Abstract
Bakery products are a food appreciated by consumers all over the world. There is a great opportunity to incorporate more bioactive compounds to enhance its quality. The objective of this study was to utilize the advantage of CTE in the production of sponge cake. The five different levels of CTE (0, 5, 10, 15 and 20%, w/w) was incorporated into sponge cake. The sponge cakes were evaluated for physicochemical (color, volume, water activity, total phenolic content, and antioxidant properties) and texture characteristics as well as consumer acceptance. Addition of CTE into the sponge cakes increased the polyphenol content and antioxidant activity concomitant with reduced lipid peroxidation. Increasing hardness, adhesiveness, gumminess, and chewiness and decreasing cohesiveness, springiness and resilience of cakes were seen when increasing percentage of CTE in the cake. A significant decrease was observed in the lightness, redness and yellowness in the cake containing CTE. No differences were found in overall acceptability between the control and the cake containing CTE. The findings suggest that CTE could be a potential source for development of sponge cakes with more effective antioxidant properties.
Keywords: Clitoria ternatea extract, Sponge cake, Antioxidant, Lipid oxidation, Sensory analysis
Introduction
Bakery products, especially cakes, are the most popular and widely available consumed as a snack or an alternative meal worldwide (Zhang et al. 2012). Sponge cake, a type of air-leavened cake, is made from flour, sugar, eggs, fat and other ingredients (Lee 2015). Fat is common ingredient that enhances a softer final texture of sponge cake during the manufacturing process, however, many fats are prone to lipid oxidation by generating reactive oxygen species and free radicals when exposure to oxygen (Lu et al. 2010; St. Angelo et al. 1996). In general, lipid oxidation directly affects rancid odors, unpleasant flavors and discoloration of products, leading to a decrease in the nutritional quality and safety which may be harmful to human health over the long-term (Waraho et al. 2011).
Rapidly growing concern about health foods have led researchers to use of antioxidants for minimizing rancidity, retarding the formation of toxic oxidation products, maintaining nutritional quality, and increasing the shelf-life of food products (Lobo et al. 2010). Natural edible plants such as watermelon rinds and sharlyn melon peels (Al-Sayed and Ahmed 2013), green tea extract (Bajerska et al. 2010), tea powder (Mau et al. 2015), potato peel extract (Mohdaly et al. 2010), black rice (Mau et al. 2017), and sesame extract (Suja et al. 2005) have been recently reported to be the potential sources for prevention of lipid peroxidation in bakery products. Therefore, incorporation of antioxidants rich fruits, vegetables, and plants into sponge cakes is a new approach for prevention of the lipid peroxidation and improvement of product quality in the food industry (Shahidi and Zhong 2010).
Clitoria ternatea L. (CT), known as butterfly pea or blue pea, is a plant species belonging to the Fabaceae family. The flower petals of CT that contain abundant anthocyanin pigments have considerable potential for application as a source of natural colorants in a variety of foods and beverages (Nair et al. 2015). For example, the CT flower extract has been rationally used as the blue color in the dessert and beverages in Southeast Asia countries such as Malaysia and Thailand (Lijon et al. 2017). Moreover, the flower petals of CT have been reported as regular vegetables in India and the Philippines (Tecson-Mendoza 2007). The extract of CT not only imparts beautiful coloration to food systems but it also exhibits a wide range of pharmacological aspects for the health-promoting effects (Mukherjee et al. 2008; Zingare et al. 2013). The beneficial effects of polyphenols in the CT flower extract have been linked to their ability to scavenge free radicals (Chayaratanasin et al. 2015; Madhavarao et al. 2011; Madhu 2013). Published work has demonstrated the inhibitory effect of polyphenols in the CT extract against carbohydrate digestive enzymes (α-glucosidase and α-amylase), which may result in delayed glucose absorption and lowering of postprandial hyperglycaemia (Adisakwattana et al. 2012). The microencapsulation of CT makedly retained higher amount of polyphenols and improved antioxidant capacity, pancreatic α-amylase inhibitory activity, and bile acid binding after the gastrointestinal digestion (Pasukamonset et al. 2016). A recent study has shown the effect of the CT extract for reduction of lipid oxidation and improvement of the shelf life and color stability of cooked pork products in meat industry (Pasukamonset et al. 2017). However, knowledge of using the CT extract as active ingredients in sponge cakes is very limited. Hence, the study was aimed to develop a novel formula of sponge cake by replacement of cake flour with various CT extracts. In addition, the study was to determine the effect of sponge cakes enriched with the CT extract on antioxidant activity, physicochemical characteristics, and consumer acceptance.
Materials and methods
Materials
The ingredients used in the formula of sponge cakes were cake flour (UFM Food centre Co., Ltd., Bangkok, Thailand), sucrose (Mitr Phol Sugar Co., Ltd., Bangkok, Thailand), rice bran oil (Thai Edible Oil Co., Ltd., Bangkok, Thailand), fresh eggs (Charoen Pokphand Foods Public Co., Ltd., Bangkok, Thailand), sodium chloride (Thai Refined Salt Co., Ltd., Bangkok, Thailand), baking powder (Unilever Thai Trading Ltd., Bangkok, Thailand), vinegar (Win Chance Foods Co., Ltd., Bangkok, Thailand), and vanilla flavor (Greathill Ltd. Part., Bangkok, Thailand). The spray-dried form of Clitoria ternatea flower petal extract (CTE) was purchased from Specialty Natural Products Co., Ltd., Bangkok, Thailand. Catechin, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferrous chloride tetrahydrate, ferrozine, trichloroacetic acid, ferric chloride hexahydrate, trifluoroacetic anhydride and 6-hydroxyl-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Folin–Ciocalteau reagent was purchased from Merck (Darmstadt, FR, Germany). All other reagents used were of analytical grade and purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and Merck (Darmstadt, FR, Germany).
