ apoptotic rate in HuCC cell line |
Anti-proliferative | Salman et al. (2007) |
| 14. | Lycopene (0.0001–10 µM) |
Hep-G2 IMR-90 DU-145 A549 A431 HS-68 HS-578T |
Reduction in cell proliferation in Hep-G2 and IMR-90 | Anti-proliferative | Burgess et al. (2008) |
| 15. | Lycopene (0–10 µM) |
TNF-α induced HUVECs THP-1 monocytes |
Inhibited TNF-α induced ICAM-1 expression in HUVECs Inhibited TNF-α induced NK-кB expression, IкB phosphorylation, NF-кB p65 translocation and monocyte-endothelial interaction |
Anti-inflammatory | Hung et al. (2008) |
| 16. | Lycopene (0 and 10 µM) |
HT-29 | Inhibited cell proliferation in HT-29 Suppressed protein levels of non-phosphorylated β-catenin and Akt activation in HT-29 Reduced expression of cyclin D1 and phosphorylation levels of Rb and Akt proteins ↑ p27kip abundance (an inhibitor of nuclear cyclin-dependent kinase) |
Anti-tumorigenic Anti-proliferative |
Tang et al. (2008) |
| 17. | Green tomato extract containing tomatine (mg/100 g) |
HT-29 AGS MCF 7 HepG2 |
Inhibition of all cell lines including normal human liver cell line (Chang) | Anti-carcinogenic | Friedman et al. (2009) |
| 18. | Lycopene (0.1–20 µM) |
MDA-MB-231 H-Ras MCF 10 A |
Reduction in phosphorylated active forms of Akt and ERK1/2 in H-Ras MCF 10 A cells Inhibition of invasion, migration and proliferation |
Anti-proliferative Anti-invasive Anti-metastatic |
Koh et al. (2010) |
| 19. | Lycopene (2.5–10 µM) |
LNCaP HCT-116 HT-29 PC3 BEN |
Cell cycle arrest and apoptosis Reduction in total cholesterol by decreasing HMG-CoA reductase expression Reduction in Ras-dependent activation of NF-кB ↓ ROS, cyclin D1 and pAKT ↑ p27, p21 and p53 |
Anti-tumorigenic/anti-carcinogenic | Palozza et al. 2010a, b |
| 20. | Lycopene (0.31–10 µM) |
Canine osteosarcoma cell lines OS 2.4 D17 HMPOS |
Inhibition of cell proliferation Apoptosis in HMPOS cells primarily through truncated Bid expression ↓ AKT phosphorylation |
Anti-proliferative | Wakshlag and Balkman (2010) |
| 21. | Lycopene (1–5 µM) |
HT-29 MCF-7 T84 Hep G2 Hela DU-145 Hep-2 A 549 |
↓ no. of viable cells in HT-29, MCF-7 and T84 cancer cell lines Promoted cell cycle arrest ↑ apoptosis in HT-29, DU 145, MCF-7 and T84 |
Anti-proliferative | Teodoro et al. (2012) |
| 22. | Lycopene (0–10 µM) |
MCF-7 MCF-10 |
Inhibitory effect only in MCF-7 cells | Anti-proliferative | Uppala et al. (2013) |
| 23. | Lycopene (2.5–20 µM) |
Androgen independent DU-145 PC-3 |
↑ protein and mRNA expression of LXRα, PPARγ, ABCA1 and apo A1 protein ↓ cellular total cholesterol levels A combination of lycopene with LXRα agonist T0901317 exhibited synergistic antiproliferative effects in DU 145 cells |
Anti-proliferative | Yang et al. (2012) |
| 24. | Lycopene (0–100 µM) |
PC-3 | Biphasic effect on PC-3 cell proliferation with a modest ↑ at low conc. and ↓ at higher conc. ↑ cell death by necrosis and apoptosis in prostate cells A combination of lycopene (25 µM) with temezolomide ↓ PC-3 cell proliferation in dose-dependent manner Lycopene, in combination with PPARγ potentiated the anti-proliferative responses |
Anti-proliferative | Rafi et al. (2013) |
| 25. | Lycopene α-tocopherol (0–50 µM) |
Androgen insensitive PC-3 DU-145 |
Synergistic inhibition of prostate carcinoma cell proliferation | Anti-proliferative | Pastori et al. (1998) |
| 26. | All trans lycopene All trans β-carotene (0.5–10 µM) |
MCF-7 MDA-MB-235 MDA-MB-231 |
Both the carotenoids inhibited cell proliferation, ↑ apoptosis and arrest cell cycle in different phases | Anti-proliferative | Gloria et al. (2014) |
| 27. | Lycopene (2–3 µM) atRA (1 µM) |
MCF-7 ECC-1 MCF-7.7D1.13 |
Inhibition of mitogenic activity of IGF-I on MCF-7 and ECC-1 cancer cells through reduction of cyclin D1 and p21CIP1/WAF1 levels ↓ p130 and pRb phosphorylation |
Anti-proliferative | Nahum et al. (2006) |
| 28. | Lycopene β-carotene (3–7 µmol/l) |
KB-1 | In comparison between the two carotenoids, lycopene was found to be an effective inhibitor of KB-1 cell growth in dose dependent manner Lycopene also caused ↑ in gap-junctional communication and in connexin 43 expression |
Anti-carcinogenic | Livny et al. (2002) |
| 29. | Lycopene (1–20 µmol/l) β-carotene (10 µmol/l) |
SK-Hep-1 | Lycopene: ↑ nm23-H1 expression at both mRNA and protein levels Inhibited migration and invasion of SK-Hep-1 cells |
Anti-migration and Anti-invasive | Huang et al. (2005) |
