Abstract
Enzyme-assisted extraction has emerged as an attractive green, cost-effective and high bioactive yielding technology by which desired bioactives with preserved or better efficacy are released. In the present study, the ability of cellulase, hemicellulase and their binary mixture (cellulase:hemicellulase; 1:1) in improving the extraction of essential oils from coriander (Coriandrum sativum L.) seeds, or the residue by-products from the distillation process containing value-added phytochemicals (fatty acids and phenolics) were evaluated. Cellulase and the binary mixture improved the extraction of essential oils by 44.2 and 40%, respectively. Application of enzymes was associated with increased amount of oxygenated terpenes in the essential oils derived from enzyme-treated samples. Linalool, camphor and geranyl acetate were the prominent compounds. From the hydrodistillation residues, a better recovery of petroselinic-rich oil with a good nutritional quality was also observed in enzyme-treated seeds. They also contained an appreciable amount of polyphenols and showed an improved antioxidant activity as revealed by the DPPH, FRAP and cellular antioxidant activity assays. The results suggested that enzyme pre-treatment allowed better recovery without alteration of the essential oil composition. The hydrodistillation residues obtained could be potentially exploited for the development of functional food ingredients and nutraceuticals.
Keywords: Coriandrum sativum L., Enzyme-assisted extraction, Bioactive ingredients, Cellular antioxidant activity, Hydrodistillation by-products, Functional ingredients
Introduction
Coriander (Coriandrum sativum L.), is one of the most important spices in the world, and is of great significance in international trade. It is a multi-purpose herb mainly cultivated for its foliage and seeds which have numerous food-related biological activities and multiple functional uses (Burdock and Carabin 2009). Coriander has a long history of therapeutic use for various health problems including flatulence, diabetes, dysentery, sore throat, insomnia, anxiety, cardio-vascular and urinary disorders (Patel et al. 2012). Coriander seeds contain a wide array of health beneficial compounds such as minerals, phenolics, fatty acids and essential oils, among others (Laribi et al. 2015). Of particular interest, coriander essential oils have attracted considerable attention due to their potential biological activities and their application in fragrance, cosmetic and pharmaceutical industries. These valuable components are conventionally extracted by steam distillation, hydrodistillation or solvent extraction. Nevertheless, the drawback of these methods is the low yield. To overcome such a problem, a lot of alternative methodologies such as supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), subcritical water extraction (SWE) (Sowbhagya and Chitra 2010), coupled extruser-dynamic headspace, microwave-assisted extraction (MWE) and ultrasound-assisted extraction (USE) has been developed. More recently, enzyme-assisted extraction of essential oil is gaining momentum and its efficiency as well as its eco-friendly characters are largely proved (Puri et al. 2012).
This innovative process is based on the application of cell wall hydrolyzing enzymes such as cellulase, hemicellulase and pectinase that are able to opens up the cell, facilitating hence the release of essential oils (Hosni et al. 2013; Boulila et al. 2015). There are several reports on the successful application of enzyme to extract volatile oils from different plant species like garlic (Sowbhagya et al. 2009), celery (Sowbhagya et al. 2010), cumin seeds (Sowbhagya et al. 2011), black pepper, cardamom (Chandran et al. 2012), rosemary, thyme (Hosni et al. 2013) and bay leaves (Boulila et al. 2015). Due to their numerous advantages (high effectiveness, mild and easy operating conditions, high extract quality and environmental-compatibility), enzymes are widely used in food industries for the extraction of a wide array of valuable compounds such as volatile oils, flavours, pigments (Puri et al. 2012), polysaccharides, purine alkaloids (Rosellό-Soto et al. 2015), and polyphenols (Putnik et al. 2017).
