Abstract
This study was carried out to determine the effect of different concentrations of Bacillus subtilis (0, 1, 3, 5, and 7%) on the antioxidant potential and biochemical constituents of traditional Korean fermented soybean, Cheonggukjang (CKJ). The antioxidant capacity was studied using the reducing power, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) assays and the total phenolic contents (TPC) were measured using the Folin–Ciocalteu method. CKJ prepared using 1% B. subtilis revealed the highest TPC (5.99 mg/g), total amino acids (7.43 mg/g), DPPH (94.24%), and ABTS (86.03%) radical-scavenging activity and had the highest value of palmitic acid (11.65%), stearic acid (2.87%), and linolenic acid (11.76%). Results showed that the calcium, iron, sodium, and zinc contents increased in the CKJ prepared using 7% B. subtilis from 1481.38 to 1667.32, 41.38 to 317.00, 48.01 to 310.07, and 32.82 to 37.18 mg/kg respectively. In conclusion, the present results indicate that the fermentation of soybean with B. subtilis (KCTC 13241) significantly augments the nutritional and antioxidant potential of CKJ and it can be recommended as a health-promoting food source.
Keywords: Fermented soybean, Antioxidants, Functional food, Metabolite, Cheonggukjang
Introduction
Fermented foods provide health benefits when they are consumed. The microorganisms used in fermentation are associated with human and animal health (Hill et al. 2014). The benefits to human health are reduction in cholesterol levels, control of blood pressure, improved immune system (Reid et al. 2003), and allergy prevention (Cuello-Garcia et al. 2015). They are also a source of vitamins including vitamin B12, vitamin K, and folic acid (Molina et al. 2009). In food markets, they are generally sold as dairy products containing ‘live microbes’ and are available in drug stores as tablets or capsules consisting of lyophilized preparations of bacteria which enhance human health. Numerous Bacillus-containing products are consumed as functional foods with claims for promoting the well-being of consumers and restoring natural microflora to the intestine (Hong et al. 2005).
Soybeans (Glycine max) and its by-products play a notable role in the world’s economy. Soybean is famous as a novel food with high contents of protein, amino acids, minerals, vitamins, free sugars, fatty acids, total polyphenols, and isoflavones. Soybean can also serve as a substitute for meat protein (Xu et al. 2015). It is used in two forms, either fermented (e.g. soy sauce, cheonggukjang, tempeh, tofu, miso, and natto) or unfermented (e.g. soybean oil, soy butter, and fried soybeans) (Sanjukta and Rai 2016). Fermentation is used to preserve these foods, with the idea to save the food materials for periods of food scarcity. Currently, fermentation is used for increasing biologically active compounds to improve health. During the fermentation of soybeans, large, complex molecules are broken into smaller metabolites when the right microbes are present along with the optimum conditions for their growth. These microbes improve the physiological and biological properties of the fermented soybeans and also increase their nutritional value (Sanjukta et al. 2015).
Fermented soybean is very famous in many Asian countries. It includes doubanjiang and douche in China, miso, natto, and tofu in Japan, tempeh in Indonesia, and kinema, tungrymbai, and hawaijar in India. Cheonggukjang (CKJ), kanjang, doenjang, and meju are popular fermented soybean products in Korea (Sanjukta and Rai 2016). CKJ is considered as an excellent source of proteins, lipids, amino acids, vitamins, and minerals and is used commonly due to its health benefits. CKJ is also identified by its unique fibrous texture due to γ-aminobutyric acid that is released during fermentation (Yang et al. 2006). It promotes antioxidant, antigenotoxic, antimicrobial, anticancer, and antidiabetic activities as well as controlling cholesterol levels in the body (Omura et al. 2005). It has been found that CKJ is antimutagenic. The effect of CKJ on HT-29 colon cancer cells and AGS human gastric adenocarcinoma cells has been measured (Zhao et al. 2013).
On the basis of the above-mentioned characteristics of CKJ, this study was conducted to identify and determine a suitable concentration of Bacillus subtilis KCTC 13241 (Korean Collection for Type Cultures) for producing CKJ containing high fatty acid, amino acid, polyphenol, and mineral contents, as well as other parameters. In addition, we also investigated the in vitro antioxidative activity of CKJ to assess its role in reducing oxidative stress. For these objectives, we selected five different concentrations of B. subtilis (0, 1, 3, 5, and 7%) for incubation of soybean.
Materials and methods
Chemicals
HPLC-grade water, methanol, and acetonitrile were purchased from Fisher Scientific (Fairlawn, USA). Glacial acetic acid, Folin–Ciocalteu phenol reagent, 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (ABTS), potassium persulfate, ferric chloride, sodium acetate, 2,4,6-tripyridyl-s-triazine, and rutin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All were analytical grade chemicals.
Microorganism and soybean material
Previously isolated strain Bacillus subtilis KCTC 13241 (Lee et al. 2008) was used for the preparation of CKJ. B. subtilis was maintained in the laboratory on nutrient agar slants. For inoculum preparation, the activated culture was streaked onto nutrient agar slants and incubated at 37 °C for 18 h. The cells were harvested in sterile distilled water, and after adjusting to a concentration of 107–108 total cells mL−1, the suspension was used to inoculate cooked soybeans for fermentation. Soybean cultivar (Aga 3) was obtained for this experiment from the Genetics and Plant Breeding Lab, School of Applied Biosciences, Kyunpook National University, Daegu, South Korea.
Preparation of Cheonggukjang
A systematic representation of CKJ processing is shown in Fig. 1. Preparation of CKJ was carried out by the method of Ali et al. 2017 with some modifications. Soybeans (500 g) were sorted, washed, soaked in water for 12 h at room temperature. After draining, soybeans were heated for 30 min at 121 °C. The cooked soybeans were cooled to approximately 40 ± 3 °C and divided into five parts (100 g each). These five flasks were inoculated with 0, 1, 3, 5, and, 7% (w/w) B. subtilis culture medium (7.43 log CFU/mL) and fermented in an incubator for 60 h at 42 ± 1 °C. This experiment was repeated three times.
Fig. 1.