| 30. | Lycopene β-carotene (0.5–40 µM) |
AtT-20 | Lycopene and β-carotene: ↓ cell viability and clonogenic ability of AtT-20 cells Induced apoptosis Modulated cell cycle ↑ expression of phosphorylated connexin 43 Blocked intercellular communication ↓ p27kip1, Skp2 protein levels and ACTH secretion |
Anti-proliferative | Haddad et al. (2013) |
| 31. | Lycopene α-carotene β-carotene (0–8 µM) |
Ishikawa NCI-H226 MCF-7 |
Lycopene inhibited the growth of all the cell lines and also found to suppress IGF-1 | Anti-proliferative | Levy et al. (1995) |
| 32. | Tomato ketchup extract Lycopene, ascorbic acid and α-tocopherol (7.5 µM, 55 µM 1.4 µM resp.) |
HUVEC | Tomato ketchup extracts ↓ release of pro-inflammatory cytokines IL-8 and TNF-α whereas it ↑ release of IL-10 an anti-inflammatory cytokine | Anti-inflammatory | Hazewindus et al. (2014) |
| 33. | Carotenoids: Lycopene β-Carotene Canthaxanthin (1, 3, 7, 10 and 20 µmol/l) Retinoids: All-trans-retinol All-trans-retinoic acid 9-cis-and 13-cis-retinoic acid (10 nmol/l, 100 nmol/l and 1 µmol/l) |
MCF-7 MDA-MB-231 Hs578T |
β-carotene caused reduction in growth of Hs578T and MCF-7 cells Lycopene caused inhibition of growth of MDA-MB-231 and MCF-7 cells 9-cis-and all-trans-retinoic acid caused reduction in growth of both Hs578 T and MCF-7 cells All-trans-retinol and 13-cis-retinoic acid led to reduction effect only on MCF-7 cells |
Anti-proliferative | Prakash et al. (2001) |
| 34. | tlcy-b Tomato (20, 50 and 100 ml/l) |
HT-29 | Inhibited cell growth in dose-dependent manner, arrest cell cycle progression and induced apoptosis ↓ expression of Bcl-2, Bcl-xl and cyclin D1 |
Anti-tumorigenic | Palozza et al. (2008) |
| 35. | Tomato digestate (20–100 ml/l) |
HT-29 HCT-116 |
Inhibited growth of both HCT-116 and HT-29 cells in a conc. dependent manner by arresting cell cycle progression and inducing apoptosis ↓ exp. of Bcl-2, Bcl-xl and cyclin D1 |
Anti-tumorigenic | Palozza et al. (2007) |
HL-60, human leukemic cell line; MCF-7, human mammary cancer cell line; IGF-I, insulin-like growth factor-I; ECC-1, human endometrial cancer cell lines; cdk4, cdk2, cyclin-dependent kinases; LNCaP, human prostrate cancer cell lines; PrEC: normal human prostrate epithelial cell line; SK-Hep1, human hepatocellular carcinoma cell line; MMP-2, MMP-9, matrix metalloproteinases; Hep3B, human hepatoma cell line; GJIC, gap junction intercellular communication; SK-Hep-1, hepatocellular carcinoma cell line; Sp1, stimulatory protein-1; NF-κB, nuclear factor-kappa B; IGF-1R, insulin-like growth factor-1 receptor; PC3, prostrate cancer cell line; AKT, protein kinase B alpha; IGFBP, insulin-like growth factor binding protein; EHEB, B chronic lymphocytic leukemia cell line; K562, human erythroleukemia cell line; HuCC, human colon carcinoma cell line; Raji, a prototype of Burkitt lymphoma cell line; Hep-G2, Liver adenocarcinoma cell line; IMR-90, noncancerous lung cell line; DU-145, prostrate carcinoma cell line; A549, lung carcinoma cell line; A431, skin carcinoma cell line; HS-68, noncancerous skin cell line; HS-578T, breast carcinoma cell line; HUVECs, human umbilical endothelial cell line; TNF-α, tumour necrosis factor alpha; ICAM-1, intercellular adhesion molecule-1; HT-29, human colon cancer cell line; Rb, retinoblastoma tumor suppressor protein; AGS, human gastric cancer cell line; MDA-MB-231, MDA-MB-235 and Hs578T, human breast adenocarcinoma cell line; H-Ras MCF10A, H-Ras-transformed MCF-10A human breast epithelial cell line; ERKs, extracellular signal-regulated kinases; HCT-116, human colon carcinoma cell line; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; BEN, human lung carcinoma cell line; T-84, human colon carcinoma cell line; Hela, human cervical cancer cell line; Hep-2, human laryngeal carcinoma cell line; LXRα, liver X receptor α; PPARγ, peroxisome proliferator-activated receptor-γ; ABCA1, ATP-binding cassette transporter; atRA, all-trans retinoic acid; ECC-1, endometrial cancer cell line; KB-1, human oral tumor cell line; nm23-H1, metastasis suppressor gene; AtT-20, mouse corticotroph tumour cell line; Skp2, S-phase kinase-associated protein 2; ACTH, adrenocorticotropic hormone; Ishikawa, endometrial cancer cell line; NCI-H226, squamous lung cancer cell line; IL-8, interleukin; Bcl-2, B cell lymphoma-2; Bcl-xl, B-cell lymphoma 2 extra large
Table 3.
