Abstract
The objective of this study was to investigate the effect of variable deep-frozen temperatures storage (− 11, − 18, − 26 and − 37 °C) on quality characteristics of Solenocera crassicornis. Results for all frozen storage temperatures indicated that sensory quality, pH and colour change of frozen shrimp had high correlation with storage temperature, as a lower deep-frozen temperature was more effective in minimizing the sensory quality loss, pH and colour change. A kinetic analysis for total volatile basic nitrogen (TVB-N) and salt-soluble protein which was performed in this study, showed reaction rates inversely proportional to the deep-frozen temperature. Lipid oxidation in shrimp was quantified by determining the thiobarbituric acid reaction substances, and microbial growth were also monitored during the frozen storage. Among all groups, storage at − 37 °C was the most effective in controlling lipid oxidation and reducing aerobic bacterial count in shrimp. A comparison between different temperatures showed that qualities of shrimp stored at − 26 and − 37 °C were significantly better than those stored at − 11 and − 18 °C during frozen storage. In conclusion, the results are important to allow better management and optimization of the cold chain from manufacture to consumption.
Keywords: Solenocera crassicornis, Deep-frozen storage, Quality changes, Kinetics
Introduction
Solenocera crassicornis, also known as Solenocera Melantho, is an important marine-trawling shrimp. It is an economically important species, and has become the main aquatic product for export in China. Processed shrimp products have been exported to Japan, South Korea and other developed countries. Shrimp are an excellent source of amino acids, peptides, protein, fatty acids and other useful nutrients (Mastromatteo et al. 2010; Carrión-Granda et al. 2016). Wet peeled prawns of Solenocera crassicornis contain nearly 80–86% moisture, 11–17% protein, 0.7–1.3% fats and 0.9–1.6% ash. Eighteen kinds of fatty acids containing ω-6 PUFA and ω-3 PUFA at a ratio of 1:5 (w/w) are extracted from peeled prawns. Furthermore, Solenocera crassicornis contains rich mineral element such as calcium, potassium, sodium, magnesium, phosphorus, copper, iron, zinc, etc.
Shrimp are highly perishable and susceptible to browning after death due to high water content and nutritional ingredients (Nirmal and Benjakul 2010; Wu 2014; Dai et al. 2016). During post-mortem storage of shrimp, melanosis is a serious problem, which results in the loss of nutritional and commercial values (Kim et al. 2002; Yuan et al. 2016). Melanosis is mainly originated by an enzymatic reaction which oxidizes colorless phenols into quinones by polyphenoloxidase (PPO). Subsequently quinones undergo non-enzymatic polymerization generating insoluble dark pigments (Nirmal and Benjakul 2011; Gonçalves and de Oliveira 2016).
Freezing is an effective method to limit enzymatic and microbial activity which causes deterioration of shrimp, and to extend their shelf life (Hatha et al. 2003; Tsironi et al. 2009). However, quality deterioration in terms of color, texture and flavor occurred during frozen storage (Boonsumrej et al. 2007; Wachirasiri et al. 2016). The extent of quality loss could relate to many factors, such as storage temperature, temperature fluctuations, transportation and the rate of freeze–thaw. Especially it is important to prevent temperature fluctuations and avoid thawing and re-freezing. Moreover, storage temperature mainly determines quality loss rates and final quality of frozen shrimp, and so it is extremely essential to estimate and model the effect of variably frozen storage temperatures on the quality changes of frozen shrimp.
In this study, the deep-frozen temperature is defined as temperature being or below − 10 °C. Up to present, many researches on frozen shrimp have been conducted (Gonçalves and Junior 2009; Tsironi et al. 2009; Abd-El-Aziz and Moharram 2016). However, there is no report on the shelf life and quality changes of Solenocera crassicornis under different deep-frozen temperature conditions. Consequently, the objective of this study was to investigate the effect on quality characteristics of Solenocera crassicornis at the deep-frozen temperatures storage (− 11, − 18, − 26 and − 37 °C) immediately after harvest.
Materials and methods
Raw material
Solenocera crassicornis caught in coastal area (water depth: 20–60 m) of East China Sea in spring 2017, was provided by a commercially operated company (Dongqing marine fisheries co-operatives, Zhoushan, Zhejiang Province, China) which possesses and operates the fishing boats and frozen storage facilities. All shrimp samples came from the same fishnet were slaughtered by immersing in ice-cold water, and then quickly frozen in an hour by a conventional air-blast freezing (− 30 °C) carried out in a shipboard refrigeration system. The shrimp were immediately transported frozen to laboratory within 12 h from the moment of harvest.
The shrimp samples were divided into four groups to be frozen and stored in controlled temperature cabinets (DW-40L420F, Haier Co., Ltd., Qingdao, China) at constant temperatures (− 11, − 18, − 26 and − 37 °C). Samples were taken in 20 day intervals for kinetic analysis of quality deterioration. Quality indicators as described in the following sections were selected for analyses. Shrimp samples deheaded and peeled were analyzed immediately after thawing at 4 °C until the core temperature of shrimp reached 0 °C. Experiments were conducted within 4 month period and data were analyzed and modeled.
