Abstract
During the deep fat food frying process, the frying media, oil, continuously degenerates when exposed to high temperature, oxygen and moisture. This leads to physical and chemical changes including the formation of hydrolysis products such as free fatty acids (FFAs) which are associated with undesirable darkening in colour, off-flavouring and a lowering of the smoke point. This study was aiming to develop a method capable of identifying and quantifying individual free fatty acids within oil using a small sample size (100 mg of oil). We used liquid/liquid extraction technique to separate FFAs from the rest of the oil followed by esterification using boron trifluoride (BF3) and then gas chromatography analysis. Various extraction conditions were tested. A mixture of 0.02 M phosphate buffer at pH 12 and acetonitrile at solvent: buffer ratio larger than 2:1 showed the highest efficiency in extraction of FFAs. The method was capable of producing accurate fatty acid profiles of FFAs and showed good precision on medium rancidity oil samples. It also captured the differences induced by adding free fatty acids to samples. An interesting discrepancy was found between the new method and the traditional titration method in terms of overall FFA content, which suggests further optimisation and investigation are required.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3232-9) contains supplementary material, which is available to authorized users.
Keywords: FFA extraction, Oil degradation, Hydrolysis, Rancid oil
Introduction
The global production of vegetable oil was estimated to hit 187 million metric tons for 2016/17 (source: www.statista.com). The quality of edible oil is of primary importance to both manufacturers and consumers of deep fried food products. Although the roles of saturated fats in a healthy diet are the subject of renewed debate (Parodi 2016), there are still concerns linked to the level of saturated fatty acids in food (Dawczynski et al. 2015). Edible oil is subject to significant chemical changes in use and storage (Choe and Min 2007), while numerous studies have demonstrated that some degradation products (Mozaffarian et al. 2006; Oomen et al. 2001) are hazardous to human health. Therefore, monitoring oil degradation has been an important topic.
The main processes that reduce the quality of oil during frying can be broadly categorised into hydrolysis, oxidation and polymerization (Bordin et al. 2013). These result in the generation of FFAs, aldehydes, ketones, acids, and many other products (Fritsch 1981). Many quality control methods have been developed, each responding to a different subset of the complex array of oil degradation products. These include FFA value (FFA), total polar material (TPM), iodine value (IV), peroxide value (PV), ρ-anisidine value (ρ-AnV) and thiobarbituric acid reactive substances (TBARS) (Shahidi and Zhong 2005).
This work focuses on the analysis of FFAs. Hydrolysis of the ester linkage of triacylglycerols (TAG) to produce FFAs, diglycerides (DAG), monoglycerides (MAG), and glycerol (Choe and Min 2007) leads to various undesirable changes, including production of off-flavours, decrease of smoke point and acceleration of further hydrolysis (Choe and Min 2007, Frega et al. 1999). Hydrolysis also leads to a drop of surface tension of the oil thereby, increasing oxygen accessibility during frying and thus promoting oxidative degradation of oil (Choe and Min 2007). The acid–base titration of FFA, using phenolphthalein for endpoint determination has been the most commonly used in routine assessment of frying oil quality. Although the method was significantly improved following the introduction of technologies such as potentiometric endpoint determination, it still suffers from several drawbacks, including requirements for large amounts of organic solvents and a large sample size. The classic FFAs test requires 20 g of oil and 150 ml of solvent (IUPAC 1979).
Another limitation of the FFA test is that it measures an overall level of titratable acids and does not identify the profile of FFAs. Such additional information would help develop further understanding of the kinetics of the reaction and could lead to significant improvements to quality control in fried food production. GC of fatty acid methyl esters (FAMEs) produced using a catalyst such as BF3 is a classic method for analysis of fatty acid profiles in edible oils (Wirasnita et al. 2013). However, this also cleaves ester bonds if TAG, DAG or MAG are present (O’Keefe and Pike 2010) which produces difficulties if the focus of analysis was on FFA. Therefore, a suitable FFA extraction process is needed. Researchers have used ultrafiltration (Keurentjes et al. 1992), solid phase extraction (SPE) (Paik et al. 2009), thin layer chromatography (TLC) (Sampels and Pickova 2011), supercritical fluid extraction (SFE) (Cao and Ito 2003), and selective esterification (Kail et al. 2012). These studies had different degrees of success, but all were relatively complicated and expensive to operate. Therefore, simpler liquid–liquid extraction (LLE) may be an attractive option for many. For example, Shah and Venkatesan (1989) used aqueous isopropyl alcohol as a solvent, while Rodrigues and Meirelles (2008) used ethanol and water. This study further investigated the feasibility of extracting FFA using a simple LLE system supported by GC analysis of extracted and unextracted fractions.
