Abstract
It is less than 20 years since nanotechnology found applications in food packaging. The new packaging materials have featured various improved characteristics such as antimicrobial activity and active packaging. However, there is a great controversy about the production cost, safety and suitability of nanocomposite materials to come in contact with foodstuffs. To this end, we critically summarize the literature in order to provide the overview of the current status in the field. A scientometric evaluation is presented for the first time in order to illustrate the state of the art. The USA and the Asian countries are the leaders, while the EU countries follow. Additionally, as the analysis of nanomaterials in food matrices is still in early stage, there is an emerging demand to review the analytical techniques which are capable for the monitoring of nanomaterials. Microscopy, spectroscopy, separation and mass spectrometry techniques show advantages and drawbacks which are discussed. FFF-ICP-MS and sp-ICP-MS have the greatest potential for the detection of inorganic nanoparticles in food. In conclusion, the difficulty of analyzing nanoparticles is increased by the lack of standard solutions, reference materials, standard methods and the limited number of available inter-laboratory proficiency tests.
Keywords: Nanomaterials, Nanoparticles, Food packaging, Analytical techniques, Scientometric evaluation, FFF-ICP-MS
Introduction
The main goal of packaging is the protection of the product and the maintenance of its shelf life. The package should keep food content safe but also qualitatively acceptable. The food packaging industry has managed to accomplish this mission. However, the food industry is constantly seeking for new technologies that will further improve critical parameters such as quality, safety, traceability and shelf life of food. In recent years, there is an effort to improve the properties of existing packing material with the introduction of nanotechnology in food industry. The advent of nanotechnology has created opportunities for the development and use of new materials such as nanomaterial, on food packaging. Nanomaterials (NMs) have size, at least in one of the three dimensions, smaller than 100 nm and they are used in various food science applications. To begin with, the functionalization of NMs is one approach that should be reported. This approach utilizes NMs for a definite function or purpose. Nanomaterials can be functionalized through various routes, non-covalent or covalent to obtain complex hybrid systems (Yang et al. 2016). A typical example NMs functionalization is the use of quantum dots (QDs) for the assessment of pesticides using biosensors (Dey et al. 2017; Wu et al. 2017; Yang et al. 2017). The implementation of NMs in the sector of food safety may start a new era of on-site pesticide residues monitoring. Concerning food packaging, NMs find more and more applications in food packaging (Reig et al. 2014). The encapsulation of natural NMs like halloysite nanotubes (HNTs; Jang et al. 2017), for improved food packaging has drawn the attention of EU-funding with the new NanoPack Horizon 2020 research program (Segal 2017). Research and development departments (R&D) of food industries along with research institutes and projects at academic level are trying to improve the quality of packaging with the use of NMs in the following fields: mechanical properties (Bradley et al. 2011; Lei et al. 2006; ShengdaTech 2008), barrier properties (Šimon et al. 2008; Memiş et al. 2017; Scarfato et al. 2017; Scrinis and Lyons 2010; Smolander and Chaudhry 2010; Staroszczyk et al. 2017) and antimicrobial activity (Adepu and Khandelwal 2018; Bradley et al. 2011; Castro-Mayorga et al. 2018; Chorianopoulos et al. 2011; Espitia et al. 2016; Hannon et al. 2017; Li et al. 2011; Mlalila et al. 2017; Pal et al. 2007). In addition, the use of NMs allow realization of bold but innovative projects such as active (Downing-Perrault 2005; Grattan and Gilberg 1994; Taylor 2008) and recently innovative & intelligent packaging (Khan et al. 2018; Mlalila et al. 2016). Some of these properties are already a reality (mostly in the USA and Asian Countries) while others are still in research scale. In any case, great advances are anticipated in this field, which will change the role of packaging (Chaudhry et al. 2008). The packaging applications along with the most commonly used NMs in each case are briefly documented in Table 1.
Table 1.
Applications and features of NM-based food packaging
| Application | Type of NM | Feature |
|---|---|---|
| Mechanical properties | Carbon nanotubes | A relatively low loading, < 6%, can imrove polymer properties without impact on density, transparency and other properties |
| Barrier properties | Nano-clay | Improved gas-barrier and barrier properties against visible and UV light |
| Antimicrobial activity | Silver nanoparticles | Biocidal action based on size and shape-dependent interaction with microorganisms |
| Active packaging | Inorganic nanoparticles such as iron | Oxidation of iron instead of food constituents such as myoglobin in meet products |
| Intelligent packaging | Cellulosic NMs | Monitoring of pH changes in foods and controlled release of bioactive agents |
However, potential contamination of food in NM-based containers is crucial due to potential migration (Bumbudsanpharoke and Ko 2015; Gallocchio et al. 2016; Huang et al. 2015; Störmer et al. 2017). Thus, there is a need for new analytical methodologies to specifically assess NMs. The lack of toxicity data as well as the controversial ability of migration between the package and the food, makes analytical assessment of nanomaterial in food necessary. In this way, we critically summarize the state of the art of NMs and the analytical methods that have the potential to accurately determine them.