Preparation of sponge cakes
The recipe of control sponge cake in this study was adapted from a previous study (Mau et al. 2015). The Clitoria ternatea extract (CTE) was used to substitute 0, 5, 10, 15 and 20% (w/w) of wheat flour to make various sponge cakes, assigned as control, 5%CTE, 10%CTE, 15%CTE, and 20%CTE, respectively. The recipes of sponge cakes are described in Table 1. For the sponge cake preparation, egg white was whipped into cream using a hand mixer (HR1574, Philips, Japan) on the low-medium speed setting for 20 s. Sucrose and vinegar were added and continue whipped into cream using a hand speed 6 setting for 80 s. Then, egg yolk, oil, sodium chloride, vanilla flavor and CTE were poured into the mixer on the speed 2 setting for 20 s. Ingredients were later mixed by hand with a plastic scraper until smooth. The cake mixer (25 g) was immediately poured into round cake pans (20 cm in diameter, 5 cm in depth) and baked at 180 °C for 15 min in a preheated electric oven (KXS-4G+H, Salvia Industrial S.A., Lezo, Spain). After baking, the cakes were allowed to cool for 30 min at room temperature and then were removed from their pans. The cake samples were packed in polypropylene bags before analyses of physicochemical, antioxidant and sensory characteristics.
Table 1.
Formulation of sponge cakes
| Ingredients (per 100 g sponge cake) | Control | 5%CTE | 10%CTE | 15%CTE | 20%CTE |
|---|---|---|---|---|---|
| Cake flour | 25.00 | 23.75 | 22.50 | 21.25 | 20.00 |
| Clitoria ternatea extract | – | 1.25 | 2.50 | 3.75 | 5.00 |
| Baking powder | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 |
| Egg yolk | 25.35 | 25.35 | 25.35 | 25.35 | 25.35 |
| Egg white | 54.06 | 54.06 | 54.06 | 54.06 | 54.06 |
| Rice bran oil | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Sodium chloride | 0.41 | 0.41 | 0.41 | 0.41 | 0.41 |
| Sugar | 30 | 30 | 30 | 30 | 30 |
| Vinegar | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| Water | 3.25 | 3.25 | 3.25 | 3.25 | 3.25 |
| Vanilla flavour | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
Control, 5%CTE, 10%CTE, 15%CTE and 20%CTE: prepared with 0, 5, 10, 15 and 20% replacement of cake flour with Clitoria ternatea extract (CTE), respectively
Physical characteristics of sponge cakes
The physical characteristics of cakes were determined according to the method described previously (Lu et al. 2010). The moisture content of sponge cakes (1 g) was determined using an Infrared Moisture Determination Balance (FD-610-Kett Electric Laboratory, Tokyo, Japan) at 160 °C for 7 min. The water activity of the sponge cake was measured by the chilled mirror technique using a water activity meter (AquaLab model CX-2, Decagon Devices, Inc., Pullman, WA, USA).
Texture profile analysis (TPA) was carried out using a TA-TXT plus texture analyzer (Stable Microsystem, Godalming, U.K.) and the Texture Exponent Lite 32 software (version 4.0.8.0, Stable Microsystems). The test was performed in 4 cubes (1.5 inch side) taken from the central crumb of each cake. Texture profile analysis was performed using a test speed of 1 mm/s with a strain of 40% of the original cube height and a 5 s interval between the 2 compression cycles. A trigger a force of 5 g was selected. The double compression test was performed with a 50-mm diameter aluminium plate (P/50). The parameters obtained from the curves were hardness, chewiness, springiness, adhesiveness, and cohesiveness.
Color measurement of sponge cakes
The color determinations of cake samples (1.5 × 1.5 × 1.5 inch, crumb only and top crust) was monitored using HunterLab color analyzer (Hunter Lab Color Flex EZ 45/0°, Hunter Lab Inc., Virginia, USA) set for Hunter , , and values with a D65 illuminant at 10°. The instrument was calibrated with black and white reference tiles. The values were expressed as (whiteness or brightness/darkness), (redness/greenness) and, (yellowness/blueness) color values. Five determinations were taken for each sample. Total color difference (ΔE value) was calculated by using the formula given by Mohamad et al. (2015) as follows:
where L0, a0 and b0 are the obtained values of the cake.
Samples extraction
Samples extraction of cake extracts were determined according to the method of Mau et al. (2017) with minor modification. One gram of sponge cake sample was mixed with 6 ml of distilled water and homogenized for 30 min. It was followed by centrifugation at 4500g for 15 min at 25 °C. The mixture was held at ambient temperature for 20 min in order to separate the solid and liquid phases and centrifuged at 10,000 rpm for 15 min. The supernatant was collected and analyzed for phenolic, flavonoid and antioxidant assays immediately or stored at − 20 °C until used for analysis.
Total polyphenols, flavonoids and antioxidant activity of sponge cakes
The total phenolic content (TPC) were determined according to a previous method (Singh et al. 2015). Total flavonoid content (TFC) was determined following the method given previously (Singh et al. 2015). The antioxidant activity of sponge cakes was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging and ferric reducing antioxidant power (FRAP) assays following the protocols described previously (Prokopov et al. 2015).