Materials and methods
Materials
Palmitic acid, stearic acid, oleic acid (9-cis), linoleic acid (9,12,-cis,-cis), linolenic acid (9,12, 5-cis,-cis,-cis) (all analytical grade), their methyl esters (reference grade), potassium dihydrogen orthophosphate, dipotassium hydrogen phosphate trihydrate, and sodium hydroxide solution (1 N) (all analytical grade) were purchased from Sigma-Aldrich, UK. Boron trifluoride (12% in methanol), hexane (≥ 97% purity), hexane (GC grade) acetonitrile (Extra Pure), methanol (HPLC grade), and propan-2-ol (≥ 99%), were purchased from Fisher Scientific. Fresh rapeseed oil and two different rapeseed oil samples of differing rancidity were provided by a local food factory producing fried ethnic products. Additional fresh rapeseed oil (labelled as “vegetable oil”) was purchased from a local supermarket. The oil samples were sealed in airtight PE containers and stored in a fridge at 4 °C.
Reference standard and sample preparation
FFA standards (1 mg/ml) were prepared by dissolving 100 mg of palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid in 100 ml of hexane. A dilution series of solutions at 0.2, 0.4, 0.6 and 0.8 mg/ml was prepared for each FFA standard for calibration of GC analysis. Fresh, medium rancidity and high rancidity rapeseed oil samples (5 mg/ml) were prepared in similar way. Spiked fresh rapeseed oil samples were prepared by adding 4% oleic acid (w/w), then the spiked oils were dissolved in hexane to a concentration of 5 mg/ml. See Table 1 for a list of samples.
Table 1.
Fatty acid standards and oil samples list
| Sample | Description |
|---|---|
| SFASa-PA | 1 mg/ml palmitic acid in hexane |
| SFAS-SA | 1 mg/ml stearic acid in hexane |
| SFAS-OA | 1 mg/ml oleic acid in hexane |
| SFAS-LA | 1 mg/ml linoleic acid in hexane |
| SFAS-ALA | 1 mg/ml linolenic acid in hexane |
| Fresh rapeseed oil (FRO) | 5 mg/ml fresh rapeseed oil (from industry) in hexane |
| Medium rancidity rapeseed oil (MRRO) | 5 mg/ml medium rancidity oil in hexane |
| High rancidity rapeseed oil (HRRO) | 5 mg/ml high rancidity oil in hexane |
| Retail fresh rapeseed oil (RFRO) | 5 mg/ml fresh rapeseed oil (from retail) in hexane |
| Spiked fresh rapeseed oil (SFRO) | 5 mg/ml fresh rapeseed oil spiked with 4% oleic acid (w/w) in hexane |
aSFAS single fatty acid sample
Liquid–liquid extraction of FFAs
FFA standard solutions (10 ml) were transferred into a 50 ml separating funnel. Phosphate buffer (5 ml) and solvent (methanol, propan-2-ol or acetonitrile, see below for volumes) were added and the funnel shaken (see below for times). The layers were allowed to separate and the lower aqueous layer was recovered and labelled Fraction 1. The upper organic layer was re-extracted a further two times following the same procedure to obtain fractions, labelled Fraction 2 and Fraction 3. The final upper organic layer was also retained following extraction and labelled Un-extracted fraction. To establish optimal conditions for extraction, various solvents, volumes of solvent, pH of phosphate buffer, and extraction time (shaking time) were tested (see “Results and Discussion” for details). The chosen conditions for recovery of FFAs from oil solution in hexane (10 ml at 5 mg/ml) were determined to be extraction with a mixture of acetonitrile (10 ml) and 0.02 M phosphate buffer at pH 12 (5 ml) with an extraction time of 5 min.
Derivatisation procedure
The derivatisation procedure for Fractions 1 to 3 and Un-extracted fraction was carried out using a modified protocol based on the IUPAC Official method 2.301 (IUPAC 1979). Collected extraction fractions (1 ml) were evaporated to dryness under nitrogen at room temperature. Methanolic sodium hydroxide (1.5 ml of 0.5 M) was added to each fraction and heated at ~ 100 °C for 7 min. After cooling to room temperature, 12% BF3 in methanol (2 ml) was added and the mixture heated at ~ 100 °C for 5 min. The mixture was allowed to cool, hexane (2 ml) was added and the mixture was shaken with saturated NaCl solution (5 ml) for 5 min and allowed to separate. The upper layer was transferred into a separate container and the lower layer re-extracted using hexane (2 ml). The two upper layers were pooled and evaporated to dryness under nitrogen at room temperature. The dry extract was reconstituted with hexane (1 ml) and transferred into GC vials for analysis.