State of the art
Production cost
Utilization of nanotechnology in food packaging poses questions about whether production cost of such material is cost-efficient. A spontaneous response could be that polymers containing nanoparticles (NPs) in their composition cannot be produced in marketable quantities due to high cost. However, the situation is debatable. Two cases can be distinguished. In the first case, materials that have been released into the market and give improved properties of packaging. Such materials are polymers that contain nano-clay or which have integrated metals (or oxides of metals) at nano-dimensions or nano-cellulose. These NMs can be incorporated into conventional packaging materials such as membranes or containers and improve the properties of packaging. The increased cost of such materials is not significant, as conventional methods are used for the production, while the improved packaging properties result to increased food shelf life. On the other hand, the creation of innovative packaging with high production costs is feasible. Examples are the nano-composite multiple layers and the use of hybrid organic and inorganic nano-coatings. However, such packages cannot be placed on a commercial scale and are still under research (Bradley et al. 2011).
The impact of nanotechnology in food industry is clear from available market reports, applications for patents and the growing number of products available in the market. In accordance with the market analysis done by the Helmut Kaiser Consultancy, the USA dominated in efforts to import nanotechnology in food sector. Asian countries follow, with China as a pioneer in the field, with predictions (2010) that the Asian market will be the largest in the world. The total size of nanotechnology market in food was estimated about 7 billion US $ in 2006, while it increased reaching 20 billion US $ in 2015 (Smolander and Chaudhry 2010). According the consulting firm Cientifica, applications of nanotechnology in food are estimated to be about 410 million US $. Specifically, nanocomposite packaging materials occupy more than 50% of the market (210 million US $), followed by food processing and food ingredients applications with 100 million US $, respectively. Finally, around 400 companies deal with nanotechnology applications in food science field.
Scientometric evaluation of the field
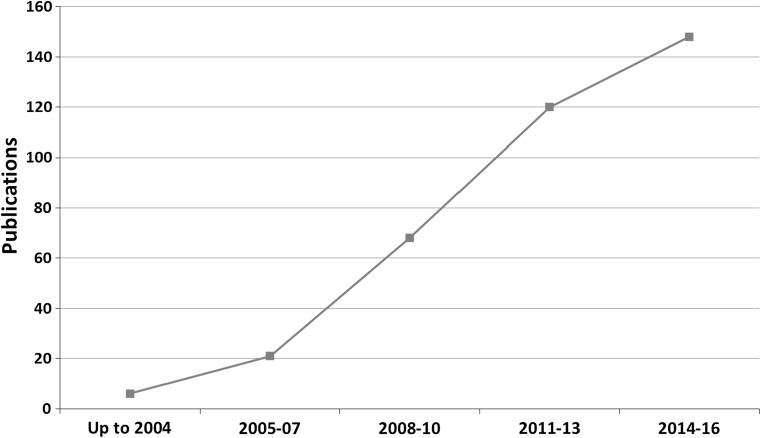
The use of nanotechnology in the field of packaging attracts more and more research interest. Figure 1 shows the temporal evolution of publications related to the subject. The search was performed using Scopus database and (nanomaterial or nanomaterials or nanotechnology and “food packaging”) were used as keywords. A continuous increase in publications number was observed indicating the emerging interest of scientific community towards NMs in food packaging. Furthermore, we should notice that the first study which reported in Scopus database was published in 2003 (Childs 2003)! Since 2005–2007 period, 10 years ago, relative publications presented seven-fold increase. This shows that the sector is in its infancy and there is place for rapid growth.
Fig. 1.
Temporal evolution of published work (Scopus, 4-2017)
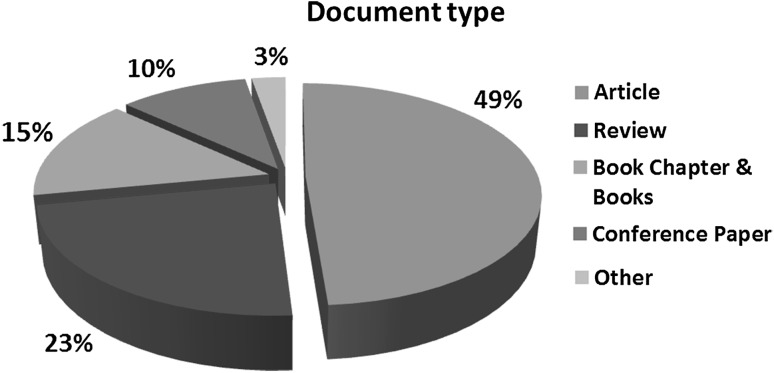
Three hundred sixty-four studies have been published until the end of 2016 as it is depicted in Fig. 2. Almost the half of them are original works which were published in research journals, 178 articles, while review articles stand for the 23%, 84 articles, of the total. Furthermore, book chapters and books counted about 15% indicating the increased public and scientific interest. It is also important to notice that all of them have been published after 2009.
Fig. 2.
Published research per document type
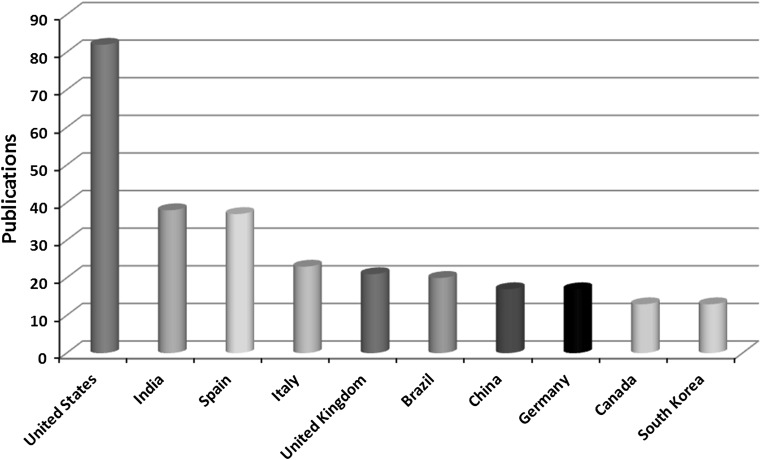
Figure 3 shows the top ten countries in publications related to nanotechnology and food packaging. Two hundred eighty-one publications, 77% of the total, originate from these ten countries. It can be highlighted that the USA dominate the field, while dynamic is the presence of Asian countries (India, China and South Korea). Concerning European countries, active research is made in the UK, Spain, Italy and Germany.