In the light of the above considerations, enzyme-assisted extraction appears to be particularly attractive for extracting bioactive added value compounds. Therefore, the aim of this study was to evaluate the effects of enzyme pre-treatment on yields and chemical composition of the essential oil of coriander seeds. It also investigates the phytochemical profile of the residual hydrodistilled seeds (a by-product of the hydrodistillation process). A special attention was paid to their lipid content, fatty acid profile, total phenols and flavonoid contents as well as their antioxidant activity. To the best of our knowledge, this is the first report on enzyme-assisted extraction of coriander essential oils. This is also the first example on phytochemicals from the residual hydrodistilled seeds and their bioactivity. Results are envisaged to pave a way for full exploitation of the raw material, and the development of novel, low cost nutritious food products and food formulations.
Materials and methods
Plant material, enzymes and chemicals
Air dried coriander seeds were purchased from the local market (Menzel Temim, Tunisia). Cellulase (E.C. 3.2.1.4, 8.9 U/mg) from Trichoderma viridie and hemicellulase (H-7649, 13.8 U/mg) from Aspergillus niger, were purchased from Sigma-Aldrich (St. Louis. MO, USA). Methanol, ethyl acetate, petroleum ether and diethyl ether were purchased from VWR Chemicals (Fontenay, France). Analytical grade hexane and chloroform were obtained from Carlo Erba (Val de Reuil, France). Phosphate buffered saline (PBS) was purchased from Gibco (Life Technology, France) and RPMI was obtained from Gibco (BRL, Paisley, UK). Anhydrous sodium sulphate (Na2SO4) and n-alkanes series were purchased from Fluka Chemicals (Buchs, Switzerland). The 2,2-diphenyl-1-picrylhydrazyl (DPPH), aluminium chloride (AlCl3), sodium methylate, ferric chloride (FeCl3), 2,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), and 2′,7′-dichlorofluorescin diacetate (DCFH-DA), 2,2′-azobis (2-amidinopropane) (ABAP), quercetin and gallic acid were purchased from Sigma-Aldrich (Steinheim, Germany).
Enzyme pre-treatment, essential oil extraction and analysis
Prior to extraction, the dried seeds were ground by using a Retsch blender Mill (Normandie-Labo, Normandy, France), and sifted through 0.5 mm mesh screen to obtain uniform particle size. For enzymatic treatment, 100 g of ground seeds were mixed with 500 mL distilled water containing 10 mg of single enzyme (cellulase and hemicellulase) or 20 mg of the binary enzyme mixture (cellulase:hemicellulase 1:1), stirred for 1 h at 40 °C, and thereafter, the whole mixture was subjected to hydrodistillation for 2 h using a Clevenger-type apparatus (Boulila et al. 2015). Control samples were subjected to hydrodistillation without any treatment. The oils were recovered, weighed, dried over Na2SO4 and stored in amber and airtight sealed vials at − 20 °C until analyzed. The hydrodistillation solid residues (by-product of the distillation process) were recovered, oven dried at 40 °C and stored at − 20 °C for further uses.
Separation and identification of the essential oil components were carried out using GC-FID and GC–MS. The GC apparatus was an HP 6890 (II) gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionisation detector (FID), an apolar HP-5 (5% phenylmethylsiloxane) and a polar Innowax (100% PEG) capillary columns (60 m × 0.25 mm, 0.25 µm film thickness). Nitrogen of chromatographic grade was used as carrier gas at a flow rate of 1.2 mL/min. The inlet was operated in the split mode with a split ratio of 60:1. The temperature program used was 50 °C for 1 min, increasing by 2 °C/min to 280 °C, and kept at this temperature for 4 min. Injector and detector temperature were set at 250 and 300 °C, respectively.
The GC–MS analyses were carried out using an HP 6890 N gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with a fused silica capillary column HP-5MS (30 m × 0.25 mm, 0.25 mm film thickness) and coupled with an HP 5975 mass selective detector (MSD) from the same company. The temperature program was initiated at 40 °C, and linearly increased to 280 °C at a rate of 5 °C/min. Gas helium at a flow rate of 2 mL/min was used as carrier. The injector port and the ion source were maintained at 270 and 230 °C, respectively. Positive electron impact spectra were recorded at 70 eV in the range m/z 50–550.
