Abstract
This study investigated the effects of various plasticizer types [glycerol (GLY), sorbitol (SOR), and polyethylene glycol (PEG)] on the properties of fish myofibrillar protein (FMP) film. FMP films plasticized with GLY showed the greatest elongation at break (116.53%). It also showed the greatest water vapor permeability (1.43 × 10−10 g m−1 s−1 Pa−1). The film plasticized with SOR exhibited the highest tensile strength (12.56 MPa) and film solubility (62.59%). PEG plasticized film showed to have yellowish colour as indicated by the high b* value and low light transmission at 280 nm. Furthermore, FMP films containing PEG and SOR possessed lower moisture content than films with GLY. FT-IR and electrophoretic properties were not affected by any types of plasticizer. The appearance of the FMP film was similar to that of the PVC film. It was concluded that plasticizers had major effects on FMP films. They not only plasticize the protein film, but also affected other major film properties.
Keywords: Biodegradable film, Fish myofibrillar protein, Plasticizer
Introduction
In recent years, biodegradable films have become increasingly popular as they are more biodegradable and environmentally friendly. They may possibly minimize or even replace other typical commercial film made from plastics, which would then reduce the environmental impact from packaging waste. Film-forming materials are commonly used for biodegradable film production. These materials include hydrocolloids (e.g. polysaccharide, protein), lipids (e.g. fatty acids, waxes), and their composites (both hydrocolloid and lipid). Proteins are widely used for preparing biodegradable films due to their high nutritional value, abundance, and film-forming ability (Lee et al. 2015a; Riquelme et al. 2015). Fish myofibrillar proteins (FMP) are frequently used as a startng material for producing biodegradable film (Kaewprachu et al. 2016a; Nuanmano et al. 2015). FMP consists of proteints with a high molecular weight, and this produces strong film-forming characteristics (Nuanmano et al. 2015). However, a stand-alone FMP film is very brittle and weak. This is due to the extensive protein–protein chain interactions that firmly stabilize the film network that is further reinforced by disulfide bonds, hydrogen bonding, hydrophobic bonding, and/or electrostatic interactions. As a consequence, the properties are not elastic enough for commercial application. To overcome this problem, plasticizers can be added into the biodegradable film in order to decrease the films’ brittleness. The addition of plasticizers could increase the films’ extensibility and toughness by lowering the forces between the protein–protein chains (Nuanmano et al. 2015). However, plasticizers generally decrease the strength of the films as well as the water barrier properties.
Hydrophilic plasticizers are widely added into protein-based films. Most commonly, these include glycerol (GLY), glucose (GLU), fructose (FRUC), sucrose (SU), sorbitol (SOR), and polyethylene glycol (PEG) (Aguirre et al. 2013; Lee et al. 2015a, b). The efficiency of each plasticizer to function in the film network depends on molecular size, shape, structure, and water binding (Sothornvit and Krochta 2001). Several studies have monitored the properties of protein-based films with various added plasticizer types including jellyfish protein (Lee et al. 2015a), gelatin (Riquelme et al. 2015), triticale protein (Aguirre et al. 2013), chicken feet protein (Lee et al. 2015b), canola protein isolate (Chang and Nickerson 2014), and whey protein (Pérez et al. 2016). However, there remains little information about plasticizers in FMP films. Therefore, the aim of this investigation was to produce FMP film plasticized with various plasticizer types (GLY, SOR, and PEG). Their properties were compared with a polyvinyl chloride (PVC) wrap film.
Materials and methods
Materials
Sodium dodecyl sulfate (SDS), coomassie brilliant blue R-250, and β-mercaptoethanol were all purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Glycerol (GLY), and other analytical grade reagents were obtained from Merck (Darmstadt, Germany). Sorbitol (SOR) and polyethylene glycol (PEG, average MW ~ 400) were purchased from Union Science Co., Ltd. (Chiang Mai, Thailand). Polyvinyl chloride wrap films (PVC) of 10 μm thickness (Quick Pack Pacific, Thailand) were used as the commercial wrap film in this study.
Preparation of fish myofibrillar protein (FMP)
Firstly, minced fish (fresh tilapia; Oreochromis niloticus) was added with five volumes of 50 mM NaCl. They were homogenized with a homogenizer (Ultra Turrax T25, IKA®-Werke GmbH, Staufen, Germany) for 2 min at 11,000 rpm. The mixtures were then centrifuged (Legend X1R, Thermo Fisher Scientific, Osterode, Germany) at 10,000×g for 10 min at 4 °C. This process was repeated twice (Kaewprachu et al. 2016a). Finally, the FMP was freeze-dried (FD-8, Heto Dry Winner, Denmark), packed into a plastic bag with a zipper and kept at − 20 °C until use.
Preparation of FMP film
Firstly, the FMP was added with distilled water to obtain the final protein concentration of 1% (w/v). The mixture was homogenized at 11,000 rpm for 1 min. Twenty-five percent of GLY, SOR, and PEG (w/w, based on protein content) were added and then stirred for 30 min at room temperature. After stirring, the pH of the mixture was adjusted to 3 using 1 N HCl. The solutions were centrifuged at 3000×g for 10 min at room temperature. The obtained supernatant was used for film casting (Kaewprachu et al. 2016a) by adding 4 g of film-forming solution (FFS) onto a rimmed silicone resin plate (5 cm × 5 cm), and it was evaporated for 24 h at room temperature prior to drying in a ventilated oven environmental chamber (Model H110K-30DM, Seiwa Riko Co., Tokyo, Japan) at 50 ± 5% relative humidity (RH) and 25 ± 0.5 °C for 24 h. Finally, the obtained dry film was peeled and used for determining its properties.
