Abstract
Starch and chitosan are biodegradable polymers from renewable sources that can be used to overcome the serious environmental problem caused by improper disposal of synthetic plastic materials, non-biodegradable, derived from petroleum sources. The starch–chitosan based films manufactured allow improving the better characteristics of each one, adding their good characteristics and compensating for some limitations. In this work, it was studied: two sources of starch (corn and cassava), two different modes of chitosan addition (chitosan blended in the starch filmogenic solution and chitosan as coating), and the effect of glutaraldehyde as crosslinking agent. All films were prepared by casting using glycerol as a plasticizer and were characterized by their physicochemical (water vapor permeability, water contact angle, and FTIR), mechanical, and antimicrobial properties. The properties analyzed were influenced by all variables tested. Moreover, the principal component analysis was also conducted in order to relate and describe the variables analyzed. The antimicrobial activity of the corn starch-based films containing chitosan was confirmed, and these films have potential for development of active packaging.
Keywords: Corn starch, Cassava starch, Chitosan, Glutaraldehyde, Blend, Coating
Introduction
Starch is a semi-crystalline polymer composed of two polysaccharides: amylose and amylopectin. Amylose, a mostly linear chain, typically consists of up to 3000 glucose molecules interconnected primarily by α-1,4 glycosidic linkages and is reported to contain a few branched networks. Amylopectin is a large branched polymer with α-1,4 linkages that serve as the backbone and α-1,6 bridges at the branching points (Copeland et al. 2009). Furthermore, chitosan is a natural carbohydrate polymer obtained by the deacetylation of chitin [poly-β-(1 → 4)-N-acetyl-d-glucosamine], a major component of crustacean shells such as crab, shrimp and crawfish, which could be obtained from fish industry wastes (Demarger-Andre and Domard 1994). These polymers are biodegradable from renewable sources and both can be used for film and coating production. This can be the solution to minimize the high generation of non-biodegradable solid waste from packaging. One of the advantages of a faster material degradation in landfill is that the volume of waste to be compacted would be also reduced. A decrease in the degradation time, even of a few days, represents a significant reduction of waste volume and hence, it leads to a very important benefit with regard to environmental precaution (Medina Jaramillo et al. 2016).
Starch based films have been particularly considered for the reason that they exhibit physical characteristics similar to synthetic polymers, besides having biodegradable properties (Mali et al. 2010). However, the hydrophilic and semi-crystalline starch nature can difficult their processing since the material is more susceptible to moisture and temperature changes, and has greater structural rigidity than conventional synthetic polymers. Thus, the relatively more hydrophobicity of chitosan film could be expected to improve the moisture barrier properties and water resistance including dimensional stability of starch films. The combination of hydrogen bonding, opposite charge attraction between chitosan cations and negatively charged starch film surface, hydrophilicity, and compatible water activities provided a good adherence between starch and chitosan film (Bangyekan et al. 2006).
According to Chen et al. (2014) polymer blends using chitosan surprisingly can minimize the individual limitations of the other biopolymers being a potential replacement of petroleum-based non biodegradable polymers in some applications. Croisier and Jérôme (2013) cite that chitosan is a unique bio-source polymer that exhibits exceptional properties, beside biocompatibility and biodegradability, mainly due to the presence of primary amines. Furthermore, some authors have already verified that chitosan is a functional biopolymer with intrinsic antimicrobial and antioxidant properties and consequently, it has high potential to be used as an alternative biodegradable active food package (Bangyekan et al. 2006; Vásconez et al. 2009; Shen et al. 2010; Van Den Broek et al. 2015).
Holley et al. (2000), Devlieghere et al. (2004) and Fernandez-Saiz et al. (2009) have demonstrated antimicrobial activity of chitosan films against fungi and bacteria. As verified by these studies only the soluble protonated fraction of chitosan that is released from the solid film upon liquid phase contact (on antimicrobial test) is capable of acting as a biocide agent. These researchers have observed antimicrobial activity of chitosan films produced by casting because in this process occurs a previous dissolution of chitosan in acid medium when filmogenic solution is prepared. This action protonates the NH2 groups of chitosan enhancing its solubility. In this case, the positively charged molecules may interact with the negatively charged membranes of bacteria, resulting in membrane rupture and cell death.
Antimicrobial activity of chitosan have been also evaluated in chitosan–starch films; Shen et al. (2010) studied the antimicrobial effect of sweet potato starch based films incorporated with chitosan in. The authors observed the inhibition effect of Escherichia coli and Staphylococcus aureus when was utilized the chitosan amount of 5 and 10%, respectively. Vásconez et al. (2009) evaluated interactions between chitosan-tapioca starch with/without potassium sorbate and two different techniques for chitosan addition (coating and blend). In this work, it was verified that antibacterial action depended on the application technique, because chitosan is more available in a coating solution than in a film matrix. Besides the antimicrobial activity, the physical properties of the films were also affected. Bangyekan et al. (2006) also produced chitosan-coated cassava starch films using an automatic coater and observed that these samples presented better mechanical properties and a notable reduction of wettability and water vapor permeability.
The use of antimicrobial films has become very attractive for several applications in the food industry, particularly due to the successful results obtained. The direct incorporation of a biocide into the packaging material could provide several advantages, such as the maintenance of a high concentration of the active agent directly on food surface, with low migration; the decrease of chances of active substance inactivation by food constituents; and the avoidance of the use of this substance as a food additive (Pelissari et al. 2009).
Therefore, the objective of this work was to evaluate the effect of the addition of chitosan in corn and cassava starch films. For this, two techniques were tested: starch and chitosan blend films and starch based films with chitosan coating. Moreover, it was investigated two botanical sources of starch (corn and cassava) and the use of a crosslinking agent (glutaraldehyde) in the chitosan coating solution. It is noteworthy that the starch based films were made by casting using glycerol as plasticizer; lactic acid was used to solubilize the chitosan and to produce filmogenic solutions based on this material. The lactic acid was chosen based on the results of Velásquez-Cock et al. (2014) and Niamsa and Baimark (2009) who highlighted that the lactic acid acts as a plasticizer originating films more flexible and extensible when compared to those prepared with acetic acid.