Thiobarbituric acid reaction substances (TBARS)
Lipid peroxidation was measured according to previously published method with minor modifications (Moraes et al. 2010). Briefly, the supernatant (250 µL) was mixed with 250 µL of 20% TCA (w/v) and 12.5 µL of 7.2% Butylated hydroxytoluene (w/v) and centrifuged at 10,000 rpm for 15 min at 4 °C. The collected supernatant (250 µL) was mixed with an equal volume (500 µl) of 20 mM thiobarbituric acid and vigorously vortexed. After that, the test tubes were placed in a 105 °C dry bath for 10 min. After cooling, the absorbance was measured at 532 nm using a spectrophotometer. The results were calculated from a standard curve of malondialdehyde (0–40 µM). The TBARS values were expressed as number of nmol of malondialdehyde (MDA) per g of sponge cake.
Sensory analysis
Thirty undergraduate students from the Chulalongkorn University were recruited to be panelists. The panelists were trained before initiation in the experiment by using matching, ordering and ranking tests. The samples were assessed in a standardized tasting room equipped with individual booths. Each consumer received 5 pieces of cakes (2.5 cm cubes cake samples). The pieces of cakes were coded using 3-digit random numbers. The pieces of cakes were served at room temperature in random order. Water was supplied to clean the consumers’ mouths between each sample. Cakes were evaluated on the basis of appearance, color, sponginess, texture, aroma, flavor, taste and overall acceptability by a nine-point hedonic scale (9 = like extremely, 8 = like very much, 7 = like moderately, 6 = like slightly, 5 = neither like nor dislike, 4 = dislike slightly, 3 = dislike moderately, 2 = dislike very much, and 1 = dislike extremely).
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM), n = 6. Data were subjected to one-way analysis of variance (ANOVA) using the Statistical Package for Social Sciences (SPSS) version 18.0 (SPSS Inc., Chicago, IL, USA). When Tukey’s multiple comparison test was performed to determine significant differences within groups at P < 0.05.
Results and discussion
Physicochemical characteristics
The physicochemical characteristics of sponge cakes supplemented with different levels of CTE are described in Table 2. The weight, moisture and water activity of all sponge cakes showed no significant differences between all the five tested groups. The baking process of the sponge cake leads to the expansion of liquids when temperature increases, resulting in increasing generation of gas together with vapor pressure (Kim et al. 2012). When gas diffuses into the cake, it produces the structural damage and the loss in the volume of cake (Lee 2015). As shown in Table 2, there were no statistically significant difference in the values of the baking loss rate between the cakes enriched CTE (5–20%) and a control cake, suggesting that addition of CTE did not affect the baking loss of the sponge cake. Therefore, CTE can maintain a stable volume and moist texture and the structural transformation of the cake during the baking process. Our results are consistent with a previous study of the sponge cakes enriched with cheonnyuncho powder (Kim et al. 2012). However, our results show some disagreement with the study of Lee et al. suggesting that baking loss rate was increased with increasing level of Rubus coreanus powder (Lee 2015). As shown in Table 2, the hardness value of the CTE enriched cakes (10–20%) was significantly increased when compared to the control cake. This may due to the interaction of hydrogen bonding and hydrophobic interaction between protein molecules and phenolic groups, which causes structural and conformational changes of the protein in food (Yuksel et al. 2010; Jakobek 2015). It indicates that cakes enriched CTE become harder and the loss of flexibility than control cake. Cohesiveness is defined as the ability of the food products to resist deformation or compression between the teeth before the breaking down of the internal structure of foods (Lu et al. 2010). The results demonstrated a statistically significant decrease in the cohesiveness of cakes with added CTE (10–20%) when compared to the control cake. This reduction indicates that the cakes formulated with CTE have low ability to resist before the cake structure deformed under the teeth. A similar trend of decreased cohesiveness was also seen in the cake added cheonnyuncho powder (Kim et al. 2012). Adhesiveness represents the ability of cake to adhere to the teeth when chewed, therefore, the greater negative value of adhesiveness results in a greater adhesive (Lu et al. 2010). Adhesiveness values of produced cakes enriched CTE ranged between − 3.12 ± 0.20 and − 1.55 ± 0.11 g/s. Addition of CTE (10–20%) into the cakes noticeably decreased adhesiveness when compared to the control cake. Furthermore, addition of CTE (15–20%) diminished the resilience of produced cakes, whereas reduced springiness was observed for 20% CTE. Noticeable differences of gumminess and chewiness were seen in the samples containing 15–20% CTE. Springiness value is described as the extent of recovery between the first and second compression indicates the elasticity of cakes (Lu et al. 2010). Resilience is defined as the ability to bounce back of cake after first compression is relieved, whereas gumminess measures the force for masticating the cake until it is ready for swallowing (Lu et al. 2010). Chewiness represents the amount of energy needed to disintegrate a food for swallowing, indicating the rate of cake breakdown (Lu et al. 2010). Overall results are in agreement with the previous studies, indicating that sponge cake containing green tea (Lu et al. 2010), cheonnyuncho powder (Kim et al. 2012) and tea powder (Mau et al. 2015) caused an increase in hardness, adhesiveness, gumminess, and chewiness and a decrease in cohesiveness, springiness, and resilience. Previous study reported that the significantly decreased springiness, cohesiveness, and resilience of anthocyanin-rich black rice extract powder (ABREP) fortified bread might be due to the weaker and less elastic gluten structure of the bread caused by the anthocyanins within ABREP (Sui et al. 2016). Therefore, the significant decrease in springiness, cohesiveness, and resilience of cakes containing CTE might be due to the weaker and less elastic gluten structure of the cakes caused by the polyphenols within CTE. However, there were no significant values on the hardness, cohesiveness, adhesiveness, springiness, gumminess, resilience, and chewiness with the cakes containing 5% CTE.