Film thickness
Film thickness was determined by using a hand-held micrometer (Bial Pipe Gauge, Peacock Co., Tokyo, Japan). The film samples were randomly measured at six locations around the film. Experiments were repeated ten times.
Mechanical properties
A film sample was cut into sections that were 2 cm wide and 5 cm long, and then conditioned at 50 ± 5% RH at 25 °C for 48 h prior to testing. A Universal Testing Machine (Lloyd Instrument, Hampshire, UK) was used to measure tensile strength and elongation at break according to the ASTM standard method D882-97 (1999). The conditions of testing were 1 kN load cell, 3 cm of initial grip separation with the cross-head speed at 30 mm/min. The measurement was performed until the film finally broke. Experiments were repeated ten times.
Water vapor permeability (WVP)
A modified ASTM E96-80 standard method (1989) was used to evaluate the WVP as described in Kaewprachu et al. (2016a). Measurements were conducted at 30 °C at 50 ± 5% RH. The weight was recorded at hourly intervals for 8 h. Experiments were repeated in triplicate and expressed as g m−1 s−1 Pa−1.
Film solubility
The films’ solubility was measured following the method described in Sai-Ut et al. (2015), and calculated by substracting the weight of un-dissolved debris from the initial weight of the dry matter. The films’ solubility was expressed as a percentage of the total weight. Experiments were repeated in triplicate.
Appearance, color, light transmittance, and transparency
After the films were dried and conditioned at 50 ± 5% RH and 25 °C for 48 h, the visual aspects of the film samples were examined by using a digital camera (Fujifilm Finepix S4900; acquired from Fujifilm Thailand Co. Ltd., Bangkok, Thailand).
Color Quest XE (Hunter Lab, Virginia, USA) was used to determine the color attributes of the film and expressed as L*, a* and b*.
A UV–Vis spectrophotometer (G105 UV–VIS, Thermo Scientific Inc., Massachusetts, USA) was used to measure the light transmission of the films. A film sample was cut into a rectangular shape (4 cm × 4 cm). Measurements were performed at wavelengths between 200 and 800 nm (Jongjareonrak et al. 2006).
A film sample was cut to a rectangular shape (4 cm × 4 cm) and measured at a wavelength of 600 nm using a spectrophotometer. The transparency of the film was calculated as follows (Han and Floros 1997):
| 1 |
where T600 is transmittance (%) at 600 nm, and x is the film thickness (mm).
Fourier transform infrared spectroscopy (FT-IR)
FT-IR was performed as described in Limpan et al. (2010). Measurements were examined at 25 °C in the range of 4000–650 cm−1 with 64 scans and 4 cm−1 resolution by using a FT-IR Spectrum GX (PerkinElmer Inc., California, USA).
Electrophoretic analysis
A film sample was mixed with 5% SDS and blended for 1 min. It was then stirred continuously at room temperature for 24 h. After, it was centrifuged at 3000×g for 5 min. The obtained supernatants were used to examine sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was executed according to the method described in Laemmli (1970) that used a 10 and 4% running gel and stacking gel, respectively.
Moisture sorption isotherm
A film sample was cut into pieces 2.5 cm wide and 5 cm long. They were kept in a desiccator containing dry silica gel for 7 days at 25 °C prior to testing. Seven different RH conditions (21 ± 2%, 36 ± 2%, 52 ± 2%, 63 ± 2%, 74 ± 2%, 86 ± 2%, and 96 ± 2%) were prepared by using saturated salt solutions of LiCl, MgCl2, Mg(NO3)2∙6H2O, NaNO2, NaCl, KCl, and KNO3, respectively. Experiments were repeated three times. The percentage equilibrium moisture content of the film was calculated as follows (Srinivasa et al. 2003):
| 2 |
Statistical analysis
Analysis of variance (ANOVA) was used for statistical analysis and the differences between means were carried out by DMRT. An SPSS package (SPSS Inc., Chicago, IL, USA) was used as a tool for statistical analysis.
Results and discussion
Film thickness
The thickness of FMP films containing different plasticizer types compared to the PVC film is shown in Table 1. No significant differences were observed in FMP films plasticized with various plasticizer types (p > 0.05). This is consistent with Chang and Nickerson (2014) who found that canola protein isolate films plasticized with various plasticizer types had similar thickness (0.07–0.10 mm). Nuanmano et al. (2015) concluded that types of plasticizes (GLY and gelatin hydrolysate) had no effect on the thickness of FMP films (0.035–0.040 mm). Pérez et al. (2016) also reported that whey protein concentrate films plasticized with GLY or trehalose did not show significant differences in the thickness (0.126–0.138 mm). Generally, the film thickness affects properties such as light transmission, transparency, water vapor permeability (WVP), and mechanical properties (tensile strength; TS and elongation at break; EAB). Galdeano et al. (2013) concluded that the films’ EAB and optical properties were proportional to the film thickness. However, as compared with the PVC film, all plasticized films provided higher thickness values (by approximately one time).
Table 1.