Summary of literature on protective action of tomato products/active constituents against various mutagens
| S. no. | Name of bioactive compound (concentration) | Cell lines used | Chemical agents/mutagens/to which cell lines were exposed (concentration/dose) | Effect on biomarkers | Bioactivity | References |
|---|---|---|---|---|---|---|
| 1. | Lycopene (0.5–2.0 µM) |
RAT-1 immortalized fibroblasts | Cigarette smoke condensate (TAR) 25 µg/ml |
Inhibited cell growth in concentration and time-dependent manner by promoting apoptosis and arresting cell cycle progression in cells exposed to TAR ↓ cyclin D1 and phosphorylation of Bad and AKT Inhibited expression of hsp 90 and COX-2 |
Anti-carcinogenic | Palozza et al. (2005) |
| 2. | Lycopene (2.5–10 µM) |
RAW 264.7 | LPS: 0.5 µg/ml | ↓ NO production and inhibited iNOS expressions | Anti-inflammatory | Rafi et al. (2007) |
| 3. | Lycopene (10, 25 and 50 µM) |
CHO K-1 | H2O2: 0.6 mM MMS: 80 µg/ml 4-NQO: 0.01 µM |
↓ no. of aberrant cells and DNA lesions | Anti-genotoxicity/anti-mutagenicity | Scolastici et al. (2007) |
| 4. | Lycopene (1.86, 9.31 and 18.62 µM) |
Rat hepatocytes | γ-radiations 1, 2 and 4 Gy |
↓ TBARs and DNA damage | Radio-protective | Srinivasan et al. (2007) |
| 5. | Lycopene (10, 25 and 50 µM) |
HepG2 | H2O2: 0.1 mM DEN: 5 µg/ml |
↓ no. of aberrant cells and DNA lesions | Anti-genotoxicity/anti-mutagenicity | Scolastici et al. (2008) |
| 6. | Lycopene (0–25 µg/ml) |
Calu-3 Airway epithelial cells |
Rhinovirus-43 (MOI = 0.32) LPS: 1 µg/ml |
↓ release of IP-10 and IL-6 in cells treated with RV infection and also reduced Rhinovirus-1B replication ↓ IL-6 release in cells exposed to LPS |
Anti-inflammatory | Saedisomeolia et al. (2009) |
| 7. | Lycopene (1, 5 and 10 µM) |
RAW 264.7 | LPS: 1 µg/ml | Inhibition of IL6 and NO; IкB phosphorylation, NF-кB translocation and IкB degradation Blocking phosphorylation of p38 MAP kinase and ERF1/2 |
Anti-inflammatory | Feng et al. (2010) |
| 8. | Lycopene (0.01–20 µM) | HUVECs | LPS: 100 ng/ml | Suppressed NF-кB, TNF-α, TLR-4 and CD14 Inhibited leukocyte adhesion, vascular barrier permeability, transendothelial migration and expression of cell adhesion molecules |
Anti-inflammatory | Bae and Bae (2011) |
| 9. | Lycopene (0.5–2 µM) |
THP-1 macrophages | 25-OHC: 2–4 µM 7-KC: 4–16 µM |
↓ pro-inflammatory cytokines secretions Inhibited ROS production, NF-кB activation and protein kinase phosphorylation |
Anti-atherogenic | Palozza et al. (2011) |
| 10. | Lycopene (0.5–2 µM) |
THP-1 macrophages | CSE (cigarette smoke extract) (0.5%) |
Suppressed NF-кB/p65 nuclear translocation, NF-кB DNA binding and phosphorylation of IкBα and IKKα ↓ NOX-4 expression and ROS production Inhibited phosphorylation of JNK, p38 MAPK, ERK1/2 and IL-8 expression ↑ PPARγ levels |
Anti-inflammatory | Simone et al. (2011) |
| 11. | Lycopene (0–20 µM) |
HUVECs THP-1 Human monocyte cell line |
LPS: 100 ng/ml | Inhibition of LPS-mediated HMGB1 release by HUVECs, HMGB1-mediated TNF-α expression, expression of sPLA2-IIA and pro-inflammatory signaling mediated by HMGB1 -↓ expression of CAMs, TLR-2 and -4 and HMGB1 receptors |
Anti-inflammatory | Lee et al. (2012) |
| 12. | Lycopene (3–20 µM) |
