Abstract
Thermal inactivation kinetics of peroxidase (POD), polyphenol oxidase (PPO), pectin methyl esterase (PME) and ascorbate oxidase (AO) were studied over the temperature range of 45–85 °C along with the degradation kinetics of ascorbic acid (AA) and lycopene in Psidium guajava pulp. POD, PPO, PME and AO followed first-order kinetics whereas AA degradation data was explained by pseudo first-order kinetics. Lycopene degradation was suitably fitted in an exponential model, indicating continuous degradation of lycopene and higher degradation at higher temperature. Activation energy (Ea) of POD, PPO, PME, and AO was 63.79 ± 1.28, 60.36 ± 1.21, 63.22 ± 1.06 and 106.33 ± 8.51 kJ/mol, respectively. AA had Ea (95.82 ± 1.92 kJ/mol) higher than lycopene (54.92 ± 1.10 kJ/mol). PME (Z = 39.4 ± 0.1 °C) showed highest heat stability while AO (Z = 14.3 ± 1.1 °C) was least stable amongst the enzymes studied. AA (Z = 23.5 ± 0.5 °C) was weakest amongst the phytoconstituents in guava pulp and its retention was challenged during thermal processing. The thermal resistance of quality deteriorating enzyme of guava was found to be higher than that of the common spoilage organisms such as Saccharomyces cerevisiae and Lactobacillus plantarum reported for processed fruit products. Thus, this research hints towards the need for more robust thermal processing for inactivation of quality deteriorating enzymes.
Keywords: Psidium guajava, Pectinmethylesterase, Thermal inactivation, Lycopene, Z-value
Introduction
Pink guava (Psidium guajava L.) is a very popular tropical fruit. It is a good source of micronutrients such as vitamins A, B, C and also a rich source of soluble fiber, phosphorous and nicotinic acid (Morton 1987). Being rich source of nutrients, fruits and their products are likely to be attacked by microorganisms. Beside microbial degradation of quality, deteriorative enzymes such as peroxidase (POD), polyphenol oxidase (PPO), pectin methyl esterase (PME) and ascorbate oxidase (AO) can also lead up to the loss of quality of guava fruit and its products. These endogenous enzymes may adversely affect sensory and nutritional attributes if not inactivated during processing. POD and PPO bring about browning by reducing the diphenols to o-quinones, affecting the acceptability. PME brings about hydrolysis of methylester groups of pectin and is responsible for loss of texture or viscosity of fruit products. Like POD and PPO, AO also belongs to the family of oxidoreductase and it oxidizes AA to dehydroascorbic acid. Hence, the inactivation of these quality-deteriorating enzymes becomes equally essential as the inhibition of microbial growth for quality fruit products.
Various processing industries involved in manufacturing of fruit products use thermal energy [high-temperature short-time (HTST)] to achieve preservation. Thermal processing though allows efficient inactivation of spoilage microorganisms and endogenous enzymes, results in loss of nutritional quality parameters (Ali et al. 2011). AA being an antioxidant and essential nutrient being unstable can easily degrade under non-challenging conditions (Burdurlu et al. 2006). Lycopene has been reported to have health benefits in preventing oxidative stress and carcinogenesis. Pink guava is a good source of lycopene but during the processing of raw products it undergoes degradation resulting in lowered sensory quality and decreased health benefits of the finished products (Shi et al. 2002).
Though POD has received considerable attention due to its use as an indicator of the efficacy of heat treatment, understanding the thermal stability of other enzymes and nutritional components is also essential. Thermal processing is generally performed by heating to temperatures from 50 to 150 °C, depending upon the pH of the product (Thongsook and Barrett 2005). These heat treatments can hamper the nutritional quality of the food product and the extent of this is specific to cultivar, physiological state and environmental conditions. Optimization of a product’s quality requires control over inactivation of enzymes and deactivation of nutritional components. Many researchers have studied thermal inactivation of enzymes at various stages of purification, mainly because the enzymatic behavior is environment dependent (Terefe et al. 2013). Others have reported the effect of heat on degradation of nutritional components such as AA (Patras et al. 2009) and lycopene (Evoli et al. 2013).
The present work aims to understand the effect of heat on inactivation kinetics of quality deteriorating enzymes such as POD, PPO, PME and AO in pink guava pulp. Further, the effect of this thermal treatment on degradation of AA and lycopene in pink guava pulp was investigated.
Materials and methods
Materials
Fully ripened ‘Lalit’ guava (Psidium guajava L.) fruits (Skin brightness, L value = 60.81 ± 4.18; a* = 3.30 ± 0.47) were harvested from guava orchard located at Pune, India and brought to the research laboratory in cardboard boxes (at temperature of 25 °C). Bovine serum albumin-V, hydrogen peroxide was procured from Himedia laboratories Pvt Ltd., India Acetone, butylated hydroxytoluene, ethanol, hexane, guaiacol, metaphosphoric acid, acetic acid, sulphuric acid, ammonium molybdate, pectin, phenol red, L-AA and catechol were purchased from SD Fine Chemicals Pvt. Ltd., India. Bradford reagent was obtained from Bio-Rad, US. All chemicals used in the analysis were of analytical grade.