Materials and methods
The corn (Delaware Company, RS, Brazil) and cassava (Natal Public Market, RN, Brazil) starches were used to prepare filmogenic solutions. Glycerol used was analytical grade (Nuclear, SP, Brazil). Additionally, the commercial high viscosity chitosan (degree of deacetylation above 75%), obtained from crab shell (Sigma-Aldrich, Missouri, EUA) and lactic acid (85%) (Synth, SP, Brazil) were used for films manufacture. For crosslinking chitosan, a 25% glutaraldehyde solution (Vetec, SP, Brazil) was utilized.
Solutions preparation
Chitosan 0.5 g (w/w) was dispersed in 100 mL of 1% (v/v) lactic acid solution under stirring for 24 h for complete chitosan solubilization, according the experimental planning presented in Table 1. For crosslinked chitosan solutions, 10% of glutaraldehyde (related to biopolymer weight) was added to the solution under vigorous stirring for 15 min to ensure a complete mixture.
Table 1.
Experimental conditions to corn, cassava and chitosan based films preparation
| Formulation | Corn starch (g/ 100 mL) | Cassava starch (g/ 100 mL) | Glycerol (g/ 100 mL) | Water (mL) | Chitosan (g) | Chitosan coating (mL) |
|---|---|---|---|---|---|---|
| Corn starch film (M) | 3 | – | 0.9 | 96.1 | – | – |
| Cassava starch film (C) | – | 3 | 0.9 | 96.1 | – | – |
| Chitosan film (Q) | – | – | – | – | 0.5 | – |
| Corn starch–chitosan blended film (MQ) | 3 | – | 0.9 | 96.1 | 0.5 | – |
| Cassava starch–chitosan blended film (CQ) | – | 3 | 0.9 | 96.1 | 0.5 | – |
| Corn starch film coated with chitosan (EM) | 3 | – | 0.9 | 96.1 | – | 5 |
| Cassava starch film coated with chitosan (EC) | – | 3 | 0.9 | 96.1 | – | 5 |
| Corn starch film coated with crosslinked chitosan (ERM) | 3 | – | 0.9 | 96.1 | – | 5 |
| Cassava starch film coated with crosslinked chitosan (ERC) | – | 3 | 0.9 | 96.1 | – | 5 |
In MQ and CQ formulations 10 mL of 1% (v/v) lactic acid solution was added to the filmogenic solution after gelatinization to ensure an acidic pH
Corn and cassava starch at concentration of 3 g/ 100 mL, glycerol (0.9 g/ 100 mL) and distillated water were used to prepare the filmogenic solutions. The suspensions were heated in a water bath at 80 °C for 35 min under stirring, to accomplish a complete starch gelatinization. The samples were identified by the letters M and C, for corn and cassava starch-based films, respectively.
Preparation of blended starch/chitosan-based films
After cooling of the filmogenic solutions, 10 mL of a solution 1% (v/v) of lactic acid was added to starch solutions in order to ensure the acidic pH and prevent chitosan precipitation. Subsequently, the chitosan solution was poured into the starch filmogenic solutions, mixed and stirred until complete homogeneity. The blend solution was spread on petri dishes (0.3 g cm−2). The drying was carried out in a food dryer (DeLeo A5AFD/0915, Brazil) with forced convection for 24 h at 35 °C. The samples were identified with the letters MQ for corn starch/chitosan blend and with the letters CQ for cassava starch/chitosan blend.
Preparation of starch-based films coated with chitosan
After cooling, the starch filmogenic solution was spread in petri dishes by casting (0.3 g cm−2) and then, the samples were placed in a food dryer (DeLeo A5AFD/0915, Brazil) with forced convection for 24 h at 35 °C. After drying, the coating chitosan solution (5 mL) was overspread on the starch film surface. Finally, the coated films were dried for 15 h. The samples were identified with the letters EM for corn starch films coated with chitosan and with the letters EC for cassava starch films coated with chitosan.
Preparation of starch casting films coated with crosslinked chitosan
The same procedure described in “Preparation of starch-based films coated with chitosan” section was followed. However, the chitosan solution coating used in this stage was crosslinked using glutaraldehyde. The samples were identified by the letters ERM for the corn starch films coated with crosslinked chitosan and by the letters ERC for the cassava starch films coated with crosslinked chitosan.
Film characterization
The biodegradable starch–chitosan based films were characterized by physicochemical (water vapor barrier, water contact angle measurements), mechanical and antimicrobial properties, with the aim to evaluate their suitability as possible materials for the food packaging sector. The films were conditioned under 60% relative humidity (RH) at 25 °C at least 48 h before analyses.
Thickness measurement
Film thickness was measured by a digital micrometer (Mitutoyo IP 65, Japan). Six thickness values were taken along the length of the filmstrip (at least 5 samples for each formulation) and the mean value was used for mechanical properties and water vapor permeability calculation.
Water vapor permeability analysis
The water vapor permeability (WVP) analysis was done according to the standard test method ASTM E96 (2002). For this, samples were superposed on permeation cells filled with silica (RH = 0%). The films water vapor permeability (WVP) was determined in triplicate using Eq. (1):
| 1 |
where WVP is the water vapor permeability (g mm m−2 h−1 kPa−1); w is the water mass which permeated through the film (g); e is the average film thickness (mm); A is the permeation area (m2); t is the permeation time (h) and Δp is the water vapor pressure (kPa).