BV-2 microglia Rat primary cultured microglia Mouse primary cultured microglia |
LPS: 100 ng/ml | Inhibition of LPS induced COX-2 expression, inflammation mediators, NF-кB and AP-1 DNA binding activity ↑ phosphorylation of LKB1, AMPK-α1 and CaMKII |
Anti-neuroinflammatory | Lin et al. (2014) |
| 13. | β-carotene (10–50 µM) |
RAW 264.7 Peritoneal macrophages |
LPS: 1 µg/ml | Inhibition of iNOS expression, PGE2, IL-1β, TNF-α, COX-2, nuclear translocation of NF-кB 65, IкBα phosphorylation and degradation Blocked intracellular accumulation of ROS Suppressed NF-кB activation |
Anti-inflammatory Antioxidant |
Bai et al. (2005) |
| 14. | Lycopene β-carotene (10 and 20 µM) |
Hs68 | AMVN: 50 mM Fe/NTA: 1 mM AAPH: 50 mM |
Both lycopene and β-carotene ↑ TBARS induced by AMVN whereas only lycopene at 20 µM ↓ levels of TBARS induced by Fe/NTA significantly | Antioxidative/pro-oxidative | Yeh and Hu (2000) |
| 15. | Lycopene (0.5–2 µM) β-carotene (5Z)-Lycopene |
THP-1 macrophages | 7-KC: 10–25 µM | Lycopene caused: ↓ 8-OHdG formation and ROS production in dose dependent manner Prevention in ↑ expressions of hsp70, hsp90, NOX-4, p53 and p21, phosphorylation of JNK, ERK1/2 and p38 Prevented arrest at G0/G1 cell cycle phase Among these 3 carotenoids, lycopene and its isomer (5Z-Lycopene) were found to be more effective than β-carotene in ↓ ROS production, cell growth and preventing apoptotic induction |
Anti-atherogenic | Palozza et al. 2010a, b |
| 16. | Lycopene (30 and 300 µg/plate) and (14, 28 and 56 µg/plate) Tomato puree: 100, 200 and 400 µl/plate |
Histidine deficient TA-98 and TA-100 strains of Salmonella typhimurium | AFB1: 0.1, 1 and 10 µg/plate for both TA98 and TA 100 IQ: 0.1, 1 and 10 µg/plate; TA 100 IQ: 0.1, 0.01 and 0.001 µg/plate; TA 98 MNU: 10, 100 and 1000 µg/plate; TA 100 |
Both lycopene as well as tomato puree showed antimutagenic effects in a dose-dependent manner by reducing the no. of micronuclei in cells | Anti-mutagenic | Polívková et al. (2010) |
hsp, heat shock protein; COX-2, cyclooxygenases-2; RAW 264.7, mouse macrophage cell line; LPS, lipopolysaccharide; NO, nitric oxide; iNOS, inducible nitric oxide synthase; CHO K-1, Chinese hamster ovary cell line; MMS, methylmethanesulphonate; 4-NQO, 4-nitroquinoline-1-oxide; TBARS, thiobarbituric acid reactive substances; DEN, n-nitrosodiethylamine; MOI, multiplicity of infection; IP-10, interferon-gamma induced protein-10; MAP kinase, mitogen activated protein kinase; ERF1/2, eukaryotic release factor 1; TLRs, toll like receptors; CD14, cluster of differentiation 14; 25-OHC, 25 hydroxycholestrol; 7-KC, 7-ketocholestrol; JNK, c-Jun N-terminal kinase; IKK, IκB kinase; HMGB1, high mobility group box 1; sPLA2-IIA, secretory phospholipase A2; CAMs, cell adhesion molecules; AP1, activator protein; LKB1, liver kinase B1; AMPK-α1, adenosine monophosphate-activated protein kinase-α1; CaMKII, calmodulin-dependent protein kinase II; PGE2, prostaglandin E2; AMVN, (2,2′-azobis [2,4-dimethylvaleronitrile]; Fe/NTA, ferric nitrilotriacetate; 8-OH-dG, 8-hydroxy-2′-deoxyguanosine; AAPH, (2,2′-azobis [2-amidinopropane]dihydrochloride); AFB1, aflatoxin B1; IQ, 2-amino-3-methylimidazo[4,5-f]quinoline; MNU, N-nitroso- N-methylurea
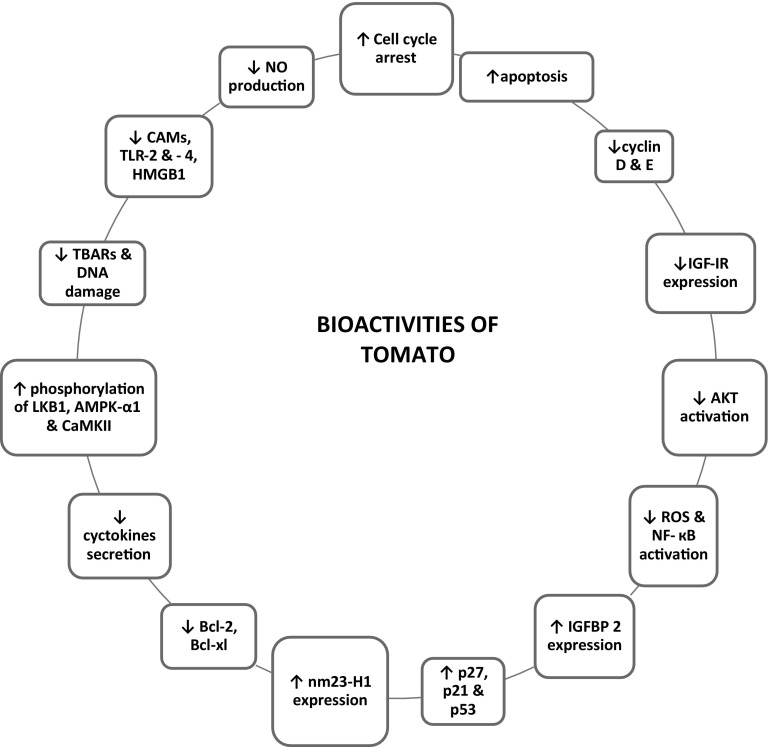
Fig. 2.