Gas chromatography (GC)
GC analysis was performed using a Shimadzu GC 2010 with a flame ionization detector (FID) and automatic sampler. Samples were separated using a BP 5 capillary column (i.d 0.25 mm, 29 m in length) obtained from Perkin Elmer. Helium was used as the carrier gas at a flow rate of 1 ml per min. The initial temperature was set to 60 °C, then increased to 230 °C at a rate of 15 °C per min followed by increasing to 325 °C at a rate of 25 °C per min. The injector temperature was set at 300 °C with a split ratio of 20:1 and the detector temperature was set at 360 °C. Each solution (1 µl) was injected onto the column. Hexane was used as a blank. Reference standard methyl esters of fatty acids were used to identify the peaks.
Titration of FFAs
The FFA values of oil samples were determined using a Metrohm Titrino 848 autotitrator equipped with an optrode for photometric titration (Metrohm UK Ltd). The method was based on “Determination of the acid value (AV)” in Metrohm Application Bulletin 141/4 e modified to suit smaller sample size. Oil samples (1–2 g) were mixed with 70 ml of propan-2-ol and 1–2 drops phenolphthalein were added prior to loading into the autotitrator. The endpoints were determined by changes in absorbance at 595–625 nm. The test was performed in triplicate for each of the oil samples.
Statistical analysis
Data sets were analysed utilising Minitab® 17 (Minitab Ltd. UK) and SPSS Statistics 23 (IBM Corporation, US) using various models of analysis of variances (ANOVA).
Results and discussion
Factors affecting extraction rates
A sequence of experiments was designed to optimise four factors: (i) choice of extraction solvent, (ii) solvent volume, (iii) pH, and (iv) extraction time. Stages (i) and (ii) were carried out using 10 mg/ml oleic acid in hexane to represent the common long chain fatty acids which dominate the rapeseed oil fatty acid profile (Orsavova et al. 2015). Confirmatory tests suggested that the five most common fatty acids behave similarly in extraction. Thus palmitic acid was used in stages (iii) and (iv) as it is more resistant to oxidation (Min and Boff 2002)and less expensive. Extraction of free fatty acid standards and oil samples were triplicated.
Selection of solvent
Acetonitrile, methanol and propan 2-ol were selected as potential dispersive solvents for FFA extraction based on polarity and miscibility (Bayne and Carlin 2010). ANOVA followed by Tukey test confirmed the statistical significances of solvent as a factor and all three selected solvents performed differently at the point of first extraction (Fig. 1a) although all achieved a similar total extraction rate (> 99%) when all three fractions were combined. The highest concentration of FFAs in the first extract was achieved with acetonitrile. It was observed that methanol resulted in relatively cloudy layers which led to more difficult layer separation. When using propan 2-ol, the aqueous phase consistently lost volume to the hexane phase, which might explain the lower extraction efficiency. Shah and Venkatesan (1989) described similar findings and suggested this might be due to mutual solubility of oil and aqueous propan-2-ol. Based on this result, acetonitrile was used as the dispersive solvent in subsequent experiments.
Fig. 1.
Factors affecting extraction rates. All data were processed using one-way ANOVA (Analysis of variance) followed by post hoc test (Tukey). The extraction rates of all 3rd fractions were below limited of quantifications, therefore were excluded. Error bars were standard errors. a Oleic acid was extracted by using 10 ml selected solvent and 5 ml 0.02 M phosphate buffer at pH 8 with 15 min extraction time, For both fractions, p < 0.01. Tukey test error teams were mean square 2.81 for 1st fraction and 0.004 for 2nd fraction. b Oleic acid was extracted by using 5–10 ml of acetonitrile and 5 ml 0.02 M phosphate buffer at pH 8 with 15 min extraction time. For both fractions, p < 0.01. Tukey test error teams were mean square 0.59 for 1st fraction and 0.013 for 2nd fraction. c Palmitic acid was extracted by using 10 ml of acetonitrile and 5 ml of 0.02 M phosphate buffer at various pH with 15 min extraction time. For all fractions, p < 0.01. Tukey test error teams were mean square 0.181 for 1st fraction and 0.044 for 2nd fraction. d Palmitic acid was extracted by using 10 ml of acetonitrile and 5 ml of 0.02 M phosphate buffer at pH 12 with various extraction time. For all fractions, p < 0.01. Tukey test error teams were mean square 0.683 for 1st fraction and 0.73 for 2nd fraction
Volume of solvent
With the aim of keeping solvent volumes low for environmental and cost reasons, small volumes (5–10 ml of acetonitrile) were tested. As expected, the results (Fig. 1b) clearly showed that increasing volume has a positive effect on concentrations of FFAs found in Fraction 1. While it may be possible to further increase extraction efficiency by using larger volumes of acetonitrile, the extraction efficiency was already deemed to be satisfactory following three extractions and so the possibility was not investigated further.