Sensory evaluation
The sensory quality of shrimp samples were evaluated by a panel of six trained panelists which have been trained and mastered basic sensory knowledge and skills. Appearance, texture, odor and flavor were analyzed as sensory parameters. A rating was assigned separately for each parameter by using the 9-point descriptive hedonic scale (1, the lowest score to 9, the highest score) (Wu 2014; Farajzadeh et al. 2016). The scores were calculated as the average values, and comprehensive scores below 5 for the sensory attribute were considered unacceptable.
Color analysis
Color analysis of shrimps was performed using a Hunter Lab colorimeter (SC-80C, Kangguang Optical Instrument Co., Ltd., Beijing, China). Shrimps were deheaded and peeled, and middle section of peeled prawns was selected for color measurements. Color was quantified based on the measurement of the CIE Lab values (“L”: lightness, “a”: redness and greenness, “b”: yellowness and blueness) (Papadakis et al. 2000). All measurements were performed in triplicate. Total color difference (TCD) was also used to evaluate the color change during storage period (Yuan et al. 2016). TCD value (ΔE) was calculated using following equation:
where L0, a0 and b0 are the values of L*, a*, and b* color parameters at the beginning of frozen storage (namely day 0).
pH measurement
The pH values were determined referring to reported methods (Sundararajan et al. 2011; Chouljenko et al. 2017). Ten grams of shrimp samples was homogenized with 40 mL distilled water for 60 s. Then the mixture was transferred to a beaker and sonicated for 1 min at 4 °C, subsequently centrifuged at 8000 r/min for 20 min. The pH value of the supernatant was measured with a Sartorius PB-10 pH meter (Sartorius scientific instruments (Beijing) Co., Ltd., China).
Total volatile basic nitrogen (TVB-N)
TVB-N content was measured according to Chinese National Standard (GB 5009.228-2016) (2016). 20 g of shrimp samples was taken into a 250 mL conical flask and blended with 100 mL distilled water. The mixture was stirred evenly and sonicated for 1 min at 4 °C. Homogenate was impregnated still for 30 min at room temperature and then filtered through the filter paper. 10 mL of 20 g/L boric acid and 5 drops of mixture indicators (The volume ratio of methyl red ethanol solution and bromine cresol green ethanol solution is 1:5) were added to receiving flask. 10 mL of filtrate was poured slowly into reaction chamber using a glass rod, which was carried out again after washing the beaker with 10 mL distilled water. 5 mL of 10 g/L magnesia (MgO) was subsequently added. Steam distillation was performed for 5 min using a Kjeldahl distillation unit. The resulting solution was titrated with HCl (0.01 mol/L) using a micro-burette until the color turned into fuchsia. Blank assay was performed simultaneously and all measurements were done in triplicate. TVB-N content was calculated using the following equation:
where X is the value of TVB-N content (mg/100 g), V1 is the titration volume of HCl (mL), V2 is the titration volume of blank (mL), and c is the actual concentration of HCl (mol/L), m is the weight of shrimp sample (g).
Salt-soluble protein (SSP)
The salt-soluble protein (SSP) was measured according to the method of Al-Bulushi et al. (2013). 6.67 g of shrimp samples was blended with 100 mL chilled NaCl (5%) and pH was adjusted to 7–7.5. The mixture was homogenized for 2 min. Then homogenate was washed with chilled NaCl (5%) into centrifuge tubes, and centrifuged at 4000 r/min for 30 min. The supernatant was collected and the precipitate was washed again with 10 mL chilled NaCl (5%), centrifuged as above. The aliquot of the combined supernatant was mixed with 10 mL chilled trichloroacetic acid (15%, TCA) and centrifuged as above to remove non-protein nitrogen compounds. The final SSP concentration was determined by the biuret method (Gornall et al. 1949) using bovine serum albumin as the standard. All steps were carried out at less than 10 °C.
Thiobarbituric acid reaction substances (TBARS)
The degree of lipid oxidation present in shrimp was measured by TBARS assay according to the method of Tsironi et al. (2009) with slight modification. 5 g of shrimp samples was homogenized with 15 mL distilled water. 1 mL homogenate was mixed with 2.5 mL of thiobarbituric acid (TBA) solution which contained 0.375% TBA, 15% trichloroacetic acid (TCA) and 0.25 mol/L HCl. The mixture was reacted in boiling water bath for 10 min, and centrifuged at 4000 r/min for 20 min after cooling. The supernatant was collected and absorbance was measured at 532 nm with a digital spectrophotometer (Cary 50, Varian Australia Pty Ltd., Australia). A standard curve was established on the basis of 1,1,3,3 tetraethoxypropane in the gradient concentrations of 0–10 μg/mL. The malondialdehyde (MDA) content was expressed as mg/kg.