Methods
Preparation of fruit pulp
Fresh fruit were cut open and deseeded manually and then it was crushed in a blender to obtain the pulp. Each retortable pouch (100 mm × 75 mm made up of 12 µ polyester, 9 µ aluminum foil, 15 µ nylon, 70 µ food-grade cast polypropylene) was filled with 3 mL pulp (pH = 3.8 ± 0.3; Total soluble solids = 19.8 ± 0.8 °Brix).
Heat treatment
Thermal processing of fruit pulp (3 mL) packed in mini retortable heat-sealed pouches was performed in a thermostat controlled water bath (MetaLab Scientific Instruments, Mumbai, India). Samples for POD, PPO and PME were treated in the temperature range of 45 and 85 °C (temperature interval of 10 °C); samples for AO were treated in the temperature range of 45 and 65 °C (temperature interval of 5 °C); whereas, samples for AA and lycopene were treated in the temperature range of 55 and 85 °C (temperature interval of 5 °C). The come-up time (CUT) of < 35 s for the retort pouches was determined using a dummy pouch filled with 3 mL pulp. Heated samples were cooled immediately (< 20 s) in ice-water (≤ 4 °C), and the residual enzymatic activity was evaluated.
Total lycopene and Ascorbic acid content
Lycopene from pulp was extracted in hexane and absorbance of the extract was measured at 503 nm using UV–Vis spectrophotometer (UV-1800, Shimadzu Corporation, Japan) against hexane blank (Vishwasrao and Ananthanarayan 2016). AA was extracted and estimated using colorimetric assay involving a reduction reaction with ammonium molybdate (Vishwasrao and Ananthanarayan 2016). Absorbance was measured at 760 nm against a reagent blank using µQuant spectrophotometer (BioTek, India).
Enzyme extraction
The pulp was mixed in 200 mM potassium phosphate buffer (1:3 w/v) of pH 7.0 (Ortuño et al. 2013) and stirred for 30 min in ice bath. The solution was then centrifuged (Beckman Coulter J2-MC, Brea, US) at 12,064×g, 4 °C for 30 min. The supernatant was filtered (Whatman #1 filter paper) under vacuum. The filtrate was used as crude enzyme extract for estimation of POD, PPO and AO activities. PME was extracted from the pulp in 88 g/L NaCl solution (4:15 w/v) of pH 7.5 and the filtrate was used as crude enzyme extract for estimation of PME activity (Vishwasrao and Ananthanarayan 2016).
Protein determination
The protein content was determined by using coomassie brilliant blue G-250 dye by the dye- binding method. Bovine serum albumin-V was used to prepare standard curve.
Estimation of POD, PPO, AO and PME activity
Hydrogen peroxide (30 µM) and guaiacol (40 mM) in potassium phosphate buffer (50 mM/L) of pH 6.5 was used as a substrate solution for estimating POD activity. PPO was estimated using catechol (10 mM) in 50 mM/L Tris HCl buffer of pH 8.0. One unit activity of POD and PPO was defined as change in absorbance of 0.001 units (at 475 and 420 nm, respectively) per minute.(Vishwasrao and Ananthanarayan 2016) AO activity was measured using a reaction mixture composed of 500 µL phosphate buffer (200 mM, pH 6.5) containing 0.5 µM L-AA and 100 µL of the enzyme extract. After 10 min, the reaction was stopped by the addition of 1.48 mL of 0.2 M HCl, and the absorbance was measured in a spectrophotometer at 245 nm. The control was prepared with the inactivated extract by adding 0.2 M HCl before addition of the substrate (Gomez and Lajolo 2008). One unit of AO activity was defined as the oxidation of 1 mM AA min−1 at 25 °C considering an extinction coefficient for AA of 9249/M cm at 265 nm (Al-Madhoun et al. 2003). PME activity was determined using 5 g/L pectin as substrate and 0.1 g/L phenol red solution (0.003 mol/L potassium phosphate buffer) and adjusting the pH of the final solution to 7.5 (Hagerman and Austin 1986). The substrate mixture was incubated at 25 °C for 15 min before usage. One unit activity of PME was defined as change in absorbance of 0.001 units (at 545 nm) per minute. Further, specific activities of all enzymes were calculated as activity per mg protein and was used for kinetic data analysis.
Kinetic data analysis
Rate constants (k) were calculated for inactivation of POD, PPO, PME and AO in samples treated at specified temperatures and times. Similarly, k were calculated for deactivation of lycopene and AA by heating pulp to various temperatures for specified time in water bath. ‘k’ is the slope of a plot of the natural log of residual activity versus time of heat exposure.
| 1 |
where, ‘a’ and ‘a0’ is the enzyme activity/concentration of lycopene/concentration of AA at time ‘t’ and ‘t0’.