Table 2.
Physical characteristics of sponge cakes prepared with Clitoria ternatea extract (CTE) replacement in cake flour
| Control | 5%CTE | 10%CTE | 15%CTE | 20%CTE | |
|---|---|---|---|---|---|
| Weight (g) | 22.54 ± 0.11a | 22.63 ± 0.08a | 22.85 ± 0.09a | 22.86 ± 0.10a | 22.44 ± 0.09a |
| Moisture (%) | 35.13 ± 0.32a | 35.03 ± 0.36a | 35.26 ± 0.48a | 34.75 ± 0.31a | 35.24 ± 1.07a |
| Water activity | 0.94 ± 0.01a | 0.95 ± 0.01a | 0.95 ± 0.01a | 0.95 ± 0.01a | 0.95 ± 0.02a |
| Baking loss (%) | 11.12 ± 0.08a | 10.92 ± 0.05a | 10.33 ± 0.08a | 10.43 ± 0.07a | 11.72 ± 0.09a |
| Texture properties | |||||
| Hardness (g) | 128.24 ± 4.48a | 139.68 ± 5.46a,b | 149.35 ± 7.68b | 166.83 ± 4.26c | 187.30 ± 7.81d |
| Cohesiveness | 0.82 ± 0.01a | 0.82 ± 0.01a | 0.80 ± 0.01b | 0.80 ± 0.01b | 0.79 ± 0.01b |
| Adhesiveness (g/s) | − 3.12 ± 0.20a | − 2.72 ± 0.10a,b | − 2.23 ± 0.18b | − 2.07 ± 0.25b,c | − 1.55 ± 0.11c |
| Springiness | 0.89 ± 0.01a | 0.88 ± 0.01a | 0.88 ± 0.01a | 0.87 ± 0.01a | 0.83 ± 0.02b |
| Resilience | 0.46 ± 0.01a | 0.45 ± 0.01a | 0.43 ± 0.01a,b | 0.42 ± 0.01b,c | 0.40 ± 0.01c |
| Gumminess (g) | 105.89 ± 4.02a | 114.96 ± 5.72a | 122.02 ± 5.96a,b | 137.21 ± 4.15b,c | 142.26 ± 8.44c |
| Chewiness (g) | 99.50 ± 2.86a | 101.83 ± 4.53a | 107.83 ± 4.84a | 121.60 ± 4.13b | 131.96 ± 5.17b |
| Crust color | |||||
| 53.69 ± 0.43a | 48.77 ± 0.89b | 39.82 ± 0.22c | 38.81 ± 0.39c | 35.65 ± 0.38d | |
| 16.53 ± 0.19a | 12.64 ± 0.42b | 11.95 ± 0.06b,c | 11.49 ± 0.04b,c | 10.63 ± 0.38c | |
| 34.23 ± 0.20a | 28.03 ± 0.17b | 26.33 ± 0.11c | 25.21 ± 0.19c | 22.14 ± 0.23d | |
| Chroma value | 38.01 ± 0.09a | 31.08 ± 0.27b | 28.94 ± 0.16c | 27.81 ± 0.28c | 24.48 ± 0.43d |
| E index | 65.81 ± 0.54a | 57.84 ± 0.74b | 49.22 ± 0.23c | 47.74 ± 0.31c | 43.24 ± 0.51d |
| Crumb color | |||||
| 70.58 ± 0.17a | 57.38 ± 0.84b | 48.80 ± 0.26c | 45.49 ± 0.44d | 40.32 ± 0.07e | |
| 5.53 ± 0.21a | − 3.45 ± 0.22b | − 5.28 ± 0.13c | − 6.17 ± 0.12c,d | − 7.01 ± 0.35d | |
| 36.40 ± 0.24a | 21.46 ± 0.28b | 18.07 ± 0.36c | 14.80 ± 0.17d | 13.37 ± 0.10e | |
| Chroma value | 36.82 ± 0.21a | 21.73 ± 0.27b | 18.96 ± 0.46c | 16.01 ± 0.15d | 15.19 ± 0.05d |
| E index | 79.61 ± 0.31a | 61.35 ± 0.88b | 52.49 ± 0.05c | 48.23 ± 0.38d | 43.09 ± 0.06e |
Each value is expressed as mean ± S.E.M. (n = 6). Means with different capital letter within a row are significantly different (P < 0.05)
Color characteristics
The effects of CTE addition on the color characteristics of sponge cakes are presented in Table 2. The results were expressed by Hunter , and values corresponding to lightness, redness, and yellowness, respectively. Significant differences of the crust and crumb colors were observed between the control cake and the cake substituted with CTE (P < 0.05). The crust color of the control sponge cake gave higher , and values than the cakes substituted with CTE (P < 0.05). The crust color of the control was lighter and more reddish and yellowish than any of the other CTE cakes. The crumb color of control samples was also significantly different from the cakes containing CTE (P < 0.05). When the level of CTE substitution was increased, the reduction of the , and values was observed. It suggests that a darker color of cakes including less red and yellow is obtained. Therefore, the crust and crumb colors of sponge cakes were greatly affected by the CTE levels. The appearance of cakes containing CTE is shown in Fig. 1. Incorporation of CTE significantly decreased (P < 0.05) chroma value of both crust and crumb colors. The increased CTE content in sponge cakes resulted in a decrease in chroma values. These results are in agreement with a study of chiffon cake prepared with tea powders (Mau et al. 2015) and black rice (Mau et al. 2017). It suggests that the darker baked cakes was due to the color of the used substituted materials and their interactions (Al-Sayed and Ahmed 2013). For a comprehensive analysis combining , and values together, E index was introduced to describe the color change. E index calculated by the equation E = (L * 2 + a * 2 + b * 2)1/2, which is mostly influenced by the brightness (Peng et al. 2010). Cakes fortified with CTE had lower E values than the control cake. The findings also showed that a trend of E values was inversely related to the levels of CTE in the cake. These observations are indicative of a loss in brightness of cake caused by CTE. Therefore, the color change of sponge cakes fortified with CTE could be attributed to purple pigments in CTE. Previous study also reported the discoloration of pork patties with addition of natural antioxidants like CTE (Pasukamonset et al. 2017). Additionally, the similar color characteristics have also been detected in other studies of cake with added green tea (Lu et al. 2010) and black rice (Mau et al. 2017).
