Abstract
Various innovations have so far been devised to extract cholesterol from foods. Achieving a supercritical fluid is perhaps one of the greatest human successes in the field of extraction from foodstuffs in last 2 decades. Supercritical fluid extraction (SFE) offers a rapid, environment-friendly and selective method for extracting cholesterol from foods. This review aims at investigating the application of supercritical fluids in extraction of cholesterol. Various factors affecting the SFE, collection systems, examples of cholesterol extraction and SFE benefits are some of the issues discussed in this study.
Keywords: Supercritical fluid, Cholesterol, Lipid, Extraction method, SFE
Introduction
The increasing concerns about the environmental issues caused by organic solvents have obligated the food industry to think about the “green chemistry” idea (Poliakoff and Licence 2015; Sarrade and Seaudea 2014). Application of supercritical fluids, especially supercritical CO2 is an excellent alternative to chemical solvents.
Supercritical fluid extraction (SFE) was an epidemic issue during 1980s, so that some articles named it magic (Fjeldsted and Lee 1984). A liquid or a gas becomes supercritical fluid if the temperature and pressure increase above their critical point (Guiochon and Tarafder 2011). In supercritical region, the surface of demarcation between gas and liquid disappears and emerges a homogeneous fluid (McHugh and Krukonis 2013). Supercritical fluids have a density and diffusivity between gas and liquid. Density of these fluids, in opposite of liquids, varies by change in temperature and pressure values, therefore a slight increase in pressure can lead to a large increase in fluid density (Pourmortazavi et al. 2014). Selectivity is the other advantage of SFE. Changing the pressure or temperature affects the solubilizing potency of the fluid. This makes it possible to extract complex compounds (Zougagh et al. 2004). Baron Cagniard de la Tour was the first person to devise the SFE in 1822 (Carles 2010), then in 1879 Hannay and Hogarth investigated on the solubility of solids in supercritical fluids (Marr and Gamse 2000). Various compounds have already been used as supercritical fluid which some of them are given in Table 1. Among these compounds, carbon dioxide is more used. Critical point of carbon dioxide reported by Dr. Andrew in 1975 (30.92 °C and 74.0 bars) is near to the currently accepted value (31.1 °C and 73.8 bars). Most of supercritical fluids are inert, inexpensive, pure and nontoxic. Also, this method is suitable for heat-sensitive materials because of lower critical temperature and pressure of fluids such as CO2 and N2O (Marr and Gamse 2000). Use of CO2 as a supercritical solvent has other advantages like environmentally friendly, nonflammable, inexpensive and nontoxic features. It also exists naturally around the world (Keskin et al. 2007).
Table 1.
The most important fluids used as supercritical fluid
(reproduced with permission from Herrero et al. 2006)
| Solvent | Critical property | |||
|---|---|---|---|---|
| Temperature (°C) | Pressure (atm) | Density ρSCF (g/mL) | Solubility ρSFC (cal−1/2 cm−3/2) | |
| Carbon dioxide | 31.2 | 72.9 | 0.470 | 7.5 |
| Ethane | 32.4 | 48.2 | 0.200 | 5.8 |
| Ethene | 10.1 | 50.5 | 0.200 | 5.8 |
| Methanol | − 34.4 | 79.9 | 0.272 | 8.9 |
| Nitrous oxide | 36.7 | 71.7 | 0.460 | 7.2 |
| n-Butene | − 139.9 | 36.0 | 0.221 | 5.2 |
| n-Pentane | − 76.5 | 33.3 | 0.237 | 5.1 |
| Sulfar hexafluoride | 45.8 | 37.7 | 0.730 | 5.5 |
| Water | 101.1 | 217.6 | 0.322 | 13.5 |
A SFE system is composed of pump, restrictor, oven, extractor, stainless steel and collector (Ong et al. 1990). Trapping of extracting materials is the most important part of the process. SFE is designed in two forms: off-line and on-line. The off-line collection includes solvent collection and solid phase trapping. In solvent collection method, effluent (CO2-analyte mixture) is connected with the solvent via the restrictor or depressurized into a distinct solvent (Lang and Wai 2001). The system is widely used for extracting from environmental samples.
The solid phase collection is designed in three approaches, including cryogenic trapping, empty vial collection and adsorption on trapping solids (Nahar and Sarker 2005). Empty vial has poor collection efficiency in comparison with other trapping methods (Turner et al. 2002). For instance, Shen et al. first, removed ~ 75% of the terpene hydrocarbons from orange oil loaded onto a silica gel, then used SC-CO2 at 35 °C, 13.1 MPa, and 2 kg/h until achieved a maximum recovery of flavor compounds (Shen et al. 2002).
The on-line method directly combines the SFE system with analytical instruments such as gas chromatography (GC) or liquid chromatography (LC) columns. Online coupling removes the sample handling, sample loss and reduces the analysis time. The main disadvantage is the need of the experts to handle the process.
Cholesterol is one of the main parts in human biomembranes and vessels. However, a strong relationship between high blood cholesterol and coronary heart disease is its biggest problem (Shepherd et al. 2006; Zhang et al. 2003). The American Heart Association has recommended to reduce the daily intake of saturated fatty acid and cholesterol (Lichtenstein et al. 2006). So, extraction of cholesterol always was considered as an important issue in food industries. Nowadays, SFE of cholesterol has attracted the attention of researchers as an alternative method for organic solvent extraction. This method enables extracting the cholesterol from beef, fish muscles, egg yolk, milk fat and other foods.
Advantages of SFE
SFE is a promising alternative technique to classic and conventional solvent extractions and it has various distinctive properties. The main advantage is possibility for easy manipulating of fluid density. The density of supercritical fluids is easily adjustable by small changes in pressure and temperature. In other words, SFE method has high selectivity. For example, it is possible to launch the extraction at low CO2 density (for example, 0.29 g/cm3) then continue the second extraction stage at high CO2 density (for example, 0.87 g/cm3). Volatile and soluble compounds like essential oils are extracted during the first stage (Aghel et al. 2004; Pourmortazavi et al. 2004, 2005) and the fewer soluble substances are isolated in the subsequent step (for examples, lipids or antioxidants) (Grigonis et al. 2005). Another unique feature of SFE is its low time and solvent consuming property (Boselli et al. 2001, 2002). This technique impressively reduces the extraction time to about 10–60 min (Sapkale et al. 2010). But, for the Soxhlet extraction method with n-hexane as the solvent, time required for lipid and then cholesterol extraction is more than 7 h. Supercritical fluids (SCFs) have rather high diffusivity and low viscosity. So, they can penetrate a solid matrix more effectively than liquid solvents.
Another advantage of SCFs such as N2O or CO2 is the gaseous form of them at room pressure and temperature, which makes the recovery of components simple and fast. SFE usually works at mild condition (low temperature and pressure), so it is suitable to extract thermolabile compounds (Scalia et al. 1999). Another feature of SFE is its low organic solvent consumption compared with classic extraction methods (Hubbard et al. 2004). In comparison with 20–100 g of required matrices in liquid–solid extraction methods, 0.5–1.5 g of the sample is required in SFE method (Sahena et al. 2009). Sample preparation in classic extraction techniques is tedious and needs more time, but SFE requires short time for sample handling and preparation.