The values of activation energy required for inactivation of the enzymes were obtained from Arrhenius plots. An Arrhenius Law normally describes the temperature dependence of the rate constant as:
| 2 |
where, Ea is the activation energy (KJ/mol), R is the gas constant (8.314 J/mol K) and T and Tref are the temperatures in K at which rate constant k and kref are considered.
The decimal reduction time (D-value), time (min) needed for a tenfold reduction of the initial activity at a given temperature and was calculated using following equation:
| 3 |
where, k is reaction constant for first-order kinetics at given temperature.
The Z-value is the temperature increase that is needed to achieve a tenfold decrease in D-value. The Z-value is the slope of a log of D-value plotted against absolute temperature.
| 4 |
where D1 and D2 are the decimal reduction time at temperatures T1 and T2, respectively.
Statistical analysis
All determinations were conducted at least in triplicates. Analysis of variance (ANOVA) and Duncan’s multiple range test was employed to determine the statistical significance of the differences between the means (p ≤ 0.05) by using IBM SPSS statistics 20.
Results and discussion
Thermal processing in the form of HTST plays a major role in providing shelf-stable food with acceptable quality by inactivating enzymes, reducing the microbial load and maintaining the overall product quality. Enzymes could be used as bio-indicators in such processes as most enzymes have D and Z-values within a range similar to that for microorganisms (Smelt and Brul 2014). Guava pulp being low pH food has potential susceptibility to spoilage by Saccharomyces cerevisiae (yeast) and Lactobacillus plantarum (lactic acid bacteria) with Z-value of 3.8–6.1 and 5.3–6.7 °C, respectively (Lemgo Database). These are therefore inactivated during normal heat processing (Fellows 2009). However, some heat resistant enzymes can pose a problem in the processing of acidic foods. They may not be completely denatured by the relatively short heat treatments and temperatures used for microbial destruction.
Thermal inactivation of POD
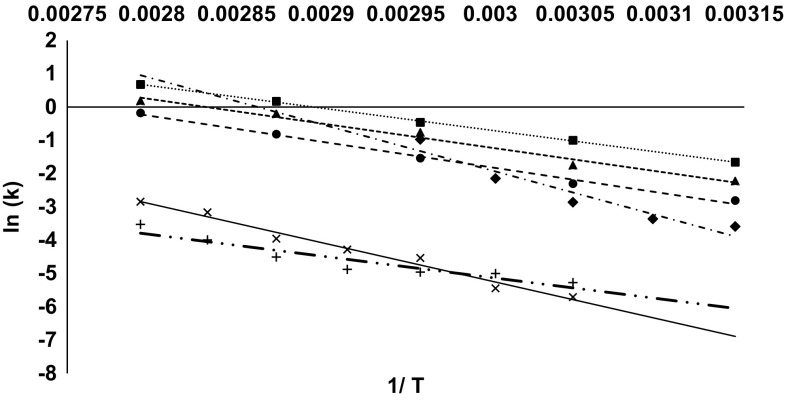
POD, one of the most thermostable enzymes found in fruits and vegetables, has a role in causing quality deterioration. It is commonly used as an indicator for inactivation of endogenous enzymes during thermal processing for its simple and rapid assay technique. We studied the thermal stability of guava POD to determine its efficacy as an indicator enzyme during guava processing. The semilog plot of the residual activity of guava POD (Fig. 1a) versus heating time was linear in the temperature range of 45–85 °C (R2 > 0.95), which is consistent with inactivation by means of a simple (monophasic) first-order process. The presence of labile and resistant forms of POD are reported for fruits and vegetables such as butternut squash and pineapple (Agüero et al. 2008; Lee et al. 2009). However, in this study only the resistant fraction was observed which, may be due to rapid inactivation of heat-labile fraction during the early phase of heat treatment (Agüero et al. 2008). D-values were obtained in the range of 38.2–2.8 min. Arrhenius plots constructed from these inactivation rate constants were also linear (R2 = 0.99) (Fig. 2). D-values of POD at various temperatures have been specified in Table 1.
Fig. 1.