The volatile compounds were identified by comparison of their retention indices (RI) relative to (C7–C20) n-alkanes (Adams 2001), and their mass spectra with those of authentic standards when available and components of known essential oils, as well as those from Wiley 275.L library, mass finder 3, and a homemade MS library. The relative percentages of components were calculated from the peak-area percent of the GC-FID data.
Total lipid content, fatty acid profiles and lipid quality of intact and hydrodistilled residual seeds
Total lipid content from intact (NTNH: neither treated nor hydrodistilled) and those of untreated (NTH: not enzymatically treated but hydrodistilled) and enzymatically treated and hydrodistilled (ETH) seeds were extracted according the procedure of Bligh and Dyer (1959). Briefly, 1 g of raw material was crushed in a mortar with a chloroform/methanol mixture (2:1; v/v), and the homogenate was centrifuged at 3000 g for 15 min. The lower chloroform phase containing the total lipids was recovered and dried under a stream of nitrogen.
The fatty acid methyl esters (FAMEs) of the total lipids were prepared according to the procedure described by Cecchi et al. (1985). Briefly, a 200 µL aliquot of the oil was dissolved in 2 mL hexane, then 0.5 mL of sodium methylate (3% in methanol), a known amount of nonadecanoic acid (C19:0) as the internal standard, 0.2 mL of 1 N H2SO4 and 1.5 mL of sodium chloride were added and the reaction mixture was thoroughly agitated for 1 min. The hexane phase containing FAMEs was recovered and concentrated under a stream of nitrogen, prior to analysis.
The FAMEs analysis was carried out using an HP 6890 gas chromatograph series II (Agilent Technologies, Palo Alto, California, USA) equipped with flame ionisation detector (FID) and an electronic pressure control injector (EPC). Separation of individual FAMEs was performed on a polar TR-FAME (60 m × 0.32 mm, 0.25 µm; Thermo Scientific, Wöhlerstraße, Mainz, Germany).
The oven temperature was initially held at 150 °C for 1 min, raised to 200 °C at a rate of 15 °C/min, held for 3 min then ramped to 242 °C at a rate of 2 °C/min. The temperature of the injector and FID detector were maintained at 250 and 275 °C, respectively. Identification of FAMEs was made by comparing their retention time with those of reference standards mix purchased from Sigma-Aldrich Inc (Steinheim, Germany). The FAMEs compositions refer to the percentage ratio of each component to total fatty acids.
The double bond index (DBI) was calculated as follow (Gignon et al. 2004):
The iodine values (IV) were calculated from fatty acid percentages using the following formula (Cecchi et al. 2011):
In addition to the unsaturated fatty acid/saturated fatty acid (UFA/SFA) ratio, artherogenic index (AI), thrombogenic index (TI) as well as the calculated oxidizability value (Cox), the oxidative susceptibility (OS) and peroxidisability index (PI) were used to evaluate the lipid quality of C. sativum seeds.
Atherogenic index (AI) and thrombogenic index (TI) were calculated according to the following equations (Ulbrich and Southgate 1991):
where MUFA is the sum of monounsaturated fatty acid, and PUFA is the sum of polyunsaturated fatty acids.
The Cox value was calculated using the following equation (Fatemi and Hammond 1980):
The oxidative susceptibility (OS) was calculated using the following formula (Cecchi et al. 2011):
The peroxidisability index (PI) was calculated using the following formula (Kang et al. 2005):
Extracts preparation from intact and hydrodistilled residual seeds
Intact (NTNH) and hydrodistilled residual seeds from untreated (NTH) and enzymes treated (ETH) residual seeds (1 g) were mixed with 20 mL of organic solvents with different polarity (petroleum ether, ethyl acetate, methanol, 80% methanol and water) and shaken mechanically for 48 h at room temperature in an orbital shaker. The supernatants were next filtered through Wattman #1 filter paper (Bärenstain, Germany) and concentrated under reduced pressure in a Heidolph rotary evaporator (Schwabach, Germany). The water extract was frozen and lyophylized in a Christ-Alpha 2-4 freeze drier (Osterode, Germany).