SFE enables direct coupling with assessment instruments such as GC or LC. Therefore, it provides qualitative and quantitative detection of substances. Among several solvents utilized for SFE, CO2 is the most widely used because of its low critical pressure and temperature, also it is nonflammable, nontoxic and is convenient to handle. The on-line coupling of a supercritical fluid extractor to an analytical instrument provides other advantages like possibility to flow the large amount of extracted analytes through the extractors, also samples can be protected from light and air.
Limitations of SFE
Despite the fabulous advantages of SFE, but some flaws remain. The first and the main problem of SC-CO2 is its low tendency to dissolve highly polar components. Therefore, it is mandatory to add an organic solvent as modifier to SC-CO2. Also, modifiers, in spite of increasing the yield of extraction, need to be separated in the clean-up step. On the other hand, they cause problems to adsorbents used in the collection step (Brondz et al. 2017). The necessity of having very expensive equipment and experts to handle the SFE process is the other problem. Although, SFE is a fast extraction system, but the cost of buying the equipment, initiating and running the process is high. In addition, the high pressure utilized in the SFE requires that all transfer lines, valves and vessels be fabricated as unfired pressure vessels according to the application code (Khosravi-Darani 2010). The SFE technique likewise requires the clean-up steps repeatedly (De Castro and Jiménez-Carmona 2000). In some cases the extracts are contaminated with unwanted components, therefore the clean-up steps are compulsory, particularly when the analytes are fat-soluble.
Cholesterol
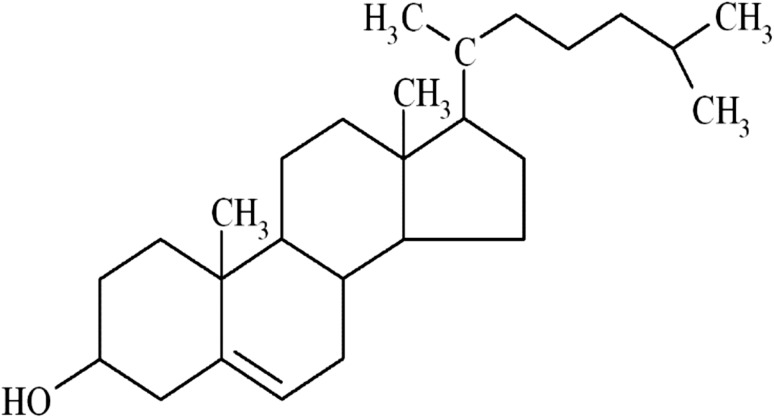
Cholesterol is an organic molecule, belonging to the sterol family (Fig. 1). All animal cells can synthesize cholesterol which is essential for cell membranes. Cholesterol keeps the membrane fluidity and integrity. Cholesterol enables animal cells to change their shape. Poulletier de la Salle in 1769 firstly discovered the cholesterol in gallstones and bile (Dam 1958) and subsequently Chevreul in 1815 who named it cholesterine (Olson 1998).
Fig. 1.
The scheme of cholesterol chemical structure
There are two different sources of cholesterol. In the body, the liver produces all necessary cholesterol. But eating a diet with high content of saturated fatty acids obliges liver to produce more cholesterol (Ikonen 2008). Also, the main food sources of cholesterol are poultry, egg, fish, meat and full fat dairy products.
Cholesterol is slightly soluble in water (Jocz and Savage 2016) so, it tends to be transferred into lipoprotein structure. There are five types of lipoproteins in the body (blood). Chylomicrons, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), intermediate density lipoprotein (IDL) and high-density lipoprotein (HDL) and among them, LDL particles have the most cholesterol content. A cell with high cholesterol will block its cholesterol receptor. Afterwards, excess cholesterols form atherosclerotic plaques (Weingärtner et al. 2010) which cause the serious diseases such as stroke or heart attack.
Solubility of cholesterol
Solubility has an important role in SFE owing to the interaction between supercritical fluid and substance which is extracted. SC-CO2 is a proper solvent for most nonpolar and slightly polar molecules which have low molar mass.
Cholesterol (C27H46O) has an OH polar functional group that gives cholesterol a dipole moment of nearly 1.9 D, but it is a chiefly hydrocarbon compound. Thus, cholesterol is more soluble in the solvents such as carbon dioxide or ethane. Also, it has been shown that, cholesterol solubility in pure supercritical ethane is more than in CO2. Moreover, solubility degree is under influence of pressure and temperature. For example, at 130 bars and 333.1 K, the ratio of solubility in ethane to CO2 is around 15.3 while, at 190 bars and 313.1 K is 4.3 (Singh et al. 1993). Also, solubility of a hydrocarbon solid is exponentially proportionate to the density value. The ability to predict the solubility of cholesterol in supercritical fluids is important in the food industry. For this purpose, Peng–Robinson equation (Babaei et al. 2006; Bozorgmehr and Housaindokht 2006), density-based correlation (Huang et al. 2004), statistical association fluid theory (SAFT) equation (Aghamiri and Nickmand 2010) and Van Der Waals mixing rules (Ksibi and Moussa 2007) have been employed.
Concerning the effects of pressure on solubility of SCFs, it is noteworthy that, the density of SC-CO2 has the direct relationship with pressure, so that as the pressure is increased, the density of carbon dioxide increases and so does the solubility. Other important factor affecting the solubility of the substances in SCF is the system temperature. The effect of temperature on the solubility is not as simple as pressure. It depends on two competing factors: SCF solvent density and solute sublimation. As temperature increases, the vapor pressure of solute also increases while the density of solvent decreases, resulting in the opposite effects. An increase in the vapor pressure makes the solute more soluble, while a decrease in the solvent density makes it less soluble. Thus, a prediction about the impact of pressure on the solubility of solutes in SFC requires an accurate investigation on physical properties of SCF and cholesterol such as critical constants, vapor pressure, molar volume and acentric factor.
Effective parameters in SFE
There are several factors affecting the yield of supercritical fluid (SCF) extraction. The physical form of the materials (i.e., particle size, surface area, shape, porosity), variation of temperature and pressure, moisture content, existence of modifiers, initial oil content, swelling of the food, fluid flow rate and time of extraction are some most important of them.
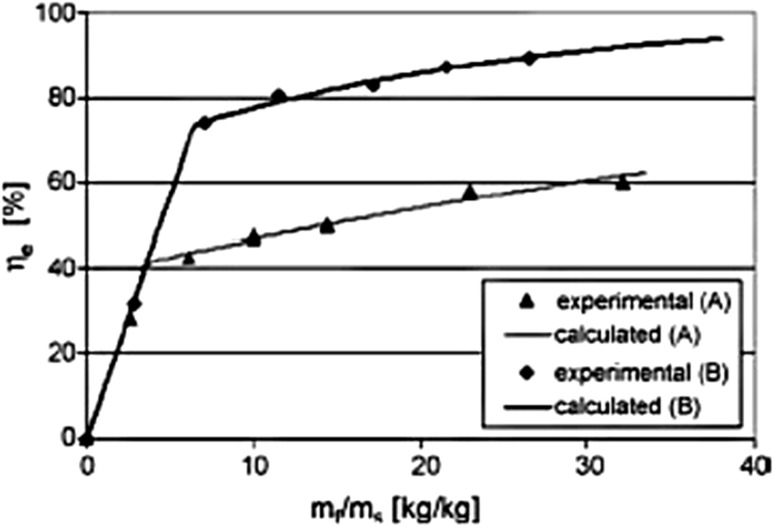
Extraction efficiency increases with decreasing the particle size. Also, decreasing the particle size of matrices leads to the creating a larger surface area. Asep et al. (2008) showed that the yield of extraction from the smaller particle size of cocoa nibs (especially 0.074 mm cocoa nibs) was significantly higher than whole cocoa nibs (Fig. 2). In a similar survey, Nagy et al. displayed that milling and decreasing the size of paprika to 0.1–0.7 mm, easily removed the oil from samples (Nagy and Simándi 2008).