Potential bioactivities of tomato demonstrated in different studies
Numerous in vitro studies have shown protective effects of lycopene obtained from tomato against different types of cancer including mammary gland (Levy et al. 1995, Karas et al. 2000, Gloria et al. 2014), lung (Levy et al. 1995), colon (Salman et al. 2007, Tang et al. 2008), endometrial (Levy et al. 1995), leukemia (Amir et al. 1999), liver (Hwang and Lee 2006) and prostate (Palozza et al. 2010a, b; Pastori et al. 1998). Lycopene and other bioactive compounds cause cell cycle arrest and inhibit the growth of many cancerous cell lines in a dose-dependent manner (Table 2). Lycopene supplementation (2.5–10 µM) also causes a reduction in total cholesterol by decreasing HMG-CoA reductase expression (Palozza et al. 2010a, b). Studies reported dose-dependent anti-proliferative and inhibitory effects of lycopene on various cancer cell lines viz. K562, HuCC, Lymphoma and Mammary cancer cell lines (Salman et al. 2007; Uppala et al. 2013; Teodoro et al. 2012). Antiproliferative effect was also observed by the action of all-trans β-carotene (0.5–10 µM) on mammary cancer cell lines i.e., MCF-7, MDA-MB-235 and MDA-MB-231 (Gloria et al. 2014). The antioxidant potential of lycopene is associated with the ability to scavenge reactive oxygen species and modulation of phase I and II enzymes thus showing protective effects against various types of cancer. Tomatine present in green tomato extract is known to inhibit the growth of human breast, colon, liver, and stomach cancer cell lines. But, it was observed that tomatine can also inhibit normal human liver cell line (Friedman et al. 2009). Livny et al. (2002) compared anti-carcinogenicity of two carotenoids; lycopene and β-carotene at same concentrations (3–7 µmol/l), and reported that lycopene was a more effective inhibitor of KB-1 cell growth by increasing connexion-43 expression.
Human body encounters the attack of various toxins in the course of life. The exposure to these mutagens/xenobiotics is being increased as the civilization is progressing. Implications of exposure to these toxic compounds are evident in many ways like chromosomal aberrations, increase in ROS and induction of tumor. Some recent literature on protective effects of tomato against the action of various mutagens is summarized in Table 3. Treatment of different cell lines with various chemical mutagens like LPS (lipopolysaccharide), H2O2 (hydrogen peroxide), MMS (methyl methanesulphonate), 4-NQO (4-nitroquinoline-1-oxide), DEN (n-nitrosodiethylamine), AMVN (2,2′-azobis [2,4-dimethylvaleronitrile]) etc. triggered inflammatory responses. However, the addition of bioactive compounds from tomato along with these mutagens exhibited protective actions against them (Feng et al. 2010; Palozza et al. 2011; Lin et al. 2014). Srinivasan et al. (2007) treated rat hepatocytes with γ-radiations along with lycopene at three different concentrations (1.86, 9.31 and 18.62 µM) and reported lycopene as a radio-protectant as it reduces γ-radiation-induced DNA damage.
Health benefits
Carotenoids (lycopene) and vitamins (ascorbic acid and α-tocopherol) of tomato have a role in reducing oxidative stress and minimizing the risk of cancer and CVD (Tables 2, 3). Lycopene content in blood is known to be inversely proportional to the incidence of heart diseases (Sesso et al. 2004). The consumption of tomatoes is inversely correlated with the risk of inflammatory disorders such as atherosclerosis (Hazewindus et al. 2014). Oxidative modulation of low-density lipoprotein (LDL) plays a major role in protection against atherosclerosis and CVD. Modulation of atherogenic processes in endothelial cells by the action of lipophilic compounds of tomato on LDLs prevents CVD (Viuda-Martos et al. 2014). Studies have shown the protective effects of tomato products against various cancers, including prostate and lung cancer (Hwang and Bowen 2004; Palozza et al. 2010a, b; Tan et al. 2010). Polyphenols and carotenoids of tomato are known to obstruct tumor formation by interfering with initiation, promotion or progression of cancer (Martí et al. 2016). Quercetin helps in the remodeling of chromatin, thus inhibits epigenetic alterations during cancer progression (Martí et al. 2016).
Tomato is rich in carotenoids and high carotenoid intake in the human diet is known to be associated with low risk of chronic diseases. Carotenoids modulate the immune response, stimulate intercellular signaling (gap junction) pathways, possess pro-vitamin A activity, regulate cell cycle and apoptosis, and modulate many physiological processes, thus provide resistance to various diseases (Rao and Rao 2007). α and β-carotene and β-cryptoxanthin act as precursors to vitamin A and decrease in the content of these carotenoids in the blood lead to vitamin A deficiency (Fernández-García et al. 2012). Availability, absorption, breakdown, and storage of carotenoids are influenced by a number of factors. Mainly type, amount and association of carotenoids with other compounds influence their bioavailability in the human body. Lycopene in tomato occurs in microcrystalline form making it difficult for the absorption as compared to other carotenoids. Studies have revealed that heating food items leads to disruption of the cell wall and thus making the easy release of lycopene (Fernández-García et al. 2012). Also, the factors like gender, human health and age influence the carotenoids absorption. Alternation of fat absorption and presence of some drugs like aspirin in human body directly influences the carotenoid absorption. Carotenoids like β-Carotene and Lutein also interact and compete with each other during absorption. Considering health benefits of tomato, various breeding strategies to increase the level of beneficial phytochemicals in tomato have been carried out throughout the world (Saavedra et al. 2017). Improving the content of bioactive compounds would have commercial benefits in the production of drug supplements from tomato. This review suggested that tomatoes are carriers of compounds beneficial in managing and preventing many important health problems.
Conclusion and future perspective
The complex matrix of compounds present in tomato pose many health benefits for the human race, it is difficult to mention one particular constituent. It is the complex formulation in nature which provides all these benefits. Various studies have established the opposing link between intake of tomatoes/tomato products against the incidence of diseases. There is a need to understand the possible mechanism of action against various diseases. Whole fruit has more protective effects as compared to the derived compounds. Bioavailability of lycopene increases after cooking of the tomatoes. There is need to understand various pathways of action of bioactive compounds of tomato and their role in preventing invasion and metastasis of cancer.
Acknowledgements
We are grateful to Council of Scientific and Industrial Research, New Delhi (F.No 38 (1399)/14/EMR-II) and University Grants Commission, New Delhi for providing the financial assistance under MRP, UPE, CPEPA and DRS schemes.