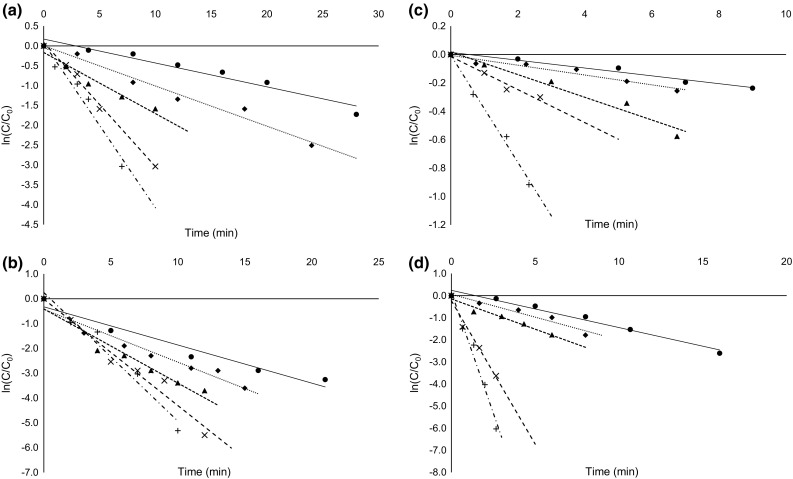
Thermal inactivation curves of guava a POD at 45 °C (circle), 55 °C (diamond), 65 °C (triangle), 75 °C (multiple), 85 °C (plus), b PPO at 45 °C (circle), 55 °C (diamond), 65 °C (triangle), 75 °C (multiple), 85 °C (plus), c AO at 45 °C (circle), 50 °C (diamond), 55 °C (triangle), 60 °C (multiple), 65 °C (plus), d PME at 45 °C (circle), 55 °C (diamond), 65 °C (triangle), 75 °C (multiple), 85 °C (plus)
Fig. 2.
Arrhenius plots of thermal deactivation of POD (circle), PPO (triangle), PME (square), AO (diamond), AA (multiple), lycopene (plus)
Table 1.
Kinetic parameters (D-value, Z-value and activation energy) for guava POD, PPO, PME, AO, ascorbic acid and lycopene in fruit pulp at atmospheric pressure
| POD | PPO | PME | AO | Ascorbic acid | Lycopene | |
|---|---|---|---|---|---|---|
| D values (min) | ||||||
| 45 °C | 38.3 ± 0.8m | 21.4 ± 0.4k | 12.1 ± 1.0h | 83.4 ± 6.7p | – | – |
| 50 °C | – | – | – | 66.6 ± 5.3° | – | – |
| 55 °C | 23.1 ± 0.5 l | 13.3 ± 0.3i | 6.3 ± 0.5f | 40.3 ± 3.2m | 697.9 ± 14.0y | 451.6 ± 9.0w |
| 60 °C | – | – | – | 19.8 ± 1.6j | 535.6 ± 10.7x | 343.7 ± 6.9v |
| 65 °C | 10.7 ± 0.2g | 5.0 ± 0.1e | 3.7 ± 0.3d | 6.1 ± 0.5f | 215.2 ± 4.3s | 329.0 ± 6.6u |
| 70 °C | – | – | – | – | 166.9 ± 3.3r | 303.0 ± 6.1t |
| 75 °C | 5.2 ± 0.1e | 2.8 ± 0.1c | 1.9 ± 0.2b | – | 120.6 ± 2.4q | 209.4 ± 4.2s |
| 80 °C | – | – | – | – | 54.6 ± 1.1n | 125.2 ± 2.5q |
| 85 °C | 2.8 ± 0.1c | 1.9 ± 0.0b | 0.8 ± 0.1a | – | 39.7 ± 0.8m | 78.3 ± 1.6p |
| Z values (°C) | 34.1 ± 0.7C | 36.2 ± 0.7D | 39.4 ± 0.1E | 14.3 ± 1.1A | 23.5 ± 0.5B | 41.7 ± 0.8F |
| Ea (kJ/mol) | 63.79 ± 1.28H | 60.36 ± 1.21G | 63.22 ± 1.06H | 106.33 ± 8.51J | 95.82 ± 1.92I | 54.92 ± 1.10F |
Data represents Mean ± SD of three independent experiments
Means among each set of data labelled by the same letter are not significantly different (p < 0.05) by Duncan’s multiple range test
Lower Ea values indicate more stability of the enzyme to temperature change. Ea of guava POD was observed to be 63.79 ± 1.28 kJ/mol. Similar results were observed for heat labile fractions of POD (Ea = 68.79 kJ/mol) extracted from pineapple, whereas, heat resistant fraction of pineapple showed higher Ea of 93.23 kJ/mol (Lee et al. 2009). Ea of seedless guava variety was reported to be much higher (101.46 ± 3.00 kJ/mol) (Ali et al. 2011) than that of the Lalit guava variety in the present study. However, lower Ea values of 14.023, 15.794 kJ/mol have also been reported for heat labile and heat sensitive fractions of butternut squash (Agüero et al. 2008). Higher Z-values reflect greater thermal stability of enzyme. Z-value for guava POD was calculated as 34.1 ± 0.7 °C. Z-value observed was higher than that reported for POD from mature coconut water (Tan et al. 2014). However, it was found to be similar to the Z-value obtained for POD from mangosteen pericarp (Deylami et al. 2014). Z-value from this study suggests that POD from Lalit guava had higher thermal stability compared to those from other sources.
Thermal inactivation of PPO
While POD is one of the most thermostable enzyme found in fruits and vegetables this may not stand true in every case. From the present studies on guava PPO, it was found that the thermal stability of PPO was slightly higher than POD. The semilog plot of the residual activity of guava PPO (Fig. 1b) versus heating time was consistent with inactivation by means of a monophasic first-order process similar to guava POD. Heat-labile fraction of PPO was not observed and the D-values obtained were in the range of 21.4–1.9 min (Table 1).