Microbiological analysis
Microbiological analysis was performed according to the Chinese National Standard (GB 4789.2-2016) (2016) by measurement of total aerobic plate counts (TPC). Twenty-five grams of samples were collected in sterile lab blender bags with 225 mL of phosphate buffered saline (PBS), and homogenized using a germfree homogenizer for 10 Hz, 2 min. Sample homogenate was serially diluted in dilution tubes filled with 9 ml of sterile PBS. Three suitable gradients were selected and 1 ml dilution homogenate was spread in sterile petri dishes using a sterile pipette, then covered with 15-–0 ml of PCA maintained at 46 °C. All plating was done in duplicate. The plates were incubated at 30 °C for 72 h. An ideal count ranged from 30 to 300 colonies was selected and the final CFU/g value of TPC was calculated using the following equation:
where N is the TPC value of sample (CFU/g). C is the TPC of all enumerated plates. The n1 and n2 are the numbers of flat plate corresponding with first and second dilution gradient, respectively. The d is the dilution factor of first dilution gradient.
Statistical analysis
Protein changes of shrimp during frozen storage are complex reactions, and can be considered as failure/formation phenomena. Thus, TVB-N and SSP values were modelled with apparent first order equations to explain the protein changes (Tsironi et al. 2009). The equation was shown as follows:
where A, A0 is the values of TVB-N or SSP at frozen storage times t and zero, respectively. k is the kinetic rate at temperature T. t is the frozen storage time.
Statistical analysis was performed using the software SPSS 19.0 for Windows. One-way analysis of variances (ANOVA) was used to test for significant differences and equivalence among the different samples. All statements regarding significance differences were based on a significance level of p = 0.05.
Results and discussion
Sensory analysis
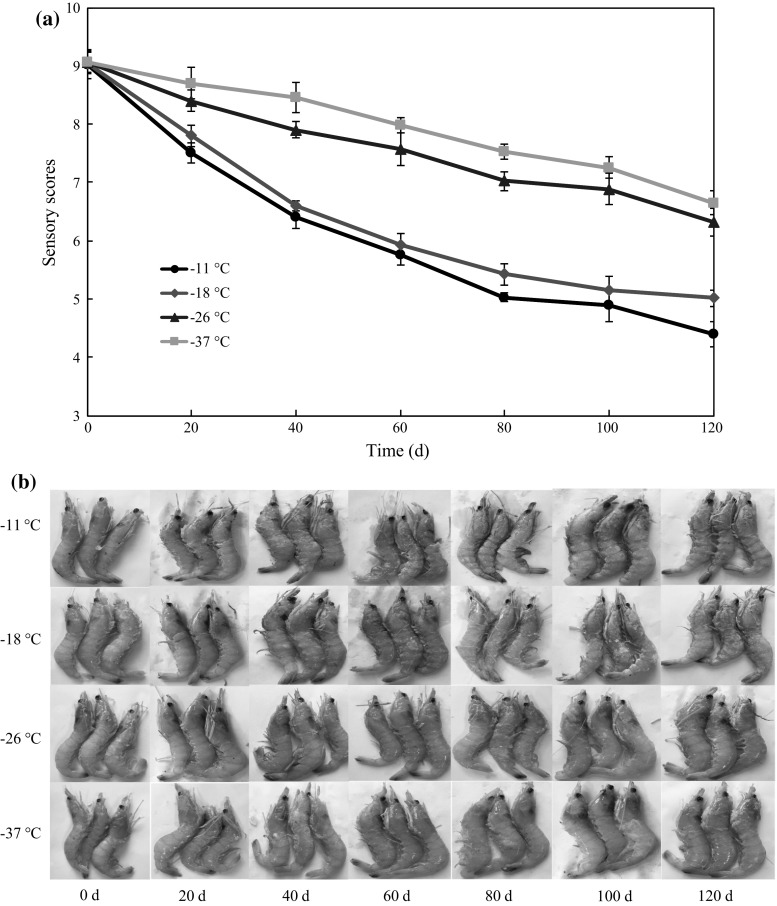
The sensory quality of Solenocera crassicornis was evaluated by the changes in appearance, texture, odor and flavor on 20, 40, 60, 80, 100 and 120 days. The “overall quality” plotted against the storage time at different deep-frozen temperatures is shown in Fig. 1a. The sensory scores showed decline with storage time and temperature. At the beginning, all groups of samples had the score higher than 9 and no differences in likeness of samples were found. For samples stored at − 11 and − 18 °C, a significant decrease of sensory scores was seen at day 40, and the head and tail of shrimp darkened slightly (Fig. 1b). At day 60, black centers were clear in head and tail regions. Then the sensory scores were 5.7 and 5.9 respectively, which indicated the shrimps were still acceptable. At day 100, the head of shrimps stored at − 11 °C was almost completely dark, and the muscle became soft with a slight odor. Then a score of 4.9 indicated that the shrimps had been unfit for consumption. The shrimps stored at − 18 °C had a score of 5.0 at day 120, which suggested that the shrimps were still acceptable. In addition, the decrease of sensory scores in shrimp stored at − 26 and − 37 °C was significantly lower than that stored at − 11 and − 18 °C (p < 0.05). At day 120, the head and tail of shrimps stored at − 26 and − 37 °C darkened slightly (with a score of 6.3 and 6.6, respectively), which were well identified as edible. In particular, the shrimps stored at − 37 °C showed highest overall quality value. The results suggested that a lower deep-frozen temperature was more effective in minimizing the sensory quality loss of Solenocera crassicornis. Tsironi et al. (2009) reported the sensory scores of shrimp showed decline with storage time during variable frozen temperature conditions, and the results for temperature dependence were in agreement with the ones in this study.