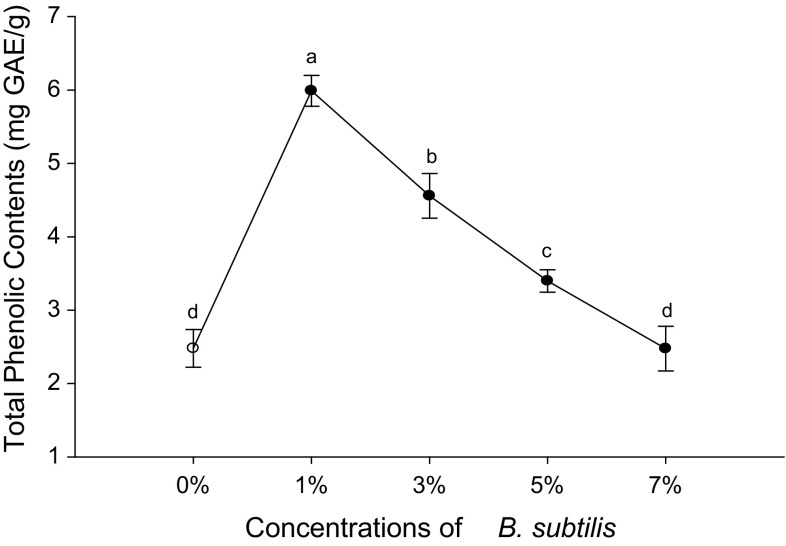
Total phenolic contents of Cheonggukjang using five different concentrations of the B. subtilis (KCTC 13241). Values are means of three independents experiments. The letters a, b, c, and d indicate significant differences (p ≤ 0.05)
Sample extraction
Extractions were carried out by the method of Xu and Chang (2005) with some modifications. Freeze-dried CKJ was finely ground using a grinding machine. The ground, freeze-dried sample was extracted using 5 g of sample and 50 mL extraction solvent (80% v/v methanol) and incubated at 25 °C for 24 h. The incubated extracts were centrifuged at 1660g for 20 min. The supernatants were filtered through a 0.45-μm Millipore PVDF filter (Schleicher & Schuell, GmbH, Dassel, Germany). The residue was then extracted with two additional treatments using 50 mL 80% methanol. The combined 150 mL methanol extract of each sample was then rotary evaporated at 40 °C to dryness and kept in the dark at 4 °C for further analysis.
Total polyphenol content (TPC)
Total polyphenol content was measured by the method of Cho et al. (2011) with slight modifications. One gram of powder CKJ was extracted with 10 mL methanol and incubated at 25 °C for 24 h at 150 rpm. The mixture was centrifuged at 1660g for 15 min and the supernatant liquid was filtered through a 0.2-µm syringe filter (Water, Milford, MA, USA). A methanolic extract (50 µL) was added to 1 mL of a 2% sodium carbonate solution and was left for 3 min. The mixture was added to 50 µL of 1 N Folin–Ciocalteu reagent and left in the dark for 30 min at room temperature. Quantification was performed using a linear regression equation derived from a gallic acid standard curve. Five gallic acid standard solutions of 100, 250, 500, 750, and 1000 mg/L were prepared in deionized water. The absorbance was read at 750 nm using a Multiskan GO Microplate Spectrophotometer (Thermal Fischer Scientific, Finland). The standard calibration curve was obtained by plotting concentration against absorbance.
DPPH radical-scavenging activity
The antioxidant activity of the CKJ extracts was assayed based on the scavenging activity of the stable 2,2-diphenyl-1-picrylhydrazyl free radical, as described by Bilal et al. (2016), with some modifications. Briefly, a 0.1 mM solution of DPPH in methanol (99.9% purity) was prepared. Subsequently, 4 mL of the sample solution in deionized water at different concentrations (250, 500, 750, and 1000 µg/g) was added to 1 mL of this solution. The solution was shaken vigorously and kept in the dark at room temperature for 30 min until stable absorption values were obtained. The absorbance was measured at 517 nm using a spectrophotometer (Thermo Fischer Scientific, Finland). Trolox was used as the control. The DPPH radical-scavenging activity was calculated by the following equation:
ABTS radical-scavenging activity
The spectrophotometric assay of ABTS radical-scavenging activity was measured as described in our previous study (Bilal et al. 2016). Briefly, the ABTS cation radical was generated by reacting a 7 mM ABTS solution with 140 mM potassium persulfate followed by incubation in the dark at room temperature for 14 h. Before use, the solution was diluted to achieve an absorbance of 0.7 ± 0.02 at 734 nm with 50% ethanol. To determine scavenging activity, the ABTS reagent was mixed with the samples (250, 500, 750, and 1000 µg/g) and the absorbance was measured at 734 nm using 50% ethanol as the blank. Trolox was used as a positive control. The scavenging capability of the ABTS radical was calculated using the following equation:
Estimation of reducing power
The reducing activity of the extracts was measured using the method described by Balakrishnan et al. (2011). Briefly, 100 µL of the extract was added to 900 µL of phosphate buffer (0.2 M, pH 6.6) and 900 µL of 1% potassium ferric cyanide. The solution was mixed thoroughly and incubated at 50 °C for 20 min. Nine hundred microliters of 10% TCA were added, mixed, and centrifuged at 1660g for 10 min. From the supernatant, 900 µL of the solution was mixed with 900 µL of distilled water and 900 µL of 0.1% FeCl3. The solution was mixed and reducing activity was measured at 700 nm using a spectrophotometer.
Free amino acid and fatty acid content analysis
The free amino acid composition of freeze-dried CKJ samples was determined according to the method of Shahzad et al. (2017). Briefly, the amino acid composition in all treatments was determined with a Hitachi Amino Acid Analyzer (L-8900, Hitachi, Japan) after hydrolysis of 100 mg protein with 6 M HCl at 110 °C for 24 h. An amino acid standard mixture solution for automatic amino acid analysis (Type H, Wako Pure Chemical Industries Ltd., Japan) was used for the quantification of endogenous amino acid content. All of the samples were run in triplicate and expressed in mg/g dry weight.