Fig. 3.
Top ten countries in the field
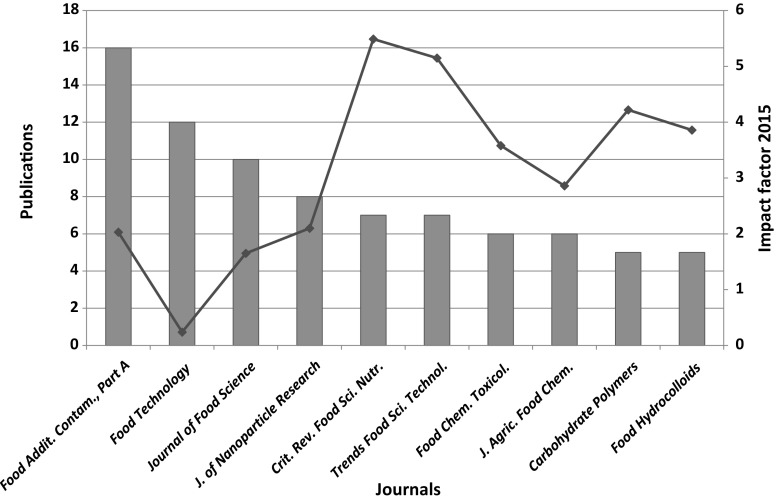
Publications on nanomaterial and food packaging were scattered in more than 140 journals! This fact could be explained due to the multidisciplinary character of the field and the appreciation by a variety of journals. Journals which concern food analysis and food chemistry are mostly chosen such as “Food additives and contaminants part A; Chemistry analysis control exposure and risk assessment”, “Journal of food science”, “Food and chemical toxicology”, “Journal of agricultural and food chemistry”, “Food and chemical toxicology” and finally “Food hydrocolloids” (Fig. 4). Also in the top ten are two reputed “review” journals “Critical reviews in food science and nutrition” and “Trends in food science and technology”. Also, all journals in the top ten present an impact factor higher than 2 from “Food technology” and “Journal of food science”. All in all, NMs is at the spotlight of high-level scientific research.
Fig. 4.
Top ten journals along with their impact factor (red line) and the number of publications (blue bars) (color figure online)
Safety assessment
Application of nanotechnology in food packaging is an innovative approach. Therefore, the possible migration of NMs should be assessed in order to protect consumers from harmful health effects. EU and USA require through their competent authorities, European Food Safety Authority (EFSA) and Food & Drug Administration (FDA) respectively, specific approval for each packaging material that is going to be released on the market (Chau et al. 2007; Raj and Matche 2012). The safety assessment is based on the chemistry and toxicity data submitted and must follow the requirements of the legislation. However, there were not any requirement for the size of packaging materials until recently. Consequently, NMs were approved without any special risk assessment. In 2011, FDA requirements revised resulted in consideration of the materials dimensions used in food packaging (Bradley et al. 2011). In 2009, EFSA published the potential risks that may arise from the use of nanotechnology in food. EFSA is not a regulatory authority but it gives scientific opinions on which the regulations adopted in the European Parliament usually comply with. Risk assessment should be performed by a public authority in each case and the classification of NMs intended for packaging cannot be a choice. This comes in accordance to EC/1935/2004 regulation, in which it is mentioned that any material coming in contact with foodstuffs or animal feeding stuffs must be considered separately as to whether it is safe. The current status in other countries of the world is well discussed and presented by Bumbudsanpharoke et al. (2015).
The doubts of regulatory bodies on the safety of NMs is based on the controversy that can be found in published research (Gismondi et al. 2016; Qian et al. 2013). This statement is reflected by the following two examples for graphene oxide and inorganic nanoparticles such as silver and gold nanoparticles (Ag NPs. Au NPs). Nguyen et al. (2015) showed the low toxicity of graphene oxide against selected intestinal bacteria. Cells were unaffected at all selected graphene oxide concentrations for 24 h, and the dose-dependent cytotoxicity of graphene oxide was not observed. On the other hand, Wang et al. (2014) stated the in vitro cytotoxic effect of graphene oxide to cultured RPMI 8226 cells. The observed cytotoxic effect was dose-dependent and associated with increased oxidative stress. Regarding Au NPs seems not to be inherently toxic to human cells, despite being taken up into cells (Connor et al. 2005). Data published by Haase et al. (2011) demonstrated that even low doses of Ag NPs exerted adverse effects in human macrophages. A recently published article in Nature, McClements and Xiao (2017), concludes that many NMs are unlikely to have adverse effects on human health, but there is evidence that some of them could have harmful effects and that future studies are required. Taking all the above facts into consideration, safety assessment of NMs is still in an early stage and more effort should be made.