Thickness, tensile strength, elongation, water vapor permeability, and solubility of FMP films plasticized with different plasticizers
| Plasticizer | Thickness (mm) | Tensile strength (MPa) | Elongation (%) | WVP (× 10−10 g m−1 s−1 Pa−1) | Film solubility (%) |
|---|---|---|---|---|---|
| GLY | 0.013 ± 0.0014a | 3.52 ± 0.35d | 116.53 ± 8.40b | 1.43 ± 0.78a | 33.73 ± 3.63c |
| PEG | 0.014 ± 0.0026a | 6.73 ± 0.22c | 96.59 ± 11.47c | 0.47 ± 0.53b | 47.02 ± 3.06b |
| SOR | 0.014 ± 0.0014a | 12.56 ± 1.10b | 65.81 ± 4.05d | 0.30 ± 1.34c | 62.59 ± 7.07a |
| PVC | 0.010 ± 0.0008b | 46.92 ± 2.16a | 268.31 ± 7.27a | 0.23 ± 0.01c | ND |
GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride, ND not detected
Different superscripts in each column are significantly difference (p < 0.05)
Mechanical properties
Plasticizers are used to reduce the films’ strength or brittleness. They are inserted between the polymer molecules in order to lower the interaction between polymer–polymer (Nuanmano et al. 2015), which consequently, increases the films’ flexibility. The mechanical properties of FMP films plasticized with different plasticizers were compared to the PVC film. All films exhibited significant differences in mechanical properties (p < 0.05) (Table 1). Among the plasticized films, the FMP film plasticized with SOR exhibited the highest TS (12.56 MPa), however this value was lower than the PVC film by about 4 times (p < 0.05). This would be consistent with Chang and Nickerson (2014) who reported that canola protein isolate film showed a stronger film network in the presence of SOR. This might be because SOR are molecules with ring conformation, which may make it difficult to insert between the protein–protein chains. This would then give it less ability to destroy the protein network (Yang and Paulson 2000). Rezaei and Motamedzadegan (2015) reported that fish gelatin film containing SOR had greater TS than film plasticized with GLY. They also suggested that the incorporation of GLY into films could increase the movement of the molecules in the matrix, so the TS lowered more than the SOR plasticized film. This is also consistent with recent study, Brzoska et al. (2018) reported that the use of SOR improved the TS of sodium caseinate based emulsion films, compared with those films containing GLY.
FMP films plasticized with GLY (116.53%) showed greater EAB compared to other plasticizer types (SOR: 65.81% and PEG: 96.59%) (p < 0.05), even though it was lower than the PVC film by about 2 times (p < 0.05). The is consistent with Jongjareonrak et al. (2006) who concluded that among plasticizers used (SOR, ethylene glycol, PEG 200, and PEG 400), gelatin film containing GLY showed greater EAB. These results are also in accordance with triticale protein plasticized films (Aguirre et al. 2013), where GLY plasticized films showed greater EAB over SOR plasticized films by around 99%. They also concluded that films containing the lower MW of plasticizer exhibited the higher EAB. Lee et al. (2015b) also found that the chicken feet protein film plasticized with GLY had higher EAB (13.69%) compared to the film plasticized with SOR (1.01%). Brzoska et al. (2018) also reported that sodium caseinate-emulsion films containing GLY showed higher EAB (~ 40%) than films containing SOR (~ 8%). GLY is the smallest molecule, so it is able to be positioned between the polymer–polymer chains, thereby causing the mechanical properties to change (Jongjareonrak et al. 2006). According to Aguirre et al. (2013), glycerol and water plasticized synergistically, resulting in more elastic of the films. The addition of plasticizer could improve the mechanical properties in protein based films by increasing the mobility of protein chains. The molecules of plasticizer and polymeric sidechains of the protein could form the interaction by hydrogen bonding, thus decreasing protein–protein interactions (Brzoska et al. 2018). In addition, the difference in nature, composition, size, concentration, and structure as well as the compatibility between protein molecule and plasticizer typically affects both the TS and the EAB of the resulting films (Aguirre et al. 2013; Jongjareonrak et al. 2006).
As compared with the PVC film, the FMP films plasticized with different plasticizer types were still around 73–92% and 57–75% less, both in strength and in flexibility, than the PVC film, respectively. Further research is required for enhancing the mechanical properties of the FMP film by using a combination of plasticizers at different ratios. The synergistic effect of the mixed plasticizers may play a role for improving the plasticity of the developed films.
Water vapor permeability (WVP)
The WVP of FMP films plasticized with different plasticizers compared to the control PVC film is shown in Table 1. The WVP of the developed FMP films were in a range of 0.30–1.43 × 10−10 g m−1 s−1 Pa−1. Among all of the plasticized films, the film plasticized with SOR (0.30 × 10−10 g m−1 s−1 Pa−1) had the lowest WVP compared to the films plasticized with PEG (0.47 × 10−10 g m−1 s−1 Pa−1) and GLY (1.43 × 10−10 g m−1 s−1 Pa−1). This result suggests that SOR had less ability to bind water than PEG and GLY, which resulted in a lower WVP. However, FMP films exhibited significant differences in WVP (p < 0.05) when various plasticizer types were added. The difference in WVP values might be related to the hygroscopic nature of the plasticizers. This is consistent with Chang and Nickerson (2014) who observed that canola protein isolate film plasticized with GLY showed the highest WVP. Rezaei and Motamedzadegan (2015) reported that gelatin film plasticized with GLY (13 g mm−1 kPa−1 h−1 m2) showed greater WVP compared to gelatin film plasticized with SOR (2.1 g mm−1 kPa−1 h−1 m2). Lee et al. (2015b) also reported that the lowest WVP was found in chicken feet protein film plasticized with SOR (2.90 × 10−9 g m−1 s−1 Pa−1). The hydrophilic and hygroscopic nature of GLY allows it to easily attract water molecules, which could forme a complex between water-plasticizers, resulting in more water passing through the film network (Nuanmano et al. 2015). As a consequence, the WVP value of the film increased. From this result, the FMP films showed to have higher WVP values than the PVC film, especially in FMP films plasticized with GLY and PEG. Notably, the WVP of FMP film plasticized with SOR is comparable to the PVC film (p > 0.05). Protein based films generally have poorer water barrier properties than commercial wrap films because of the high degree of hydrophilicity of proteins and the hydrophilic plasticizers contained in protein-based films. The addition of plasticizers into polymeric films could cause structural modification to the polymer network by increasing the space in the polymeric matrix and the movement of polymer chains, thus lowering the films’ density and increasing water permeability (Nuanmano et al. 2015).