Ea value of 60.36 ± 1.21 kJ/mol makes PPO equally stable to temperature change like POD. Lower Ea values were observed for soluble PPO extracted from starking apple (Ea = 53.76 kJ/mol), Izmir grape (Ea = 51.88 kJ/mol) and Victoria grape must (Ea = 53.34 kJ/mol) (Onez et al. 2008; Soysal 2008). Also, higher Ea value of 146.1 ± 10.8 kJ/mol has been reported for PPO from Thompson seedless grape (Zheng et al. 2012). The calculated Z-value for guava PPO (36.2 ± 0.7 °C) was higher than guava POD (34.1 ± 0.7 °C). A similar phenomenon of higher Z-value for PPO over POD has been observed for mature coconut water (Tan et al. 2014). Z-value obtained for guava PPO was similar to that for PPO of sterile coconut water (39.5 °C), however, it was found to be higher than heat labile and heat resistant fractions of PPO extracted from mature coconut water (Tan et al. 2014). Z-value from this study suggests that PPO from guava has higher thermal stability compared to guava POD.
Thermal inactivation of PME
A first-order kinetic model in the temperature range of 45–85 °C could accurately describe thermal inactivation of guava PME. Semilog plot of the residual activity of guava PME (Fig. 1d) versus heating time resulted in D-values in the range of 12.1-0.8 min. Table 1 presents the kinetic parameter estimates for thermal inactivation of guava PME. In the temperature range studied, Arrhenius plot defined Ea value of 63.22 ± 1.06 kJ/mol for guava PME which is in close accordance with 64.5 kJ/mol reported for Paluma cultivar of Brazilian guava varieties (da Silva Cerqueira Leite et al. 2006). Ea for other fruits were reported to be as low as 12.39 kJ/mol for Malatya apricot (Ozler et al. 2008) to as high as 206.1 kJ/mol for Alyanak apricot (Ünal and Şener 2013) and 300 kJ/mol in apple and cloudberry juice (Wilińska et al. 2008).
Z-value of 39.4 ± 0.1 °C resembles to that of heat resistive fraction of orange PME and was found to be much higher than the other reported Z-values for PME from Alyanak apricot, tomato juice.(Fachin et al. 2002; Ünal and Şener 2013) Such high Z-value defines the higher stability of the enzyme when challenged with thermal processing. Guava PME has higher Z-value than the other enzymes studied, making it a potential candidate as an indicator enzyme for thermal processing.
Thermal inactivation of AO
In-situ thermal inactivation of AO from pink guava also followed first-order kinetics with an Arrhenius dependence (Fig. 1c). A linear Arrhenius plot between inactivation rate constants [ln (k)] and absolute temperature (1/T) is depicted in Fig. 2. Kinetic parameters (D, Z, and Ea values) for AO inactivation have been summarized in Table 1. AO inactivation was enhanced with increase in treatment temperature. Based on the detection limit of the assay used in this study, a complete inactivation of AO can be defined to occur at temperatures > 80 °C. A semilog plot of the residual activity of guava AO (Fig. 1c) versus heating time resulted in D-values in the range of 83.4–6.1 min (Table 1).
Ea (106.33 ± 8.51 kJ/mol) obtained from the Arrhenius plot was higher than 10 kJ/mol as reported for AO purified from Cucurbita pepo medullosa (Maccarrone et al. 1993) and lower than 128.45, 167.36, 266.57 kJ/mol reported for cucumber, squash, broccoli florets (Itoh et al. 1995; Munyaka et al. 2010). The lower activation energy observed in the present study can be attributed to the inherent property of enzyme. Z-value of 14.3 ± 1.1 °C obtained for guava AO was lower than the range reported for peach and other vegetables (Gokmen 2010). Z-value of guava AO was found to be lowest amongst the guava enzymes studied in this work, suggests it to be the most heat labile enzyme.