Analytical prospects and gaps
Applications of nanomaterials become more and more popular arising concerns whether it is possible to accurately detect them in complex food matrices. The analytical measurement of NMs in food is even more needed when one taking into consideration the questionable NMs toxicity and the controversial migration ability between the package and the food (Grieger et al. 2016; Picó 2016). The main analytical problem concerning NMs in food is the complex nature of the matrix. The characterization and identification of NMs would be much easier if they were separated entirely from the matrix. Another bottleneck of NMs detection is that their physicochemical properties are highly connected with the food matrix. In other words, a universal analytical approach is not available and the development of new methods in each matrix is necessary (Blasco and Picó 2011). Although a great effort is made on NMs analysis in food, still the analytical techniques which have the potential to improve current status are undefined. To this end, we summarize which techniques have been used or proved “fit for purpose” concerning the detection of NPs emerging in foodstuffs from packaging.
Microscopy
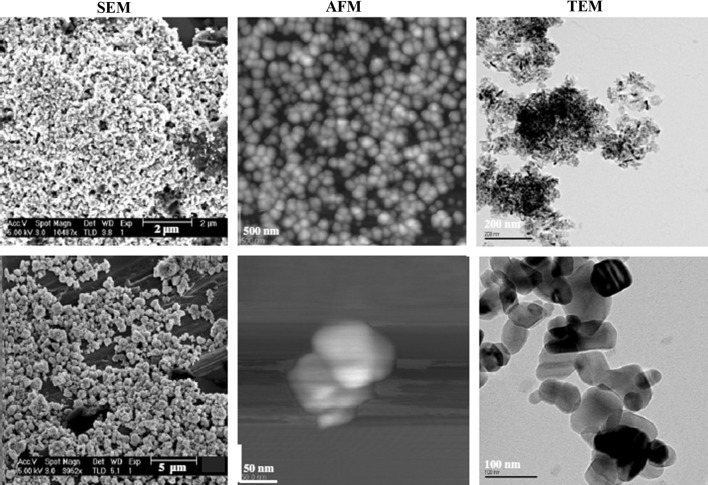
Qualitative determination of metals found in nano-dimensions can be achieved by electron microscopy (EM). Determination using EM is feasible even in complex matrices such as food or biological tissues. However, the detection is possible when NMs are in a high concentration as a high magnification is required because of their very small size. Using techniques such as atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), can not only visualize NMs but also infrom about their size, shape and structure (Mavrocordatos et al. 2004). Figure 5 compares the images extracted using techniques AFM, SEM and TEM (Tiede et al. 2008). Nevertheless, food packages contain very small amounts of nanocomposite materials and consequently high analytical sensitivity is needed. Last but not least, the time-consuming analysis using microscopy techniques is another limiting factor which have to be improved in order to incorporate this kind of analysis in routine analysis.
Fig. 5.
ZnO nanoparticles (1st line) and TiO2 (2nd line) identified in water with the SEM, AFM, TEM techniques respectively with permission of Taylor and Francis (www.tandfonline.com; Tiede et al. 2008)
Spectroscopy
EM techniques can also identify carbon based NMs such as carbon nanotubes and fullerenes. However, the determination is very slow compared to inorganic nanostructures, as aforementioned. In this case Raman spectroscopy (RS) is a much appropriate analytical choice. Raman spectroscopy is a cheap, non-destructive and quick technique while it can also be applied for in situ determinations (Fantini et al. 2004; Tiede et al. 2008). On-site detection of food contaminants is a big trend as it can improve the current status in food safety. Except from RS, carbon nanotubes and fullerenes have been successfully detected using ultraviolet–visible spectroscopy (UV–Vis) and Fourier transformation–Infrared spectroscopy (FT–IR) (Andrievsky et al. 2002). In addition, a common technique for the determination of particle size is the dynamic light scattering (DLS), which is widely used for carbon-based NMs. The main drawback of DLS is that it cannot distinguish properly NMs size in samples with high heterogeneity as well as discriminate inorganic and organic NMs that can be found in the determined material (Farré and Barceló 2012). Notable is the use of nuclear magnetic resonance spectroscopy (NMR) which can determine the three-dimensional structure of a solid or a suspension, such as a food matrix. In addition, various applications of X-ray spectroscopy could potentially assist both the qualitative and the quantitative determination of NPs. Thus, X-ray diffraction (XRD) is a non-destructive method that can determine the crystalline structure and elemental composition of various materials. Indeed, XRD has been used for determination of Fe nanoparticles (Nurmi et al. 2005). Finally, X-ray fluorescence (XRF) is another non-destructive technique used for qualitative and quantitative determination of elements in solid samples, powders and liquid samples. The XRF is used widely in the quality control of materials (Tiede et al. 2008).