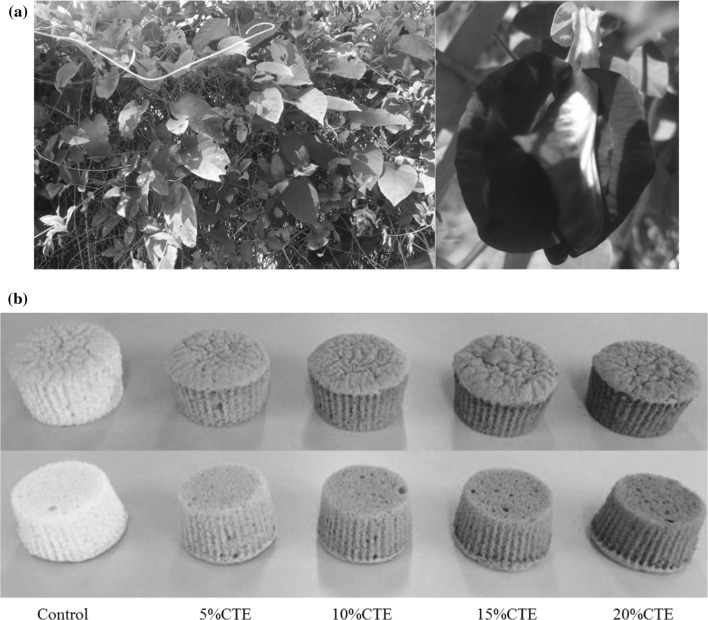
Fig. 1.
a The flower petal of Clitoria ternatea L. b The appearance of Clitoria ternatea extract sponge cakes. Control, 5%CTE, 10%CTE, 15%CTE and 20%CTE represent sponge cakes manufactured with 0, 5, 10, 15 and 20% (w/w) replacement of cake flour by Clitoria ternatea extract (CTE), respectively
Total polyphenols, total flavonoids and antioxidant properties
The contents of total polyphenol in the cake ranged from 585.32 ± 42.49 to 1068.61 ± 36.38 µg gallic acid equivalent/g sponge cake, whereas total flavonoid contents of cake samples ranged from 38.48 ± 5.32 to 63.22 ± 3.82 µg catechin equivalent/g sponge cake (Table 3). These results showed that total polyphenol and flavonoid contents of sponge cake significantly increased with 10, 15 and 20% replacement of CTE as compared to the control (P < 0.05). The highest amount of polyphenol and flavonoid contents was found in cake prepared with 20% replacement of cake flour with CTE. After baking, total polyphenol and total flavonoid contents remaining in the CTE sponge cakes. Similar results were also reported in chiffon cakes fortified with various tea powders (Mau et al. 2015), cakes incorporated with gilaburu fruit (Viburnum opulus) pomace (Şeker et al. 2016) and chiffon cake prepared with black rice as replacement for wheat flour (Mau et al. 2017) that total phenols were increased proportionally with the level of compound incorporation in cakes. The antioxidant properties of the cake containing CTE are summarized in Table 3. The results exhibited that adding CTE greatly enhanced antioxidant properties of the sponge cakes. The FRAP value of sponge cake significantly increased from 90.50 ± 3.54 to 324.06 ± 4.96 µM FeSO4/g sponge cake when increasing incorporation with CTE (5–20%), respectively (P < 0.05). The ability of CTE sponge cakes to scavenge the DPPH radical was 91.55 ± 6.35 to 186.96 ± 1.81 mg/mL. The findings indicate that sponge cakes added with 10, 15 and 20% CTE significantly exhibits strong antioxidant as compared with the control cake (P < 0.05). This implies that polyphenolic compounds in CTE might contribute to their radical scavenging activity.
Table 3.