Water contact angle analysis
The water contact angle (WCA) was measured by the sessile drop method using the Krüss equipment (Hamburg, Germany). Deionized water (3 μL) was dripped on the films surface with a microsyringe; the photographic images acquisitions were taken with Drop Shape Analysis (DSA4) software. The water contact angles values were measured immediately after to drip the water drop on the film surface. The measurements were done in triplicate.
Mechanical properties analysis
The mechanical properties of the films were evaluated using a texturometer (Texture analyzer TA.XT2i) according to ASTM D882 method (ASTM, 2012), used for films with a thickness below 1 mm. For this, the films were placed in the machine with an initial grip separation of 50 mm and operating speed of 0.8 mm s−1.
Chemical structure analysis
The Fourier Transform Infrared Spectroscopy (FTIR) using a Perkin Elmer Spectrum Model 1000 was made to acquire the spectra of chemical structure of the samples. The spectra were obtained in ATR mode, with a resolution of 4 cm−1 and average of 32 scans.
Antimicrobial activity
Antimicrobial activities of the starch and chitosan films were analyzed by agar diffusion method (Silva et al. 2013) using the microorganisms naturally present in a commercial cooked ham. Samples present on the cooked ham slice surface were collected using cotton swabs sterilized at 121° C for 15 min in an autoclave. The swab was moistened in 0.1% of bacteriological peptone solution (Bacteriological Peptone—BD), rubbing at a 30° angle to the ham surface, in the “zig-zag” shape, by the diagonal directions at the collection area (5 cm × 5 cm) and transferred to a test tube containing 10 mL of 0.1% bacteriological peptone solution. After, 100 μL of the inoculum (bacteriological peptone solution suspension, 0.1% and collected microorganisms) were plated by spreading in the plate count agar (PCA, Merck).
Subsequently, film disks samples (2.5 cm of diameter) previously sterilized by UV for 30 min were placed in the Petri dishes, containing agar and inoculum, and were incubated at 30 °C for 48 h. In order to compare the results, control plates containing only the solution of agar and inoculum, without film sample, were also inoculated in the same conditions. The assays were performed in duplicate, with two or three film samples for each Petri dish. After the incubation period, the aerobic mesophilic bacteria growth inhibition zones were evaluated.
Statistical analysis
The Tukey’s test was performed on STATISTICA 8.0 software (Statsoft Inc., Tulsa, USA) with confidence level of 95% (p < 0.05). For comparison purposes, commercial films based on polyvinyl chloride (PVC), low density polyethylene (LDPE) and corn starch (CS) were also characterized.
In order to compare the mechanical properties and permeation results, a Principal Components Analysis (PCA) using a covariance matrix was also performed using STATISTICA 8.0 software (Statsoft Inc., Tulsa, USA). The physicochemical (water vapor permeability and permeation) and mechanical properties (tensile strength, elongation at break, and Young’s modulus) of films were used as active variables in the derivation of the principal components, and the different formulation samples (corn, cassava, chitosan blended or coated and with or without glutaraldehyde films) were projected onto the factor space.
Results and discussion
By visual examination, the corn and cassava starch–chitosan films obtained were colorless, uniforms, thin, and homogeneous. Moreover, the films were easily removed from the Petri dishes after drying due to their flexibility and they did not contain bubbles or surface cracks. It should be noted that chitosan films were translucent, unlike the corn and cassava starch films that were slightly opaque.
Physicochemical and water barrier properties
Thickness measurements are presented in Table 2; it could be seen that these results ranged between 0.08 and 0.13 mm for all formulations, except to chitosan film that have 0.04 mm. These results are very similar to Bangyekan et al. (2006) and Vásconez et al. (2009) who developed chitosan-coated cassava starch films and chitosan-tapioca starch based edible films and coatings, respectively.
Table 2.
Thickness, water vapor permeability and water contact angle measurements analysis
| Film | Thickness (mm) | Water vapor permeability (g mm m−2 h−1 kPa−1) | WCA (°) |
|---|---|---|---|
| Corn starch film (M) | 0.13 ± 0.01A | 0.26 ± 0.02C | 31 ± 22G |
| Cassava starch film (C) | 0.11 ± 0.02B | 0.27 ± 0.04C | 49 ± 1EF |
| Chitosan film (Q) | 0.04 ± 0.01E | 0.06 ± 0.007E | 104 ± 2A |
| Corn starch–chitosan blended film (MQ) | 0.09 ± 0.003CD | 0.19 ± 0.01D | 43 ± 3F |
| Cassava starch–chitosan blended film (CQ) | 0.08 ± 0.007D | 0.17 ± 0.01D | 60 ± 3D |
| Corn starch film coated with chitosan (EM) | 0.10 ± 0.01BC | 0.30 ± 0.06BC | 87 ± 3B |
| Cassava starch film coated with chitosan (EC) | 0.11 ± 0.01B | 0.36 ± 0.05B | 52 ± 7E |
| Corn starch film coated with crosslinked chitosan (ERM) | 0.11 ± 0.01B | 0.31 ± 0.01B | 79 ± 5C |
| Cassava starch film coated with crosslinked chitosan (ERC) | 0.12 ± 0.03AB | 0.41 ± 0.01A | 74 ± 2C |
| PVC based film | 0.008 ± 0.0005E | 0.03 ± 0.003E | – |
| LDPE based film | 0.008 ± 0.0005E | 0.003 ± 0.0001E | – |
| Corn starch (CS) commercial film | 0.011 ± 0.0005E | 0.04 ± 0.01E | – |
Different letters in the columns indicate that there are statistically significant differences (p < 0.05) between samples through the Tukey’s multiple range tests
The water vapor permeability (WVP) and water contact angle (WCA) measurements for all samples are also displayed in Table 2. It was possible to verify that the water vapor permeability of chitosan films was ten times lower than others films. The hydrophobic acetyl groups of incompletely deacetylated chitosan caused a notable reduction of water vapor permeability which is preferable for packaging film application (Bangyekan et al. 2006; Bourtoom and Chinnan 2008). This behavior also can be related to the intense hydrogen bond interactions between NH2 and OH functional groups of chitosan (Thakur et al. 2017). The technique of chitosan addition (blended in the film matrix or coated on the starch film surface) promoted changes in the surface properties. A significant increase in water contact angle values of the films with chitosan blended or coated was obtained, indicating a lower wettability of starch–chitosan films in comparison with starch films. Probably, this occurred because the hydrophobic nature of chitosan film which presented a WCA of 104° ± 2°.