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Balwinder Singh, Email: bbs171@rediffmail.com.
Avinash Kaur Nagpal, Email: avnagpal@yahoo.co.in.
References
- Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. Can Med Assoc J. 2000;163(6):739–744. [PMC free article] [PubMed] [Google Scholar]
- Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, Sharoni Y. Lycopene and 1, 25-dihydroxyvitamin d3 cooperate in the inhibition of cell cycle progression and induction of differentiation in hl-60 leukemic cells. Nutr Cancer. 1999;33(1):105–112. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- Bae JW, Bae JS. Barrier protective effects of lycopene in human endothelial cells. Inflamm Res. 2011;60(8):751–758. doi: 10.1007/s00011-011-0330-9. [DOI] [PubMed] [Google Scholar]
- Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kim YM. [beta]-carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-[kappa] B activation. Exp Mol Med. 2005;37(4):323. doi: 10.1038/emm.2005.42. [DOI] [PubMed] [Google Scholar]
- Burgess LC, Rice E, Fischer T, Seekins JR, Burgess TP, Sticka SJ, Klatt K. Lycopene has limited effect on cell proliferation in only two of seven human cell lines (both cancerous and noncancerous) in an in vitro system with doses across the physiological range. Toxicol In Vitro. 2008;22(5):1297–1300. doi: 10.1016/j.tiv.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel R, Singh N, Sharma S, Kaur A. Beneficial phytochemicals in potato—a review. Food Res Int. 2013;50(2):487–496. doi: 10.1016/j.foodres.2011.04.025. [DOI] [Google Scholar]
- FAOSTAT (2014) Global tomato production. http://faostat.fao.org/. Accessed on June–July
- Feng D, Ling WH, Duan RD. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-κB in macrophages. Inflamm Res. 2010;59(2):115–121. doi: 10.1007/s00011-009-0077-8. [DOI] [PubMed] [Google Scholar]
- Fernández-garcía E, Carvajal-lérida I, Jarén-galán M, Garrido-fernández J, Pérez-gálvez A, Hornero-méndez D. Carotenoids bioavailability from foods: from plant pigments to efficient biological activities. Food Res Int. 2012;46(2):438–450. doi: 10.1016/j.foodres.2011.06.007. [DOI] [Google Scholar]
- Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of human breast cell line (MCF-7) Toxicol In Vitro. 2007;21(2):217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Friedman M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α -tomatine, and tomatidine in pure form and in fresh and processed tomatoes. Agric Food Chem. 2013;61(40):9534–9550. doi: 10.1021/jf402654e. [DOI] [PubMed] [Google Scholar]
- Friedman M, Levin CE, Lee SU, Kim HJ, Lee IS, Byun JO, Kozukue N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J Agric Food Chem. 2009;57(13):5727–5733. doi: 10.1021/jf900364j. [DOI] [PubMed] [Google Scholar]
- Gloria NF, Soares N, Brand C, Oliveira FL, Borojevic R, Teodoro AJ. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014;34(3):1377–1386. [PubMed] [Google Scholar]
- Haddad NF, Teodoro AJ, de Oliveira FL, Soares N, de Mattos RM, Hecht F, de Carvalho DP. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS ONE. 2013;8(5):e62773. doi: 10.1371/journal.pone.0062773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazewindus M, Haenen GR, Weseler AR, Bast A. Protection against chemotaxis in the anti-inflammatory effect of bioactives from tomato ketchup. PLoS ONE. 2014;9(12):e114387. doi: 10.1371/journal.pone.0114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Shih MK, Chuang CH, Hu ML. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J Nutr. 2005;135(9):2119–2123. doi: 10.1093/jn/135.9.2119. [DOI] [PubMed] [Google Scholar]
- Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem. 2007;18(7):449–456. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hung CF, Huang TF, Chen BH, Shieh JM, Wu PH, Wu WB. Lycopene inhibits TNF-α-induced endothelial ICAM-1 expression and monocyte-endothelial adhesion. Eur J Pharmacol. 2008;586(1–3):275–282. doi: 10.1016/j.ejphar.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. J Med Food. 2004;7(3):284–289. doi: 10.1089/jmf.2004.7.284. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Lee HJ. Inhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cells. Exp Biol Med. 2006;231(3):322–327. doi: 10.1177/153537020623100313. [DOI] [PubMed] [Google Scholar]
- Ivanov NI, Cowell SP, Brown P, Rennie PS, Guns ES, Cox ME. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and-independent prostate cancer cell lines. Clin Nutr. 2007;26(2):252–263. doi: 10.1016/j.clnu.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kanagaraj P, Vijayababu MR, Ravisankar B, Anbalagan J, Aruldhas MM, Arunakaran J. Effect of lycopene on insulin-like growth factor-I, IGF binding protein-3 and IGF type-I receptor in prostate cancer cells. J Cancer Res Clin Oncol. 2007;133(6):351–359. doi: 10.1007/s00432-006-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, Sharoni Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36(1):101–111. doi: 10.1207/S15327914NC3601_14. [DOI] [PubMed] [Google Scholar]
- Kaur C, Walia S, Nagal S, Walia S, Singh J, Singh BB, Jaggi S. Functional quality and antioxidant composition of selected tomato (Solanum lycopersicon L.) cultivars grown in Northern India. LWT Food Sci Technol. 2013;50(1):139–145. doi: 10.1016/j.lwt.2012.06.013. [DOI] [Google Scholar]