Fatty acid content of CKJ was analysed by the method described in Bilal et al. (2016). The freeze-dried ground powdered CKJ from each sample was extracted with n-hexane (10 mL). The samples were then placed in a shaking incubator at 50 °C. These samples were centrifuged and supernatants were transferred to new tubes. Hexane was removed by using a continuous flow of nitrogen. The extracted material from each sample was placed in a screw-capped vial and 5 mL of methylation solution (H2SO4/methanol/toluene 01:20:10 mL) was added. The sealed vial was heated in a water bath (100 °C) for 60 min and allowed to cool to room temperature. Then, 5 mL of water was added and the mixture was shaken. The mixture was separated into two layers; the upper layer was removed using a Pasteur pipette and dried using anhydrous sodium sulphate for 5 min. The GC–MS analysis was conducted with an Agilent Model 7890A Series system (Agilent, Dover, DE, USA) equipped with an Agilent 5975C MS detector and an Agilent 7683B autosampler. MS ChemStation A.03.00 was used. The GC–MS device was equipped with a DB-5MS capillary column (30 m × 0.25 mm i.d. × 0.25-µm film thickness; J&W Scientific-Agilent, Folsom, CA, USA) and helium was used as a carrier gas with a flow rate of 0.6 mL/min in split mode (1:50). Each sample (1 µL) was directly injected into the GC using an automatic sampler (Agilent 7683B). The injector and detector temperatures were 120 and 200 °C respectively. The column temperature was programmed from 50 to 200 °C at 10 °C min−1 and then held at 200 °C for 5 min. The mass conditions were as follows: ionisation voltage, 70 eV; scan rate, 1.6 scan/s; mass range 30–450; and ion source temperature, 180 °C. The components were identified based on their relative retention time and mass spectra compared with standards, Wiley7 N, NIST library data of the GC–MS system, and data from the literature.
Determination of minerals and carbohydrates
Minerals were identified and quantified as per the method described by Andualem and Gessesse (2014), with slight modifications. Fifteen millilitres of HNO3 was added to 0.5 g of a freeze-dried CKJ sample. An equal volume of distilled water was added for dilution. A plasma-atomic emission spectrometer (ICP AES: Varian Vista, Varian Australia, Victoria, Australia) was used to determine the concentrations of different minerals. Standards for each mineral were used for calibration of the instrument.
Carbohydrates in the samples were identified and quantified according to the method followed by Kang et al. (2014). Ground dried samples were homogenized with liquid nitrogen and sugars were extracted with aqueous ethanol. The ethanol was evaporated using a rotary evaporator. The residues were dissolved in water and the filtrate was injected into a Waters HPLC system (Millipore, Waters Chromatography, Milford, MA, USA). The sugar signals were detected using a Waters refractive index detector. De-ionized water was used as the mobile phase and the flow rate was 0.5 ml/min at 90 °C. Glucose and fructose were measured on the basis of peak areas and comparison with a calibration curve obtained with corresponding standards.
Statistical analysis
The experiment comprised five treatments (control with 0, 1, 3, 5, and 7% B. subtilis) with three replications. Collected data were subjected to analysis of variance using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). The mean values were compared using Duncan’s multiple range tests at (p ≤ 0.05) employing the statistical software program SAS (version 9.2, Cary, NC, USA). The results were expressed as mean ± SD (standard deviation) of three replicates. SigmaPlot was used for graphical presentations.
Results and discussion
Total phenolic content
The TPC of CKJ produced using different concentrations of B. subtilis (0, 1, 3, 5, and 7%) is shown in the Fig. 1 and expressed as milligram gallic acid equivalents (GAE) per gram of sample. Unfermented soybean and CKJ fermented by 7% B. subtilis showed the lowest value of TPC. CKJ prepared with 3 and 5% B. subtilis had TPC of 4.56 ± 0.3 and 3.39 ± 0.15 mg/g, respectively. TPC includes secondary metabolites that are present in all types of plants and have beneficial biological activities. Free radical-scavenging activity is based on hydrogen donating ability. Phenolic compounds are found as forms conjugated through hydroxyl groups with glycosides in plants. The release of phenolic compounds from soybeans may lead to enhancement of sugar and glycoside compounds during fermentation by microbes (Hwang et al. 2013). The results were comparable to previous studies on fermented soybeans and CKJ. One prior study showed that the TPC of fermented soybean (natto) was 637.62 ± 21.38 and 644 ± 11.20 μg/g after 0 and 48 h fermentation, respectively (Hu et al. 2010). Sanjukta et al. (2015) found that TPC in unfermented yellow soybean and black soybean were 1.93 and 1.64 mg/g, respectively, which increased to 8.4 and 7.5 mg/g, respectively, after 24 h of fermentation. However, the values for TPC in our CKJ samples were higher than natto and lower than that of yellow and black soybeans. These changes happened due to the different concentrations of B. subtilis that were used as a starter for soybean fermentation.
Antioxidant activities of CKJ
All samples of CKJ in our study exhibited DPPH radical-scavenging activity. The DPPH radical-scavenging activity of CKJ prepared using the five different concentrations of B. subtilis changed in a dose-dependent manner, as shown in Fig. 2a. CKJ fermented with 1% B. subtilis showed the highest percentage (94.24 ± 0.83%) of DPPH radical-scavenging activity, with a maximum concentration of 1 mg/mL. CKJ prepared using 0, 3, 5, and 7% B. subtilis showed 89.98 ± 0.58, 92.10 ± 0.29, 90.73 ± 0.89, and 88.41 ± 0.52% respectively. According to the findings observed in our study, it can be concluded that the 1 and 3% samples had the strongest DPPH radical-scavenging activity. Based on our findings, it was concluded that all samples, especially the 1 and 3% samples, had strong DPPH radical-scavenging potential by trapping ions, forming stable radicals, and terminating radical chain reactions. Previous studies showed that black soybeans Cheongja, Cheongja#3, Geomjeong#5, and Ilpumgeomjeong showed 62, 83, 70, and 80% DPPH radical-scavenging activity, respectively, after 48 h of fermentation (Haque et al. 2016). Our results showed higher activity than did Haque et al. (2016) and that may be due to different cultivars or microorganisms. Our findings indicated that all of the CKJ samples, particularly at 1%, can be used as the best source of natural antioxidants.