The total polyphenol content, the total flavonoid content, and the antioxidant activity of Clitoria ternatea sponge cakes
| Control | 5% | 10% | 15% | 20% | |
|---|---|---|---|---|---|
| TPC | 585.32 ± 42.49a | 738.16 ± 60.40a,b | 821.95 ± 39.29b,c | 956.89 ± 26.89c,d | 1068.61 ± 36.38d |
| TFC | 38.48 ± 5.32a | 53.09 ± 5.49a | 57.30 ± 5.50a,b | 62.80 ± 4.63a,b | 63.22 ± 3.82b |
| FRAP | 90.50 ± 3.54a | 150.99 ± 4.80b | 199.90 ± 4.22c | 264.09 ± 5.80d | 324.06 ± 4.96e |
| DPPH IC50 | 186.96 ± 1.81a | 179.99 ± 3.95a | 148.61 ± 19.77b | 120.78 ± 4.49c | 91.55 ± 6.35d |
TPC total polyphenol contents (µg gallic acid equivalent/g sponge), TFC total flavonoid contents (µg catechin/g sponge). FRAP (µM FeSO4/g sponge), DPPH IC50 (mg sponge/mL). Each value is expressed as mean ± S.E.M. (n = 6). Means with different capital letter within a row are significantly different (P < 0.05)
Oxidative stability
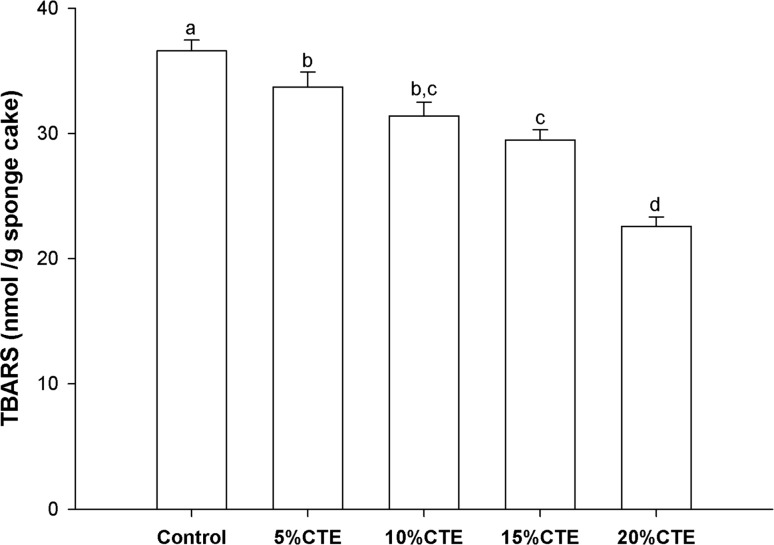
Oxidation of lipids is one of the most common processes in cakes enriched lipids contents. Lipid hydrolytic degradation is predominant precursors to oxidative instability of bakery products during high baking temperature (Kamkaen and Wilkinson 2009). Several lipid peroxidation compounds such as aliphatic aldehydes and 1-octanol was reported to be detected in the sponge cake during the baking process (Zamora and Hidalgo 2016). The levels of lipid oxidation products expressed as TBARS in CTE supplemented sponge cakes are shown in Fig. 2. The addition of CTE (5–10%) significantly reduced the TBARS values in sponge cakes (7.8–38.23%) when compared with the control sponge cake (P < 0.05). These results showed that CTE at low concentration of 5% caused the reduction of lipid oxidation in sponge cake. Several studies have documented that CTE has antioxidant activity including the TEAC, FRAP and DPPH assays (Chayaratanasin et al. 2015; Ismail et al. 2016). Previous study has also shown that CTE (0.02–0.16% w/w) containing the phenolic compounds imparts characteristic inhibition of lipid oxidation in meat products (Pasukamonset et al. 2017). This study proved that a high concentration of CTE may provide a better inhibition effect of TBARs than a low concentration of CTE. By comparison of the results shown above, it can be concluded that the antioxidant activity of the CTE may be varied depending on the level of concentrations. A positive correlation between the phenolic compound content in Clitoria ternatea extract and inhibition of lipid oxidation in cooked pork patty model has been described (Pasukamonset et al. 2017). According to these findings, the reduced TBARS of cakes might be due to the high content of phenolic compounds in CTE, which act as a free radical scavenger.
Fig. 2.
The effects of Clitoria ternatea extract on the TBARS values of sponge cakes. The results are expressed as mean ± S.E.M. (n = 6). Means with different superscripts differ significantly (p < 0.05). Control, 5%CTE, 10%CTE, 15%CTE and 20%CTE: prepared with 0, 5, 10, 15 and 20% replacement of cake flour with Clitoria ternatea extract (CTE), respectively
Sensory evaluation
Sensory evaluation on a nine-point hedonic scale of the sponge cakes is summarized in Table 4. The sensory analysis showed no statistically significant differences in appearance, sponginess, texture, aroma, flavor, and taste scores between the control cake and caked enriched CTE (5–20%). The color scores of cakes plus CTE 5, 10, 15 and 20%, on the other hand, had a lower score than the control cake (P < 0.05). It suggests that the bright blue color of CTE is attributed to sensory acceptance which are consistent with the reduction of the , and values when increased concentration of CTE. Furthermore, the result of overall acceptability of those cakes added CTE was in the range of 5.70–6.10, indicating that these cakes were moderately acceptable. These scores are in agreement with previous studies regarding the moderately acceptable score on a seven-point hedonic scale of cakes with green tea extract (Lu et al. 2010) and black rice extract (Mau et al. 2017) which were in the range of 5.6–6.0 and 5.1–5.8, respectively. According to the sensory characteristics together with the obtained results from physicochemical property that a partial replacement of cake flour with 5% CTE in sponge cakes is considerably more satisfactory than cake flour with 10, 15, and 20% CTE.