Fig. 1.
Changes in sensory scores (a) and appearance (b) of Solenocera crassicornis during frozen storage at − 11, − 18, − 26 and − 37 °C. Error bars represent the standard error of three replicates
Color changes
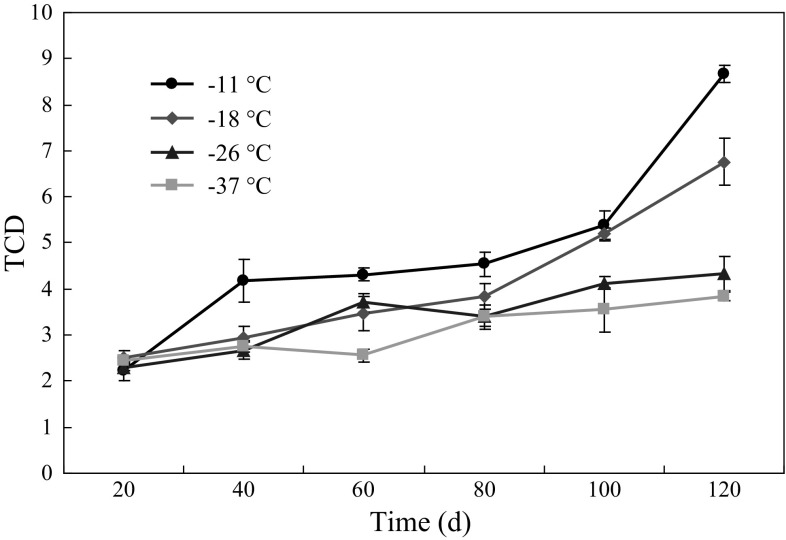
Color is one of the important quality parameters for freshness. The changes in TCD values (ΔE) of Solenocera crassicornis stored at different deep-frozen temperatures are shown in Fig. 2. There was a gradual increase in the TCD values of shrimp with increasing the storage period, but TCD values of shrimp stored at − 11 °C had a fastest increasing rate and that stored at − 37 °C had a slowest increasing rate. The TCD values of shrimp stored at − 11 °C were always higher than those of other groups during frozen storage. For shrimps stored at − 18, − 26 and − 37 °C, there was no significant difference (p > 0.05) on the TCD values in the first 80 days,and significant difference (p < 0.05) was observed between samples stored at − 18 °C and two other groups after day 80. Moreover, the TCD values of shrimps stored at − 26 and − 37 °C were significantly lower (p < 0.05) than that stored at − 11 and − 18 °C at day 120. Whereas the difference between samples stored at − 26 and − 37 °C was insignificant (p > 0.05). Thus, Solenocera crassicornis stored at − 26 and − 37 °C could better delay the change of color in the present study. The present findings are in agreement with that of Tsironi et al. (2009) who reported the colour degradation rates were high for the higher frozen temperatures.
Fig. 2.
TCD values (ΔE) of Solenocera crassicornis during frozen storage at − 11, − 18, − 26 and − 37 °C. Error bars represent the standard error of three replicates
Effect of deep-frozen storage on pH
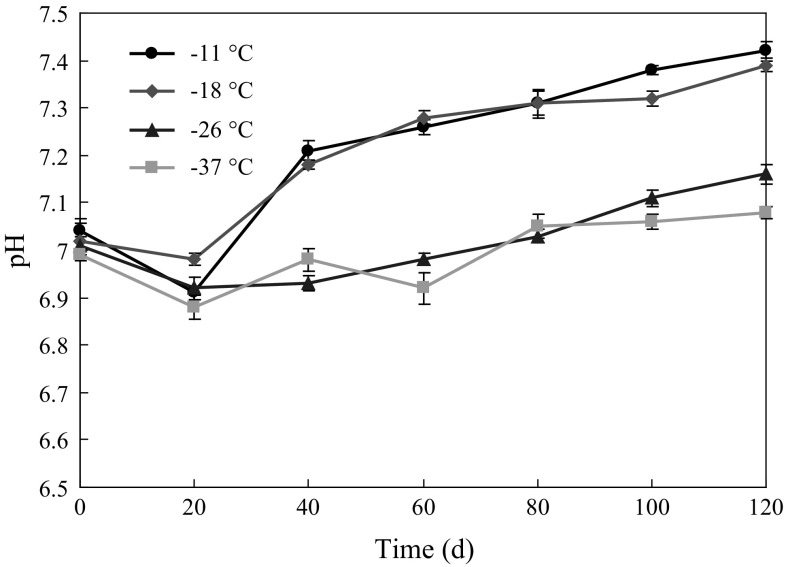
The pH changes of Solenocera crassicornis stored at different deep-frozen temperatures are shown in Fig. 3. The initial pH of all samples was about 7.02, and no significant differences were found. Interesting, the pH value decreased at the beginning, and then increased during frozen storage. Previous study (Dai et al. 2016) has reported that the decrease in pH could result from glycogen glycolysis of shrimp body for an hour after death. The increase in pH value is related to the accumulation of alkaline molecules (ammonia and amines, etc.) produced from the metabolism of amino acids, peptides and protein. However, a continuous increase in pH during variable frozen temperature conditions was observed by Tsironi et al. (2009). The results disagreeing with the present study may be due to difference of frozen temperatures.