pH
Extraction with phosphate buffer (0.02 M) at three pH values (7, 8 and 12) showed that highest concentration of palmitic acid in the first extract was obtained with pH 12 buffer (Fig. 1c). Long chain FFA have been reported to exhibit pKa values in the range 8–10 (Kanicky and Shah 2002). Bayne and Carlin (2010) have emphasised that the pH of the aqueous phase should be well above the dissociation constant (pKa) for the FFA to be ionised and migrate more completely from the organic phase, and this is in agreement with the observation that the best extraction was obtained at the highest pH. As result of this test, the phosphate buffer was adjusted to pH 12 in subsequent experiments.
Extraction time
Four extraction times in the range 5–20 min were tested, with the result (Fig. 1d) suggesting that shorter extraction times are essential. No times less than 5 min have been tested so far. The reduced extraction efficiency might be due to the partial solubility of the buffer/acetonitrile layer in the hexane layer, perhaps exacerbated by the tendency for FFA to form alkali soaps at high pH, which would be more significant with prolonged agitation during extraction.
Summary
A simple optimisation sequence has suggested that short extractions of FFA from solutions of lipids in hexane proceeds best with an aqueous phase of phosphate buffer (0.02 M) at pH 12 modified with a relatively high proportion of acetonitrile (solvent: buffer ratio > 2:1). The benefits of high pH and high proportion of modifier is predictable, but the less predictable benefits of short extraction time and the optimum for buffer concentration require a more extensive investigation.
FFA profiles of industrial oil samples
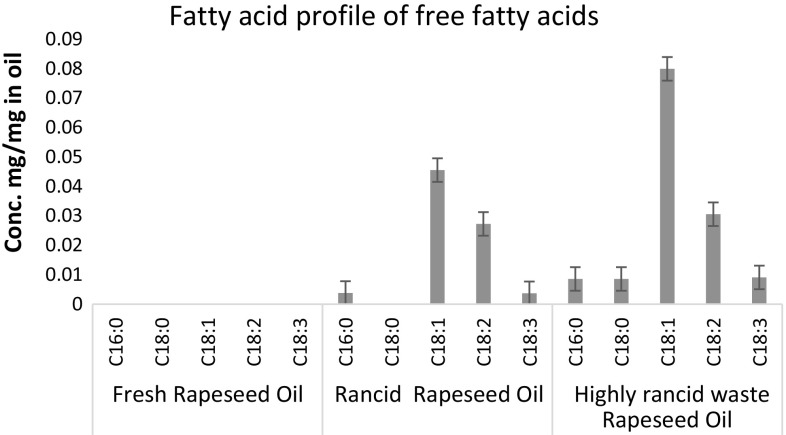
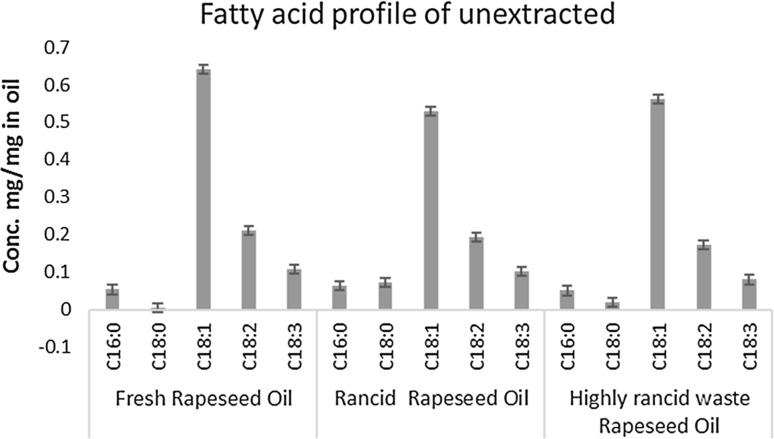
Three rapeseed oil samples with different rancidity levels were obtained from local food industry sources and were analysed using LLE-GC method and titration methods. A Two-way ANOVA followed by Tukey test were carried out for fatty acid concentrations of extracted FFAs. Fresh sample data and stearic acid were excluded from the analysis as they were below the limit of quantification (LoQ). The LoQ benchmarks were 0.80 µg/ml for palmitic acid, 0.72 µg/ml for stearic acid, 1.10 µg/ml for oleic acid, 0.89 µg/ml for linoleic acid, and 1.03 µg/ml for linolenic acid, adopted from Zhang et al. (2015). The same tests were carried out for un-extracted fatty acids, with data transformed using logarithm base 10 to achieve normal distribution. Summary of means are illustrated in Figs. 2 and 3. The fatty acids profile of fresh oil (Fig. 3) shows oleic acid accounted 63.16% of the fatty acids in oil, followed by 20.72% for linoleic acid, 10.53% linolenic acid, and 5.26% stearic acid. Palmitic acid was the smallest component, at 0.33% of the fatty acids in oil. This was in agreement with the literature (Orsavova et al. 2015, Sakhno 2010).