Similar results were presented by Bangyekan et al. (2006) who evaluated the chitosan coating solutions (varying from 1 to 4 wt %) addition in cassava starch films and by Jantanasakulwong et al. (2016) after blending of thermoplastic starch, natural rubber and chitosan system. The chitosan addition permitted to obtain samples with higher water contact angle (low wettability) in comparison with the control sample, this result was suggested to miscibility of hydrophobic chitosan with thermoplastic cassava starch phase. Furthermore, hydrogen bond interactions between starch and chitosan may reduce the availability of the hydrophilic groups, diminishing their interactions with water molecules (Vásconez et al. 2009). This fact has been confirmed by the significant lower values of the water vapor permeability of the starch–chitosan blended films (CQ and MQ). Thus, starch–chitosan blended films presented a better barrier to water vapor and the chitosan coating in starch films can be considered a less efficient technique to prevent moisture transfer between food and the surrounding atmosphere. Chillo et al. (2008) and Lopez et al. (2014) tested the effect of chitosan addition on tapioca and corn starch-based film properties by blend solutions previously to casting and thermo-compression process, respectively, as manufactured techniques and observed that the chitosan presence promoted a decrease in WVP values; similar water barrier results were found in present work. These authors explain this effect by the increase of interactions between chitosan and corn starch (hydrogen bonding type), which decrease availability of the hydrophilic groups, therefore, the water vapor transmission rate of the matrix decreased.
Moreover, similar to the result found in present work, no considerable differences in water barrier properties were observed by Li et al. (2013) comparing chitosan/starch composite films after and before crosslinking (using glutaraldehyde), even when the amount of crosslinking agent increases.
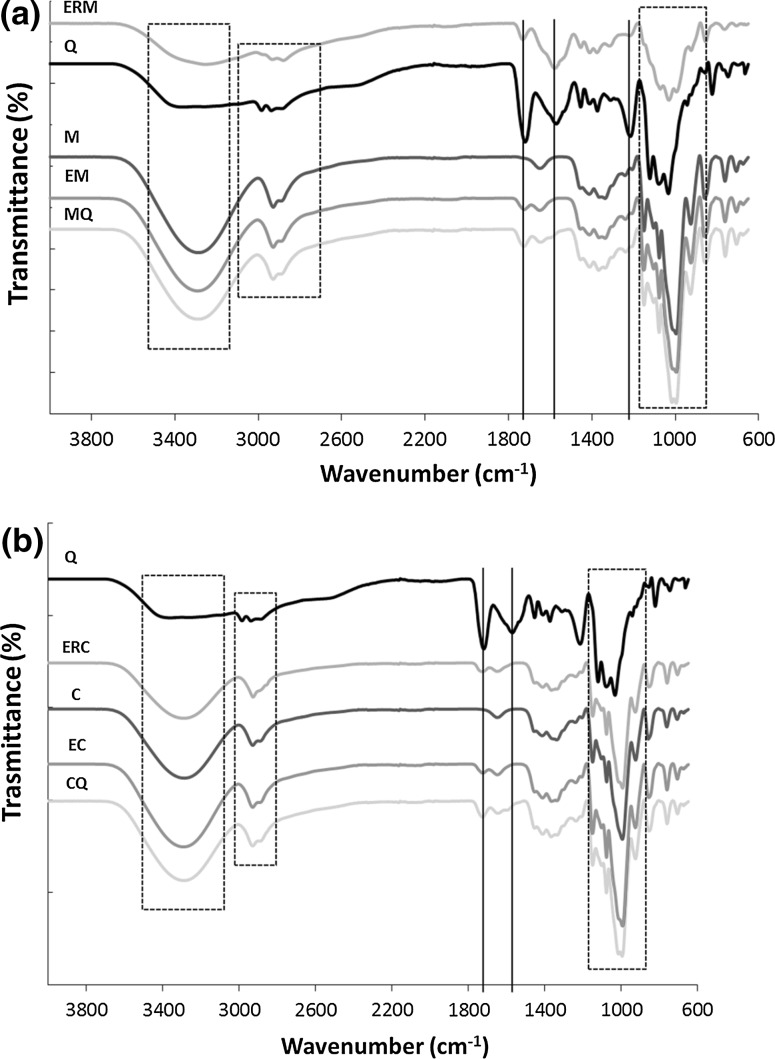
The chemical structure of corn and cassava starch films and starch–chitosan films were analyzed by Fourier transformed infrared spectroscopy (FTIR) and these results are presented in Fig. 1a, b, respectively. In general, the FTIR spectrum analysis shows the similar behavior for the starch and chitosan–starch films. It is possible to observe for all samples the region peak 3500–3000 cm−1 that corresponds to OH–, the C–H stretching in region peak 2980–2860 cm−1, and the characteristics of polysaccharides peaks in the region around 1000 cm−1, as highlighted with dashed lines in the graphs. The corn and cassava starch powder had peaks positioned at 1020 cm−1 (higher intensity) and 995 cm−1. According Singh et al. (2010), these peaks are consistent with a semicrystalline material since fully crystalline materials show similar intensity peaks centered around 1020 and 1006 cm−1.
Fig. 1.