Effect of thermal treatment on AA and lycopene
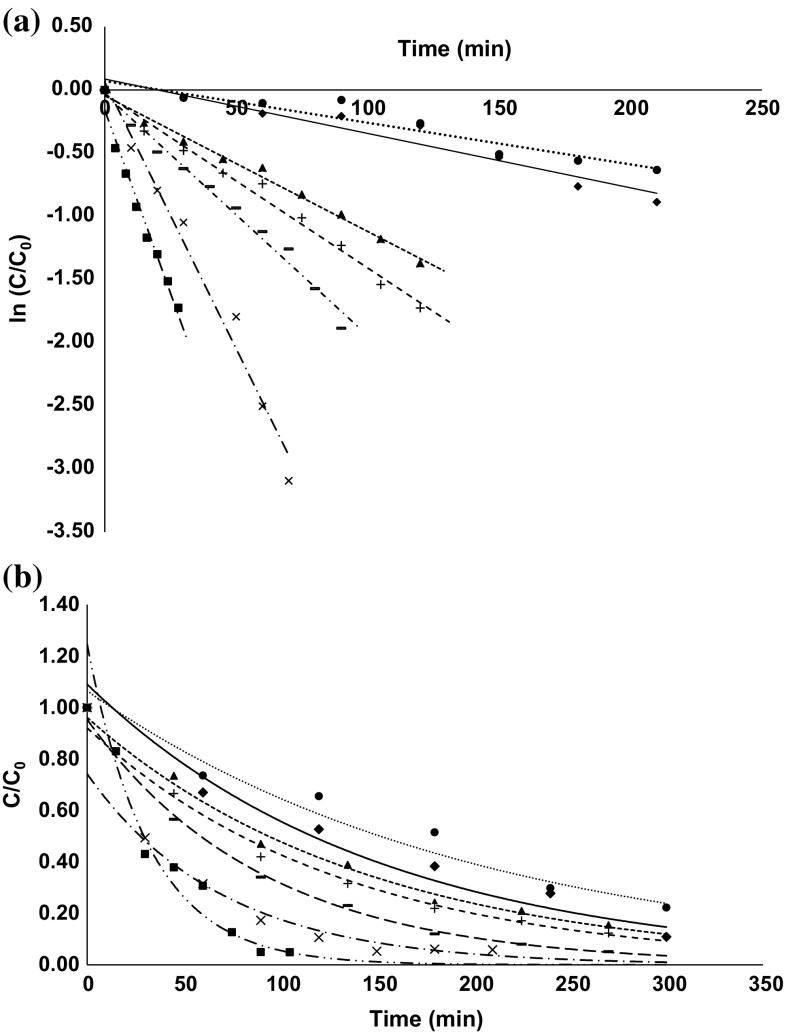
Guava is a rich source of AA and the content varies depending upon the geographical location, horticultural practices, season of harvest and cultivar (Ali et al. 2011). The total AA content of fresh Lalit guava (untreated) was found to be 290.31 ± 3.60 mg/100 g of edible flesh. The AA degradation as a function of time and temperature was analysed to define the kinetics of the degradation reaction (Fig. 3a). Though, first-order equation gave a proper fit, the reaction must be a pseudo first-order reaction as many factors and pathways would be involved in degradation of AA (Uddin et al. 2002). D-values for AA degradation are reported in Table 1. It was observed that longer treatment time at lower temperatures led to AA losses similar to that for higher temperatures in shorter treatment time, attributed to instability of AA at high temperature (Ali et al. 2011).
Fig. 3.
Thermal inactivation curves of a ascorbic acid and b lycopene of guava at 55 °C (circle), 60 °C (diamond), 65 °C (triangle), 70 °C (plus),75 °C (line), 80 °C (multiple) and 85 °C (square)
Arrhenius plots constructed from inactivation rate constants were found to be linear (R2 = 0.9796) and gave Ea of 95.82 ± 1.92 kJ/mol. Ea values for inactivation of AA has been reported in the range of 53.43, 76.86, 76.86, 79.24 and 105.27 kJ/mol for lemon, grapefruit, watermelon, tangerine and orange, respectively (Burdurlu et al. 2006; Tola and Ramaswamy 2015). Z-value of 23.5 ± 0.5 °C calculated for degradation of AA in guava pulp (present study) was similar to Z-value reported for AA degradation in orange juice of 24.4 °C (Vikram et al. 2005). Apart from this, the other reported Z-values for thermal destruction of L-AA in squeezed oranges and tomatoes were 27.15 and 30.15 °C, respectively (Van Den Broeck et al. 1998). The results of Ea and Z-value suggests that AA from Lalit guava variety was weakly resistance to thermal processing than the AA reported in other fruits.
Pink guava is a rich source of lycopene which thereby possesses a risk of being degraded (via isomerization and oxidation) during processing, reducing the health benefits (Tola and Ramaswamy 2015). Lycopene degradation as a function of time and temperature fitted well in an exponential model, the model indicating continuous degradation of lycopene and higher degradation at increased temperature (Fig. 3b). However, k value from this model did not represent the reaction rate constant. Therefore, the data was fitted into the first order model to compare the effect of different temperature treatments. The reaction rate constant and hence D-values were obtained from the semilog plot of the residual activity of total lycopene at various temperatures (Table 1).
An Arrhenius plot between natural logarithm of apparent k versus inverse of absolute temperature for lycopene loss during heat treatment followed a linear relationship (R2 = 0.90). The Ea obtained for lycopene degradation was 54.92 ± 1.10 kJ/mol which was lower than the Ea values (97.7 kJ/mol) reported for watermelon Juice (Tola and Ramaswamy 2015). The Z-value for lycopene in guava pulp (41.7 °C) was higher than Z-value (24.5 °C) reported for lycopene from watermelon juice (Tola and Ramaswamy 2015) as well as Z-value of the quality deteriorating enzymes studied in this work, which suggests that lycopene in guava pulp can survive thermal processing.