Fig. 2.
Profile of Free Fatty Acids extracted to buffer. Data displayed were means of three replicates. Error bars were standard errors. Two-way ANOVA (exclude fresh rapeseed oil sample and C18:0 data) shown p < 0.01 for factor “oil sample” and factor “fatty acid”, and p < 0.05 for their interaction
Fig. 3.
Fatty Acids Profile of remaining non-free fatty acids. Data displayed were means of three replicates. Error bars were standard errors. Two-way ANOVA shown p < 0.01 for all factors and their interaction
FFAs extracted from fresh oil sample were all below the limit of quantification, which agreed with FFA titration test. It might not be common for edible oils in retail to have such low FFA value, however oil used in food industry requires higher quality and so would be expected to have a low FFA value (stated by an industrial expert via personal communication 2013). The FFA values from titration method and calculated from results of LLE-GC method shows positive correlation with increased frying time, which is in line with current knowledge (Lalas 2009). Comparing FFA profiles of the oil samples, the largest difference was the concentration of oleic acid (C18:1), which was 74% higher in HRRO (high rancidity rapeseed oil) comparing to MRRO (medium rancidity rapeseed oil). As oleic acid was the biggest component in the fatty acids profile, this difference clearly contributed most to the variation of FFA values, which was 63% higher in HRRO for LLE-GC calculated FFA. Increases of other fatty acids were also found, with the most noticeable being the percent of stearic acid (C18:0) in HRRO free fat acid profile, which was absent in MRRO. Simultaneously, the increase of linoleic acid (C18:2) was much lower, at 14.8%, compared to other fatty acids. This indicated that the increase of free stearic acid could be at least partially contributed by loss of unsaturation of linoleic acid. Although not tested for FFA, similar changes were reported in vegetable oils during frying process (Sharoba and Ramadan 2012, Valantina et al. 2015).
The precision of FFA quantification using LLE-GC method was not consistent across samples and fatty acids. Table 2 summarises the means and relative standard deviation of concentrations within the replicates. It appears that, as expected, the precision of detection of a fatty acid with relatively larger quantity is better than a fatty acid with smaller quantity as the quantification of later could be closer to the limitation of the instrument/method. Also, clear separation of linoleic acid and linolenic acid from oleic acid proved to be challenging. This might be improved by using an alternative temperature program or by using a different column such as DB-FFAP (nitroterephthalic acid modified polyethylene glycol) capillary GC column (Zhang et al. 2015) or ionic liquid (IL) columns (Weatherly et al. 2016). There might be other solvents, or combination of solvents, more effective in extraction such as hexane/diethyl ether at 50/50 (Bravi et al. 2017), could use porous materials to improve LLE (Bravi et al. 2014, 2017). Temperature of extraction environment could also be optimised to improve extraction (Ansolin et al. 2013). The method was promising in terms of evaluated total amount of FFA. For MRRO, the relative standard deviation (RSD) was 1.8%, which was lower than the titration method (Table 2). However, for HRRO, the RSD was 30.93%. This requires further investigation. While it could be caused by interference from other compounds produced in oxidation (Frega et al. 1999) this high level of variability is unexpected.