Separation
Various separation techniques are utilized to isolate an analyte from complex matrices. In the case of NMs, successful separations can be achieved by size exclusion chromatography (SEC), capillary electrophoresis (CE) and field flow fractionation (FFF) techniques (Luykx et al. 2008). Concerning SEC, a column of certain porosity is used, which means that within the network only specific-sized particles may enter. Particles larger than the gel porous cannot enter the network and are eluted directly from the column. The next step is the fractionation of NPs which can fit into the gel (based on their size). Ideally, particles size should be the unique separation criterion. However, interactions between stationary phase and the analytes are often observed due to adsorption mechanisms. Furthermore, the limited variety of columns for the separation of NPs is still a problem as the pores of most columns are large compared to the size of NPs. The SEC technique has successfully used for the separation of carbon nanotubes (Ziegler et al. 2005). Additionally, capillary electrophoresis can be used. CE has the advantage of not encountering interactions with stationary phase. This does not mean that there are no obstructions as the analyte interacts with mobile phase. The separation is based on the charge and size of the ingredients. A big drawback is that since separation is not based only on the size, data interpretation becomes difficult. In this way, Au and Ag have been successfully separated (Lin et al. 2007). The chemical characterization of these NP species was achieved within 4 min using diode array detection (DAD). Field flow fractionation (FFF) is a promising separation technique. It is similar to chromatographic techniques, but the separation is achieved by physical partitioning without any interaction of the analyte with the stationary phase. Particles are separated depending on how they are affected by an applied field, which may be the centripetal force or a hydrodynamic flow. The field controls the speed of the particles within a thin channel. The FFF is capable of separating particles whose size ranges from 1 nm to 1 μm and therefore is suitable for separating nanoparticles (Tiede et al. 2008; Von der Kammer et al. 2011).
Mass spectrometry
NPs used in packaging materials can be both inorganic (metal nanoparticles) and organic compounds such as carbon nanotubes that confer improved mechanical properties in the package. Techniques such as inductively coupled plasma-optical emission spectrometry (ICP-OES) and inductively coupled plasma-mass spectrometry (ICP-MS) are preferred for the determination of the inorganic NMs. ICP-MS is an excellent option for simultaneous determination of elemental content to levels that reach up to ppt (Georgiou and Danezis 2015). While, usually, sample preparation includes use of concentrated acids and microwave-assisted digestion, in the case of NMs such a procedure does not fit the analytical purpose. Digestion is used when the overall elemental content of the matrix should be determined. In this case, only the inorganic NPs have to be assessed, so a separation technique is combined such as LC (Carneado et al. 2015) and FFF (Gimbert et al. 2007). In cases of very low abundant elements, such as gold, wet digestion procedure is satisfactory, as it is accepted that the determined gold comes almost exclusively from the nanomaterial (Blasco and Picó 2011). Another significant feature of FFF-ICP-MS is that can distinguish NPs based on their diameter. A promising technique for the determination of NPs is the single particle-ICP-MS (sp-ICP-MS). Single particle-ICP-MS can determine the average diameter of a nanoparticle. This is possible because of short dwell time, time necessary for identifying m/z, at the level of msec. Low dwell time allows better separation between NPs, while, in each dwell time, a single NP is measured. In addition, sp-ICP-MS technique allows distinguishing between nanoparticles of natural and artificial origin (Lee et al. 2014). Unfortunately, these techniques cannot determine organic NPs as they are destroyed in the process of digestion.
The current situation about the techniques that can detect organic NPs in food is in an early stage. The need for organic NMs determination has led scientists to evaluate available feasible analytical approaches. Thus, TiO2 nanoparticles have been identified using time of flight mass spectrometry (TOF-MS) combined with matrix assisted desorption ionization (MALDI; Guan et al. 2007). Αnalysis of NMs in environmental samples is in a more advanced level compared to food and should serve as a guide on how the analysis in food science field can be improved. Fullerenes have been identified in waste water using liquid-chromatography paired with successive mass spectrometers (quadropole and ion trap; Farré et al. 2010) as well as the determination of their toxicity by LC-MS (Isaacson et al. 2007).
Problems in the analysis of NMs
Except the difficulties that have already been mentioned, such as the possible complex matrix, it should also be emphasized that there are no standard solutions, reference methods, protocols and procedures for the analysis of NPs in food. In the case of food contaminants, the existence of internal standards is very important for quantification. Currently there is an available internal standard of fullerene C60 highlighted with 13C. Also, the Joint Research Centre, Institute of Reference Materials and Measurements (IRMM) released quality control material for silica NPs, the IRMM-304, while the National Institute of Standards and Technology (NIST) has reference materials for gold NPs (NIST RM 8011, 8012 and 8013) and polystyrene spheres (NIST SRM 1963a and 1964). A great gap in the analytical process is that there are no inter-laboratory studies, which are useful for the validation of the methods. Quality criteria such as the accuracy and the limits of detection are indispensable for reliable analytical results. The main difficulty for the inter-laboratory studies lies in the fact that there are many different techniques and methods without any validation. Therefore, the obtained results cannot be easily compared and evaluated between different laboratories (Blasco and Picó 2011; Picó 2016).
Conclusions
The use of NMs in food packaging annually draws greater attention by the scientific community. These packaging applications have already become part of the market in the case of the USA and Asian countries. On the other hand, EU countries have not adapted yet these innovative technological features. In fact, Europeans prove their perception to utilize materials that are fully examined for safety issues in order to be used as packaging agents. One way or another, nanocomposite materials should be studied extensively in order to meet safety and quality standards. Analytical chemists should act as pioneers and develop powerful instrumentation and suitable methodologies for an accurate and sensitive determination of NPs. Nevertheless, the lack of standard solutions, reference methods, reference materials, and standardized protocols is a great analytical bottleneck of high importance for the analysis of NPs in food matrices. The developed methods should be fully validated. In any other case, quantitative measurement of NMs in food matrices cannot be precise.