Film solubility
The solubility of FMP films plasticized with different plasticizers compared to the control PVC film is presented in Table 1. The solubility of the film plasticized with various plasticizers was in the range of 33.73–62.59% (p < 0.05). A lowered film solubility was observed in the GLY plasticized film (33.73%), while the PVC film could not be dissolved in water (0%). The films’ solubility of FMP plasticized films were relatively higher than reported in FMP (~ 25–30%) (Cuq et al. 1997), Tara gum (13.62–29.72%) (Antoniou et al. 2014), and mung bean protein (~ 18–51%) (Wittaya 2013). However, the film solubility in this study showed to be lower than others reported. Nuanmano et al. (2015) found that the solubility of FMP films containing GLY and gelatin hydrolysate were in the range of 79.08–82.23%. They concluded that the water solubility of films depended on the hydrophilicity of each plasticizers. In this study, the highest film solubility was observed in the film plasticized with SOR (62.59%). This might be because SOR has a ring and a high molecular weight, which may block them from positioning between the protein–protein chains. GLY is a small molecule with straight chains, which can be easily inserted between the protein chains (Yang and Paulson 2000). Water solubility is normally used to indicate the hydrophilicity of the films. Moreover, the films’ solubility is a main factor for designing their applications. In some application, the films should have higher solubility. For instance, sachets containing food ingredients that need to be dissolved before consumption benefit from films with higher solubility. Sometimes it should be low to provide water resistance and improve food integrity. However, biodegradable films are mostly sensitive to water, and it means the film had lower water resistance.
Color, light transmission, and transparency
The color attributes of FMP films plasticized with different plasticizers compared to the control PVC film are shown in Fig. 1. The plasticized FMP films had smooth surfaces, and they were homogenous without any pores or cracks. The L* and a* values of the FMP films plasticized with different types of plasticizers seemed not significantly difference (p > 0.05). The GLY plasticized film showed to have a* and b* values closer to the PVC film (GLY-PVC; a* = (− 1.17)–(− 1.18), b* = 0.44–0.41), while all of the FMP plasticized films showed to have lower L* values (90.62–90.64) when compared to the PVC film. A similar trend was found for mung bean protein films (Wittaya 2013). They reported that the mung bean protein films containing various plasticizers showed no significant differences in L* and a* values. Pérez et al. (2016) also concluded that no significant differences in total color difference (ΔE) were observed between whey protein concentrate films plasticized with GLY and trehalose. Plasticizers are normally colorless; therefore they have no effect on the films’ color. The sources and concentrations of proteins have greater effect on the color of films rather than the types of plasticizers (Kaewprachu et al. 2016a, b; Pérez et al. 2016).
Fig. 1.

Visual aspect of FMP films plasticized with different plasticizers. GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride
Transmission of UV and visible light at selected wavelengths of 200–800 nm, as well as the transparency of all films, are presented in Table 2. Films from FMP act generally well as barriers for light transmission in the UV ranges (200–280 nm) compared to commercial wrap film. Light transmission in the UV ranges for the films were in the range of 0.04–84.68%, while the light transmission in the range of 82.95–91.85% were observed in the visible ranges. The PVC film had the highest light transmission in UV–Vis ranges, while the PEG plasticized film showed the lowest values. This suggests that the FMP plasticized films in this study could prevent the transmission in the UV ranges greater than the PVC film. Moreover, the film plasticized with PEG showed to have lower light transmission at 280 nm than other plasticizer types. This would be consistent with Jongjareonrak et al. (2006) who found that a lowered light transmission in the UV ranges were observed in the skin gelatin film containing PEG 200 and PEG 400. The difference in size, molecular weight, nature, and composition typically affects the light transmission of the resulting films. As a result, the light transmission at 280 nm was different (Orliac et al. 2003). Leerahawong et al. (2011) reported that the squid mantle muscle films showed the similar light transmission values, though they contain different plasticizer types (GLY, SOR, GLU, and FRUC). They also reported that the UV–Vis light transmission was in the range of 0.3–83.8%, which was higher than the films observed in this study, especially in the UV ranges.
Table 2.