Fig. 3.
pH changes of Solenocera crassicornis during frozen storage at − 11, − 18, − 26 and − 37 °C. Error bars represent the standard error of three replicates
In the first 20 days, the differences of pH values among all samples were not significant (p > 0.05). At later frozen storage time, the pH values of shrimps stored at − 11 and − 18 °C increased fast, and then the difference between the pH values of shrimps stored at − 11, − 18 °C and two other groups was significant (p < 0.05). At day 120, the pH values were 7.42, 7.39, 7.16 and 7.08 at − 11, − 18, − 26 and − 37 °C, respectively. In general, the pH 7.7 (being or below) is the scope for the shrimps to keep good freshness (Buyukcan et al. 2009; Mu et al. 2012). In the present study, the pH values of all samples were lower than pH 7.7, which suggested that storage at deep-frozen temperature could effectively slow down the pH change of shrimps and inhibitory effect was more significant with a lower deep-frozen temperature.
Effect of deep-frozen storage on TVB-N
Kinetic curves (R2 > 0.90 for all experiments) corresponding to TVB-N are shown in Fig. 4. The results showed the changes in TVB-N values of Solenocera crassicornis in following 120 days. At the beginning, no significant differences (p > 0.05) of the TVB-N values among all samples were found, and the values subsequently increased with frozen storage time, whereas the increase of TVB-N values varied among all groups. The increasing rate in TVB-N values was fastest in shrimp stored at − 11 °C (k = 0.0033), and slowest in shrimp stored at − 37 °C (k = 0.0009). In addition, the TVB-N values in shrimp stored at − 26 and − 37 °C were significantly lower than those stored at − 11 and − 18 °C at day 120 (p < 0.05), and then the TVB-N values were 18.7, 17.2, 13.8 and 13.5 mg/100 g at − 11, − 18, − 26 and − 37 °C, respectively. The results also showed the difference of the TVB-N values between samples stored at − 26 and − 37 °C was insignificant (p > 0.05). Previous study (Tsironi et al. 2009) has shown that TVB-N values increased with storage time during variable frozen temperatures and were modelled with apparent first order equations. In the present study, TVB-N values were modelled with exponential equation.
Fig. 4.
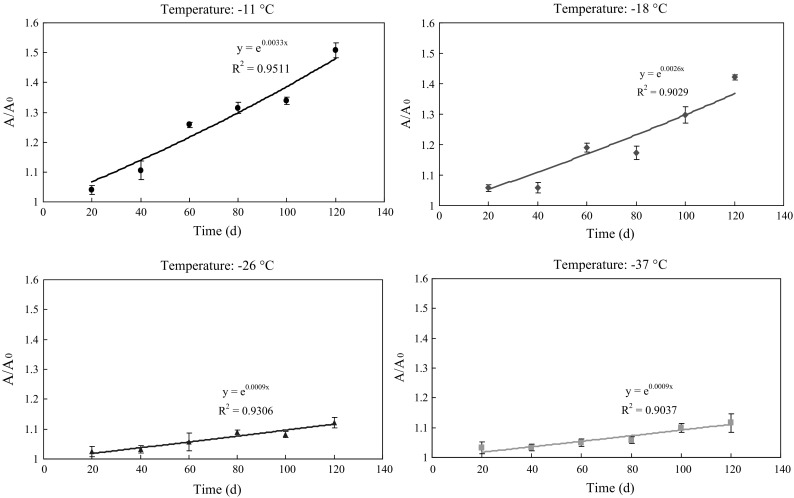
Kinetic curves of TVB-N at different storage temperatures (− 11, − 18, − 26 and − 37 °C). Error bars represent the standard error of three replicates
According to the Chinese National standard (GB 2733-2015) (2015), the TVB-N value of fresh shrimp should be ≤ 30 mg/100 g. In the present study, the TVB-N values of all samples were lower than 30 mg/100 g. Above results suggested that storage at deep-frozen temperature could effectively inhibit the increase in TVB-N values of shrimp. In general,the increase of TVB-N level in shrimp is related to the activity of spoilage bacteria (Mu et al. 2012; Sun et al. 2014). Thus, the changes of TVB-N values can be explained as that deep-frozen temperatures reduce the activity of spoilage microorganisms and endogenous enzymes, and the metabolism of protein is effectively restrained, then the increase of TVB-N values is slowed down.