Fig. 2.
The Effect of the particle size on the extraction efficiency. Particle size 1.5 mm (A), 0.7 mm (B)
Moisture content of raw materials has different efficacy in the extraction yield. In other words, low moisture content can act as a modifier, so increases the solubility. For example, Ling et al. (1999) found that nearly 10% of moisture was enough to improve the solubilizing power of the fluid to maximum limit in SFE of herbal medicines. But, higher amount of moisture was undesirable because of clogging problems in the restrictor. Temperature and pressure conditions of restrictor may create the ice from the moisture and subsequently clog the restrictor. The ways to avoid such problems are: (1) mixing the sample with sodium sulfate that can save the moisture effectively (Brannegan et al. 2011); (2) using the silica gel to absorb moisture from analytes (Brannegan et al. 2011); and (3) heating the restrictor to an acceptable temperature (Beňová et al. 2010).
Low primary oil content in foods has positive effect on the extraction. But, in high quantities, it does not have significant effect. The reason is that a little amount of oil could act as a co-solvent, particularly for low-soluble compounds (Nagy and Simándi 2008).
Matrix swelling is another index which aids to improve the extraction efficiency. The supercritical fluid easily diffuses into the food or polymer, when the sample is swelled (Zhang et al. 1997). Changing in temperature, pressure and modifiers can regulate the swelling. For instance, Sun and Temelli (2006) mentioned that canola oil (as a modifier) penetrated the structure of dried carrots and makes it swell. Therefore, it became easier for SC-CO2 to extract carotenoids from cells (Sun and Temelli 2006).
Temperature, pressure and flow rate are the most discussed factors among parameters affecting the extraction of cholesterol (Hou et al. 2010; Liu et al. 2011; Vedaraman et al. 2004). The velocity of supercritical fluid through foodstuff is one of the most effective factors in the extraction of cholesterol. Decreasing the fluid rate of supercritical fluids causes it to penetrate deeper in the matrix. So, it has the positive effect on extraction efficacy due to an increase in contact area between SCF and sample. Flow rate is a changeable feature relevant to the restrictor. For example, SC-CO2 extracted better the lycopene at a flow rate of 2.5 mL/min than 15 mL/min. For flow rates more than 10 mL/min, the yield of recovery was less than 8%, whereas the percentage of 2.5 mL/min flow rate was 38.8% (Rozzi et al. 2002). In recent years likewise, it has been shown that the liquid volume expansion is another effective thermodynamic criterion in cholesterol micronization and solubility. In this regard, Wang et al. (2012) indicated that a good size distribution of the cholesterol particles could be achieved when the liquid volume expansion is lower than 80% (Wang et al. 2012).
Application of modifiers in SFE of cholesterol
Modifiers (entrainers) are co-solvents which improve the efficiency of the SCF extraction by increasing the solubility of analyte. Also, modifiers extend the range of extractable materials. Some common modifiers are methanol, ethanol, dichloromethane, 2-propanol and acetonitrile. Since the SC-CO2 is fairly nonpolar, it is necessary to add polar modifiers such as ethanol to enhance the solubility of more polar and higher weight molecules. Besides, application of modifiers reduces the temperature needed for the SFE (Paul et al. 2016). Although, adding a modifier to CO2 transform it from supercritical to subcritical and makes difficulties such as needing for separating the modifiers from subcritical fluid after extraction process, however a well selected modifier improves the yield of extraction.
The role of modifiers in increasing the yield of SFE has been mentioned in the following examples. Shen et al. (2008) used co-solvent-modified SC-CO2 to extract the cholesterol from soft-shell turtle fish egg powder. They obtained 70.1% yield, using ethanol as a modifier (Shen et al. 2008). Vedaraman et al. compared the effect of 2-propanol, acetone and ethanol as modifiers in different amounts on the extraction of the cholesterol from cattle brain. It can be inferred that, the use of propane with supercritical ethane at a density of 3.5 mol% has better effect on the solubility of cholesterol than CO2 as co-solvent (Vedaraman et al. 2008). Another result of the research showed that adding a little amount of CO2 to the ethane has a stronger effect on the solubility of cholesterol than adding ethane to CO2. Kang et al. (2005) demonstrated that the use of ethanol as an entrainer at 1.5% w/w SC-CO2 could significantly improve the recovery of cholesterol from the fish oil.
SCF extraction of cholesterol
Investigation of literatures suggests that the SFE technique presumably is the only efficient method to extract the large amount of cholesterol with no need for lipid extraction stage. The high percentage extraction or recovery of cholesterol from foods in supercritical fluid extractor, needs considering the factors such as temperature, pressure, density and flow rate of the supercritical fluid. Although such factors vary according to the type of food, however an optimum range for all of them can be imagined. Results obtained from several studies related to the SFE of cholesterol, indicated that the optimum temperature, pressure and density used in the extractor were 40–50 °C, 330–383 bars, 0.9–0.93 g/cm3, respectively. Concerning the flow rate of supercritical fluids a wide range of 2 mL/min–12 L/min has been utilized. The following are examples of cholesterol extraction by SFE method.
Vedaraman et al. indicated that a maximum of 52% of the cholesterol could be extracted from cow brain using SC-CO2. In their survey, the extraction yield was studied by varying the parameters such as temperature, pressure, and the mass flow of SC-CO2. Also, they studied the structure of extracted cholesterol by the nuclear magnetic resonance spectroscopy (NMR), differential scanning calorimetry (DSC), and Fourier-transform infrared spectroscopy (FT-IR) and also, they investigated the purity of samples by the GC method. GC analysis showed that the purity of extracted cholesterol is 86% and the FT-IR, DSC, and NMR results displayed that the cholesterol spectra was matched well with the standard cholesterol. Their other findings showed, although the increase in the pressure from 230 to 270 bar was not effective on the extraction yield, but the increase in the temperature and the flow rate of SC-CO2 from 50 to 70 °C and 2 to 4 kg/h, marginally increased the rate of extraction. The effective diffusion coefficient was likewise found to increase with the temperature and the flow rate of the SCF. In general, the effective diffusion coefficient was between 1.9 and 2.8 × 10−13 m2/s for the whole range of experiments utilized in the their study. (Vedaraman et al. 2005).