Light transmission and transparency of FMP films plasticized with different plasticizers
| Plasticizer | Transmittance (%) at wavelength (nm) | Transparency* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| GLY | 0.04 | 16.34 | 85.25 | 86.71 | 87.51 | 88.42 | 88.98 | 89.17 | 3.84 ± 0.00003b |
| PEG | 0.05 | 1.10 | 82.97 | 84.29 | 85.53 | 86.68 | 87.10 | 87.41 | 3.79 ± 0.00004c |
| SOR | 0.05 | 8.05 | 82.95 | 83.73 | 86.75 | 86.92 | 87.98 | 88.06 | 3.76 ± 0.00007d |
| PVC | 12.06 | 84.68 | 88.04 | 89.40 | 90.35 | 91.29 | 91.61 | 91.85 | 3.95 ± 0.00017a |
GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride
*Values are given as mean ± SD from triplicate determinations
Different superscripts in each column are significantly difference (p < 0.05)
The FMP plasticized films were more transparent than the PVC film as indicated by their lower transparency value (Table 2). Pérez et al. (2016) observed that whey protein film containing both GLY and trehalose were bright and transparent. They suggested that those films can be used for food packaging, particularly for food products that need to be displayed. SOR plasticized film (3.76) showed to be slightly transparent when compared to the films plasticized with PEG (3.79) and GLY (3.84). Leerahawong et al. (2011) reported that film containing GLY (4.67) showed to be less transparent than those films containing SOR (3.29), GLU (3.19), and FRUC (3.42). They concluded that GLY had higher molarity than SOR, GLU, and FRUC, resulting in films being less transparent. The difference in the transparency might be due to the different characteristics of the plasticizers used (Orliac et al. 2003). Chang and Nickerson (2014) reported that the canola protein isolate film plasticized with GLY showed greater transparency followed by SOR and PEG plasticized films, respectively. Riquelme et al. (2015) reported that types of plasticizer (GLY, GLU, and trehalose) could not affect the visible properties of the film. The difference in source of protein, FFS, and the film-making procedures typically have effects for transparency values. In this study, it can be concluded that FMP films plasticized with various plasticizers were clear enough and can be used as see-through packaging.
Fourier transformed infrared spectrometry (FT-IR)
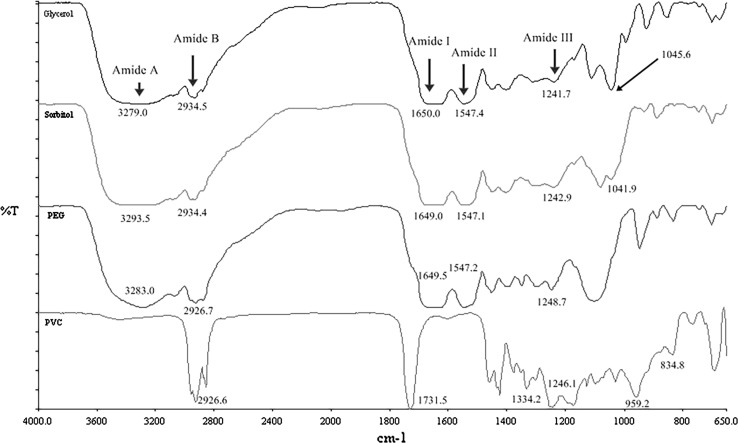
The FT-IR spectrum of FMP films plasticized with different plasticizers compared to the PVC film is shown in Fig. 2. Similar patterns of the spectra were observed in FMP films containing various plasticizer types. The FMP plasticized films showed the peaks of amide-A, which represents N–H and/or O–H stretching at ~ 3270 cm−1, and amide-B that is assigned to C–H stretching at ~ 2931 cm−1. An Amide-I (C=O stretching) and amide-II (N–H bending and C–N stretching) were observed in the FMP plasticized films at ~ 1650 and ~ 1547 cm−1, respectively. Singh et al. (2009) and Nie et al. (2017) also found amide-I and amide-II peaks of protein at 1630–1685 and 1530–1550 cm−1. The FMP plasticized films also exhibited a peak of amide-III, which is related to vibrations in the plane of C–N and N–H groups of bound amide, or of vibrations of CH2 groups of glycine at ~ 1242 cm−1 (Hoque et al. 2010; Kaewprachu et al. 2016b). The peak located around 1045 cm−1 was associated with the plasticizer (OH groups of GLY, SOR, and PEG) (Bergo and Sobral 2007), which is clearly observed in the film plasticized with GLY. The slightly shift to higher wavenumber of amide-A peak was found in the films plasticized with PEG (3283 cm−1) and SOR (3293 cm−1) when compared with the GLY plasticized films (3279 cm−1). This indicates that a lower formation of hydrogen bonds. Therefore, the free hydrogen groups of PEG and SOR plasticized films could form a few hydrophilic bonding with water. This result was associated with the mechanical and water barrier properties of the resulting films (Table 1). According to the spectra, amide-I, amide-II, and amide-III peaks of all FMP plasticized films showed a similar in vibrational wavenumber. However, this result was not consistent with earlier study, Singh et al. (2009, 2010) who found that amide-I and amide-II peaks of zein films were shifted to lower wavenumber when GLY was added. They concluded that the addition of GLY could change the chemical environment of the β-sheets, resulting in reduction of protein–protein interaction. As a consequence, more random coils and/or α helices formation (Singh et al. 2010). The spectra of the film plasticized with PEG that was situated at around 1200–900 cm−1 was inconsistent with the spectra that was observed in the GLY and SOR plasticized films. This might indicate that PEG induced some conformational changes in the polymer (Haq et al. 2014). The C–H stretching at 2926 cm−1, the carbonyl groups at ~ 1731 cm−1, CH2 groups deformation at ~ 1334 cm−1, out-of-plane angular deformation (pCH) at ~ 1246 cm−1, out-of-plane trans deformation (ωCH) at ~ 959 cm−1, C–Cl stretching at ~ 835 cm−1 were all observed in the PVC film. In addition, FT-IR results used to reconfirm the interaction between the FMP and the plasticizers in the film matrix.
Fig. 2.