- Kim L, Rao AV, Rao LG. Effect of lycopene on prostate LNCaP cancer cells in culture. J Med Food. 2002;5(4):181–187. doi: 10.1089/109662002763003320. [DOI] [PubMed] [Google Scholar]
- Koh MS, Hwang JS, Moon AR. Lycopene inhibits proliferation, invasion and migration of human breast cancer cells. Biomol Ther. 2010;18(1):92–98. doi: 10.4062/biomolther.2010.18.1.092. [DOI] [Google Scholar]
- Lee W, Ku SK, Bae JW, Bae JS. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem Toxicol. 2012;50(6):1826–1833. doi: 10.1016/j.fct.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either α-carotene or β-carotene. Nutr Cancer. 1995;24:257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- Lin HY, Huang BR, Yeh WL, Lee CH, Huang SS, Lai CH, Lu DY. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol Aging. 2014;35(1):191–202. doi: 10.1016/j.neurobiolaging.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr. 2002;132(12):3754–3759. doi: 10.1093/jn/132.12.3754. [DOI] [PubMed] [Google Scholar]
- Martí R, Roselló S, Cebolla-cornejo J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers. 2016;8(6):58. doi: 10.3390/cancers8060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí R, Leiva-Brondo M, Lahoz I, Campillo C, Cebolla-Cornejo J, Roselló S. Polyphenol and l-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem. 2018;239:148–156. doi: 10.1016/j.foodchem.2017.06.102. [DOI] [PubMed] [Google Scholar]
- Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, Sharoni Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27Kip1 in the cyclin E-cdk2 complexes. Oncogene. 2001;20(26):3428. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- Nahum A, Zeller L, Danilenko M, Prall OW, Watts CK, Sutherland RL, Sharoni Y. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr. 2006;45(5):275. doi: 10.1007/s00394-006-0595-x. [DOI] [PubMed] [Google Scholar]
- Obermüller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, Van der Vliet A, Valacchi G, Packer L. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J Nutr. 2003;133(11):3356–3360. doi: 10.1093/jn/133.11.3356. [DOI] [PubMed] [Google Scholar]
- Palozza P, Sheriff A, Serini S, Boninsegna A, Maggiano N, Ranelletti FO, Cittadini A. Lycopene induces apoptosis in immortalized fibroblasts exposed to tobacco smoke condensate through arresting cell cycle and down-regulating cyclin D1, pAKT and pBad. Apoptosis. 2005;10(6):1445–1456. doi: 10.1007/s10495-005-1393-2. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Boninsegna A, Bellovino D, Lucarini M, Monastra G, Gaetani S. The growth-inhibitory effects of tomatoes digested in vitro in colon adenocarcinoma cells occur through down regulation of cyclin D1, Bcl-2 and Bcl-xL. Br J Nutr. 2007;98:789–795. doi: 10.1017/S0007114507746883. [DOI] [PubMed] [Google Scholar]
- Palozza P, Bellovino D, Simone R, Boninsegna A, Cellini F, Monastra G, Gaetani S. Effect of β-carotene-rich tomato lycopene β-cyclase (tlcy-b) on cell growth inhibition in HT-29 colon adenocarcinoma cells. Br J Nutr. 2008;102(2):207–214. doi: 10.1017/S0007114508169902. [DOI] [PubMed] [Google Scholar]
- Palozza P, Colangelo M, Simone R, Catalano A, Boninsegna A, Lanza P, Ranelletti FO. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31(10):1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- Palozza P, Simone R, Catalano A, Boninsegna A, Böhm V, Fröhlich K, Ranelletti FO. Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell cycle arrest and apoptosis in human macrophages. J Nutr Biochem. 2010;21(1):34–46. doi: 10.1016/j.jnutbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Palozza P, Simone R, Catalano A, Monego G, Barini A, Mele MC, Ranelletti FO. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. J Nutr Biochem. 2011;22:259–268. doi: 10.1016/j.jnutbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B human hepatoma cells. BioFactors. 2005;23(3):129–139. doi: 10.1002/biof.5520230302. [DOI] [PubMed] [Google Scholar]
- Pastori M, Pfander H, Boscoboinik D, Azzi A. Lycopene in association with α-tocopherol inhibits at physiological concentrations proliferation of prostate carcinoma cells. Biochem Biophys Res Commun. 1998;250(3):582–585. doi: 10.1006/bbrc.1998.9351. [DOI] [PubMed] [Google Scholar]
- Peralta IE, Spooner DM. History, origin and early cultivation of tomato (Solanaceae) In: Razdan MK, Mattoo AK, editors. Genetic improvement of solanaceous crops. United States: Tomato, Science Publishers, Enfield; 2007. pp. 1–27. [Google Scholar]
- Perveen R, Suleria HAR, Anjum FM, Butt MS, Pasha I, Ahmad S. Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—a comprehensive review. Crit Rev Food Sci Nutr. 2015;55(7):919–929. doi: 10.1080/10408398.2012.657809. [DOI] [PubMed] [Google Scholar]
- Polívková Z, Šmerák P, Demová H, Houška M. Antimutagenic effects of lycopene and tomato purée. J Med Food. 2010;13(6):1443–1450. doi: 10.1089/jmf.2009.0277. [DOI] [PubMed] [Google Scholar]
- Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J Nutr. 2001;131(5):1574–1580. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- Rafi MM, Yadav PN, Reyes M. Lycopene inhibits LPS-induced proinflammatory mediator inducible nitric oxide synthase in mouse macrophage cells. J Food Sci. 2007;72(1):S069–S074. doi: 10.1111/j.1750-3841.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Rafi MM, Kanakasabai S, Reyes MD, Bright JJ. Lycopene modulates growth and survival associated genes in prostate cancer. J Nutr Biochem. 2013;24(10):1724–1734. doi: 10.1016/j.jnutbio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Saavedra TM, Figueroa GA, Cauih JGD. Origin and evolution of tomato production Lycopersicon esculentum in México. Ciência Rural. 2017;47(3):1–8. doi: 10.1590/0103-8478cr20160526. [DOI] [Google Scholar]