Table 2.
Concentrations of extracted free fatty acid and remaining non-free fatty acids, and their tested FFA values and calculated FFA values based on the fatty acid concentrations
| Oil Sample | Fatty Acids | Extracted | Not extracted | Calculated FFA value** | Tested FFA value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (mg/mg of oil sample)* | S.D.* | RSD (%) | Conc. (mg/mg of oil sample)* | S.D.* | RSD (%) | %FFA | S.D. | RSD % | %FFA | S.D. | RSD % | ||
| Fresh rapeseed oil (FRO) | C16:0 | Too low to be detected | N/a | N/a | 0.053 | 0.004 | 6.75% | Close to zero | N/a | N/a | Too low to be detected | N/a | N/a |
| C18:0 | Too low to be detected | N/a | N/a | 0.003 | 0.003 | 57.75% | |||||||
| C18:1 | Too low to be detected | N/a | N/a | 0.640 | 0.027 | 4.30% | |||||||
| C18:2 | Too low to be detected | N/a | N/a | 0.210 | 0.008 | 3.77% | |||||||
| C18:3 | Too low to be detected | N/a | N/a | 0.107 | 0.022 | 21.50% | |||||||
| Sum | Close to zero | N/a | N/a | 1.013 | N/a | N/a | |||||||
| Medium rancid rapeseed oil | C16:0 | 0.004 | 0.001 | 13.58% | 0.064 | 0.007 | 10.80% | 8.059 | 0.145 | 1.80% | 3.615 | 0.09% | 2.58 |
| C18:0 | Too low to be detected | N/a | N/a | 0.072 | 0.047 | 64.58% | |||||||
| C18:1 | 0.046 | 0.001 | 2.78% | 0.529 | 0.005 | 1.02% | |||||||
| C18:2 | 0.027 | 0.001 | 3.51% | 0.193 | 0.006 | 2.94% | |||||||
| C18:3 | 0.004 | 0.000 | 10.14% | 0.101 | 0.012 | 11.69% | |||||||
| Sum | 0.080 | N/a | N/a | 0.959 | N/a | N/a | |||||||
| Highly rancid waste rapeseed oil | C16:0 | 0.009 | 0.009 | 101.43% | 0.050 | 0.005 | 10.77% | 13.177 | 4.076 | 30.93% | 7.731 | 0.85% | 0.85 |
| C18:0 | 0.009 | 0.010 | 114.86% | 0.019 | 0.006 | 32.88% | |||||||
| C18:1 | 0.080 | 0.016 | 19.77% | 0.562 | 0.044 | 7.88% | |||||||
| C18:2 | 0.031 | 0.014 | 45.16% | 0.171 | 0.012 | 6.79% | |||||||
| C18:3 | 0.009 | 0.012 | 126.47% | 0.080 | 0.016 | 20.42% | |||||||
| Sum | 0.137 | N/a | N/a | 0.882 | N/a | N/a | |||||||
*Means and standard deviation of three extraction replicates
* *Theoretical FFA values were calculated by converting (weight/weight mg/mg oil) concentrations of individual fatty acids into molecule concentration (mol/mg oil) based on following molecule weight of fatty acids: Stearic Acid 284.48 g/mol, Palmitic Acid 256.43 g/mol, Oleic Acid 282.47 g/mol, Linoleic Acid 280.45 g/mol, and Linolenic Acid 278.436 g/mol. Therefore, the amount of 0.1 M KOH required to naturalise these fatty acids within a certain sample can be calculated, which can be converted to FFA value expressed as Oleic acid using method described in IUPAC method 2.201 (IUPAC 1979)
Interestingly, FFA values obtained from titration were about 5% lower than calculated FFA values based on LLE-GC method. To investigate this discrepancy, FRO (fresh rapeseed oil from retail) samples were spiked with 4% oleic acid, then the FFA values of spiked oil samples were calculated following analysis using LLE-GC method as well as analysed using the titration method (Table 3). Data showed that both methods capture the increase of oleic acid, however, 0.26% higher for titration method and near 1% higher than expected for LLE-GC method, which might be caused by the challenges of LLE-GC method discussed above. Also, the LLE-GC method yielded higher FFA value again, which suggest either the titration method might underestimate FFA value, or LLE-GC method might overestimate FFA. Berezin et al. (1996) completed a similar study shown similar acid values (AV) of extracted oil from oilseed and extracted FFAs from oilseed, however, the AV were both determined by titration method. In their study, FFAs were analysed by GC after BF3 derivatisation, however the quantification information was not provided. Therefore, it is difficult to determine whether they would observe similar trend if they produced calculated FFA values based on GC results. It is clear that further investigations are also required to explain this finding.