Determination of total phenolic (TPC) and total flavonoid (TFC) contents
Total phenolic contents (TPC) were measured by the Folin–Cieucalteu (FC) method (Singleton and Rossi 1965) with some modifications. The extract was dissolved in methanol to give a concentration of 1 mg/mL (w/v). A 100 µL aliquot was mixed with 500 µL of freshly diluted tenfold FC reagent and 1 mL of 20% sodium carbonate solution. After 1 h in the dark and at room temperature, the decrease in absorbance was measured at 760 nm using a Jasco V-630 UV–Vis spectrophotometer (Tokyo, Japan). The final results were expressed as microgram of gallic acid equivalents per g of extract (µg GAE/g of extract).
The colorimetric aluminium chloride (AlCl3) method was used to determine total flavonoid (TFC) contents according to the procedure proposed by Chang et al. (2002). A 500 µL aliquot was mixed with 1.5 mL methanol, 0.1 mL of 10% AlCl3 solution, 0.1 mL of potassium acetate (1 M), and 2.8 mL of distilled water. After 30 min incubation at room temperature, the absorbance was read at 415 nm. The TFC was expressed as microgram of quercetin equivalents per g of extract (µg QE/g of extract).
Antioxidant activity
DPPH assay
The DPPH radical scavenging activity (RSA) and the reducing ability of extracts were estimated according to the procedure of Sharma and Bhat (2009). Briefly, 100 µL of different extracts was added to 100 µL of 0.05 mg/mL DPPH solution in methanol, and the mixture was incubated for 30 min in the dark, at room temperature. Thereafter, the absorbance was measured at 517 nm using a microplate spectrophotometer reader (ThermoLab systems, Franklin, MA). The assay vehicle without extract served as negative control, whereas quercetin and trolox were used as positive controls. The RSA against DPPH* was calculated as the following formula:
where A0 is the absorbance of the control and As is the absorbance of the sample. The concentration having 50% radical inhibition activity (IC50) expressed as µg extract/mL was determined from the graph of the free RSA (%) against the extract concentration.
FRAP assay
The antioxidant activity based on the reducing ability of extracts was estimated according to the procedure of Benzie and Strain (1996) with some modifications. The working FRAP reagent was freshly prepared by mixing 10 mL of 300 mM acetate buffer (pH 3.6) with 1 mL of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM of hydrochloric acid and 1 mL of FeCl3·6H2O. Extracts (5 µL) diluted in 20 µL of distilled water were added to the FRAP reagent. After 8 min, absorbance of the mixture was measured using microplate spectrophotometer reader at 594 nm. Ascorbic acid and trolox were used as positive controls. The percentage FRAP reduction was calculated as the following formula:
where As is the absorbance of the sample and Ac is the absorbance of the control. The effective concentration having 50% FRAP reduction (EC50) expressed as µg extract/mL was determined from the graph of the FRAP reduction (%) against the extract concentration.
Cellular antioxidant activity (CAA) assay
The CAA of different extracts was assessed using the method of Wolfe and Liu (2007). Briefly, splenocytes (100 µL in RPMI 1640 medium) isolated from BALB/c mice were seeded onto 96-well microplates at a density of 5 × 106/well. After 24 h incubation, the growth medium was removed, and the wells were washed with PBS (pH 7.4). Wells were treated in triplicate for 1 h at 37 °C with 95 µL of extract and 5 µL of 25 mM DCFH-DA. After additional 1 h incubation, 100 µL of 600 mM ABAP in RPMI was applied to all wells except for the blank ones, and the fluorescence was measured using a fluorescence microplate reader (Biotech, Winooski, USA) for 1 h (5 min intervals) at 538 nm emission and 485 nm excitation. Each plate included triplicate control and blank wells. Control wells contained cells with DCFH-DA and RPMI without ABAP, whereas control wells contained cells with DCFH-DA and ABAP. The area under the curve of fluorescence versus time was integrated to calculate the CAA value of each sample after a blank subtraction from the fluorescent reading.
where ∫SA and ∫CA are the integrated areas for sample and control curves, respectively (Wolfe and Liu 2007).