- Saedisomeolia A, Wood LG, Garg ML, Gibson PG, Wark PA. Lycopene enrichment of cultured airway epithelial cells decreases the inflammation induced by rhinovirus infection and lipopolysaccharide. J Nutr Biochem. 2009;20(8):577–585. doi: 10.1016/j.jnutbio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomed Pharmacother. 2007;61(6):366–369. doi: 10.1016/j.biopha.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Scolastici C, de Lima RA, Barbisan LF, Ferreira ALDA, Ribeiro DA, Salvadori DMF. Lycopene activity against chemically induced DNA damage in Chinese hamster ovary cells. Toxicol In Vitro. 2007;21(5):840–845. doi: 10.1016/j.tiv.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Scolastici C, de Lima RA, Barbisan LF, Ferreira ALDA, Ribeiro DA, Salvadori DMF. Antigenotoxicity and antimutagenicity of lycopene in HepG2 cell line evaluated by the comet assay and micronucleus test. Toxicol In Vitro. 2008;22(2):510–514. doi: 10.1016/j.tiv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr. 2004;79(1):47–53. doi: 10.1093/ajcn/79.1.47. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in food: cancer prevention and apoptosis induction. Curr Med Chem. 2017 doi: 10.2174/0929867324666171006144208. [DOI] [PubMed] [Google Scholar]
- Simone RE, Russo M, Catalano A, Monego G, Froehlich K, Boehm V, Palozza P. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARγ signalling in cigarette smoke-stimulated macrophages. PLoS ONE. 2011;6(5):e19652. doi: 10.1371/journal.pone.0019652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits: a review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Singh N, Kaur A. Saponins in pulses and their health promoting activities: a review. Food Chem. 2017;233:540–549. doi: 10.1016/j.foodchem.2017.04.161. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Phenolic composition and antioxidant potential of grain legume seeds: a review. Food Res Int. 2017;101:1–16. doi: 10.1016/j.foodres.2017.09.026. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol. 2017;54(4):858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: a review. Food Chem. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Lycopene as a natural protector against γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta. 2007;1770(4):659–665. doi: 10.1016/j.bbagen.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Tan H, Thomas-ahner JM, Grainger EM, Wan L, Francis DM, Schwartz SJ, Clinton SK. Tomato-based food products for prostate cancer prevention: what have we learned? Cancer Metastasis Rev. 2010;29(3):553–568. doi: 10.1007/s10555-010-9246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FY, Shih CJ, Cheng LH, Ho HJ, Chen HJ. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol Nutr Food Res. 2008;52(6):646–654. doi: 10.1002/mnfr.200700272. [DOI] [PubMed] [Google Scholar]
- Teodoro AJ, Oliveira FL, Martins NB, de Azevedo Maia G, Martucci RB, Borojevic R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012;12(1):36–40. doi: 10.1186/1475-2867-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas M, Beekwilder J, Hall RD, Sagdic O, Boyacioglu D, Capanoglu E. Industrial processing versus home processing of tomato sauce: effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017;220:51–58. doi: 10.1016/j.foodchem.2016.09.201. [DOI] [PubMed] [Google Scholar]
- Uppala PT, Dissmore T, Lau BH, Andacht T, Rajaram S. Selective inhibition of cell proliferation by lycopene in MCF-7 breast cancer cells in vitro: a proteomic analysis. Phytother Res. 2013;27(4):595–601. doi: 10.1002/ptr.4764. [DOI] [PubMed] [Google Scholar]
- USDA (2016) United States Department of Agriculture, Agricultural Research Service, National Nutrient Database for Standard Reference Release 28. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/. Accessed 26 July 2017
- Viuda-Martos M, Sanchez-Zapata E, Sayas-Barberá E, Sendra E, Pérez-Álvarez JA, Fernández-López J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: a review. Crit Rev Food Sci Nutr. 2014;54(8):1032–1049. doi: 10.1080/10408398.2011.623799. [DOI] [PubMed] [Google Scholar]
- Wakshlag JJ, Balkman CE. Effects of lycopene on proliferation and death of canine osteosarcoma cells. Am J Vet Res. 2010;71(11):1362–1370. doi: 10.2460/ajvr.71.11.1362. [DOI] [PubMed] [Google Scholar]
- Watada AE, Aljlenbach BB, Worthington JT. Vitamins A and C in ripe tomatoes as affected by stage of ripeness at harvest and by supplementary ethylene. J Food Sci. 1976;41(4):856–858. doi: 10.1111/j.1365-2621.1976.tb00738_41_4.x. [DOI] [Google Scholar]
- Yang CM, Lu YL, Chen HY, Hu M. Lycopene and the LXRα agonist T0901317 synergistically inhibit the proliferation of androgen-independent prostate cancer cells via the PPARγ-LXRα-ABCA1 pathway. J Nutr Biochem. 2012;23(9):1155–1162. doi: 10.1016/j.jnutbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Yeh SL, Hu ML. Antioxidant and pro-oxidant effects of lycopene in comparison with β-carotene on oxidant-induced damage in Hs68 cells. J Nutr Biochem. 2000;11(11–12):548–554. doi: 10.1016/S0955-2863(00)00117-0. [DOI] [PubMed] [Google Scholar]