Table 4.
Sensory evaluation scores of sponge cake prepared supplemented with various levels of Clitoria ternatea extract (CTE)
| Attributes | Control | 5% | 10% | 15% | 20% |
|---|---|---|---|---|---|
| Appearance | 5.87 ± 0.28a | 5.63 ± 0.21a | 5.60 ± 0.28a | 5.40 ± 0.25a | 5.40 ± 0.30a |
| Color | 6.60 ± 0.23a | 4.86 ± 0.28b | 4.50 ± 0.29b,c | 3.90 ± 0.25c | 4.30 ± 0.42b,c |
| Sponginess | 6.50 ± 0.20a | 6.16 ± 0.19a | 6.47 ± 0.20a | 6.36 ± 0.22a | 6.17 ± 0.26a |
| Texture | 6.36 ± 0.19a | 6.03 ± 0.24a | 6.13 ± 0.22a | 5.87 ± 0.28a | 5.83 ± 0.29a |
| Aroma | 6.36 ± 0.22a | 6.03 ± 0.24a | 6.33 ± 0.26a | 6.47 ± 0.27a | 6.20 ± 0.24a |
| Flavor | 6.30 ± 0.24a | 6.03 ± 0.21a | 6.30 ± 0.24a | 6.40 ± 0.24a | 6.20 ± 0.21a |
| Taste | 6.56 ± 0.22a | 6.53 ± 0.18a | 6.20 ± 0.20a | 6.06 ± 0.28a | 6.53 ± 0.26a |
| Overall acceptability | 6.70 ± 0.18a | 6.10 ± 0.18a,b | 5.70 ± 0.24b | 5.80 ± 0.27b | 5.96 ± 0.26b |
Each value is expressed as mean ± S.E.M. (n = 30). Means with different capital letter within a row are significantly different (P < 0.05)
Conclusion
The replacement of wheat flour with CTE (5–20%) in the sponge cake formula increases the polyphenol content and antioxidant activity as well as the inhibition of lipid peroxidation. Taken together with the texture analysis and acceptability, CTE would be an alternative and potential source for development of sponge cakes as a functional food.
Acknowledgments
PP was supported by Overseas Research Experience Scholarship for Graduate Student, Graduate school and Faculty of Allied Health Sciences, Chulalongkorn University. This work was supported by Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University.
References
- Adisakwattana S, Ruengsamran T, Kampa P, Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal alpha-glucosidase and pancreatic alpha-amylase. BMC Complement Altern Med. 2012;12:110. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sayed HM, Ahmed AR. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann Agric Sci. 2013;58:83–95. [Google Scholar]
- Bajerska J, Mildner-Szkudlarz S, Jeszka J, Szwengiel A. Catechin stability, antioxidant properties and sensory profiles of rye breads fortified with green tea extracts. J Food Nutr Res. 2010;49:104–111. [Google Scholar]
- Chayaratanasin P, Barbieri MA, Suanpairintr N, Adisakwattana S. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement Altern Med. 2015;15:1. doi: 10.1186/s12906-015-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail T, Akhtar S, Riaz M, Hameed A, Afzal K, Sattar Sheikh A. Oxidative and microbial stability of pomegranate peel extracts and bagasse supplemented cookies. J Food Qual. 2016;39:658–668. doi: 10.1111/jfq.12231. [DOI] [Google Scholar]
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Kamkaen N, Wilkinson JM. The antioxidant activity of Clitoria ternatea flower petal extracts and eye gel. Phytother Res. 2009;23:1624–1625. doi: 10.1002/ptr.2832. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HJ, Lee HS, Lim EJ, Imm JY, Suh HJ. Physical and sensory characteristics of fibre-enriched sponge cakes made with Opuntia humifusa. LWT Food Sci Technol. 2012;47:478–484. doi: 10.1016/j.lwt.2012.02.011. [DOI] [Google Scholar]
- Lee JH. Physicochemical and sensory characteristics of sponge cakes with Rubus coreanus powder. Prev Nutr Food Sci. 2015;20:204–209. doi: 10.3746/pnf.2015.20.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijon MB, Meghla NS, Jahedi ME, Rahman A, Hossain L. Phytochemistry and pharmacological activities of Clitoria ternatea. Int J Nat Soc Sci. 2017;4:1–10. [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TM, Lee CC, Mau JL, Lin SD. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010;119:1090–1095. doi: 10.1016/j.foodchem.2009.08.015. [DOI] [Google Scholar]
- Madhavarao B, Sabithadevi K, Vinnakoti A. In vitro antimicrobial and free radical scavenger assay of two medicinal plants Clitoria ternatea and Cardiospermum halicacabum. Int J Chem Anal Sci. 2011;2:1253–1255. [Google Scholar]
- Madhu K. Phytochemical screening and antioxidant activity of in vitro grown plants Clitoria ternatea L. using dpph assay. Asian J Pharm Clin Res. 2013;6:38–42. [Google Scholar]
- Mau JL, Lu TM, Lee CC, Lin LY, Cheng CH, Lin SD. Physicochemical, antioxidant and sensory characteristics of chiffon cakes fortified with various tea powders. J Food Process Preserv. 2015;39:443–450. doi: 10.1111/jfpp.12249. [DOI] [Google Scholar]