Fig. 2.
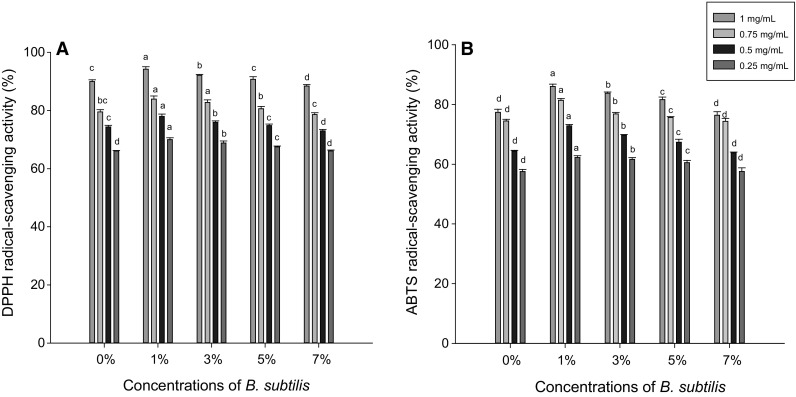
a DPPH radical-scavenging activity and b ABTS radical-scavenging activity of Cheonggukjang prepared using different concentrations of B. subtilis (KCTC 13241). Different colours show the different concentrations (1, 0.75, 0.5, and 0.25 mg/mL) in the samples. All values are the average of three separate experiments. The letters a, b, c, and d indicate significant differences (p ≤ 0.05)
ABTS results for the CKJ samples prepared using the five different concentrations of B. subtilis were moderately to strongly dose dependent, as shown in Fig. 2B. CKJ prepared using 1% B. subtilis exhibited the highest ABTS radical-scavenging activity (86.03 ± 0.81) with a concentration of 1 mg/mL in the sample, which was significantly (p ≤ 0.05) greater than that of the other samples. At the highest concentration (1 mg/mL), 1% CKJ exhibited 1.13-, 1.3-, 1.05-, and 1.11-fold greater inhibition percentages than did the 0, 3, 5, and 7% B. subtilis concentrations, respectively. Our findings were in agreement with those of the previous studies of Shin et al. (2014), who inoculated soybeans with B. subtilis W42 and B. amyloliquefaciens. Another study also mentioned that CKJ prepared from Seoritae and Seormoktae showed 91.06, and 81.12% ABTS free radical-scavenging activities after 48 h of fermentation (Hwang et al. 2013). These results strongly support our findings.
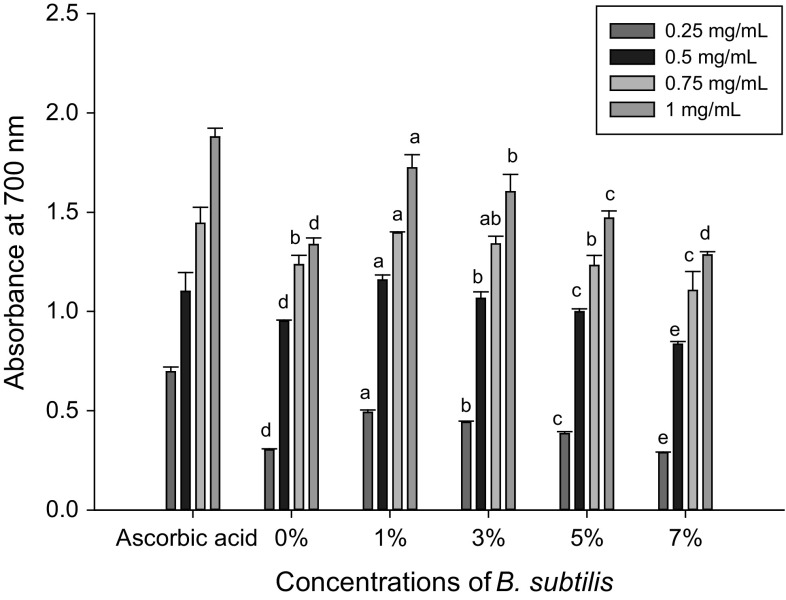
Reducing power activity is related to the presence in the samples of reductones, which are responsible for the termination of radical chain reactions. These compounds are hydrogen donors and can reduce oxidised intermediates during lipid peroxidation (Duh 1998). The reducing power potential of the CKJ samples prepared using the five different concentrations of B. subtilis and ascorbic acid as a standard are shown in Fig. 3. The CKJ samples (0, 1, 3, 5, and 7%) had 1.34, 1.72, 1.60, 1.47, and 1.28 nm reducing power, respectively, at 700 nm at maximum concentration dependent (1 mg/mL). The results were statistically significant (p ≤ 0.05). CKJ samples prepared using 1% B. subtilis is noted as the most prominent for reducing power potential. Past studies support our findings on the enhanced reducing power potential of fermented soybeans (Chung et al. 2002; Lin et al. 2006). Sanjukta et al. (2015) also reported that cooked yellow soybean and fermented yellow soybean prepared using B. subtilis MTCC 1747 had 6.75 ± 0.42 and 18.27 ± 0.18 mg AAE/g reducing power potential, respectively, as measured in methanol extracts. In addition, the peptides of the B. subtilis starter microbes, their electron-donating capacity, and intracellular antioxidant activity may be responsible for the enhancement of reducing power potential (Yang et al. 2000). Our results demonstrated that the CKJ samples, especially those prepared using 1 and 3% B. subtilis, had reducing power as potent as ascorbic acid. Therefore, these can be consumed as a cheaper, readily available source of antioxidants for the food, pharmaceutical, and cosmetic industries. Phenolic compounds are responsible for the higher values of antioxidant activities (Haque et al. 2016) and our findings corroborate well with that report. The total antioxidant activity of the extract cannot be expected on the basis of its TPC alone, a synergism of phenolic compounds, with one another, and other components present in CKJ may contribute to the overall detected antioxidant activity.