FTIR spectra (a) of chitosan film (Q), corn starch film (M), corn starch–chitosan blended film (MQ) and corn starch film coated with chitosan (EM) and crosslinked chitosan (ERM); b cassava starch film (C) and cassava starch–chitosan blended film (CQ) and cassava starch film coated with chitosan (EC) and crosslinked chitosan (ERC)
Nevertheless, when two components are mixed, the physical blends versus chemical interactions are affected by changes in the characteristic spectra peaks (Guan et al. 1997; Yin et al. 1999; Xu et al. 2005). Comparing the starch–chitosan blended and coated films with the films containing only chitosan or starch, it was observed a displacement in some characteristics peaks that can indicate interactions between these biopolymers (Mendes et al. 2016). Because of that, changes in the starch–chitosan films FTIR spectrums were detected when compared with those presented by the starch films. It was observed peaks in the regions of 1740 cm−1 referring to typical amide I (C=O) and in the region of 1620–1530 cm−1 referring to amines and secondary amides (C–N) stretching band groups (Silva-Pereira et al. 2015), which are characteristics from chitosan and lactic acid solution. Similar changes were founded by Bourtoom and Chinnan (2008) after the rice starch–chitosan biodegradable blend film preparation. In addition, it is necessary to highlight that contrary to others chitosan/starch based films, the ERM (corn starch film coated with crosslinked chitosan) film displayed a FTIR spectrum more similar to chitosan film rather than to corn starch film, probably because of the more homogenous corn starch surface layer. On the contrary, the ERC (cassava starch film coated with crosslinked chitosan) presented more similar characteristics with cassava starch film. It can be related with the gummy aspect (touch perceptible) of the cassava starch film that could have favored the solution-diffusion of chitosan through the sample, becoming similar to the blended film.
Mechanical properties
The mechanical properties results: tensile strength [MPa], maximum percentage of elongation [%] and elastics modulus [MPa] for all samples are presented in Table 3. From mechanical properties analysis it could be observed that in comparison with the other samples, the chitosan films presented the lower elastic modulus, i.e., lower rigidity, and also higher percentage of elongation. Furthermore, it is possible to say that this characteristic migrated to blended and coated films, which had lower elastic modulus compared to the starch films. Pelissari et al. (2009) also related that the chitosan addition in starch films led to a significant reduction of the Young’s modulus (p < 0.05) and, therefore, the formation of less rigid structures. Another explanation for this behavior is the plasticizer characteristic of lactic acid (Niamsa and Baimark 2009), which helps to promote higher percentage of elongation to the films.
Table 3.
Mechanical properties analysis
| Film | Tensile strength (MPa) | Elongation (%) | Elastic modulus (MY) (MPa) |
|---|---|---|---|
| Corn starch film (M3) | 5.0 ± 0.1C | 62 ± 4CDE | 79 ± 9BC |
| Cassava starch film (C3) | 3.0 ± 0.5DE | 96 ± 27BCDE | 90 ± 42B |
| Chitosan film (Q) | 1.1 ± 0.1F | 202 ± 32A | 0.8 ± 0.07E |
| Corn starch–chitosan blended film (MQ) | 5.3 ± 0.8C | 108 ± 7BC | 44 ± 11BCDE |
| Cassava starch–chitosan blended film (CQ) | 3.2 ± 0.1DE | 146 ± 9AB | 13 ± 2E |
| Corn starch film coated with chitosan (EM) | 2.7 ± 0.2E | 77 ± 2CDE | 33 ± 1BCDE |
| Cassava starch film coated with chitosan (EC) | 2.2 ± 0.3E | 110 ± 20BC | 23 ± 4DE |
| Corn starch film coated with crosslinked chitosan (ERM) | 2.9 ± 0.2E | 46 ± 9DE | 48 ± 5BCDE |
| Cassava starch film coated with crosslinked chitosan (ERC) | 2.3 ± 0.2E | 43 ± 6E | 28 ± 2CDE |
| PVC based film | 17.1 ± 0.7A | 45 ± 6E | 81 ± 2BC |
| LDPE based film | 3.9 ± 0.4D | 99 ± 50BCD | 71 ± 7BCD |
| Corn starch (CS) commercial film | 10.1 ± 0.2B | 98 ± 13BCD | 205 ± 45A |
Different letters in the columns indicate that there are statistically significant differences (p < 0.05) between samples through the Tukey’s multiple range tests
According Shen et al. (2010), the chitosan–starch hydrogen bonding interaction is the intrinsic factor which determines the mechanical and physical properties of the films. Analyzing the results for maximum percentage of elongation, the blended starch and chitosan films presented the higher deformation. This result suggested that there was synergic compatibility between starch and chitosan biopolymers after film formation, once the individual elongation percentage values were lower than the blended starch and chitosan films. Moreover, the elongation of cassava starch films, independently of chitosan presence, was higher in comparison with corn starch films. Contrary, the chitosan used as coating did not present significant differences in the elongation percentage when compared with starch films, but presented lower values in comparison with pure chitosan (Q) film.
In addition, the crosslinked ones presented the lower elongation values; this result is a direct consequence of crosslinking chemical modification, which combines and/or creates new bonds, leading to a decrease in the mobility of polymer chains and, consequently, the films becomes less deformable.
The corn and cassava starch–chitosan films presented higher tensile strength (2.2–5.3 MPa) and elongation (43–146%) values composed to those presented by Ren et al. (2017). These authors developed starch and chitosan (dissolved in acetic acid solution) films by casting and the mechanical properties were evaluated; the films reaching the values of 3 MPa for tensile strength, 38 MPa for MY and 58% for elongation, similar to results displayed here for corn and cassava starch films coated with crosslinked chitosan. Moreover, similar to results displayed by Ren et al. (2017) the incorporation of chitosan resulted in an increase in elongation at break. Some differences in the mechanical properties values can be due to the different amount of glycerol used in film production. However, the mechanical properties results in the present work were higher than the values published by Llanos and Tadini (2018) after production of biocomposite films based on cassava native starch and chitosan (tensile strength = 1.6 MPa; MY = 25 MPa and elongation = 27%), corroborating that the films developed have potential for use in the packaging sector.