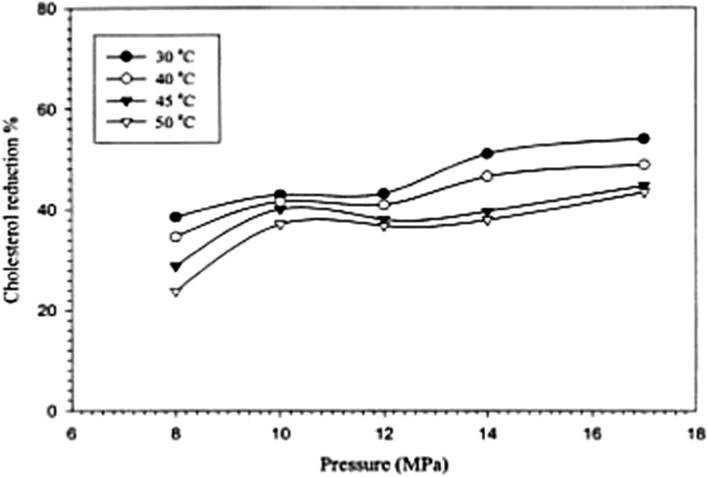
SFE of cholesterol from aquatic sources are of interest to researchers. In this regard, Kang et al. (2005) published important data on the solubility and extraction of lipids and cholesterol from the fish (squid) oil. Their investigation indicated that, up to 54% reduction in cholesterol content of the squid oil can be achieved at 30 °C and 17 MPa (Fig. 3).
Fig. 3.
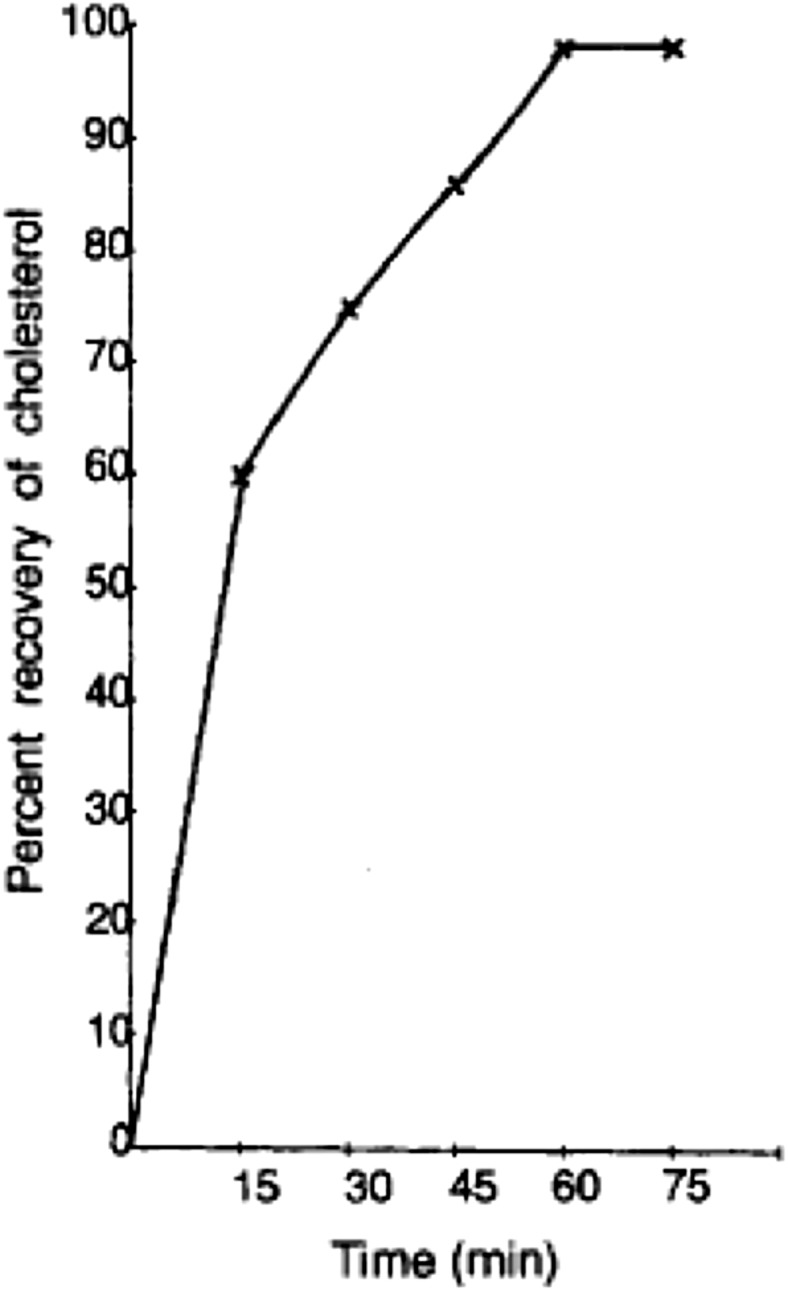
The correlation between time and the percentage recovery of the cholesterol using a spiked glass wool
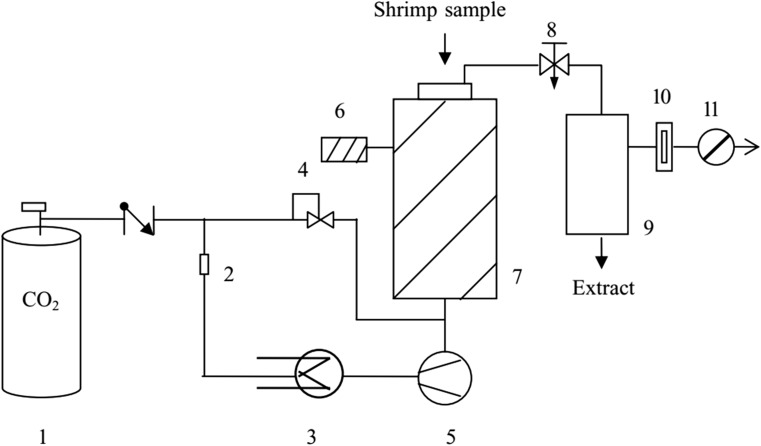
The use of experimental designs to optimize the conditions used for extracting cholesterol from foodstuffs has been likewise considered. For instance, Higuera-Ciapara et al. (2005), designed a Response Surface Analyses method to get the optimum temperature, volume and the pressure used to extract cholesterol from shrimp. They utilized a central composite rotatory design for three aforementioned variables with five levels each and the statistical significance was defined to be < 0.95%. The variables studied for pressure, volume, and temperature were 275, 289, 310, 331 and 345 bar, 250, 909, 1875, 2841 and 3500 L CO2 and 35, 36, 37, 38 and 39 °C, respectively. To extract cholesterol from shrimp, they employ a system schematically illustrated in Fig. 4. Their findings indicated that, under the optimum conditions of 37 °C, 310 bar, and 1875 L of CO2 it was feasible to obtain a low-cholesterol shrimp (Higuera-Ciapara et al. 2005).
Fig. 4.
The influence of several temperatures and pressures values on the cholesterol extraction by supercritical CO2
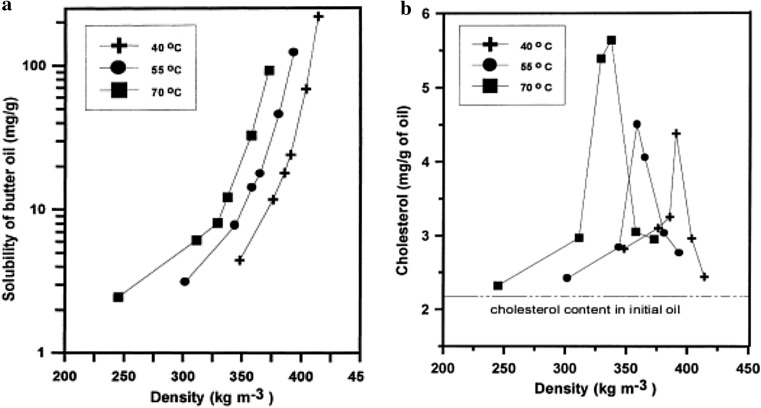
In other research, the influence of several temperatures (40, 55 and 70 °C) and pressures (8.5–24.1 MPa) in SFE of cholesterol from butter oil was studied (Mohamed et al. 2000). The results showed that combining extraction and adsorption methods using 17.2 MPa and 40 °C and an alumina as absorbent, reduced the cholesterol content from 2.2 to less than 0.1 mg/g in the butter oil. Hou et al. (2010) could successfully reduce the lipid content of freeze-dried goat placenta up to 21.02%. They reported that the application of SC-CO2 at 34.6 MPa, 35.3 °C and 29.1 min reduced the cholesterol content up to 8.46 mg/g dry basis (Hou et al. 2010).