Statistical analyses
Data were expressed as mean (± SD) and differences between means were determined by one-ANOVA, using Tukey’s post hoc test. P values < 0.05 were considered as statistically significant. All analyses were performed using SPSS ver.13 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Effects of enzyme treatment on yield and chemical composition of essential oils
The yields of essential oil from enzyme-treated and non-treated coriander seeds are shown in Table 1. As shown, the application of enzymes increased the essential oil recovery by 44.2, 33.3 and 40% for samples treated by cellulase, hemicellulase and their binary mixture, respectively. Although that the highest essential oil recovery was observed for cellulase as compared to hemicellulase and the binary mixture (cellulase/hemicellulase), there were no significant (p > 0.05) changes in quantitative amounts of essential oil yield between different treatments. These results are in good agreement with earlier reports (Sowbhagya et al. 2009, 2010, 2011; Hosni et al. 2013; Boulila et al. 2015) that indicate the high cell-wall degrading activity of T. viridie cellulase owing to its endoglucanase, exoglucanase, and β-glucosidase components (Xu et al. 2014). Thus it can be inferred that the use of cellulase alone is more appropriate for this type of application.
Table 1.
Chemical composition (% total peak area) of control (untreated) and enzyme-treated samples of coriander (Coriandrum sativum L.) seeds
| Component | RIa | RIb | Control | Cellulase | Hemicellulase | binary mixturec |
|---|---|---|---|---|---|---|
| Camphene | 950 | 1076 | 0.47a | 0.42a | 0.15b | 0.14b |
| β-Pinene | 981 | 1118 | 0.71a | 0.71a | 0.46b | 0.4b |
| β-Myrcene | 988 | 1176 | 0.73a | 0.71a | 0.69a | 0.64a |
| 4-Carene | 997 | 1149 | 0.33a | 0.34a | 0.3b | 0.29b |
| α-Phellandrene | 1002 | 1157 | – | – | 0.13a | 0.12a |
| p-Cymene | 1026 | 1288 | 2.29a | 1.36b | 1.24bc | 1.06c |
| Limonene | 1031 | 1203 | 3.89a | 2.96b | 2.63c | 2.48c |
| (Z)-β-Ocimene | 1037 | 1247 | 0.11a | 0.12a | 0.12a | 0.11a |
| (E)-β-Ocimene | 1049 | 1266 | 0.14a | 0.15a | 0.16a | 0.15a |
| γ-Terpinene | 1059 | 1255 | 12.07a | 10.25b | 9.57b | 8.81bc |
| Linalool | 1088 | 1553 | 73.34b | 76.73ab | 79.01a | 78.2a |
| Camphor | 1145 | 1553 | 3.01a | 3.05a | 3a | 2.91a |
| Isoborneol | 1159 | 1659 | 0.16a | – | – | – |
| Borneol | 1165 | 1719 | – | 0.06a | – | – |
| Tepinen-4-ol | 1178 | 1611 | 0.3a | 0.29a | 0.23b | 0.25b |
| α-Terpineol | 1189 | 1706 | 0.12b | 0.21a | 0.18a | 0.2a |
| Decanal | 1193 | 1494 | 0.16a | 0.19a | 0.13c | 0.16b |
| Citronellol | 1228 | 1772 | 0.03a | – | – | 0.03a |
| Geranyl acetate | 1383 | 1765 | 1.84b | 2.14a | 1.82b | 2.02a |
| β-Caryophyllene | 1418 | 1612 | 0.08a | 0.09a | 0.07a | 0.08a |
| 2-Dodecenal | 1444 | 1867 | 0.1a | 0.06b | – | 0.09a |
| Group components | ||||||
| Monoterpene hydrocarbons | 20.74a | 17.02b | 15.45c | 14.2c | ||
| Oxygenated monoterpenes | 78.77b | 82.48a | 84.24a | 83.64a | ||
| Sesquiterpene hydrocarbons | 0.08a | 0.09a | 0.07a | 0.08a | ||
| Others | 0.29a | 0.25b | 0.13c | 0.28a | ||
| Oxygenated/hydrocarbons ratio | 3.78d | 4.82c | 5.42b | 5.85a | ||
| Total identified | 99.88 | 99.84 | 99.89 | 98.2 | ||
| Yield (% w/w) | 0.32b | 0.46a | 0.43a | 0.45a |
RI retention index on: aHP-5 and bHP-Innowax; –: not detected
cBinary mixture (cellulase + hemicellulase 1:1)
dMean values within the same raw followed by different superscript letters are significantly (p < 0.05) different
The identity, retention index and percentage composition of the essential oil components are listed in Table 1. The essential oils are resolved into 21 compounds covering more than 98% of the total integrated GC peak area. The main contributors of the volatile profiles are oxygenated monoterpenes (78.77–84.24%) with linalool (73.3–79.01%), camphor (2.91–3.05%) and geranyl acetate (1.82–2.14%) were the major components.