Contributor Information
Aristeidis S. Tsagkaris, Phone: +30-2105294248, Email: atsagkaris@chem.uoa.gr
Georgios P. Danezis, Phone: +30-2105294248, Email: gdanezis@aua.gr
References
- Adepu S, Khandelwal M. Broad-spectrum antimicrobial activity of bacterial cellulose silver nanocomposites with sustained release. J Mater Sci. 2018;53:1596–1609. doi: 10.1007/s10853-017-1638-9. [DOI] [Google Scholar]
- Andrievsky G, Klochkov V, Bordyuh A, Dovbeshko G. Comparative analysis of two aqueous-colloidal solutions of C 60 fullerene with help of FTIR reflectance and UV–Vis spectroscopy. Chem Phys Lett. 2002;364:8–17. doi: 10.1016/S0009-2614(02)01305-2. [DOI] [Google Scholar]
- Blasco C, Picó Y. Determining nanomaterials in food. TrAC Trends Anal Chem. 2011;30:84–99. doi: 10.1016/j.trac.2010.08.010. [DOI] [Google Scholar]
- Bradley EL, Castle L, Chaudhry Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci Technol. 2011;22:604–610. doi: 10.1016/j.tifs.2011.01.002. [DOI] [Google Scholar]
- Bumbudsanpharoke N, Ko S. Nano-food packaging: an overview of market, migration research, and safety regulations. J Food Sci. 2015;80:910–923. doi: 10.1111/1750-3841.12861. [DOI] [PubMed] [Google Scholar]
- Bumbudsanpharoke N, Choi J, Ko S. Applications of nanomaterials in food packaging. J Nanosci Nanotechnol. 2015;15:6357–6372. doi: 10.1166/jnn.2015.10847. [DOI] [PubMed] [Google Scholar]
- Carneado S, Hernández-Nataren E, López-Sánchez JF, Sahuquillo A. Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem. 2015;166:544–550. doi: 10.1016/j.foodchem.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Castro-Mayorga JL, Freitas F, Reis MAM, Prieto MA, Lagaron JM. Biosynthesis of silver nanoparticles and polyhydroxybutyrate nanocomposites of interest in antimicrobial applications. Int J Biol Macromol. 2018;108:426–435. doi: 10.1016/j.ijbiomac.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Chau CF, Wu SH, Yen GC. The development of regulations for food nanotechnology. Trends Food Sci Technol. 2007;18:269–280. doi: 10.1016/j.tifs.2007.01.007. [DOI] [Google Scholar]
- Chaudhry Q, et al. Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A. 2008;25:241–258. doi: 10.1080/02652030701744538. [DOI] [PubMed] [Google Scholar]
- Childs NM. Nutraceuticals and development systems: process and content. J Diet Suppl. 2003;4:1–2. [Google Scholar]
- Chorianopoulos N, Tsoukleris D, Panagou E, Falaras P, Nychas G-J. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 2011;28:164–170. doi: 10.1016/j.fm.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Dey N, Bhagat D, Cherukaraveedu D, Bhattacharya S. Utilization of red-light-emitting CdTe nanoparticles for the trace-level detection of harmful herbicides in adulterated food and agricultural crops. Chem Asian J. 2017;12:76–85. doi: 10.1002/asia.201601302. [DOI] [PubMed] [Google Scholar]
- Downing-Perrault A. Polymer nanocomposites are the future. Menomonie: University of Wisconsin-Stout; 2005. [Google Scholar]
- Espitia PJP, Otoni CG, Soares NFF. Zinc Oxide nanoparticles for food packaging applications. In: Barros-Velazquez J, editor. Antimicrobial food packaging. 1. London: Elsevier; 2016. pp. 425–431. [Google Scholar]
- Fantini C, et al. One-dimensional character of combination modes in the resonance Raman scattering of carbon nanotubes. Phys Rev Lett. 2004;93:087401. doi: 10.1103/PhysRevLett.93.087401. [DOI] [PubMed] [Google Scholar]
- Farré M, Barceló D. Introduction to the analysis and risk of nanomaterials in environmental and food samples. In: Barcelo D, Farré M, editors. Comprehensive analytical chemistry. 1. Oxford: Elsevier; 2012. pp. 1–32. [Google Scholar]
- Farré M, Pérez S, Gajda-Schrantz K, Osorio V, Kantiani L, Ginebreda A, Barceló D. First determination of C 60 and C 70 fullerenes and N-methylfulleropyrrolidine C 60 on the suspended material of wastewater effluents by liquid chromatography hybrid quadrupole linear ion trap tandem mass spectrometry. J Hydrol. 2010;383:44–51. doi: 10.1016/j.jhydrol.2009.08.016. [DOI] [Google Scholar]
- Gallocchio F, et al. Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat. Food Addit Contam Part A. 2016;33:1063–1071. doi: 10.1080/19440049.2016.1179794. [DOI] [PubMed] [Google Scholar]
- Georgiou CA, Danezis GP. Elemental and isotopic mass spectrometry. In: Pico Y, editor. Advanced mass spectrometry for food safety and quality, comprehensive analytical chemistry. Amsterdam: Elsevier; 2015. pp. 131–243. [Google Scholar]
- Gimbert LJ, Hamon RE, Casey PS, Worsfold PJ. Partitioning and stability of engineered ZnO nanoparticles in soil suspensions using flow field-flow fractionation. Environ Chem. 2007;4:8–10. doi: 10.1071/EN06072. [DOI] [Google Scholar]