Conclusion
Heat processing in the form of HTST is necessary both to prevent microbial spoilage and to inactivate the intrinsic quality deteriorating enzymes such as POD, PPO, PME and AO. This study suggests that PME (Z-value = 39.4 ± 0.1 °C) is the most heat stable enzyme and needs to be considered while thermal processing of Lalit guava pulp. Guava enzymes studied showed higher heat stability than the reported spoilage organisms and AO was the most heat sensitive enzyme. Lycopene was found to be stable to heat processing whereas, AA showed lesser heat stability and was adversely affected in the process of inactivation of enzymes. The focus on only microbial load reduction during fruit processing hence needs to be widened to inactivate the endogenous quality-degrading enzymes.
Acknowledgements
The authors are grateful to University Grants Commission (UGC) for providing the financial support for carrying out this work.
References
- Agüero MV, Ansorena MR, Roura SI, Del Valle CE. Thermal inactivation of peroxidase during blanching of butternut squash. LWT Food Sci Technol. 2008;41:401–407. doi: 10.1016/j.lwt.2007.03.029. [DOI] [Google Scholar]
- Ali G, Russly A, Jamilah B, et al. Effect of heat and thermosonication on kinetics of peroxidase inactivation and vitamin C degradation in seedless guava (Psidium guajava L.) Int Food Res J. 2011;18:1289–1294. [Google Scholar]
- Al-Madhoun AS, Sanmartin M, Kanellis AK. Expression of ascorbate oxidase isoenzymes in cucurbits and during development and ripening of melon fruit. Postharvest Biol Technol. 2003;27:137–146. doi: 10.1016/S0925-5214(02)00090-X. [DOI] [Google Scholar]
- Burdurlu HS, Koca N, Karadeniz F. Degradation of vitamin C in citrus juice concentrates during storage. J Food Eng. 2006;74:211–216. doi: 10.1016/j.jfoodeng.2005.03.026. [DOI] [Google Scholar]
- da Silva Cerqueira Leite KM, Tadiotti AC, Baldochi D, Oliveira OMMF. Partial purification, heat stability and kinetic characterization of the pectinmethylesterase from Brazilian guava, Paluma cultivars. Food Chem. 2006;94:565–572. doi: 10.1016/j.foodchem.2004.12.008. [DOI] [Google Scholar]
- Deylami MZ, Rahman RA, Tan CP, et al. Thermodynamics and kinetics of thermal inactivation of peroxidase from mangosteen (Garcinia mangostana L.) Pericarp. J Eng Sci Technol. 2014;9:374–383. [Google Scholar]
- Evoli LD, Lombardi-boccia G, Lucarini M. Influence of heat treatments on carotenoid content of cherry tomatoes. Foods. 2013;2:352–363. doi: 10.3390/foods2030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachin D, Van Loey AM, Ly Nguyen B, et al. Comparative study of the inactivation kinetics of pectinmethylesterase in tomato juice and purified form. Biotechnol Prog. 2002;18:739–744. doi: 10.1021/bp0155080. [DOI] [PubMed] [Google Scholar]
- Fellows P. Food processing technology principles and practices. Amsterdam: Elsevier; 2009. [Google Scholar]
- Gokmen V. Enzymes in fruit and vegetable processing: chemistry and engineering applications. New York: CRC Press Taylor and Francis Group; 2010. [Google Scholar]
- Gomez PAML, Lajolo FM. Ascorbic acid metabolism in fruits: activity of enzymes involved in synthesis and degradation during ripening in mango and guava. J Sci Food Agric. 2008;88:756–762. doi: 10.1002/jsfa.3042. [DOI] [Google Scholar]
- Hagerman AE, Austin PJ. Continuous spectrophotometric assay for plant pectin methyl esterase. J Agric Food Chem. 1986;34:440–444. doi: 10.1021/jf00069a015. [DOI] [Google Scholar]
- Itoh H, Hirota A, Hirayama K, et al. Properties of ascorbate oxidase produced by Aeremonium sp. HI-25. Biosci Biotechnol Biochem. 1995;59:1052–1056. doi: 10.1271/bbb.59.1052. [DOI] [Google Scholar]
- Lee TH, Chua LS, Tan ETT, et al. Kinetics of thermal inactivation of peroxidases and polyphenol oxidase in pineapple (Ananas comosus) Food Sci Biotechnol. 2009;18:661–666. [Google Scholar]
- Maccarrone M, D’Andrea G, Salucci ML, et al. Temperature, pH and UV irradiation effects on ascorbate oxidase. Phytochemistry. 1993;32:795–798. doi: 10.1016/0031-9422(93)85207-8. [DOI] [Google Scholar]
- Morton JF (1987) Guava. In: Fruits of warm climates. Miami, pp 356–363