Chao et al. extracted the cholesterol and lipid from ground beef at two pressure values of 172 and 310 bars. They reported that the average percentage of the lipid removed at 310 bars was twice that at 172 bars, but the ratio of extracted cholesterol to lipid was higher than that found at 172 bars (Chao et al. 1991). The results observed by Chao et al. indicated that the SFE of cholesterol needs certain conditions and there is not always an equal relationship between extracted lipid and cholesterol. In another work conducted by Chitra et al. (2015), nearly 55.8% decreasing in cholesterol was achieved at the condition of 207 bar, 68 °C and flow rate of 6 L/min (Chitra et al. 2015).
Another attempt at SFE of cholesterol belongs to the Ong et al. study. They tested several pressures, temperatures and time to extract cholesterol from egg yolk and blood serum (Ong et al. 1990). They demonstrated the possibility of reaching 98% yield in the pressure of 17.7 MPa within 30 min (Fig. 5). A number of studies as to extraction of cholesterol from edible plants and foods reported since 2000 are summarized in Table 2.
Fig. 5.
Flow diagram of the supercritical fluid extraction system. (1) Gas supply; (2) filter; (3) cooler; (4) pressure regulator; (5) CO2 pump; (6) heater control; (7) extractor vessel; (8) expansion valve; (9) glass separator; (10) flow meter; (11) flow totalizer
Table 2.
Extraction conditions of cholesterol from some animal and plant edible products
| Source of extraction | Fluid | Temperature | Pressure | Flow rate | Extracted amount (%) | Time of whole process | Reference |
|---|---|---|---|---|---|---|---|
| Foods of animal origina | |||||||
| Abalone | Supercritical CO2 | 50 °C | 28 MPa | 25 L/h | 91.13 ± 1.24 | 80 min | Zhou et al. (2012) |
| Egg powder | Subcritical tetrafluoroethane | 323 K | 8 MPa | 99.26 | 6 h | Yue et al. (2011) | |
| Shrimp | Supercritical CO2 | 38 °C | 331 bar | 5.5–6.2 L/min | 91.21 | Higuera-ciapara et al. (2011) | |
| Mayonnaise | Subcritical CO2 | 9 °C | 4.66 MPa | Up to 81 | Moros et al. (2002) | ||
| Buffalo butter | Subcritical CO2 | 50 and 70 °C | 10.9–40.1 MPa | 2 mL/min | More than 50 | 2 h | Fatouh et al. (2007) |
| Sthenoteuthis oualaniensis egg powder | Subcritical 1,1,1,2-tetrafluoroethane | 55.4 °C | 8.6 MPa | 99.16 | 50 min | Sui et al. (2014) | |
| Fish byproducts | Supercritical CO2 | 313 K | 25 MPa | 1 mL/min | 4.9 (wt% in oil) | Rubio-Rodríguez et al. (2012) | |
| Sea urchin | Supercritical CO2 | 50 °C | 28 MPa | 20 L/h | 89.4 | 80 min | Zhu et al. (2010) |
| Foods of plant originb | |||||||
| Black sesame | Supercritical CO2 | 40 and 60 °C | 200–400 bar | 5.9 × 10−5 kg/s | 18.70 (relative quantity to total phytosterol) | 3.5 h | Botelho et al. (2014) |
| Hazelnut | Supercritical CO2 | 308–321 K | 18–23.4 MPa | 0.2 (relative quantity to total phytosterol) | 8.7 h | Bernardo-Gil et al. (2002) | |
| Walnut | Supercritical CO2 | 308–321 K | 18–23.4 MPa | 0.068 cm/s | 0.16 (relative quantity to total phytosterol) | 150 min | Oliveira et al. (2002) |
| Acorn | Supercritical CO2 | 313 K | 18 MPa | 2.5 × 10−4 m/s | 0.27 (relative quantity to total phytosterol) | Lopes and Bernardo-Gil (2005) | |
| Oat | Supercritical CO2 | 55 °C | 450 bar | 8 L/min | 1.9 (relative quantity to total phytosterol) | 2 h | Lu et al. (2007) |
aThe extracted amounts of cholesterol from foods of animal origin are according to the percentage of extracted cholesterol per primary cholesterol of food
bThe extracted amounts of cholesterol from foods of plant origin are according to the percentage of extracted cholesterol per total extracted phytosterol
Collection of extracted cholesterol with SFE system
The collection of cholesterol could be performed in three different approaches, including (1) collection in empty vessels; (2) solvent collection and (3) solid-phase collection. Depressurizing in U-shaped empty vessel has been less successful because of forming aerosol (Turner et al. 2002). Also, it is time-consuming. However, this method is the most prevalent technique of cholesterol extraction.
Liquid solvent collection is the most widely used technique for extracting from natural substances (Herrero et al. 2006). Two approaches have been used for this system. In the first design, the restrictor is directly placed into the liquid solvent as collector (De Martinis and Lancas 2000), so SCF is depressurized in contact with the solvent. In another approach, the SCF–analyte mixture is depressurized in a glass tube then collected into the collector solvent (Pourmortazavi and Hajimirsadeghi 2007). Liquid trapping can be an efficient way to collect volatile and polyphenol substances (Palenzuela et al. 2004). The solvent trap must be compatible with the polarity of analytes, extractant and modifiers. Methanol is a proper solvent for polar compounds, while dichloromethane often is used for nonpolar analytes (Shimmo et al. 2004). Application of this method for extracting cholesterol is rare. Carmona et al. collected the cholesterol extracted by the SC-CO2 in a stainless steel while a stream of 2-propanol solvent was pumping through the trap efficiency of collection (Braida et al. 2008). For example, Mohamed et al. combined the supercritical ethane with an alumina as an absorbent material to extract the cholesterol from a butter oil. This method produced the butter oil with cholesterol content of approximately 3% of what was in the original butter oil. In this research, the influence of several temperatures (40, 55 and 70 °C) and pressures (8.5–24.1 MPa) in solubility and SFE of cholesterol was studied (Fig. 6a, b). The results showed that the combination of extraction and adsorption methods at 17.2 MPa and 40 °C using alumina as adsorbent reduces the cholesterol content from 2.2 to less than 0.1 mg/g of butter oil. Also, it has been expressed that the solubility of the butter oil and cholesterol in supercritical ethane (SC-ethane) is higher than those found in SC-CO2.
Fig. 6.
a The solubility of butter oil in SC-ethane at different pressures, temperatures and densities (2.5–4.0 g/min), b the content of cholesterol in butter oil extracts at 40, 55, and 70 °C with different pressure values
In general, application of a cooled empty U tube for separation of cholesterol from foodstuffs is the most widely used.