Changes in Salt-soluble protein
Salt-soluble protein (SSP) is an indicator of progressive denaturation in shrimp during frozen storage. Kinetic curves (R2 > 0.90 for all experiments) are shown in Fig. 5. All the values decreased linearly during 120 days frozen storage. The SSP values in shrimp decreased significantly (p < 0.05) from 17.89 to 5.15 ug/kg (at − 11 °C), and to 5.24 ug/kg (at − 18 °C) after 120 days of frozen storage. However, changes in shrimp stored at − 26 and − 37 °C were not significant (p > 0.05), which maintained the SSP values at 12.35 and 13.25 ug/kg at day 120, respectively. It is clear that the lowest SSP value was obtained at − 11 °C and this value increased as the frozen storage temperature decreased. Consequently, the results suggested that frozen storage temperature is an important factor for changes in SSP of Solenocera crassicornis. Barraza et al. (2015), in a study with kinetics of protein changes under different frozen storage temperatures (268, 264, 260 and 255 K), found SSP decreased systematically and showed reaction rates inversely proportional to the frozen temperature using the Weibullian model (Brookmire et al. 2013), which is in agreement with results of present study.
Fig. 5.
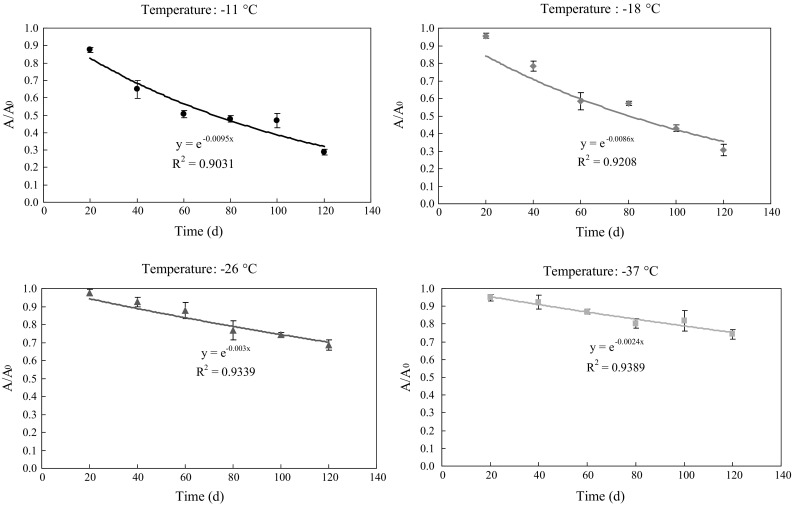
Kinetic curves of salt-soluble protein (SSP) at different storage temperatures (− 11, − 18, − 26 and − 37 °C). Error bars represent the standard error of three replicates
Frozen temperatures influenced ice crystal formation and the recrystallization processes, then determined crystal size, orientation and localization. These changes contribute to modifications in the techno-functional properties of proteins (Tahergorabi and Jaczynski 2011), which were related to warping, dehydration, denaturation, and reduction of soluble protein (Barraza et al. 2015). The lower frozen temperature could generate greater ice crystal in a shorter time period to prevent denaturation of soluble protein. Therefore, that can explain the decrease of SSP value, and the influence of frozen temperature.
Effect of deep-frozen storage on lipid oxidation
Lipid oxidation in shrimp was quantified by determining the thiobarbituric acid reactive substances (TBARS). The results are shown in Fig. 6a. The initial TBARS value of all samples was about 0.45 mg MDA/kg, and the TBARS values increased with frozen storage time. In the first 60 days, the differences of TBARS values among all samples were not significant (p > 0.05). After 60 days, the TBARS values of shrimp stored at − 26 and − 37 °C were significantly (p < 0.05) lower than those of the rest. At day 120, the TBARS values peaked, and then were 0.85 (− 11 °C), 0.82 (− 18 °C), 0.67 (− 26 °C) and 0.64 (− 37 °C) mg MDA/kg, respectively. According to Farajzadeh et al. (2016), TBA values of 1–2 mg MAD/kg are related with the unpleasant odor and taste. In the present study, TBARS values of all samples were below the maximum limit at the end of frozen storage. These results indicate that frozen storage reduced the lipid oxidation in shrimp, and a lower deep-frozen temperature has a more significant inhibitory effect. Different from this study, Tsironi et al. (2009) reported the increase of the TBARS values showed non-specific regularity and thus could not be used as a consistent quality indicator.
Fig. 6.
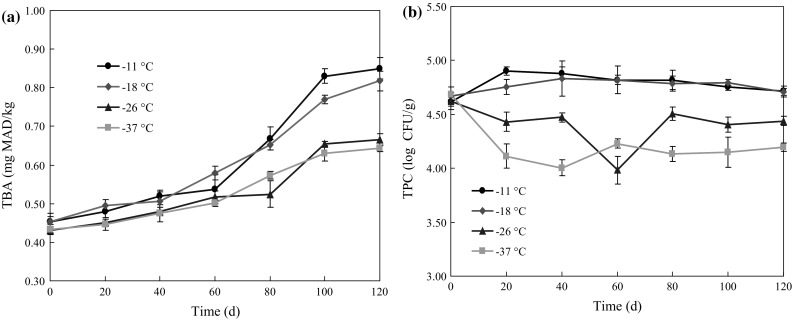
Changes in TBARS (a) and TPC (b) values of Solenocera crassicornis during frozen storage at − 11, − 18, − 26 and − 37 °C. Error bars represent the standard error of three replicates
In general,hydroxyl radical(–OH) in shrimp muscle are widespread. The chemical activity of hydroxyl radicals is strong and they easily react with biomolecules such as fatty acids, amino acids, proteins and DNA (Solval et al. 2014). A reason for effect could be that frozen storage slows down thermal motion and reduces chemical activity of hydroxyl radicals, thus inhibiting lipid oxidation.