- Munyaka AW, Makule EE, Oey I, et al. Thermal stability of l-ascorbic acid and ascorbic acid oxidase in broccoli (Brassica oleracea var. italica) J Food Sci. 2010;75:336–340. doi: 10.1111/j.1750-3841.2010.01573.x. [DOI] [PubMed] [Google Scholar]
- Onez Z, Karakus E, Pekyardimci S. Izmir grape polyphenol oxidase (Vitis vinifera L.): partial purification and some kinetic properties. J Food Biochem. 2008;32:396–414. doi: 10.1111/j.1745-4514.2008.00178.x. [DOI] [Google Scholar]
- Ortuño C, Duong T, Balaban M, Benedito J. Combined high hydrostatic pressure and carbon dioxide inactivation of pectin methylesterase, polyphenol oxidase and peroxidase in feijoa puree. J Supercrit Fluids. 2013;82:56–62. doi: 10.1016/j.supflu.2013.06.005. [DOI] [Google Scholar]
- Ozler A, Karakuş E, Pekyardimci S. Purification and biochemical characteristics of pectinesterase from Malatya apricot (Prunus armeniaca L.) Prep Biochem Biotechnol. 2008;38:358–375. doi: 10.1080/10826060802325469. [DOI] [PubMed] [Google Scholar]
- Patras A, Brunton N, Da Pieve S, et al. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov Food Sci Emerg Technol. 2009;10:16–22. doi: 10.1016/j.ifset.2008.09.008. [DOI] [Google Scholar]
- Shi J, Maguer MLE, Bryan M, Kakuda Y. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J Food Process Eng. 2002;25:485–498. doi: 10.1111/j.1745-4530.2003.tb00647.x. [DOI] [Google Scholar]
- Smelt JPPM, Brul S. Thermal inactivation of microorganisms. Crit Rev Food Sci Nutr. 2014;54:1371–1385. doi: 10.1080/10408398.2011.637645. [DOI] [PubMed] [Google Scholar]
- Soysal C. Kinetics and thermal activation/inactivation of starking apple polyphenol oxidase. J Food Process Preserv. 2008;32:1034–1046. doi: 10.1111/j.1745-4549.2008.00298.x. [DOI] [Google Scholar]
- Tan TC, Cheng LH, Bhat R, et al. Composition, physicochemical properties and thermal inactivation kinetics of polyphenol oxidase and peroxidase from coconut (Cocos nucifera) water obtained from immature, mature and overly-mature coconut. Food Chem. 2014;142:121–128. doi: 10.1016/j.foodchem.2013.07.040. [DOI] [PubMed] [Google Scholar]
- Terefe NS, Kleintschek T, Gamage T, et al. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov Food Sci Emerg Technol. 2013;19:57–65. doi: 10.1016/j.ifset.2013.05.003. [DOI] [Google Scholar]
- Thongsook T, Barrett DM. Heat inactivation and reactivation of broccoli peroxidase. J Agric Food Chem. 2005;53:3215–3222. doi: 10.1021/jf0481610. [DOI] [PubMed] [Google Scholar]
- Tola Y, Ramaswamy H. Temperature and high pressure stability of lycopene and vitamin C of watermelon Juice. African J Food Sci. 2015;9:351–358. doi: 10.5897/AJFS2014.1258. [DOI] [Google Scholar]
- Uddin M, Hawlader MN, Ding L, Mujumdar A. Degradation of ascorbic acid in dried guava during storage. J Food Eng. 2002;51:21–26. doi: 10.1016/S0260-8774(01)00031-0. [DOI] [Google Scholar]
- Ünal MÜ, Şener A. Extraction and characterization of pectin methylesterase from Alyanak apricot (Prunus armeniaca L.) J Food Sci Technol. 2013;52:1194–1199. doi: 10.1007/s13197-013-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broeck I, Ludikhuyze L, Weemaes C, et al. Kinetics for isobaric-isothermal degradation of L-ascorbic acid. J Agric Food Chem. 1998;46:2001–2006. doi: 10.1021/jf9708251. [DOI] [Google Scholar]
- Vikram VB, Ramesh MN, Prapulla SG. Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. J Food Eng. 2005;69:31–40. doi: 10.1016/j.jfoodeng.2004.07.013. [DOI] [Google Scholar]
- Vishwasrao C, Ananthanarayan L. Postharvest shelf-life extension of pink guavas (Psidium guajava L.) using HPMC-based edible surface coatings. J Food Sci Technol. 2016;53:1–21. doi: 10.1007/s13197-015-2164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilińska A, de Figueiredo Rodrigues AS, Bryjak J, Polakovič M. Thermal inactivation of exogenous pectin methylesterase in apple and cloudberry juices. J Food Eng. 2008;85:459–465. doi: 10.1016/j.jfoodeng.2007.08.009. [DOI] [Google Scholar]
- Zheng Y, Shi J, Pan Z. Biochemical characteristics and thermal inhibition kinetics of polyphenol oxidase extracted from Thompson seedless grape. Eur Food Res Technol. 2012;234:607–616. doi: 10.1007/s00217-012-1664-4. [DOI] [Google Scholar]