Comparing the results with commercial films, blended and coated films presented lower rigidity and a tensile strength (ranging between 2.2 and 5.3 MPa) similar to LDPE based film; but lower than PVC and cornstarch (CS) based commercial films. However, the starch films coated with crosslinked chitosan presented a maximum elongation similar to the PVC film and the others properties comparable to LDPE and corn starch commercial films. In general, the Young’s Modulus (3–90 MPa) were lower for all samples in comparison with commercial ones, excepted for corn and cassava starch films without chitosan that displayed values (79–90 MPa) comparable with PVC and LDPE commercial films.
Multivariate analysis
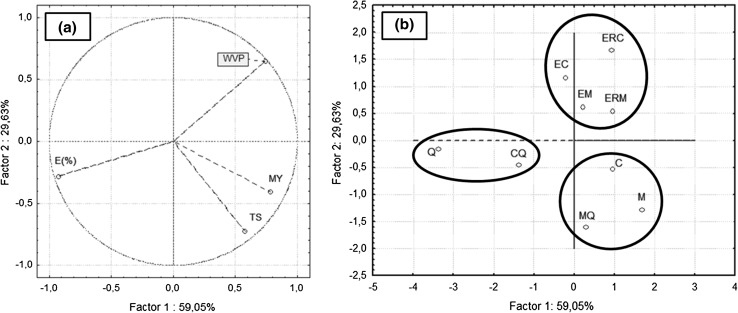
The Fig. 2 presents the results of the principal component analysis (PCA): (a) physicochemical (water vapor permeability and permeation) and mechanical properties (tensile strength, elongation at break, and Young’s modulus) of films were used as active variables in the derivation of the principal components, and (b) the different formulation samples (corn, cassava, chitosan blended or coated and with or without glutaraldehyde films) were projected onto the factor space. By this analysis it was possible to observe that the two principal components explained 88.7% of total variance.
Fig. 2.
Results of the principal component analysis (PCA): a projection of the barrier and mechanical properties in the plane defined by the two principal components. b Score plots in the PC2 vs. PC1 plane of films. Chitosan film (Q), corn starch film (M), corn starch–chitosan blended film (MQ) and corn starch film coated with chitosan (EM) and crosslinked chitosan (ERM); b cassava starch film (C) and cassava starch–chitosan blended film (CQ) and cassava starch film coated with chitosan (EC) and crosslinked chitosan (ERC)
The principal components analysis allowed separate the samples in different groups according the similar results. The samples containing only corn (M), cassava (C) or chitosan (Q) and the starch–chitosan blended samples (MQ and CQ) presented more strong correlation with mechanical properties values. However, the samples coated with chitosan, independent of the use of the crosslinking agent (EM, EC, ERM and ERC), presented more strong correlation with water vapor permeability values.
Three similar groups could be observed in the Fig. 2. The first analogous group represented by corn (M), cassava (C) starch films and corn starch–chitosan blended samples (MQ) presented the higher tensile strength (TS) and Young’s modulus (MY) values or rigidity and are located in the right side at the bottom of the chart. Other group, located in the left side at the bottom, was represented by chitosan (Q) and cassava starch–chitosan blended films (CQ) that exhibited the higher percentage of elongation values. In turn, all samples coated with chitosan (EM, EC, ERM and ERC) presented more strong correlation with water vapor permeability values and are preferentially located on the right side of the PCA graph in the top. These results suggested that the technique used for chitosan addition in starch films influenced not only the water vapor barrier but also the mechanical properties of the films. However, the glutaraldehyde used as crosslinking agent does not seem to have a strong influence on the films properties.
Antimicrobial properties
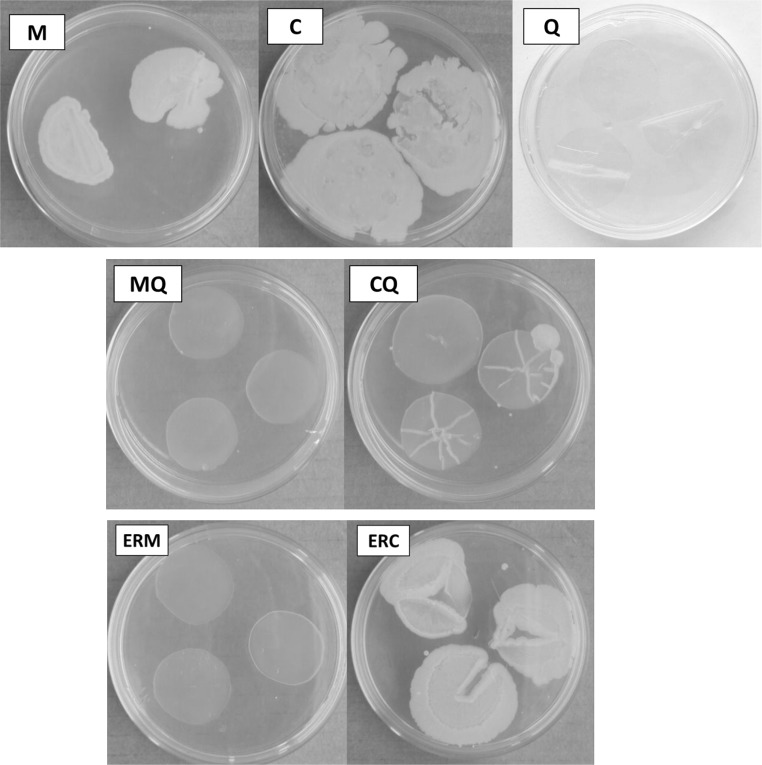
Preliminary and qualitative tests were performed in order to verify the antimicrobial activity of chitosan addition in starch films, as previously described in “Antimicrobial activity” section. By the agar diffusion method none of the samples evaluated presented the halo formation, however, it was verified an inhibitory effect against aerobic mesophilic bacteria after chitosan addition in MQ, CQ and ERM films. Furthermore, it is interesting the discussion of the aerobic mesophilic bacteria growth because is directly related to the shelf-life of the products, since the presence of these microorganisms can result in food deterioration.