Effect of deep-frozen storage on microbial growth
The changes in total aerobic plate counts (TPC) of Solenocera crassicornis during frozen storage are presented in Fig. 6b. The initial TPC value of all samples was around 4.6 log CFU/g, which indicated a high initial quality of shrimp. For shrimp stored at − 11 and − 18 °C, TPC values increased at day 20, and then decreased slowly during the frozen storage. Nevertheless, the bacterial count was significantly inhibited in shrimp stored at − 26 and − 37 °C, and a decreasing tendency was observed throughout the frozen storage time. TPC values of shrimp stored at − 26 and − 37 °C was significantly lower than of the rest at 120 day of storage (p < 0.05). Similarly, Al-Bulushi et al. (2005) reported that the initial aerobic bacterial count reduced sharply (p < 0.05) by 84–97% throughout the frozen storage at − 20 °C, which agreed with the findings of present study.
Conclusion
The present study has demonstrated that deep-frozen storage can effectively reduce the spoilage and extend the shelf life of Solenocera crassicornis. Deep-frozen storage was effective in minimizing the sensory quality loss, specially at the lowest temperature. It could decreased protein degradation and lipid oxidation, as preserved the bacteria growth. Moreover, it also assisted to improve pH and color changes for the shrimp. The qualities of shrimp stored at − 26 and − 37 °C were significantly better than those stored at − 11 and − 18 °C during frozen storage. No significantly difference of indices was observed between shrimp stored at − 26 and − 37 °C during frozen storage.
Acknowledgements
This work was supported by the Science and Technology Plan Projects of Zhejiang Province, China (2017C37009, 2017F50018).
References
- Abd-El-Aziz NA, Moharram YG. Microbiological quality of imported frozen shrimp in Egypt. Ann Agric Sci. 2016;61(1):35–40. [Google Scholar]
- Al-Bulushi IM, Kasapis S, Al-Oufi H, Al-Mamari S. Evaluating the quality and storage stability of fish burgers during frozen storage. Fish Sci. 2005;71(3):648–654. doi: 10.1111/j.1444-2906.2005.01011.x. [DOI] [Google Scholar]
- Al-Bulushi IM, Kasapis S, Dykes GA, Al-Waili H, Guizani N, Al-Oufi H. Effect of frozen storage on the characteristics of a developed and commercial fish sausages. J Food Sci Technol. 2013;50(6):1158–1164. doi: 10.1007/s13197-011-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza FAA, León RAQ, Álvarez PXL. Kinetics of protein and textural changes in Atlantic salmon under frozen storage. Food Chem. 2015;182:120–127. doi: 10.1016/j.foodchem.2015.02.055. [DOI] [PubMed] [Google Scholar]
- Boonsumrej S, Chaiwanichsiri S, Tantratian S, Suzuki T, Takai R. Effects of freezing and thawing on the quality changes of tiger shrimp (Penaeus monodon) frozen by air-blast and cryogenic freezing. J Food Eng. 2007;80:292–299. doi: 10.1016/j.jfoodeng.2006.04.059. [DOI] [Google Scholar]
- Brookmire L, Mallikarjunan P, Jahncke M, Grisso R. Optimum cooking conditions for shrimp and Atlantic salmon. J Food Sci. 2013;78(2):S303–S313. doi: 10.1111/1750-3841.12011. [DOI] [PubMed] [Google Scholar]
- Buyukcan M, Bozoglu F, Alpas H. Preservation and shelf life extension of shrimps and clams by high hydrostatic pressure. Int J Food Sci Technol. 2009;44(8):1495–1502. doi: 10.1111/j.1365-2621.2007.01628.x. [DOI] [Google Scholar]
- Carrión-Granda X, Fernández-Pan I, Jaime I, Rovira J, Maté JI. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int J Food Microbiol. 2016;232:144–149. doi: 10.1016/j.ijfoodmicro.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Chinese National Standard (GB 2733-2015) Fresh and frozen animal aquatic products. Beijing: Chinese National Hygiene Ministry; 2015. [Google Scholar]
- Chinese National Standard (GB 4789.2-2016) Microbiological examination of food hygiene: detection of aerobic plate count. Beijing: Chinese National Hygiene Ministry; 2016. [Google Scholar]
- Chinese National Standard (GB 5009.228-2016) Detection of total volatile basic nitrogen (TVB-N) in food. Beijing: Chinese National Hygiene Ministry; 2016. [Google Scholar]
- Chouljenko A, Chotiko A, Bonilla F, Moncada M, Reyes V, Sathivel S. Effects of vacuum tumbling with chitosan nanoparticles on the quality characteristics of cryogenically frozen shrimp. LWT-Food Sci Technol. 2017;75:114–123. doi: 10.1016/j.lwt.2016.08.029. [DOI] [Google Scholar]