Fig. 3.
Reducing power activities of Cheonggukjang prepared using five different concentrations of B. subtilis (KCTC 13241) and standard compound ascorbic acid. The letters a, b, c, d, and e indicate significant differences (p ≤ 0.05, n = 3)
Changes in metabolites of CKJ
The changes in free amino acid composition in CKJ samples prepared without B. subtilis and using 1, 3, 5, and 7% B. subtilis were identified and quantified. The results of 17 essential and non-essential amino acids are summarised in Table 1. Aspartic acid, threonine, serine, glutamic acid, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, lysine, histidine, arginine, and proline were identified and quantified in our study. The total free amino acid values were in the range of 3.37–7.43 mg/g of freeze-dried sample. The value of cysteine in unfermented soybean was 1.15 ± 0.63 mg/g and this value was maximum at 7% B. subtilis while the value of methionine was maximum at 1% B. subtilis. CKJ samples prepared using 1% B. subtilis contained the highest amounts (7.43 mg/g) of amino acids among all sample. As the concentration of B. subtilis increased, the number of amino acids decreased and even the 7% B. subtilis sample (3.37 mg/g) had lower amounts than those in the 0% B. subtilis sample (4.45 mg/g).
Table 1.
Free amino acid analysis of Cheonggukjang prepared using five different concentrations of the B. subtilis (KCTC 13241)
| Amino acids1 | 0% B. subtilis | 1% B. subtilis | 3% B. subtilis | 5% B. subtilis | 7% B. subtilis |
|---|---|---|---|---|---|
| Aspartic acid2 | 4.335 ± 0.10d | 5.653 ± 0.43b | 5.888 ± 0.85a | 5.180 ± 0.46c | 0.445 ± 0.06e |
| Threonine | 3.617 ± 0.37c | 4.625 ± 0.55a | 4.575 ± 0.76a | 4.575 ± 0.64b | 0.564 ± 0.87d |
| Serine | 2.737 ± 0.62d | 3.860 ± 0.43b | 4.330 ± 0.87a | 3.352 ± 1.64c | 0.108 ± 0.07e |
| Glutamic acid | 12.812 ± 0.57d | 17.205 ± 1.72b | 19.187 ± 0.65a | 15.812 ± 2.04c | 0.745 ± 1.43e |
| Glycine | 0.350 ± 0.23e | 4.460 ± 1.23b | 4.867 ± 0.92a | 4.246 ± 0.83c | 1.071 ± 1.75d |
| Alanine | 4.619 ± 0.32c | 5.777 ± 0.83a | 5.079 ± 1.65b | 5.068 ± 1.52b | 5.071 ± 1.04b |
| Cystine | 1.150 ± 0.63e | 1.372 ± 0.68c | 1.548 ± 1.43b | 1.288 ± 1.33d | 1.957 ± 1.38a |
| Valine | 5.687 ± 0.76d | 6.623 ± 0.43a | 6.074 ± 1.21b | 5.938 ± 1.69c | 2.743 ± 1.87e |
| Methionine | 0.134 ± 0.81a | 0.155 ± 0.77a | 0.144 ± 0.38a | 0.134 ± 0.90a | 0.056 ± 0.02b |
| Isoleucine | 5.317 ± 0.95d | 6.255 ± 0.75a | 5.776 ± 0.68b | 5.565 ± 0.75c | 2.230 ± 0.62e |
| Leucine | 8.414 ± 0.37d | 10.232 ± 1.32a | 9.530 ± 0.44b | 9.059 ± 0.98c | 4.148 ± 0.83e |
| Tyrosine | 3.490 ± 0.05c | 4.178 ± 1.49a | 3.751 ± 0.32b | 3.541 ± 0.41c | 1.836 ± 0.76d |
| Phenylalanine | 5.537 ± 0.18d | 6.671 ± 0.87a | 6.379 ± 1.84b | 5.958 ± 0.87c | 2.872 ± 0.87e |
| Lysine | 6.076 ± 0.25d | 7.870 ± 0.43b | 8.296 ± 1.35a | 7.290 ± 0.54c | 2.853 ± 1.43e |
| Histidine | 2.845 ± 0.32c | 3.533 ± 0.76a | 3.607 ± 1.04a | 3.182 ± 0.09b | 1.033 ± 0.07d |
| Arginine | 6.078 ± 0.54d | 7.820 ± 0.43b | 7.342 ± 1.62c | 6.977 ± 0.42a | 3.643 ± 0.74e |
| Proline | 5.627 ± 0.12d | 6.419 ± 1.32a | 6.254 ± 0.85b | 5.861 ± 0.66c | 3.371 ± 0.45e |
| Total amino acids | 4.453 ± 0.68d | 7.430 ± 0.62a | 6.060 ± 0.91b | 5.872 ± 0.43c | 3.369 ± 0.72c |
1All values are average of determinations in three independent experiments. Means with different lowercase letters (a, b, c, and d) indicate significant differences p ≤ 0.05 of different concentrations of B. subtilis by Duncan’s multiple range tests (DMRT)
2The measuring unit is mg/g
Threonine, alanine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, histidine, and proline showed the highest values in the 1% B. subtilis sample. Aspartic acid, serine, glutamic acid, glycine, and lysine had the highest values in 3% B. subtilis. Therefore, the 1 and 3% B. subtilis extracts contained the highest amounts of amino acids among the CKJ samples. The values of valine, methionine, isoleucine, leucine, tyrosine, and phenylalanine were 6.62 ± 0.43, 0.155 ± 0.77, 6.26 ± 0.75, 10.23 ± 1.32, 4.18 ± 1.49, and 6.67 ± 0.87 mg/g in the 1% B. subtilis CKJ sample, respectively. Xu et al. (2015) reported that raw yellow soybean had 0.27 ± 0.29, 0.15 ± 0.02, 0.46 ± 0.02, 0.21 ± 0.02, 1.09 ± 0.17, and 0.94 ± 0.05 mg/g for valine, methionine, isoleucine, leucine, tyrosine, and phenylalanine, respectively. After 60 h of fermentation, our findings showed higher values than did this previous study. This difference might be due to soybean cultivar, fermentation time, or microorganisms.