- Mau JL, Lee CC, Chen YP, Lin SD. Physicochemical, antioxidant and sensory characteristics of chiffon cake prepared with black rice as replacement for wheat flour. LWT Food Sci Technol. 2017;75:434–439. doi: 10.1016/j.lwt.2016.09.019. [DOI] [Google Scholar]
- Mohamad RA, Taip FS, Kamal SMM, Bejo SK. Color and volume development of cake baking and its influence on cake qualities. J Appl Sci Agric. 2015;10:63–68. [Google Scholar]
- Mohdaly AA, Sarhan MA, Smetanska I, Mahmoud A. Antioxidant properties of various solvent extracts of potato peel, sugar beet pulp and sesame cake. J Sci Food Agric. 2010;90:218–226. doi: 10.1002/jsfa.3796. [DOI] [PubMed] [Google Scholar]
- Moraes ÉA, Dantas MIdS, Morais DdC, Cod Silva, Castro FAFd, Martino HSD, Ribeiro SMR. Sensory evaluation and nutritional value of cakes prepared with whole flaxseed flour. Food Sci Technol (Campinas) 2010;30:974–979. doi: 10.1590/S0101-20612010000400021. [DOI] [Google Scholar]
- Mukherjee PK, Kumar V, Kumar NS, Heinrich M. The Ayurvedic medicine Clitoria ternatea—from traditional use to scientific assessment. J Ethnopharmacol. 2008;120:291–301. doi: 10.1016/j.jep.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Nair V, Bang WY, Schreckinger E, Andarwulan N, Cisneros-Zevallos L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J Agric Food Chem. 2015;63:6355–6365. doi: 10.1021/acs.jafc.5b00928. [DOI] [PubMed] [Google Scholar]
- Pasukamonset P, Kwon O, Adisakwattana S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016;61:772–779. doi: 10.1016/j.foodhyd.2016.06.039. [DOI] [Google Scholar]
- Pasukamonset P, Kwon O, Adisakwattana S. Oxidative stability of cooked pork patties incorporated with Clitoria ternatea extract (blue pea flower petal) during refrigerated storage. J Food Process Preserv. 2017;41:e12751. doi: 10.1111/jfpp.12751. [DOI] [Google Scholar]
- Peng X, Ma J, Cheng KW, Jiang Y, Chen F, Wang M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010;119:49–53. doi: 10.1016/j.foodchem.2009.05.083. [DOI] [Google Scholar]
- Prokopov T, Goranova Z, Baeva M, Slavov A, Galanakis CM. Effects of powder from white cabbage outer leaves on sponge cake quality. Int Agrophys. 2015;29:493–500. doi: 10.1515/intag-2015-0055. [DOI] [Google Scholar]
- Şeker İT, Ertop MH, Hayta M. Physicochemical and bioactive properties of cakes incorporated with gilaburu fruit (Viburnum opulus) pomace. Qual Assur Saf Crop. 2016;8:261–266. doi: 10.3920/QAS2014.0542. [DOI] [Google Scholar]
- Shahidi F, Zhong Y. Novel antioxidants in food quality preservation and health promotion. Eur J Lipid Sci Technol. 2010;112:930–940. doi: 10.1002/ejlt.201000044. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int J Food Sci Technol. 2015;50:1190–1197. doi: 10.1111/ijfs.12764. [DOI] [Google Scholar]
- St. Angelo AJ, Vercellotti J, Jacks T, Legendre M. Lipid oxidation in foods. Crit Rev Food Sci Nutr. 1996;36:175–224. doi: 10.1080/10408399609527723. [DOI] [PubMed] [Google Scholar]
- Sui X, Zhang Y, Zhou W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: its quality attributes and in vitro digestibility. Food Chem. 2016;196:910–916. doi: 10.1016/j.foodchem.2015.09.113. [DOI] [PubMed] [Google Scholar]
- Suja K, Jayalekshmy A, Arumughan C. Antioxidant activity of sesame cake extract. Food Chem. 2005;91:213–219. doi: 10.1016/j.foodchem.2003.09.001. [DOI] [Google Scholar]
- Tecson-Mendoza EM. Development of functional foods in the Philippines. Food Sci Technol Res. 2007;13:179–186. doi: 10.3136/fstr.13.179. [DOI] [Google Scholar]
- Waraho T, McClements DJ, Decker EA. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci Technol. 2011;22:3–13. doi: 10.1016/j.tifs.2010.11.003. [DOI] [Google Scholar]
- Yuksel Z, Avci E, Erdem YK. Characterization of binding interactions between green tea flavonoids and milk proteins. Food Chem. 2010;121:450–456. doi: 10.1016/j.foodchem.2009.12.064. [DOI] [Google Scholar]
- Zamora R, Hidalgo FJ. The triple defensive barrier of phenolic compounds against the lipid oxidation-induced damage in food products. Trends Food Sci Technol. 2016;54:165–174. doi: 10.1016/j.tifs.2016.06.006. [DOI] [Google Scholar]
- Zhang YY, Song Y, Hu X, Liao XJ, Ni YY, Li QH. Effects of sugars in batter formula and baking conditions on 5-hydroxymethylfurfural and furfural formation in sponge cake models. Food Res Int. 2012;49:439–445. doi: 10.1016/j.foodres.2012.07.012. [DOI] [Google Scholar]
- Zingare ML, Zingare P, Dubey A, Ansari M. Clitoria ternatea (Aparajita): a review of the antioxidant, antidiabetic and hepatoprotective potentials. Int J Pharm Biol Sci. 2013;3:203–213. [Google Scholar]