Table 3.
Calculated FFA values base on LLE-GC method and titration FFA values of fresh and spiked rapeseed oil
| Oil samples | Calculated FFA value** | Tested FFA value | ||||
|---|---|---|---|---|---|---|
| Mean (%) | SD (%) | RSD % | Mean (%) | SD (%) | RSD % | |
| RFRO | 2.0 | 0.1 | 2.9 | 0.1 | 0.0 | 1.8 |
| SFRO | 7.0 | 0.3 | 3.7 | 4.3 | 0.1 | 1.3 |
| Difference | 5.0 | 4.3 | ||||
*,* *See the footer of Table 2
Conclusion
A liquid–liquid extraction method for separation of FFAs from cooked vegetable oil was developed. Several optimal parameters for the extractions were determined. The method is simple, fast and cost effective. GC analysis indicated the method was able to achieve complete extraction while allowing non-FFA to remain attached to glycerol. This provided opportunities to further study regarding oil thermal hydrolysis in processing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Helen Hodgson, Paul Douglas, Daniel Eaton, Alex Atkinson, and Meez Islam for invaluable assistances in this project.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3232-9) contains supplementary material, which is available to authorized users.
Contributor Information
Naser Bazina, Email: N.Bazina@tees.ac.uk.
Jibin He, Phone: 01642 384089, Email: jibin.he@tees.ac.uk.
References
- Ansolin M, Basso RC, Meirelles AJdA, Batista EAC. Experimental data for liquid–liquid equilibrium of fatty systems with emphasis on the distribution of tocopherols and tocotrienols. Fluid Phase Equilib. 2013;338:78–86. doi: 10.1016/j.fluid.2012.09.033. [DOI] [Google Scholar]
- Bayne S, Carlin M. Forensic applications of high performance liquid chromatography. Boca Raton: CRC Press/Taylor & Francis; 2010. [Google Scholar]
- Berezin O, Turyan Y, Kuselman I, Shenhar A. Rapid and complete extraction of free fatty acids from oilseeds for acid value determination. J Am Oil Chem Soc. 1996;73:1707–1711. doi: 10.1007/BF02517976. [DOI] [Google Scholar]
- Bordin K, Kunitake MT, Aracava KK, Favaro-Trindade CS. Changes in food caused by deep fat frying—a review. Arch Latinoam Nutr. 2013;63:5–13. [PubMed] [Google Scholar]
- Bravi E, Benedetti P, Marconi O, Perretti G. Determination of free fatty acids in beer wort. Food Chem. 2014;151:374–378. doi: 10.1016/j.foodchem.2013.11.063. [DOI] [PubMed] [Google Scholar]
- Bravi E, Marconi O, Sileoni V, Perretti G. Determination of free fatty acids in beer. Food Chem. 2017;215:341–346. doi: 10.1016/j.foodchem.2016.07.153. [DOI] [PubMed] [Google Scholar]
- Cao X, Ito Y. Supercritical fluid extraction of grape seed oil and subsequent separation of free fatty acids by high-speed counter-current chromatography. J Chromatogr A. 2003;1021:117–124. doi: 10.1016/j.chroma.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72:R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Dawczynski C, Kleber ME, März W, Jahreis G, Lorkowski S. Saturated fatty acids are not off the hook Nutrition. Nutr Metab Cardiovasc Dis. 2015;25:1071–1078. doi: 10.1016/j.numecd.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Frega N, Mozzon M, Lercker G. Effects of free fatty acids on oxidative stability of vegetable oil. J Am Oil Chem Soc. 1999;76:325–329. doi: 10.1007/s11746-999-0239-4. [DOI] [Google Scholar]
- Fritsch CW. Measurements of frying fat deterioration: a brief review. J Am Oil Chem Soc. 1981;58:272–274. doi: 10.1007/BF02582355. [DOI] [Google Scholar]
- IUPAC . Standard methods for the analysis of oils, fats and derivatives. 6. Oxford: Pergamon; 1979. [Google Scholar]
- Kail B, Link D, Morreale B. Determination of free fatty acids and triglycerides by gas chromatography using selective esterification reactions. J Chromatogr Sci. 2012;50:934–939. doi: 10.1093/chromsci/bms093. [DOI] [PubMed] [Google Scholar]