There are two major types of free amino acids, essential and non-essential amino acids. The human body cannot produce the essential amino acids and they must be consumed in the daily diet (Song et al. 2013). CKJ is an excellent source of these essential amino acids because of fermentation. Glutamic acids enhance food taste, play a beneficial role in brain metabolism, and are used for the treatment of epilepsy (Vaquero and Butterworth 2006). Valine has an important role for muscle coordination and development, as well as for central nervous system stimulation. Threonine enhances the immune system and promotes the growth of the thymus glands. It is also involved in the formation of glycine and serine, which are necessary for the synthesis of muscle tissues and elastin (Genene and Winans 2004). Overall, CKJ prepared from 1 and 3% B. subtilis can be considered as the best source of free amino acids, which are recommended as key quality control parameters for the production of various products.
Palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid were detected and quantified in our study. The results of fatty acid profiles of CKJ samples prepared using the five different concentrations of B. subtilis are provided in Table 2. CKJ prepared from 1% B. subtilis had the highest value of palmitic acid (11.65 ± 0.42%), stearic acid (2.87 ± 0.12%), and linolenic acid (11.76 ± 1.04%). CKJ prepared using 7% B. subtilis contained the highest value of oleic acid (14.79 ± 0.33) and lowest value of linoleic acid (60.60 ± 1.01). Recently, Cho et al. (2017) reported that Neulchan, soybean cultivars showed palmitic acid (10.33 ± 0.44) and stearic acid (3.61 ± 0.15) after 48 h of fermentation using B. subtilis CSY19. This change is occurred due to the variation in cultivars. In particular, oleic acid, linoleic acid, and linolenic acid contributed almost 80% of the fatty acids in the profile. Glew et al. (2005) reported that oleic acid acts as an anti-inflammatory fatty acid by playing a major role in activating different immune competent cell pathways. The findings of the current study can be helpful for the preparation and development of nutrient-rich functional foods.
Table 2.
Fatty acid composition of Cheonggukjang prepared using five different concentrations of the B. subtilis (KCTC 13241), showed as % of total fatty acids
| Cheonggukjang | Palmitic (16:0) | Stearic (18:0) | Oleic (18:1) | Linoleic (18:2) | Linolenic (18:3) |
|---|---|---|---|---|---|
| 0% B. subtilis | 11.36 ± 0.16a | 2.58 ± 0.39ab | 13.02 ± 0.75bc | 61.41 ± 0.64bc | 9.92 ± 0.58b |
| 1% B. subtilis | 11.65 ± 0.42a | 2.87 ± 0.12a | 14.14 ± 0.53ab | 62.11 ± 0.48b | 11.67 ± 1.04a |
| 3% B. subtilis | 11.57 ± 0.43a | 2.33 ± 0.38ab | 13.52 ± 0.38abc | 62.16 ± 0.71b | 11.18 ± 0.72ab |
| 5% B. subtilis | 10.98 ± 0.85ab | 1.99 ± 0.33bc | 12.63 ± 1.22c | 64.20 ± 0.49a | 9.75 ± 0.76b |
| 7% B. subtilis | 10.45 ± 0.28b | 1.51 ± 0.43c | 14.79 ± 0.33a | 60.60 ± 1.01c | 10.18 ± 0.66ab |
All values are means of three separate replicates. Values with different lower case letters (a, b, and c) are significantly p ≤ 0.05 different as determined by Duncan’s multiple range tests (DMRT)
Comparison of minerals and carbohydrates in CKJ
In this study, 13 minerals were analysed and the results are shown in Table 3. The total minerals in the CKJ samples were in the range of 11,898–13,816 mg/kg. CKJ prepared from 5% B. subtilis contained the highest amounts of minerals. Ca, Fe, Mn, Mo, Na, Ni, and Zn showed a maximum in CKJ prepared using 7% B. subtilis. K and Mg had the highest amounts in the 5% B. subtilis samples, while Cu was highest in CKJ prepared without B. subtilis (0% B. subtilis). In our study, the mineral composition was determined for the first time for CKJ. Maria John et al. (2015) reported that mineral contents increased because of bacterial interaction with the breakdown of other metabolites. Moreover, Obadina et al. (2013) reported the rise in the mineral contents of fermenting soymilk compared to unfermented soymilk was an indication that the starter culture was the responsible for minerals released from the chelated complex compound during fermentation. The decrease mineral contents in CKJ prepared without B. subtilis (0% B. subtilis) was evident that the microorganisms were responsible for the higher value of minerals in CKJ. Ca plays a vital role in nerve functioning, blood clotting, and immune system function and also helps to relax and contract the muscles. Na assists in the maintenance of electrolyte balance, heart function, and specific metabolic activities. Mg helps in protein synthesis, immune system function, and constipation control, while K is needed for nerve transmission and controlling blood pressure and muscle contraction. Fe, Cu, Mn, Mo, and Zn are cofactors in many enzymes. Fe is present in red blood cells and carries oxygen throughout the body and is necessary for energy metabolism (Gharibzahedi and Jafari 2017). Due to these roles, minerals are essential for the body to remain healthy. CKJ is a source of all these minerals and can be considered as a functional and novel food to prevent rickets, diarrhoea, cardiac arrhythmias, and mental retardation. The values of glucose and fructose are represented in Table 3. CKJ prepared from 1% B. subtilis contained the highest value of glucose (12.68 mg/g) and fructose (9.54 mg/g). These free sugars were detected minimum in CKJ prepared using 0 and 7% B. subtilis. These variations in carbohydrates are due to the different concentrations of microbes used.
Table 3.