- Dai XY, Zhang MX, Wei XY, Hider RC, Zhou T. Novel multifunctional hydroxypyridinone derivatives as potential shrimp preservatives. Food Bioprocess Technol. 2016;9(7):1079–1088. doi: 10.1007/s11947-016-1694-1. [DOI] [Google Scholar]
- Farajzadeh F, Motamedzadegan A, Shahidi SA, Hamzeh S. The effect of chitosan-gelatin coating on the quality of shrimp (Litopenaeus vannamei) under refrigerated condition. Food Control. 2016;67:163–170. doi: 10.1016/j.foodcont.2016.02.040. [DOI] [Google Scholar]
- Gonçalves AA, de Oliveira ARM. Melanosis in crustaceans: a review. LWT-Food Sci Technol. 2016;65:791–799. doi: 10.1016/j.lwt.2015.09.011. [DOI] [Google Scholar]
- Gonçalves AA, Junior CSGG. The effect of glaze uptake on storage quality of frozen shrimp. J Food Eng. 2009;90(2):285–290. doi: 10.1016/j.jfoodeng.2008.06.038. [DOI] [Google Scholar]
- Gornall AG, Bardawill CJ, David MMJ. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- Hatha AM, Maqbool TK, Kumar SS. Microbial quality of shrimp products of export trade produced from aquacultured shrimp. Int J Food Microbiol. 2003;82(3):213–221. doi: 10.1016/S0168-1605(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Marshall MR, Wei C. Polyphenoloxidase in seafood enzymes: utilization and influence on postharvest seafood quality. In: Haard N, Simpson B, editors. seafood enzymes. New York: Marcel Dekker Inc; 2002. pp. 271–315. [Google Scholar]
- Mastromatteo M, Danza A, Conte A, Muratore G, Del Nobile MA. Shelf life of ready to use peeled shrimps as affected by thymol essential oil and modified atmosphere packaging. Int J Food Microbiol. 2010;144(2):250–256. doi: 10.1016/j.ijfoodmicro.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Mu HL, Chen HJ, Fang XJ, Mao JL, Gao HY. Effect of cinnamaldehyde on melanosis and spoilage of Pacific white shrimp (Litopenaeus vannamei) during storage. J Sci Food Agric. 2012;92(10):2177–2182. doi: 10.1002/jsfa.5605. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Effect of catechin and ferulic acid on melanosis and quality of Pacific white shrimp subjected to prior freeze-thawing during refrigerated storage. Food Control. 2010;21(9):1263–1271. doi: 10.1016/j.foodcont.2010.02.015. [DOI] [Google Scholar]
- Nirmal NP, Benjakul S. Inhibitory effect of mimosine on polyphenoloxidase from cephalothoraxes of Pacific white shrimp (Litopenaeus vannamei) J Agric Food Chem. 2011;59(18):10256–10260. doi: 10.1021/jf201603k. [DOI] [PubMed] [Google Scholar]
- Papadakis S, Abdul-Malek S, Kamdem E, Jam L. A versatile and inexpensive technique for measuring color of foods. Food Technol. 2000;54:48–51. [Google Scholar]
- Solval KM, Rodezno LAE, Moncada M, Bankston JD, Sathivel S. Evaluation of chitosan nanoparticles as a glazing material for cryogenically frozen shrimp. LWT-Food Sci Technol. 2014;57(1):172–180. doi: 10.1016/j.lwt.2013.12.033. [DOI] [Google Scholar]
- Sun HY, Lv H, Yuan GF, Fang XB. Effect of grape seed extracts on the melanosis and quality of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Sci Technol Res. 2014;20(3):671–677. doi: 10.3136/fstr.20.671. [DOI] [Google Scholar]
- Sundararajan S, Prudente A, Bankston JD, King JM, Wilson P, Sathivel S. Evaluation of green tea extract as a glazing material for shrimp frozen by cryogenic freezing. J Food Sci. 2011;76(7):E511–E518. doi: 10.1111/j.1750-3841.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- Tahergorabi R, Jaczynski J. Seafood proteins handbook of food proteins. Sawston: Woodhead Publishing Limited; 2011. p. 432. [Google Scholar]
- Tsironi T, Dermesonlouoglou E, Giannakourou M, Taoukis P. Shelf life modelling of frozen shrimp at variable temperature conditions. LWT-Food Sci Technol. 2009;42(2):664–671. doi: 10.1016/j.lwt.2008.07.010. [DOI] [Google Scholar]
- Wachirasiri K, Wanlapa S, Uttapap D, Rungsardthong V. Use of amino acids as a phosphate alternative and their effects on quality of frozen white shrimps (Penaeus vanamei) LWT-Food Sci Technol. 2016;69:303–311. doi: 10.1016/j.lwt.2016.01.065. [DOI] [Google Scholar]
- Wu SJ. Effect of chitosan-based edible coating on preservation of white shrimp during partially frozen storage. Int J Biol Macromol. 2014;65:325–328. doi: 10.1016/j.ijbiomac.2014.01.056. [DOI] [PubMed] [Google Scholar]
- Yuan G, Lv H, Tang W, Zhang X, Sun H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control. 2016;59:818–823. doi: 10.1016/j.foodcont.2015.07.011. [DOI] [Google Scholar]