- Kanicky JR, Shah DO. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci. 2002;256:201–207. doi: 10.1006/jcis.2001.8009. [DOI] [PubMed] [Google Scholar]
- Keurentjes JT, Sluijs JT, Franssen RJH, Van’t Riet K. Extraction and fractionation of fatty acids from oil using an ultrafiltration membrane. Ind Eng Chem Res. 1992;31:581–587. doi: 10.1021/ie00002a020. [DOI] [Google Scholar]
- Lalas S. Quality of frying oil. In: Sahin S, Sumnu SG, editors. Advances in deep-fat frying of foods. Boca Raton: CRC Press; 2009. [Google Scholar]
- Min DB, Boff JM. Lipid oxidation of edible oil. In: Akoh CC, Min DB, editors. Food lipids: chemistry, nutrition, and biotechnology. 2. Boca Raton: CRC Press; 2002. [Google Scholar]
- Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- O’Keefe SF, Pike OA (2010) Fat characterization. In: Food analysis. Springer, Boston, pp 239-260. 10.1007/978-1-4419-1478-1_14
- Oomen CM, Ocké MC, Feskens EJM, van Erp-Baart MAJ, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746–751. doi: 10.1016/S0140-6736(00)04166-0. [DOI] [PubMed] [Google Scholar]
- Orsavova J, Misurcova L, Ambrozova J, Vicha R, Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. 2015;16:12871–12890. doi: 10.3390/ijms160612871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik M, Kim H, Lee J, Brand J, Kim Y. Separation of triacylglycerols and free fatty acids in microalgal lipids by solid-phase extraction for separate fatty acid profiling analysis by gas chromatography. J Chromatogr A. 2009;1216:5917–5923. doi: 10.1016/j.chroma.2009.06.051. [DOI] [PubMed] [Google Scholar]
- Parodi PW. Dietary guidelines for saturated fatty acids are not supported by the evidence. Int Dairy J. 2016;52:115–123. doi: 10.1016/j.idairyj.2015.08.007. [DOI] [Google Scholar]
- Rodrigues CEC, Meirelles AJA. Extraction of free fatty acids from peanut oil and avocado seed oil: liquid − liquid equilibrium data at 298.2 K. J Chem Eng Data. 2008;53:1698–1704. doi: 10.1021/je7007186. [DOI] [Google Scholar]
- Sakhno LO. Variability in the fatty acid composition of rapeseed oil: classical breeding and biotechnology. Cytol Genet. 2010;44:389–397. doi: 10.3103/S0095452710060101. [DOI] [PubMed] [Google Scholar]
- Sampels S, Pickova J. Comparison of two different methods for the separation of lipid classes and fatty acid methylation in reindeer and fish muscle. Food Chem. 2011;128:811–819. doi: 10.1016/j.foodchem.2011.03.089. [DOI] [Google Scholar]
- Shah KJ, Venkatesan TK. Aqueous isopropyl alcohol for extraction of free fatty acids from oils. J Am Oil Chem Soc. 1989;66:783–787. doi: 10.1007/BF02653668. [DOI] [Google Scholar]
- Shahidi F, Zhong Y (2005) Lipid oxidation: measurement methods. In: Bailey’s industrial oil and fat products. Wiley, New York. 10.1002/047167849x.bio050
- Sharoba AM, Ramadan MF. Impact of frying on fatty acid profile and rheological behaviour of some vegetable oils. J Food Process Technol. 2012;3:161. [Google Scholar]
- Valantina SR, Kumar VM, Devasena T. Selected rheological characteristics and physicochemical properties of vegetable oil affected by heating. Int J Food Prop. 2015;19(8):1852–1862. doi: 10.1080/10942912.2015.1024849. [DOI] [Google Scholar]
- Weatherly CA, et al. Analysis of long-chain unsaturated fatty acids by ionic liquid gas chromatography. J Agric Food Chem. 2016;64:1422–1432. doi: 10.1021/acs.jafc.5b05988. [DOI] [PubMed] [Google Scholar]
- Wirasnita R, Hadibarata T, Novelina YM, Yusoff ARM, Yusop Z. A modified methylation method to determine fatty acid content by gas chromatography. Bull Korean Chem Soc. 2013;34:3239–3242. doi: 10.5012/bkcs.2013.34.11.3239. [DOI] [Google Scholar]
- Zhang H, Wang Z, Liu O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J Pharm Anal. 2015;5:223–230. doi: 10.1016/j.jpha.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.