Conclusion
Supercritical fluid extraction can be recognized as an evolution in extraction from foodstuffs in last 2 decades. This method makes it possible to extract a wide range of substances from foods with less solvent use and saving of time. This technique can easily extract the cholesterol from muscles, cells, egg yolks and milk fat. However, attainment to a high yield in extracting cholesterol, needs an accurate adjustment of temperature, pressure, density and flow rate of the supercritical fluid. It is possible to reach up to 98% yield in the cholesterol extraction by improving all conditions.
In conclusion, results obtained from several studies indicated that the SFE technique is probably the best way to extract cholesterol.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Aghamiri SF, Nickmand Z. Prediction of solubility of cholesterol in supercritical solvents. Sep Sci Technol. 2010;45:2119–2129. doi: 10.1080/01496395.2010.504448. [DOI] [Google Scholar]
- Aghel N, Yamini Y, Hadjiakhoondi A, Pourmortazavi SM. Supercritical carbon dioxide extraction of Mentha pulegium L. essential oil. Talanta. 2004;62:407–411. doi: 10.1016/j.talanta.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Asep EK, Jinap S, Tan TJ, Russly AR, Harcharan S, Nazimah SAH. The effects of particle size, fermentation and roasting of cocoa nibs on supercritical fluid extraction of cocoa butter. J Food Eng. 2008;85:450–458. doi: 10.1016/j.jfoodeng.2007.08.008. [DOI] [Google Scholar]
- Babaei MR, Jabbari A, Yamini Y. Solid–liquid extraction of fatty acids of some variety of Iranian rice in closed vessel in the absence and presence of ultrasonic waves. Asia J Chem. 2006;18:57. [Google Scholar]
- Beňová B, Adam M, Pavlíková P, Fischer J. Supercritical fluid extraction of piceid, resveratrol and emodin from Japanese knotweed. J Supercrit Fluid. 2010;51:325–330. doi: 10.1016/j.supflu.2009.10.009. [DOI] [Google Scholar]
- Bernardo-Gil MG, Grenha J, Santos J, Cardoso P. Supercritical fluid extraction and characterisation of oil from hazelnut. Eur J Lipid Sci Technol. 2002;104:402–409. doi: 10.1002/1438-9312(200207)104:7<402::AID-EJLT402>3.0.CO;2-N. [DOI] [Google Scholar]
- Boselli E, Caboni MF, Lercker G. Extraction and purification of free cholesterol from some egg-containing food by on-line supercritical fluid extraction–solid-phase extraction. Eur Food Res Technol. 2001;212:244–246. doi: 10.1007/s002170000206. [DOI] [Google Scholar]
- Boselli E, Bonoli M, Caboni MF, Lercker G. Determination of cholesterol oxidation products in the supercritical carbon dioxide extract of egg yolk powder: comparison with conventional liquid solvent extraction methods. Europ Food Res Technol. 2002;215:72–75. doi: 10.1007/s00217-002-0522-1. [DOI] [Google Scholar]
- Botelho JRS, et al. Black sesame (Sesamum indicum L.) seeds extracts by CO 2 supercritical fluid extraction: isotherms of global yield, kinetics data, total fatty acids, phytosterols and neuroprotective effects. J Supercrit Fluids. 2014;93:49–55. doi: 10.1016/j.supflu.2014.02.008. [DOI] [Google Scholar]
- Bozorgmehr MR, Housaindokht MR. Prediction of the solubility of cholesterol and its esters in supercritical carbon dioxide. Chem Eng Technol. 2006;29:1481–1486. doi: 10.1002/ceat.200600130. [DOI] [Google Scholar]
- Braida I, Mattea M, Cardarelli D. Extraction–adsorption–desorption process under supercritical condition as a method to concentrate antioxidants from natural sources. J Supercrit Fluids. 2008;45:195–199. doi: 10.1016/j.supflu.2007.08.013. [DOI] [Google Scholar]
- Brannegan D, Lee C, Wang J, Taylor L. Extraction techniques leveraging elevated temperature and pressure. In: Nickerson B, editor. Sample preparation of pharmaceutical dosage forms. Berlin: Springer; 2011. pp. 93–128. [Google Scholar]
- Brondz I, Sedunov B, Sivaraman N. Influence of modifiers on supercritical fluid chromatography (SFC) and supercritical fluid extraction (SFE). Part I. Int J Anal Mass Spectrom Chromatogr. 2017;5:17. doi: 10.4236/ijamsc.2017.52002. [DOI] [Google Scholar]
- Carles P. A brief review of the thermophysical properties of supercritical fluids. J Supercrit Fluids. 2010;53:2–11. doi: 10.1016/j.supflu.2010.02.017. [DOI] [Google Scholar]
- Chao R, Mulvaney S, Bailey M, Fernando L. Supercritical CO2 conditions affecting extraction of lipid and cholesterol from ground beef. J Food Sci. 1991;56:183–187. doi: 10.1111/j.1365-2621.1991.tb08007.x. [DOI] [Google Scholar]
- Chitra J, Deb S, Mishra HN. Selective fractionation of cholesterol from whole milk powder: optimisation of supercritical process conditions. Int J Food Sci Technol. 2015;50:2467–2474. doi: 10.1111/ijfs.12914. [DOI] [Google Scholar]
- Dam H. Historical introduction to cholesterol Chemistry. In: Cook RP, editor. Biochemistry and pathology. New York: Academic Press; 1958. pp. 1–14. [Google Scholar]
- De Castro ML, Jiménez-Carmona M. Where is supercritical fluid extraction going? TrAC Trends Anal Chem. 2000;19:223–228. doi: 10.1016/S0165-9936(99)00228-9. [DOI] [Google Scholar]
- De Martinis BS, Lancas FM. An alternative supercritical fluid extraction system for aqueous matrices and its application in pesticides residue analysis. J Environ Sci Health Part B Pestic Contam Agric Wastes. 2000;35:539–547. doi: 10.1080/03601230009373290. [DOI] [PubMed] [Google Scholar]
- Fatouh A, Mahran G, El-Ghandour M, Singh R. Fractionation of buffalo butter oil by supercritical carbon dioxide. LWT Food Sci Technol. 2007;40:1687–1693. doi: 10.1016/j.lwt.2006.12.015. [DOI] [Google Scholar]
- Fjeldsted JC, Lee ML. Capillary supercritical fluid chromatography. Anal Chem. 1984;56:619A–628A. doi: 10.1021/ac00268a004. [DOI] [Google Scholar]
- Grigonis D, Venskutonis PR, Sivik B, Sandahl M, Eskilsson CS. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloë odorata) J Supercrit Fluids. 2005;33:223–233. doi: 10.1016/j.supflu.2004.08.006. [DOI] [Google Scholar]
- Guiochon G, Tarafder A. Fundamental challenges and opportunities for preparative supercritical fluid chromatography. J Chromatogr A. 2011;1218:1037–1114. doi: 10.1016/j.chroma.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Herrero M, Cifuentes A, Ibanez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae—a review. Food Chem. 2006;98:136–148. doi: 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- Higuera-Ciapara I, Toledo-Guillen AR, Noriega-Orozco L, Martinez-Robinson KG, Esqueda-Valle MC. Production of a low-cholesterol shrimp using supercritical extraction. J Food Process Eng. 2005;28:526–538. doi: 10.1111/j.1745-4530.2005.00038.x. [DOI] [Google Scholar]