Mineral and carbohydrate composition of Cheonggukjang prepared using five different concentrations of the B. subtilis (KCTC 13241)
| Minerals (mg/kg) | 0% B. subtilis | 1% B. subtilis | 3% B. subtilis | 5% B. subtilis | 7% B. subtilis |
|---|---|---|---|---|---|
| Arsenic (As) | ND | ND | ND | ND | ND |
| Calcium (Ca) | 1481.38 ± 28c | 1435.22 ± 23d | 1392.35 ± 22e | 1505.43 ± 23b | 1667.32 ± 19a |
| Copper (Cu) | 13.86 ± 0.05a | 10.65 ± 0.04d | 10.06 ± 0.03e | 11.64 ± 0.03c | 11.83 ± 0.06b |
| Iron (Fe) | 41.38 ± 0.13cd | 40.48 ± 0.29d | 104.2 ± 0.60b | 42.91 ± 0.23c | 317 ± 2.03a |
| Mercury (Hg) | ND | ND | ND | ND | ND |
| Potassium (K) | 10,835 ± 24b | 10,262 ± 28c | 9178 ± 34.8e | 10,933 ± 47.4a | 9999 ± 99.8d |
| Magnesium (Mg) | 997.25 ± 20b | 977.32 ± 16c | 892.31 ± 15.5e | 1019 ± 17.8a | 956.12 ± 17d |
| Manganese (Mn) | 29.79 ± 0.14c | 29.71 ± 0.25c | 26.94 ± 0.16d | 32.61 ± 0.14b | 34.51 ± 0.19a |
| Molybdenum (Mo) | 1.12 ± 0.01b | 0.86 ± 0.08d | 0.85 ± 0.01d | 0.89 ± 0.003c | 1.28 ± 0.01a |
| Sodium (Na) | 48.01 ± 0.61e | 71.97 ± 0.69d | 256.7 ± 1.48b | 230.48 ± 2.42c | 310.07 ± 4.61a |
| Nickel (Ni) | 3.66 ± 0.04d | 3.60 ± 0.02e | 6.42 ± 0.02b | 3.93 ± 0.03c | 22.21 ± 0.04a |
| Lead (Pb) | ND | ND | ND | ND | ND |
| Zinc (Zn) | 32.82 ± 0.34c | 32.09 ± 0.13c | 29.80 ± 0.09d | 35.77 ± 0.21b | 37.18 ± 0.13a |
| Total Minerals | 13,485 ± 31b | 12,864 ± 26d | 11,898 ± 18e | 13,816 ± 23a | 13,358 ± 34c |
| Glucose (mg/mL) | 4.86 ± 0.99d | 12.68 ± 0.74a | 6.59 ± 0.53b | 5.64 ± 1.43c | 4.04 ± 0.95d |
| Fructose (mg/mL) | 2.26 ± 0.36c | 9.54 ± 0.48a | 5.38 ± 0.44b | 2.87 ± 0.87c | 0.65 ± 0.12d |
All values are the average of three replicates. Values with different lower case letters (a, b, and c) are significantly p ≤ 0.05 different as determined by Duncan’s multiple range tests (DMRT)
ND not detected
Conclusion
The current study evaluated the potential of various concentrations of B. subtilis (KCTC 13241) to enhance antioxidant activities and identified the most effective concentrations to affect total phenolic content, amino acids, fatty acids, proteins, minerals, and carbohydrates in CKJ. Overall, the results revealed that CKJ is a novel functional food and has natural bioactive compounds that promote human health. Among all samples, CKJ prepared using 1% B. subtilis exhibited the most remarkable results for DPPH and ABTS radical-scavenging activity assays, reducing power, and total phenolic content. This concentration also showed the highest values for total amino acids and fatty acids. Therefore, fermentation with 1% B. subtilis enhanced the metabolite and nutritional value of CKJ. As the concentration of B. subtilis increased, the potential of CKJ for antioxidants and metabolites actually decreased. These results are valuable for the selection of the best performing concentration of microbes for soybean fermentation. We concluded that CKJ fermented with 1% B. subtilis can be used as a natural source of antioxidants in the pharmaceutical and food industries with specific health benefits. Further studies on CKJ should be carried out for characterisation and isolation of the specific bioactive compounds that are responsible for the antioxidant activities.
Acknowledgements
We wish to thank Kyunpook National University Research Fund 2017, Daegu, Republic of Korea.
References
- Ali MW, Kim I-D, Bilal S, Shahzad R, Saeed MT, Adhikari B, Nabi RBS, Kyo J, Shin D-H. Effects of bacterial fermentation on the biochemical constituents and antioxidant potential of fermented and unfermented soybeans using probiotic Bacillus subtilis (KCTC 13241) Molecules. 2017;22:2200. doi: 10.3390/molecules22122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andualem B, Gessesse A. Proximate composition, mineral content and antinutritional factors of Brebra (Millettia ferruginea) seed flour as well as physicochemical characterization of its seed oil. SpringerPlus. 2014;3:298. doi: 10.1186/2193-1801-3-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Prasad B, Rai AK, Velappan SP, Subbanna MN, Narayan B. In-vitro antioxidant and antibacterial properties of hydrolyzed proteins of delimed tannery fleshings: comparison of acid hydrolysis and fermentation methods. Biodegradation. 2011;22:287–295. doi: 10.1007/s10532-010-9398-0. [DOI] [PubMed] [Google Scholar]
- Bilal S, Khan AL, Waqas M, Shahzad R, Kim ID, Lee IJ, Shin DH. Biochemical constituents and in vitro antioxidant and anticholinesterase potential of seeds from Native Korean Persimmon Genotypes. Molecules. 2016;21:893. doi: 10.3390/molecules21070893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KM, Lee JH, Yun HD, Ahn BY, Kim H, Seo WT. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J Food Comp Anal. 2011;24:402–410. doi: 10.1016/j.jfca.2010.12.015. [DOI] [Google Scholar]
- Cho KM, Lim H-J, Kim M-S, Kim DS, Hwang CE, Nam SH, Joo OS, Lee BW, Kim JK, Shin E-C. Time course effects of fermentation on fatty acid and volatile compound profiles of Cheonggukjang using new soybean cultivars. J Food Drug Anal. 2017;25:637–653. doi: 10.1016/j.jfda.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem. 2002;50:2454–2458. doi: 10.1021/jf011369q. [DOI] [PubMed] [Google Scholar]
- Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, Schünemann HJ. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Duh P-D. Antioxidant activity of burdock (Arctium lappa Linné): its scavenging effect on free-radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Genene A, Winans S. Distribution of methionine and leucine enkephalin neurons within the social behavior circuitry of the male Syrian hamster brain. Brain Res. 2004;1030:28–48. doi: 10.1016/j.brainres.2004.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Jafari SM. The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci Technol. 2017;62:119–132. doi: 10.1016/j.tifs.2017.02.017. [DOI] [Google Scholar]
- Glew RH, Ayaz FA, Millson M, Huang HS, Chuang LT, Sanz C, Golding JB. Changes in sugars, acids and fatty acids in naturally parthenocarpic date plum persimmon (Diospyros lotus L.) fruit during maturation and ripening. Eur Food Res Technol. 2005;221:113–118. doi: 10.1007/s00217-005-1201-9. [DOI] [Google Scholar]
- Haque A, Hwang CE, Lee HY, Ahn MJ, Sin E. Comparison of isoflavone contents and antioxidant effect in Cheonggukjang with black soybean cultivars by Bacillus subtilis CSY191. Korean J Environ Agric. 2016;35:62–71. doi: 10.5338/KJEA.2016.35.1.11. [DOI] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Sanders ME. Expert consensus document: The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:9. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hong HA, Le HD, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ge C, Yuan W, Zhu R, Zhang W, Du L, Xue J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J Sci Food Agric. 2010;90:1194–1202. doi: 10.1002/jsfa.3947. [DOI] [PubMed] [Google Scholar]
- Hwang CE, Seo WT, Cho KM. Enhanced antioxidant effect of black soybean by Cheonggukjang with potential probiotic Bacillus subtilis CSY191. Korean J Microbiol. 2013;49:391–397. doi: 10.7845/kjm.2013.3070. [DOI] [Google Scholar]
- Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol. 2014;54:427–433. doi: 10.1007/s12088-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Park JW, Cho IJ, Kim B, Kwon K, Hahm YT. Isolation of Bacillus spp. from Cheonggukjang and its antagonistic effect against Bacillus cereus. Korean J Food Sci Technol. 2008;40:669–673. [Google Scholar]
- Lin CH, Wei YT, Chou CC. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2006;23:628–633. doi: 10.1016/j.fm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Maria John KM, Enkhtaivan G, Lee J, Thiruvengadam M, Keum YS, Kim DH. Spectroscopic determination of metabolic and mineral changes of soya-chunk mediated by Aspergillus sojae. Food Chem. 2015;170:1–9. doi: 10.1016/j.foodchem.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Molina VC, Médici M, Taranto MP, Font de Valdez G. Lactobacillus reuteri CRL 1098 prevents side effects produced by a nutritional vitamin B deficiency. J Appl Microbiol. 2009;106:467–473. doi: 10.1111/j.1365-2672.2008.04014.x. [DOI] [PubMed] [Google Scholar]
- Obadina AO, Akinola OJ, Shittu TA, Bakare HA. Effect of natural fermentation on the chemical and nutritional composition of fermented soymilk Nono. Niger Food J. 2013;31:91–97. doi: 10.1016/S0189-7241(15)30081-3. [DOI] [Google Scholar]
- Omura K, Hitosugi M, Zhu X, Ikeda M, Maeda H. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J Pharmacol Sci. 2005;99:247–251. doi: 10.1254/jphs.FP0050408. [DOI] [PubMed] [Google Scholar]
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjukta S, Rai AK. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci Technol. 2016;50:1–10. doi: 10.1016/j.tifs.2016.01.010. [DOI] [Google Scholar]
- Sanjukta S, Rai AK, Muhammed A, Jeyaram K, Talukdar NC. Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation. J Funct Foods. 2015;14:650–658. doi: 10.1016/j.jff.2015.02.033. [DOI] [Google Scholar]
- Shahzad R, Khan AL, Bilal S, Waqas M, Kang SM, Lee IJ. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ Exp Bot. 2017;136:68–77. doi: 10.1016/j.envexpbot.2017.01.010. [DOI] [Google Scholar]
- Shin EC, Lee JH, Hwang CE, Lee BW, Kim HT, Ko JM, Cho KM. Enhancement of total phenolic and isoflavone-aglycone contents and antioxidant activities during Cheonggukjang fermentation of brown soybeans by the potential probiotic Bacillus subtilis CSY191. Food Sci Biotechnol. 2014;23:531–538. doi: 10.1007/s10068-014-0073-9. [DOI] [Google Scholar]
- Song J, Liu C, Li D, Gu Z. Evaluation of sugar, free amino acid, and organic acid compositions of different varieties of vegetable soybean (Glycine max [L.] Merr) Ind Crops Prod. 2013;50:743–749. doi: 10.1016/j.indcrop.2013.08.064. [DOI] [Google Scholar]
- Vaquero J, Butterworth RF. The brain glutamate system in liver failure. J Neurochem. 2006;98:661–669. doi: 10.1111/j.1471-4159.2006.03918.x. [DOI] [PubMed] [Google Scholar]
- Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes, sensory and nutritive qualities of food. J Food Sci. 2005;72:159. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Du B, Xu B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015;174:202–213. doi: 10.1016/j.foodchem.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Yang JH, Mau JL, Ko PT, Huang LC. Antioxidant properties of fermented soybean broth. Food Chem. 2000;71:249–254. doi: 10.1016/S0308-8146(00)00165-5. [DOI] [Google Scholar]
- Yang SO, Chang PS, Lee JH. Isoflavone distribution and beta-glucosidase activity in cheonggukjang a traditional Korean whole soybean-fermented food. Food Sci Biotechnol. 2006;15:96–101. [Google Scholar]
- Zhao X, Song J, Le Wang Q, Qian Y, Li GJ, Pang L. Comparisons of Shuidouchi, Natto, and Cheonggukjang in their physicochemical properties, and antimutagenic and anticancer effects. Food Sci Biotechnol. 2013;22:1077–1084. doi: 10.1007/s10068-013-0186-6. [DOI] [Google Scholar]