Microbial growth was observed in corn and cassava starch films (M and C, respectively), according to Fig. 3, and was not observed in chitosan (Q) films. Comparing the samples containing only starch, it can be noticed that these samples did not present antimicrobial properties; on the contrary, presented higher growth of microorganisms serving as a nutrient for their development. This effect suggested that the use of corn and cassava starch film as food packaging should be careful. Food application tests using corn and cassava starch films should take into account this result.
Fig. 3.
Results of the microbial growth in corn starch film (M), cassava starch film (C), crosslinked chitosan film (Q), corn and cassava starch chitosan blended films (MQ and CQ), corn starch film coated with crosslinked chitosan (ERM) and cassava starch film coated with crosslinked chitosan (ERC)
The antibacterial activity evaluation of the films showed that there was not microbial growth on the agar surface in contact with the corn (MQ) starch films containing chitosan, as presented in Fig. 3. However, it is noteworthy that despite some positive results, in the CQ film there was a development of microorganisms in one of the samples, as displayed in Fig. 3. This probably occurred due to a problem of homogeneity in the film-forming solution used, since it was noticeable a higher viscosity (visual aspect) when compared to MQ filmogenic solution. Silva et al. (2013) investigated chitosan film using agar diffusion method and also did not observe the halo formation (inhibition zone) in the samples, however, in the chitosan film surface was not observed bacterial growth, similar to the results obtained in this study.
Preliminary tests (data not showed) were made in order to evaluate the effect of the utilization of the glutaraldehyde as crosslinked agent on the antimicrobial properties of the chitosan solution used as coating on the surface starch films; it can be verified that there was no visual differences between samples. For this reason, second tests (swab of ham) were conducted only with the crosslinked films because these samples presented better characteristics in other parameters analyzed (for example: more facility to handle). The antimicrobial analysis for the starch films coated with crosslinked chitosan is presented in Fig. 3.
Once again, no microbial growth was observed in the corn starch films coated with crosslinked chitosan. This result was different from that found by Vásconez et al. (2009); for these authors, the antimicrobial effect of chitosan addition was more effective when applied as coating on the surface of the films than blended in filmogenic matrix, i.e., the antibacterial action of chitosan was dependent on the application technique.
However, comparing the cassava starch–chitosan films, it is possible to observe that the application technique for chitosan addition influenced in the microorganism growth. The blended samples (CQ) presented smaller microbial growth than the coated samples (ERC), probably because the blended filmogenic solution has a higher chitosan concentration than the coating solution. Furthermore, the positive and negative microbial growth results can be related with FTIR analysis presented before. The ERM spectrum showed peaks similar to chitosan films (Q) and exhibited inhibition of microorganism growth. Differently, the ERC spectrum showed a behavior more similar to cassava starch films (C) and present microorganism growth.
Conclusion
Starch/chitosan-based films were successfully prepared and their physicochemical (water vapor permeability, water contact angle and FTIR), mechanical and antimicrobial properties were determined. The results provided important information that can be used to select the better application of the films, since the type of starch, the technique of chitosan addition and the presence of the crosslinking agent caused considerable changes in the studied properties. Moreover, PCA was a useful tool to identify similarities and differences among groups of films relating them to representative parameters of water barrier and mechanical properties.
The analyses of water vapor permeability, water contact angle, mechanical properties and antimicrobial activity of the starch and chitosan based films produced could provide additional information to select the potential application of the films. Furthermore, it should be noted that the antimicrobial activity of the starch based films with chitosan addition was confirmed; these samples presented potential for development of active packaging.
Acknowledgements
The authors acknowledge the financial support received from the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Level Personnel (CAPES), and the Research Support Foundation of the State of Rio Grande do Sul (FAPERGS) of Brazil. In particular, to thank CAPES CSF-PVE’s Project, process number 88881.068177/2014-01.
Contributor Information
Cláudia Leites Luchese, Phone: +55 (51) 3308-5485, Email: claudialuchese@yahoo.com.br.
Julia Menegotto Frick Pavoni, Email: juliamfrick@gmail.com.
Nicole Zagonel dos Santos, Email: nzagonel@gmail.com.
Luci Kelin Quines, Email: kelinquines@yahoo.com.br.
Liliane Damaris Pollo, Email: liliane.pollo@ufrgs.br.
Jordana Corralo Spada, Email: jcorralospada@yahoo.com.br.
Isabel Cristina Tessaro, Email: isabel@enq.ufrgs.br.