- Gismondi A, Nanni V, Reina G, Orlanducci S, Terranova ML, Canini A. Nanodiamonds coupled with 5, 7-dimethoxycoumarin, a plant bioactive metabolite, interfere with the mitotic process in B16F10 cells altering the actin organization. Int J Nanomed. 2016;11:557. doi: 10.2147/IJN.S96614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan DW, Gilberg M. Ageless oxygen absorber: chemical and physical properties. Stud Conserv. 1994;39:210–214. [Google Scholar]
- Grieger KD, Harrington J, Mortensen N. Prioritizing research needs for analytical techniques suited for engineered nanomaterials in food. Trends Food Sci Technol. 2016;50:219–229. doi: 10.1016/j.tifs.2016.02.004. [DOI] [Google Scholar]
- Guan B, Lu W, Fang J, Cole RB. Characterization of synthesized titanium oxide nanoclusters by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom. 2007;18:517–524. doi: 10.1016/j.jasms.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Haase A, et al. Toxicity of silver nanoparticles in human macrophages: uptake, intracellular distribution and cellular responses. J Phys Conf Ser. 2011;304:012030. doi: 10.1088/1742-6596/304/1/012030. [DOI] [Google Scholar]
- Hannon JC, Kerry JP, Cruz-Romero M, Azlin-Hasim S, Morris M, Cummins E. Kinetic desorption models for the release of nanosilver from an experimental nanosilver coating on polystyrene food packaging. Innov Food Sci Emerg Technol. 2017;44:149–158. doi: 10.1016/j.ifset.2017.07.001. [DOI] [Google Scholar]
- Huang JY, Li X, Zhou W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci Technol. 2015;45:187–199. doi: 10.1016/j.tifs.2015.07.002. [DOI] [Google Scholar]
- Isaacson CW, Usenko CY, Tanguay RL, Field JA. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal Chem. 2007;79:9091–9097. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- Jang SH, Jang SR, Lee GM, Ryu JH, Park SI, Park NH. Halloysite nanocapsules containing thyme essential oil: preparation, characterization, and application in packaging materials. J Food Sci. 2017;82:2113–2120. doi: 10.1111/1750-3841.13835. [DOI] [PubMed] [Google Scholar]
- Khan A, Wen Y, Huq T, Ni Y. Cellulosic nanomaterials in food and nutraceutical applications: a review. J Agric Food Chem. 2018;66:8–19. doi: 10.1021/acs.jafc.7b04204. [DOI] [PubMed] [Google Scholar]
- Lee S, Bi X, Reed RB, Ranville JF, Herckes P, Westerhoff P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ Sci Technol. 2014;48:10291–10300. doi: 10.1021/es502422v. [DOI] [PubMed] [Google Scholar]
- Lei S, Hoa SV, Ton-That M-T. Effect of clay types on the processing and properties of polypropylene nanocomposites. Compos Sci Technol. 2006;66:1274–1279. doi: 10.1016/j.compscitech.2005.09.012. [DOI] [Google Scholar]
- Li XH, Li WL, Xing YG, Jiang YH, Ding YL, Zhang PP. Effects of nano-ZnO power-coated PVC film on the physiological properties and microbiological changes of fresh-cut “Fuji” apple. Adv Mat Res Trans Tech Publ. 2011;152–153:450–453. [Google Scholar]
- Lin K-H, Chu T-C, Liu F-K. On-line enhancement and separation of nanoparticles using capillary electrophoresis. J Chromatogr A. 2007;1161:314–321. doi: 10.1016/j.chroma.2007.05.072. [DOI] [PubMed] [Google Scholar]
- Luykx DM, Peters RJ, van Ruth SM, Bouwmeester H. A review of analytical methods for the identification and characterization of nano delivery systems in food. J Agric Food Chem. 2008;56:8231–8247. doi: 10.1021/jf8013926. [DOI] [PubMed] [Google Scholar]
- Mavrocordatos D, Pronk W, Boller M. Analysis of environmental particles by atomic force microscopy, scanning and transmission electron microscopy. Water Sci Technol. 2004;50(12):9–18. doi: 10.2166/wst.2004.0690. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Sci Food. 2017;1:6. doi: 10.1038/s41538-017-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memiş S, Tornuk F, Bozkurt F, Durak MZ. Production and characterization of a new biodegradable fenugreek seed gum based active nanocomposite film reinforced with nanoclays. Int J Biol Macromol. 2017;103:669–675. doi: 10.1016/j.ijbiomac.2017.05.090. [DOI] [PubMed] [Google Scholar]
- Mlalila N, Kadam DM, Swai H, Hilonga A. Transformation of food packaging from passive to innovative via nanotechnology: concepts and critiques. J Food Sci Technol. 2016;53:3395–3407. doi: 10.1007/s13197-016-2325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlalila NG, Swai HS, Hilonga A, Kadam DM. Antimicrobial dependence of silver nanoparticles on surface plasmon resonance bands against Escherichia coli. Nanotechnol Sci Appl. 2017;10:1–9. doi: 10.2147/NSA.S123681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Lin M, Mustapha A. Toxicity of graphene oxide on intestinal bacteria and Caco-2 cells. J Food Prot. 2015;78:996–1002. doi: 10.4315/0362-028X.JFP-14-463. [DOI] [PubMed] [Google Scholar]