- Higuera-ciapara I, Toledo-Guillén AR, Noriega-Orozco LO, Martínez-Robinson KG (2011) Low-cholesterol shrimp and method of obtaining same. Google Patents
- Hou Z, Zheng Y, Gao Y, Liu X, Yuan F, Liu G. Optimization of supercritical carbon dioxide removal of lipid and cholesterol from goat placenta using response surface methodology. Food Bioprod Process. 2010;88:298–304. doi: 10.1016/j.fbp.2009.12.001. [DOI] [Google Scholar]
- Huang Z, Kawi S, Chiew YC. Solubility of cholesterol and its esters in supercritical carbon dioxide with and without cosolvents. J Supercrit Fluids. 2004;30:25–39. doi: 10.1016/S0896-8446(03)00116-5. [DOI] [Google Scholar]
- Hubbard J, Downing J, Ram M, Chung O. Lipid Extraction from Wheat Flour Using Supercritical Fluid Extraction. 1. Cereal Chem. 2004;81:693–698. doi: 10.1094/CCHEM.2004.81.6.693. [DOI] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Jocz JN, Savage PE. Behavior of cholesterol and catalysts in supercritical water. Energy Fuels. 2016;30:7937–7946. doi: 10.1021/acs.energyfuels.6b00924. [DOI] [Google Scholar]
- Kang K-Y, Ahn D-H, Wilkinson GT, Chun B-S. Extraction of lipids and cholesterol from squid oil with supercritical carbon dioxide. Korean J Chem Eng. 2005;22:399–405. doi: 10.1007/BF02719418. [DOI] [Google Scholar]
- Keskin S, Kayrak-Talay D, Akman U, Hortacsu O. A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids. 2007;43:150–180. doi: 10.1016/j.supflu.2007.05.013. [DOI] [Google Scholar]
- Khosravi-Darani K. Research activities on supercritical fluid science in food biotechnology. Crit Rev Food Sci Nutr. 2010;50:479–488. doi: 10.1080/10408390802248759. [DOI] [PubMed] [Google Scholar]
- Ksibi H, Moussa AB. Prediction of binary interaction coefficient and critical parameters of cholesterol in supercritical carbon dioxide. Comput Aided Chem Eng. 2007;24:333. doi: 10.1016/S1570-7946(07)80079-4. [DOI] [Google Scholar]
- Lang QY, Wai CM. Supercritical fluid extraction in herbal and natural product studies—a practical review. Talanta. 2001;53:771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, et al. Diet and lifestyle recommendations revision 2006. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Ling Y-C, Teng H-C, Cartwright C. Supercritical fluid extraction and clean-up of organochlorine pesticides in Chinese herbal medicine. J Chromatogr A. 1999;835:145–157. doi: 10.1016/S0021-9673(98)01077-2. [DOI] [PubMed] [Google Scholar]
- Liu GM, Zheng YY, Xu X, Liu X, Yuan F, Gao YX. Removal of lipid and cholesterol from goat placenta by supercritical carbon dioxide extraction. J Food Process Eng. 2011;34:657–670. doi: 10.1111/j.1745-4530.2009.00417.x. [DOI] [Google Scholar]
- Lopes IM, Bernardo-Gil MG. Characterisation of acorn oils extracted by hexane and by supercritical carbon dioxide. Eur J Lipid Sci Technol. 2005;107:12–19. doi: 10.1002/ejlt.200401039. [DOI] [Google Scholar]
- Lu B, Zhang Y, Wu X, Shi J. Separation and determination of diversiform phytosterols in food materials using supercritical carbon dioxide extraction and ultraperformance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Anal Chim Acta. 2007;588:50–63. doi: 10.1016/j.aca.2007.01.067. [DOI] [PubMed] [Google Scholar]
- Marr R, Gamse T. Use of supercritical fluids for different processes including new developments—a review. Chem Eng Process. 2000;39:19–28. doi: 10.1016/S0255-2701(99)00070-7. [DOI] [Google Scholar]
- McHugh M, Krukonis V. Supercritical fluid extraction: principles and practice. Amsterdam: Elsevier; 2013. [Google Scholar]
- Mohamed RS, Saldaña MD, Socantaype FH, Kieckbusch TG. Reduction in the cholesterol content of butter oil using supercritical ethane extraction and adsorption on alumina. J Supercrit Fluids. 2000;16:225–233. doi: 10.1016/S0896-8446(99)00034-0. [DOI] [Google Scholar]
- Moros J, Franco J, Gallegos C. Rheological properties of cholesterol-reduced, yolk-stabilized mayonnaise. J Am Oil Chem Soc. 2002;79:837–843. doi: 10.1007/s11746-002-0567-6. [DOI] [Google Scholar]
- Nagy B, Simándi B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J Supercrit Fluids. 2008;46:293–298. doi: 10.1016/j.supflu.2008.04.009. [DOI] [Google Scholar]
- Nahar L, Sarker SD. Supercritical fluid extraction. In: Sarker SD, Nahar L, editors. Natural products isolation. 2. Totowa: Gray Humana Press Inc; 2005. pp. 47–76. [Google Scholar]
- Oliveira R, Rodrigues MF, Bernardo-Gil MG. Characterization and supercritical carbon dioxide extraction of walnut oil. J Am Oil Chem Soc. 2002;79:225–230. doi: 10.1007/s11746-002-0465-y. [DOI] [Google Scholar]
- Olson RE. Discovery of the lipoproteins, their role in fat transport and their significance as risk factors. J nutri. 1998;128:439S–443S. doi: 10.1093/jn/128.2.439S. [DOI] [PubMed] [Google Scholar]
- Ong CP, Ong HM, Li SFY, Lee HK. The extraction of cholesterol from solid and liquid matrices using supercritical CO2. J Micro Sep. 1990;2:69–73. doi: 10.1002/mcs.1220020205. [DOI] [Google Scholar]
- Palenzuela B, Arce L, Macho A, Munoz E, Rios A, Valcarcel M. Bioguided extraction of polyphenols from grape marc by using an alternative supercritical-fluid extraction method based on a liquid solvent trap. Anal Bioanal Chem. 2004;378:2021–2027. doi: 10.1007/s00216-004-2540-2. [DOI] [PubMed] [Google Scholar]
- Paul ID, Jayakumar C, Mishra HN. Optimization of process parameters for supercritical fluid extraction of cholesterol from whole milk powder using ethanol as co-solvent. J Sci Food Agric. 2016;96:4885–4895. doi: 10.1002/jsfa.7760. [DOI] [PubMed] [Google Scholar]
- Poliakoff M, Licence P. Supercritical fluids: green solvents for green chemistry? Philos Trans R Soc A Math Phys Eng Sci. 2015;373:2. doi: 10.1098/rsta.2015.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmortazavi SM, Hajimirsadeghi SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J Chromatogr A. 2007;1163:2–24. doi: 10.1016/j.chroma.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Pourmortazavi SM, Baghaee P, Mirhosseini MA. Extraction of volatile compounds from Juniperus communis L. leaves with supercritical fluid carbon dioxide: comparison with hydrodistillation. Flav Frag J. 2004;19:417–420. doi: 10.1002/ffj.1327. [DOI] [Google Scholar]