References
- ASTM International Standard test methods for water vapor transmission of materials 1. Astm. 2002;14:1–10. [Google Scholar]
- ASTM International ASTM D882: standard test method for tensile properties of thin plastic sheeting. ASTM Stand. 2012 [Google Scholar]
- Bangyekan C, Aht-Ong D, Srikulkit K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr Polym. 2006;63:61–71. doi: 10.1016/j.carbpol.2005.07.032. [DOI] [Google Scholar]
- Bourtoom T, Chinnan MS. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT Food Sci Technol. 2008;41:1633–1641. doi: 10.1016/j.lwt.2007.10.014. [DOI] [Google Scholar]
- Chen F, Monnier X, Gällstedt M, et al. Wheat gluten/chitosan blends: a new biobased material. Eur Polym J. 2014;60:186–197. doi: 10.1016/j.eurpolymj.2014.09.007. [DOI] [Google Scholar]
- Chillo S, Flores S, Mastromatteo M, et al. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J Food Eng. 2008;88:159–168. doi: 10.1016/j.jfoodeng.2008.02.002. [DOI] [Google Scholar]
- Copeland L, Blazek J, Salman H, Tang MC. Form and functionality of starch. Food Hydrocoll. 2009;23:1527–1534. doi: 10.1016/j.foodhyd.2008.09.016. [DOI] [Google Scholar]
- Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–792. doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- Demarger-Andre S, Domard A. Chitosan carboxylic acid salts in solution and in the solid state. Carbohydr Polym. 1994;23:211–219. doi: 10.1016/0144-8617(94)90104-X. [DOI] [Google Scholar]
- Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004;21:703–714. doi: 10.1016/j.fm.2004.02.008. [DOI] [Google Scholar]
- Fernandez-Saiz P, Lagaron JM, Ocio MJ. Optimization of the biocide properties of chitosan for its application in the design of active films of interest in the food area. Food Hydrocoll. 2009;23:913–921. doi: 10.1016/j.foodhyd.2008.06.001. [DOI] [Google Scholar]
- Guan Y, Liu X, Zhang Y, Yao K. Study of phase behavior on chitosan/viscose. J Appl Polym Sci. 1997;67:1965–1972. doi: 10.1002/(SICI)1097-4628(19980321)67:12<1965::AID-APP2>3.0.CO;2-L. [DOI] [Google Scholar]
- Holley RA, Ouattara B, Simard RE, Piette G. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol. 2000;62:139–148. doi: 10.1016/S0168-1605(00)00407-4. [DOI] [PubMed] [Google Scholar]
- Jantanasakulwong K, Leksawasdi N, Seesuriyachan P, et al. Reactive blending of thermoplastic starch, epoxidized natural rubber and chitosan. Eur Polym J. 2016;84:292–299. doi: 10.1016/j.eurpolymj.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Li H, Gao X, Wang Y, et al. Comparison of chitosan/starch composite film properties before and after cross-linking. Int J Biol Macromol. 2013;52:275–279. doi: 10.1016/j.ijbiomac.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Lopez O, Garcia MA, Villar MA, et al. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT Food Sci Technol. 2014;57:106–115. doi: 10.1016/j.lwt.2014.01.024. [DOI] [Google Scholar]
- Mali S, Grossmann MVE, Yamashita F. Filmes de amido: Produção, propriedades e potencial de utilização. Semin Agrar. 2010;31:137–156. doi: 10.5433/1679-0359.2010v31n1p137. [DOI] [Google Scholar]
- Medina Jaramillo C, Gutiérrez TJ, Goyanes S, et al. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr Polym. 2016;151:150–159. doi: 10.1016/j.carbpol.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Mendes JF, Paschoalin RT, Carmona VB, et al. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr Polym. 2016;137:452–458. doi: 10.1016/j.carbpol.2015.10.093. [DOI] [PubMed] [Google Scholar]
- Niamsa N, Baimark Y. Preparation and characterization of highly flexible chitosan films for use as food packaging. Am J Food Technol. 2009;4:162–169. doi: 10.3923/ajft.2009.162.169. [DOI] [Google Scholar]
- Pelissari FM, Pelissari FM, Grossmann MVE, et al. Antimicrobial, mechanical, and barrier properties of cassava starch–chitosan films incorporated with oregano essential oil. J Agric Food Chem. 2009 doi: 10.1021/jf9002363. [DOI] [PubMed] [Google Scholar]
- Ren L, Yan X, Zhou J, Tong J, Su X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int J Biol Macromol. 2017;105(3):1636–1643. doi: 10.1016/j.ijbiomac.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Shen XL, Wu JM, Chen Y, Zhao G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010;24:285–290. doi: 10.1016/j.foodhyd.2009.10.003. [DOI] [Google Scholar]
- Silva SS, Popa EG, Gomes ME, et al. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013;9:6790–6797. doi: 10.1016/j.actbio.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Silva-Pereira MC, Teixeira JA, Pereira-Júnior VA, Stefani R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci Technol. 2015;61:258–262. doi: 10.1016/j.lwt.2014.11.041. [DOI] [Google Scholar]
- Singh N, Belton PS, Georget DMR. The effects of iodine on kidney bean starch: films and pasting properties. Int J Biol Macromol. 2010;45(2):116–119. doi: 10.1016/j.ijbiomac.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Llanos HRJ, Tadini, CC (2018) Preparation and characterization of bio-nanocomposite films based on cassava starch or chitosan, reinforced with montmorillonite or bamboo nanofibers. Int J Biol Macromol 107(A):371–382. 10.1016/j.ijbiomac.2017.09.001 [DOI] [PubMed]
- Thakur R, Saberi B, Pristijono P, et al. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J Food Sci Technol. 2017;54:2270–2278. doi: 10.1007/s13197-017-2664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broek LAM, Knoop RJI, Kappen FHJ, Boeriu CG. Chitosan films and blends for packaging material. Carbohydr Polym. 2015;116:237–242. doi: 10.1016/j.carbpol.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Vásconez MB, Flores SK, Campos CA, et al. Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coatings. Food Res Int. 2009;42:762–769. doi: 10.1016/j.foodres.2009.02.026. [DOI] [Google Scholar]
- Velásquez-Cock J, Ramírez E, Betancourt S, et al. Influence of the acid type in the production of chitosan films reinforced with bacterial nanocellulose. Int J Biol Macromol. 2014;69:208–213. doi: 10.1016/j.ijbiomac.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Xu YX, Kim KM, Hanna MA, Nag D. Chitosan–starch composite film: preparation and characterization. Ind Crops Prod. 2005;21:185–192. doi: 10.1016/j.indcrop.2004.03.002. [DOI] [Google Scholar]
- Yin YJ, Yao KD, Cheng GX, Ma JB. Properties of polyelectrolyte complex films of chitosan and gelatin. Polym Int. 1999;433:429–432. doi: 10.1002/(SICI)1097-0126(199906)48:6<429::AID-PI160>3.0.CO;2-1. [DOI] [Google Scholar]