FT-IR spectrum of FMP films plasticized with different plasticizers. GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride
Electrophoretic
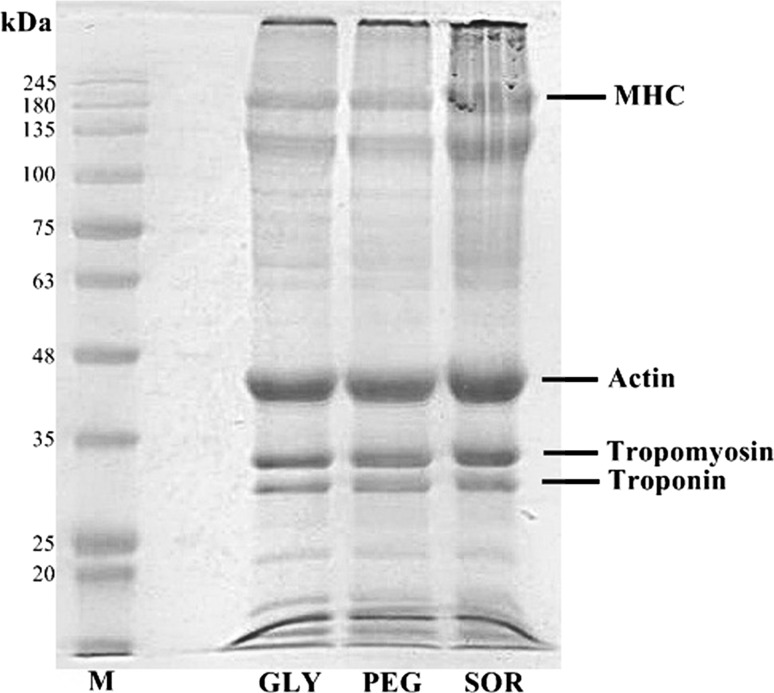
The protein patterns of FMP films containing different plasticizer types are shown in Fig. 3. The major proteins in FMP were myosin heavy chain (MHC) and actin. FMP films containing various plasticizer types showed similar protein patterns. This indicates that various plasticizer types showed no difference in non-disulfide covalent bonds. The incorporation of different plasticizers could not affect the protein pattern or protein degradation in the FMP films. However, the MHC band intensity was slightly decreased when GLY and PEG were added. This is indicated by the low TS value (Table 1). Leerahawong et al. (2011) reported that the MHC band was able to remain in all plasticized squid mantle muscle films (GLY, SOR, GLU, and FRUC). The authors suggested that the addition of plasticizers could improve EAB by increasing the films’ flexibility without affecting the protein degradation. Similar protein patterns were found in the FMP films containing GLY or protein hydrolysate, as plasticizers (Nuanmano et al. 2015). They concluded that these plasticizers were very small and could not bind with the molecules of protein in the film matrix. It was assumed that GLY was located between the protein–protein chains and was able to bind with proteins in the film matrix via hydrogen bonding, which is a weak bond and could be destroyed under the electrophoretic condition (Nuanmano et al. 2015).
Fig. 3.
Protein patterns of FMP films plasticized with different plasticizers under reducing condition. M protein makers, MHC myosin heavy chain, GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride
Moisture sorption isotherm
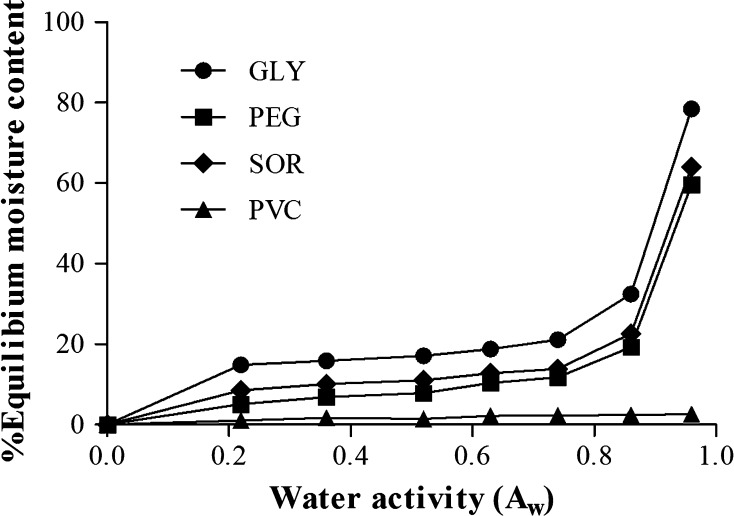
The moisture sorption isotherm (MSI) of the films has the benefit for predicting the stability of the packaged food products, and the changes of food quality during packing, storage, and transportation. MSI also has function for the design of the film application (Srinivasa et al. 2003). The MSI of FMP films plasticized with GLY, SOR, and PEG compared to the control PVC film are shown in Fig. 4. The FMP plasticized films were type-II sorption isotherm as indicated by the increased equilibrium moisture content with the coincidental increased water activity (Aw) in a sigmoidal manner. This is similar to those observed in mung bean protein (Wittaya 2013), starch-gelatin (Al-Hassan and Norziah 2012), and gelatin (Hazaveh et al. 2015). Type-II sorption isotherm are commonly found in most foods and bio-based films (Perdomo et al. 2009; Wittaya 2013). At low Aw (0.22–0.52), the moisture content of the films was determined at 25 °C and increased slowly. On the other hand, Aw between 0.63 and 0.96 moisture content increased rapidly. GLY plasticized film showed to have the highest moisture sorption followed by SOR and PEG plasticized films, respectively. This suggests that SOR and PEG were less effective in binding with water. Consequently, the film showed lower moisture content. In general, the film plasticized with GLY had the highest moisture content compared to the films plasticized with SOR and PEG. This is because GLY is more hydrophilic than SOR and PEG (Leerahawong et al. 2011). Plasticizers normally expose more active sites, especially the hydroxyl group where more molecules of water could absorb. A lower moisture content in films containing PEG and SOR have been reported by Al-Hassan and Norziah (2012). They found that the starch-gelatin film plasticized with SOR showed the lowest water binding capacity because of the molecular structure of SOR that facilitates interactions with the polymeric chains. Hazaveh et al. (2015) concluded that ribose could increase the moisture content of the gelatin film due to their hydroxyl group interactions with water when Aw was more than 0.60. The reduction of the mass transfer between the packaged food products and the environment is an important function for packaging materials. Different characteristics of the food being packaged require the different properties in the packaging material that are suitable for each individually. For example, the packaging of crackers and cookies should provide low moisture absorption and/or a high moisture barrier to prevent loss of crispness, while fruits and vegetables may require moderate-high moisture absorption. However, a low moisture barrier in packaging material properties causes a high RH inside the package, which facilitates microbial growth. Therefore, the selection of plasticizer types will be useful in determining the moisture absorption rate and moisture content of the film, thus helping to enhance the stability of the film and the food inside under varying RH conditions during storage and transportation.