- Pourmortazavi SM, Ghadiri M, Hajimirsadeghi SS. Supercritical fluid extraction of volatile components from Bunium persicum Boiss. (black cumin) and Mespilus germanica L. (medlar) seeds. J Food Commun Anal. 2005;18:439–446. doi: 10.1016/j.jfca.2004.01.003. [DOI] [Google Scholar]
- Pourmortazavi SM, Rahimi-Nasrabadi M, Hajimirsadeghi SS. Supercritical fluid technology in analytical chemistry—review. Cur Analyt Chem. 2014;10:3–28. doi: 10.2174/1573411011410010004. [DOI] [Google Scholar]
- Rozzi N, Singh R, Vierling R, Watkins B. Supercritical fluid extraction of lycopene from tomato processing byproducts. J Agric Food Chem. 2002;50:2638–2643. doi: 10.1021/jf011001t. [DOI] [PubMed] [Google Scholar]
- Rubio-Rodríguez N, Sara M, Beltrán S, Jaime I, Sanz MT, Rovira J. Supercritical fluid extraction of fish oil from fish by-products: a comparison with other extraction methods. J Food Eng. 2012;109:238–248. doi: 10.1016/j.jfoodeng.2011.10.011. [DOI] [Google Scholar]
- Sahena F, Zaidul I, Jinap S, Karim A, Abbas K, Norulaini N, Omar A. Application of supercritical CO2 in lipid extraction—a review. J Food Eng. 2009;95:240–253. doi: 10.1016/j.jfoodeng.2009.06.026. [DOI] [Google Scholar]
- Sapkale G, Patil S, Surwase U, Bhatbhage P. Supercritical fluid extraction. Int J Chem Sci. 2010;8:729–743. [Google Scholar]
- Sarrade S, Seaudea K. Supercritical fluids technologies, tools for green chemistry. Abstr Pap Am Chem Soc. 2014;247:1. [Google Scholar]
- Scalia S, Giuffreda L, Pallado P. Analytical and preparative supercritical fluid extraction of chamomile flowers and its comparison with conventional methods. J Pharm Biomed Anal. 1999;21:549–558. doi: 10.1016/S0731-7085(99)00152-1. [DOI] [PubMed] [Google Scholar]
- Shen Z, Mishra V, Imison B, Palmer M, Fairclough R. Use of adsorbent and supercritical carbon dioxide to concentrate flavor compounds from orange oil. J Agric Food Chem. 2002;50:154–160. doi: 10.1021/jf010582j. [DOI] [PubMed] [Google Scholar]
- Shen C-T, Hsu S-L, Chang C-MJ. Co-solvent-modified supercritical carbon dioxide extractions of cholesterol and free amino acids from soft-shell turtle fish egg. Sep Purif Technol. 2008;60:215–222. doi: 10.1016/j.seppur.2007.08.014. [DOI] [Google Scholar]
- Shepherd J, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes—the treating to new targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- Shimmo M, Anttila P, Hartonen K, Hyotylainen T, Paatero J, Kulmala M, Riekkola ML. Identification of organic compounds in atmospheric aerosol particles by on-line supercritical fluid extraction–liquid chromatography–gas chromatography–mass spectrometry. J Chromatogr A. 2004;1022:151–159. doi: 10.1016/j.chroma.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Singh H, Yun SJ, Macnaughton SJ, Tomasko DL, Foster NR. Solubility of cholesterol in supercritical ethane and binary gas mixtures containing ethane. Ind Eng Chem Res. 1993;32:2841–2848. doi: 10.1021/ie00023a055. [DOI] [Google Scholar]
- Sui X, Feng XM, Yue RY, Han YQ, Xue CH (2014) Optimization of Subcritical 1,1,1,2-tetrafluoroethane (R134a) removal of cholesterol from spray-dried Sthenoteuthis oualaniensis egg powder using response surface methodology. In: Gurumurthy H (ed) Advanced materials research. Trans Tech Publ, Zürich, pp 717–723
- Sun M, Temelli F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J Supercrit Fluids. 2006;37:397–408. doi: 10.1016/j.supflu.2006.01.008. [DOI] [Google Scholar]
- Turner C, Eskilsson CS, Bjorklund E. Collection in analytical-scale supercritical fluid extraction. J Chromatogr A. 2002;947:1–22. doi: 10.1016/S0021-9673(01)01592-8. [DOI] [PubMed] [Google Scholar]
- Vedaraman N, Brunner G, Muralidharan C, Rao P, Raghavan K. Extraction of cholesterol from cattle brain using supercritical carbon dioxide. J Supercrit Fluids. 2004;32:231–242. doi: 10.1016/j.supflu.2003.11.010. [DOI] [Google Scholar]
- Vedaraman N, Srinivasakannan C, Brunner G, Ramabrahmam B, Rao P. Experimental and modeling studies on extraction of cholesterol from cow brain using supercritical carbon dioxide. J Supercrit Fluids. 2005;34:27–34. doi: 10.1016/j.supflu.2004.10.004. [DOI] [Google Scholar]
- Vedaraman N, Srinivasakannan C, Brunner G, Rao P. Kinetics of cholesterol extraction using supercritical carbon dioxide with cosolvents. Ind Eng Chem Res. 2008;47:6727–6733. doi: 10.1021/ie070703q. [DOI] [Google Scholar]
- Wang Q, Guan YX, Yao SJ, Zhu ZQ. The liquid volume expansion effect as a simple thermodynamic criterion in cholesterol micronization by supercritical assisted atomization. Chem Eng Sci. 2012;75:38–48. doi: 10.1016/j.ces.2012.02.046. [DOI] [Google Scholar]
- Weingärtner O, et al. The relationships of markers of cholesterol homeostasis with carotid intima-media thickness. PLoS ONE. 2010;5(10):e13467. doi: 10.1371/journal.pone.0013467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R, Liu C, Xie J, Xue C, Han Y (2011) Study on the removal of cholesterol from Sthenoteuthis oualaniensis egg powder by subcritical 1,1,1,2-tetrafluoroethane (R134a). In: 2011 International conference on remote sensing, environment and transportation engineering (RSETE), 2011. IEEE, Washington, pp 7901–7904
- Zhang Y, Gangwani KK, Lemert RM. Sorption and swelling of block copolymers in the presence of supercritical fluid carbon dioxide. J Supercrit Fluids. 1997;11:115–134. doi: 10.1016/S0896-8446(97)00031-4. [DOI] [Google Scholar]
- Zhang X, et al. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol. 2003;32:563–572. doi: 10.1093/ije/dyg106. [DOI] [PubMed] [Google Scholar]
- Zhou DY, Tong L, Zhu BW, Wu HT, Qin L, Tan H, Murata Y. Extraction of lipid from abalone (haliotis discus hannai ino) gonad by supercritical carbon dioxide and enzyme-assisted organic solvent methods. J Food Process Pres. 2012;36:126–132. doi: 10.1111/j.1745-4549.2011.00560.x. [DOI] [Google Scholar]
- Zhu B-W, et al. Extraction of lipid from sea urchin (Strongylocentrotus nudus) gonad by enzyme-assisted aqueous and supercritical carbon dioxide methods. Eur Food Res Technol. 2010;230:737–743. doi: 10.1007/s00217-010-1216-8. [DOI] [Google Scholar]
- Zougagh M, Valcárcel M, Ríos A. Supercritical fluid extraction: a critical review of its analytical usefulness. TrAC Trends Anal Chem. 2004;23:399–405. doi: 10.1016/S0165-9936(04)00524-2. [DOI] [Google Scholar]