- Nurmi JT, et al. Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environ Sci Technol. 2005;39:1221–1230. doi: 10.1021/es049190u. [DOI] [PubMed] [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó Y. Challenges in the determination of engineered nanomaterials in foods. TrAC Trends Anal Chem. 2016;84:149–159. doi: 10.1016/j.trac.2016.06.004. [DOI] [Google Scholar]
- Qian H, Peng X, Han X, Ren J, Sun L, Fu Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci. 2013;25:1947–1956. doi: 10.1016/S1001-0742(12)60301-5. [DOI] [PubMed] [Google Scholar]
- Raj B, Matche RS. Safety and regulatory aspects of plastics as food packaging materials. In: Yam KL, Lee DS, editors. Emerging food packaging technologies: principles and practice. 1. Cambridge: Elsevier; 2012. pp. 335–357. [Google Scholar]
- Reig CS, Lopez AD, Ramos MH, Cloquell Ballester VA. Nanomaterials: a map for their selection in food packaging applications. Packag Technol Sci. 2014;27:839–866. doi: 10.1002/pts.2076. [DOI] [Google Scholar]
- Scarfato P, Di Maio L, Milana MR, Giamberardini S, Denaro M, Incarnato L. Performance properties, lactic acid specific migration and swelling by simulant of biodegradable poly (lactic acid)/nanoclay multilayer films for food packaging. Food Addit Contam Part A. 2017;34:1730–1742. doi: 10.1080/19440049.2017.1321786. [DOI] [PubMed] [Google Scholar]
- Scrinis G, Lyons K. Nanotechnology and the techno-corporate agri-food paradigm food security, nutrition and sustainability. In: Lawrence G, Lyons K, Wallington T, editors. Food security, nutrition and sustainability. 1. London: Earthscan; 2010. pp. 252–270. [Google Scholar]
- Segal E. NanoPack: state-of-the-art packaging to improve food safety and reduce food waste. Agro Food Ind Hi Tech. 2017;28:60–63. [Google Scholar]
- ShengdaTech ShengdaTech develops nano-precipitated calcium carbonate for polyethylene. Addit Polym. 2008;2008(4):2–3. [Google Scholar]
- Šimon P, Chaudhry Q, Bakoš D. Migration of engineered nanoparticles from polymer packaging to food—a physicochemical view. J Food Nutr Res. 2008;47:105–113. [Google Scholar]
- Smolander M, Chaudhry Q. Nanotechnologies in food packaging. In: Chaudhry Q, Castle L, Watkins R, editors. Nanotechnologies in food. 1. Cambridge: RSC; 2010. pp. 86–101. [Google Scholar]
- Staroszczyk H, Malinowska-Pańczyk E, Gottfried K, Kołodziejska I. Fish gelatin-nanoclay films. part I: effect of a kind of nanoclays and glycerol concentration on mechanical and water barrier properties of nanocomposites. J Food Process Preserv. 2017;41(5):e13211. doi: 10.1111/jfpp.13211. [DOI] [Google Scholar]
- Störmer A, Bott J, Kemmer D, Franz R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci Technol. 2017;63:39–50. doi: 10.1016/j.tifs.2017.01.011. [DOI] [Google Scholar]
- Taylor MR. Assuring the safety of nanomaterials in food packaging: the regulatory process and key issues, technical report. Washington: Woodrow Wilson International Center for Scholars; 2008. [Google Scholar]
- Tiede K, Boxall AB, Tear SP, Lewis J, David H, Hassellöv M. Detection and characterization of engineered nanoparticles in food and the environment. Food Addit Contam Part A. 2008;25:795–821. doi: 10.1080/02652030802007553. [DOI] [PubMed] [Google Scholar]
- Von der Kammer F, Legros S, Hofmann T, Larsen EH, Loeschner K. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. TrAC Trends Anal Chem. 2011;30:425–436. doi: 10.1016/j.trac.2010.11.012. [DOI] [Google Scholar]
- Wang Y, Wu S, Zhao X, Su Z, Du L, Sui A. In vitro toxicity evaluation of graphene oxide on human RPMI 8226 cells. Biomed Mater Eng. 2014;24:2007–2013. doi: 10.3233/BME-141010. [DOI] [PubMed] [Google Scholar]
- Wu X, Song Y, Yan X, Zhu C, Ma Y, Du D, Lin Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens Bioelectron. 2017;94:292–297. doi: 10.1016/j.bios.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Yang T, Huang H, Zhu F, Lin Q, Zhang L, Liu J. Recent progresses in nanobiosensing for food safety analysis. Sensors. 2016;16(7):1118. doi: 10.3390/s16071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fang G, Wang X, Zhang F, Liu J, Zheng W, Wang S. Electrochemiluminescent graphene quantum dots enhanced by MoS2 as sensing platform: a novel molecularly imprinted electrochemiluminescence sensor for 2-methyl-4-chlorophenoxyacetic acid assay. Electrochim Acta. 2017;228:107–113. doi: 10.1016/j.electacta.2017.01.043. [DOI] [Google Scholar]
- Ziegler KJ, Schmidt DJ, Rauwald U, Shah KN, Flor EL, Hauge RH, Smalley RE. Length-dependent extraction of single-walled carbon nanotubes. Nano Lett. 2005;5:2355–2359. doi: 10.1021/nl0510208. [DOI] [PubMed] [Google Scholar]