Fig. 4.
Moisture sorption isotherm of FMP films plasticized with different plasticizers. GLY glycerol, SOR sorbitol, PEG polyethylene glycol, PVC polyvinyl chloride
Conclusion
The results here demonstrate that the addition of different types of plasticizers into FMP films showed significant differences in their properties. Among all plasticizers used, GLY plasticized film showed to have the highest EAB, moisture content, and WVP, whereas SOR plasticized film exhibited the highest TS and solubility. However, FMP films plasticized with different plasticizer types had no effect on protein patterns and FT-IR spectra. Furthermore, FMP films showed to have good UV light barrier properties. Further research is required for improving the mechanical and barrier properties of the FMP film.
Acknowledgements
The author would like to thank Mae Fah Luang University and the Thailand Research Fund for the financial support through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0029/2555) to Ms. Pimonpan Kaewprachu. Dr. Naathakan Rugnraeng was also acknowledged for the revision preparation. This manuscript was edited for grammatical accuracy by Matthew Robert Ferguson of Mahidol University International College, Bangkok, Thailand.
References
- Aguirre A, Borneo R, León AE. Properties of triticale protein films and their relation to plasticizing–antiplasticizing effects of glycerol and sorbitol. Ind Crops Prod. 2013;50:297–303. doi: 10.1016/j.indcrop.2013.07.043. [DOI] [Google Scholar]
- Al-Hassan AA, Norziah MH. Starch–gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocolloid. 2012;26:108–117. doi: 10.1016/j.foodhyd.2011.04.015. [DOI] [Google Scholar]
- Antoniou J, Liu F, Majeed H, Qazi HJ, Zhong F. Physicochemical and thermomechanical characterization of tara gum edible films: effect of polyols as plasticizers. Carbohydr Polym. 2014;111:359–365. doi: 10.1016/j.carbpol.2014.04.005. [DOI] [PubMed] [Google Scholar]
- ASTM (1989) Standard test methods for water vapor transmission of materials. Standard designation E96-E80. Annual book of ASTM standard, Philadelphia, pp 730–739
- ASTM . Standards designations. Philadelphia: Annual book of ASTM standard; 1999. Standard test method for tensile properties of thin plastic sheeting; pp. D882–D897. [Google Scholar]
- Bergo P, Sobral PJA. Effects of plasticizer on physical properties of pigskin gelatin films. Food Hydrocolloid. 2007;21:1285–1289. doi: 10.1016/j.foodhyd.2006.09.014. [DOI] [Google Scholar]
- Brzoska N, Müller M, Nasui L, Schmid M. Effects of film constituents on packaging-relevant properties of sodium caseinate-based emulsion films. Prog Org Coat. 2018;114:250–258. doi: 10.1016/j.porgcoat.2017.10.016. [DOI] [Google Scholar]
- Chang C, Nickerson M. Effect of plasticizer-type and genipin on the mechanical, optical, and water vapor barrier properties of canola protein isolate-based edible films. Eur Food Res Technol. 2014;238:35–46. doi: 10.1007/s00217-013-2075-x. [DOI] [PubMed] [Google Scholar]
- Cuq B, Gontard N, Cuq J-L, Guilbert S. Selected functional properties of fish myofibrillar protein-based films as affected by hydrophilic plasticizers. J Agric Food Chem. 1997;45:622–626. doi: 10.1021/jf960352i. [DOI] [Google Scholar]
- Galdeano MC, Wilhelm AE, Mali S, Grossmann MVE. Influence of thickness on properties of plasticized oat starch films. Braz Arch Biol Technol. 2013;56:637–644. doi: 10.1590/S1516-89132013000400014. [DOI] [Google Scholar]
- Han J, Floros J. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J Plastic Film Sheet. 1997;13:287–298. doi: 10.1177/875608799701300405. [DOI] [Google Scholar]
- Haq MA, Hasnain A, Azam M. Characterization of edible gum cordia film: effects of plasticizers. LWT Food Sci Technol. 2014;55:163–169. doi: 10.1016/j.lwt.2013.09.027. [DOI] [Google Scholar]
- Hazaveh P, Mohammadi Nafchi A, Abbaspour H. The effects of sugars on moisture sorption isotherm and functional properties of cold water fish gelatin films. Int J Biol Macromol. 2015;79:370–376. doi: 10.1016/j.ijbiomac.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J Food Eng. 2010;96:66–73. doi: 10.1016/j.jfoodeng.2009.06.046. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocolloid. 2006;20:492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Kaewprachu P, Osako K, Benjakul S, Rawdkuen S. Effect of protein concentrations on the properties of fish myofibrillar protein based film compared with PVC film. J Food Sci Technol. 2016;53(4):2083–2091. doi: 10.1007/s13197-016-2170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewprachu P, Osako K, Benjakul S, Tongdeesoontorn W, Rawdkuen S. Biodegradable protein-based films and their properties: a comparative study. Packag Technol Sci. 2016;29:77–90. doi: 10.1002/pts.2183. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Lee J-H, Yang H-J, Won M, Song KB. Characterisation of jellyfish protein films with added transglutaminase and wasabi extract. Int J Food Sci Technol. 2015;50:1683–1689. doi: 10.1111/ijfs.12826. [DOI] [Google Scholar]