The second main chemical group of the essential oil were the monoterpene hydrocarbons (14.2–20.74%) with γ-terpinene, limonene and p-cymene as the main components. The remaining chemical classes including sesquiterpenes and miscellaneous compounds were detected with low proportions (< 1%).
It is worthy to note that application of enzymes resulted in remarkable increase of oxygenated compounds at the expense of hydrocarbons, leading to increased oxygenated/hydrocarbons ratios (from 3.78 in control to 5.85 in enzyme-treated samples). These results are consistent with those reported for cardamom (Chandran et al. 2012), thyme and rosemary (Hosni et al. 2013), and bay leaves (Boulila et al. 2015). This observation indicates an enhanced rate of oxidation following cell wall disruption on one hand, and to the greater presence of oxidase activity in the commercial enzyme preparations on the other hand (Boulila et al. 2015).
Another point to be considered is that the contents of linalool and α-terpineol were significantly (p < 0.05) higher in enzyme-treated samples suggesting that a part of these components were glycosidically bounded, and their liberation was presumably due to collateral action of β-glucosidase commonly found in the commercial preparations of cellulase and hemicellulase (Rosellό-Soto et al. 2015).
Effects of enzyme treatment on total lipids, fatty acid profiles and lipid quality of intact and hydrodistilled residual seeds
The total lipid contents, fatty acid composition and their quality indexes are depicted in Table 2. The total lipid contents were significantly (p < 0.05) higher in ETH than in NTNH (intact seeds) residues. Its content in the hydrodistilled residues was 5.94-fold higher than that obtained from the NTNH samples. Application of enzymes exacerbated the total lipid contents and the magnitude of increase was 6.22, 8.12 and 6.5 in the hydrodistilled residues from cellulase, hemicellulase and their binary mixture, respectively. From these results, the following points can be retained: (i) the recovery of total lipids was improved by heat treatment (and obviously elevated pressure) during hydrodistillation following the disintegration of cell structures, (ii) enzymatic treatment prior hydrodistillation enhanced the extent of cell disruption and markedly improved the recovery of total lipid from the residues, and (iii) the hydrodistlled residual seeds could be considered as a consolidated source of lipids.
Table 2.