- Lee J-H, Lee J, Song KB. Development of a chicken feet protein film containing essential oils. Food Hydrocolloid. 2015;46:208–215. doi: 10.1016/j.foodhyd.2014.12.020. [DOI] [Google Scholar]
- Leerahawong A, Tanaka M, Okazaki E, Osako K. Effects of plasticizer type and concentration on the physicochemical properties of edible film from squid Todarodes pacificus mantle muscle. Fish Sci. 2011;77:1061–1068. doi: 10.1007/s12562-011-0398-8. [DOI] [PubMed] [Google Scholar]
- Limpan N, Prodpran T, Benjakul S, Prasarpran S. Properties of biodegradable blend films based on fish myofibrillar protein and polyvinyl alcohol as influenced by blend composition and pH level. J Food Eng. 2010;100:85–92. doi: 10.1016/j.jfoodeng.2010.03.031. [DOI] [Google Scholar]
- Nie X, Zhao L, Wang N, Meng X. Phenolics-protein interaction involved in silver carp myofibrilliar protein films with hydrolysable and condensed tannins. LWT Food Sci Technol. 2017;81:258–264. doi: 10.1016/j.lwt.2017.04.011. [DOI] [Google Scholar]
- Nuanmano S, Prodpran T, Benjakul S. Potential use of gelatin hydrolysate as plasticizer in fish myofibrillar protein film. Food Hydrocolloid. 2015;47:61–68. doi: 10.1016/j.foodhyd.2015.01.005. [DOI] [Google Scholar]
- Orliac O, Rouilly A, Silvestre F, Rigal L. Effects of various plasticizers on the mechanical properties, water resistance and aging of thermo-moulded films made from sunflower proteins. Ind Crops Prod. 2003;18:91–100. doi: 10.1016/S0926-6690(03)00015-3. [DOI] [Google Scholar]
- Perdomo J, Cova A, Sandoval AJ, García L, Laredo E, Müller AJ. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydr Polym. 2009;76:305–313. doi: 10.1016/j.carbpol.2008.10.023. [DOI] [Google Scholar]
- Pérez LM, Piccirilli GN, Delorenzi NJ, Verdini RA. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocolloid. 2016;56:352–359. doi: 10.1016/j.foodhyd.2015.12.037. [DOI] [Google Scholar]
- Rezaei M, Motamedzadegan A. The effect of plasticizers on mechanical properties and water vapor permeability of gelatin-based edible films containing clay nanoparticles. World J Nano Sci Eng. 2015;5:178–193. doi: 10.4236/wjnse.2015.54019. [DOI] [Google Scholar]
- Riquelme N, Díaz-Calderón P, Enrione J, Matiacevich S. Effect of physical state of gelatin-plasticizer based films on to the occurrence of Maillard reactions. Food Chem. 2015;175:478–484. doi: 10.1016/j.foodchem.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Sai-Ut S, Benjakul S, Rawdkuen S. Retardation of lipid oxidation using gelatin film incorporated with longan seed extract compared with BHT. J Food Sci Technol. 2015;52:5842–5849. doi: 10.1007/s13197-014-1631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Georget DMR, Belton PS, Barker SA. Zein–iodine complex studied by FTIR spectroscopy and dielectric and dynamic rheometry in films and precipitates. J Agric Food Chem. 2009;57:4334–4341. doi: 10.1021/jf900436q. [DOI] [PubMed] [Google Scholar]
- Singh N, Georget DMR, Belton PS, Barker SA. Physical properties of zein films containing salicylic acid and acetyl salicylic acid. J Cereal Sci. 2010;52:282–287. doi: 10.1016/j.jcs.2010.06.008. [DOI] [Google Scholar]
- Sothornvit R, Krochta JM. Plasticizer effect on mechanical properties of β-lactoglobulin films. J Food Eng. 2001;50:149–155. doi: 10.1016/S0260-8774(00)00237-5. [DOI] [Google Scholar]
- Srinivasa PC, Ramesh MN, Kumar KR, Tharanathan RN. Properties and sorption studies of chitosan–polyvinyl alcohol blend films. Carbohydr Polym. 2003;53:431–438. doi: 10.1016/S0144-8617(03)00105-X. [DOI] [Google Scholar]
- Wittaya T. Influence of type and concentration of plasticizers on the properties of edible film from mung bean proteins. KMITL Sci Technol J. 2013;13:51–58. [Google Scholar]
- Yang L, Paulson AT. Mechanical and water vapour barrier properties of edible gellan films. Food Res Int. 2000;33:563–570. doi: 10.1016/S0963-9969(00)00092-2. [DOI] [Google Scholar]