Total lipid content (% w/w), fatty acid composition and lipid quality indexes of intact (NTNH: neither enzymatically treated nor hydroditilled), untreated (NTH: not enzymatically treated but hydrodistilled) and enzyme-treated samples of coriander seeds
| NTNH | NTH | Cellulase | Hemicellulase | Binary mixture | |
|---|---|---|---|---|---|
| Total lipids (% w/w) | 1.09 ± 0.06c | 6.48 ± 0.16b | 6.78 ± 0.08b | 8.86 ± 0.26a | 7.12 ± 0.14a |
| Fatty acids | |||||
| Myristic (C14:0) | 2.66 ± 0.12a | 0.71 ± 0.04c | 0.87 ± 0.02b | 0.41 ± 0.11d | 0.81 ± 0.04b |
| Palmitic C16:0) | 5.48 ± 1.11a | 2.57 ± 0.08bc | 3.14 ± 0. 15b | 2.77 ± 0.12b | 2.02 ± 0.04c |
| Palmitoleic (C16:1n-7) | 2.18 ± 0.06a | 1.32 ± 0.10c | 1.65 ± 0.15b | 1.12 ± 0.06d | 1.72 ± 0.06b |
| Margaric (C17:0) | 0.43 ± 0.04c | 0.72 ± 0.02b | 0.67 ± 0.02b | 0.47 ± 0.08c | 0.81 ± 0.06a |
| Stearic (C18:0) | 2.89 ± 0.17b | 3.05 ± 0.06a | 2.63 ± 0.15c | 2.82 ± 0.11b | 3.13 ± 0.06a |
| Petroselinic (C18:1n-12) | 71.18 ± 1.03c | 75.72 ± 0.44b | 75.67 ± 1.23b | 78.82 ± 1.34a | 76.66 ± 0.92b |
| Oleic (C18:1n-9) | 3.34 ± 0.23a | 2.93 ± 0.08b | 2.27 ± 0.29c | 1.16 ± 0.11e | 2.03 ± 0.21d |
| Linoleic (C18:2n-6) | 9.23 ± 0.32c | 10.54 ± 1.34a | 10.23 ± 0.76a | 9.07 ± 0.56c | 9.82 ± 0.31b |
| α-Linolenic (C18:3n-3) | 0.86 ± 0.06d | – | 1.68 ± 0.15a | 1.43 ± 0.06b | 1.28 ± 0.04c |
| Arachidic (C20:0) | 0.67 ± 0.04d | 1.44 ± 0.03a | 0.91 ± 0.04c | 1.16 ± 0.11b | 0.71 ± 0.06d |
| Gadoleic (C20:1n-9) | 0.71 ± 0.01b | 0.54 ± 0.04c | – | 0.77 ± 0.04a | 0.68 ± 0.02b |
| Erucic (C22:1n-9) | 0.37 ± 0.01b | 0.46 ± 0.02a | 0.28 ± 0.01c | – | 0.33 ± 0.06bc |
| Lipid quality indexes | |||||
| SFA | 12.13 ± 0.21a | 8.49 ± 0.14b | 8.22 ± 0.29b | 7.63 ± 0.15c | 7.48 ± 0.23c |
| UFA | 87.87 ± 1.56b | 91.51 ± 2.51a | 91.78 ± 1.42a | 92.37 ± 2.09a | 92.52 ± 2.39a |
| UFA/SFA | 7.24 ± 0.32c | 10.78 ± 0.29b | 11.17 ± 0.12b | 12.11 ± 0.21a | 12.37 ± 0.22a |
| (n-3)/(n-6) | 0.09 ± 0.01c | 0.00 ± 0.00d | 0.16 ± 0.01a | 0.16 ± 0.01a | 0.13 ± 0.03b |
| DBI | 98.82 ± 0.39c | 102.05 ± 0.18b | 105.37 ± 0.26a | 104.3 ± 0.29a | 104.9 ± 0.31ab |
| IV | 88.27 ± 0.41d | 91.15 ± 0.32c | 94.19 ± 0.37a | 93.39 ± 0.31b | 93.78 ± 0.44b |
| AI | 0.22 ± 0.02a | 0.09 ± 0.01b | 0.10 ± 0.01b | 0.08 ± 0.02b | 0.09 ± 0.01b |
| TI | 0.24 ± 0.03a | 0.14 ± 0.01b | 0.13 ± 0.01b | 0.12 ± 0.01b | 0.12 ± 0.01b |
| Cox | 1.88 ± 0.11c | 1.87 ± 0.09c | 2.20 ± 0.13a | 2.04 ± 0.11b | 2.07 ± 0. 41b |
| OS | 579.13 ± 5.54c | 555.27 ± 14.33d | 708.22 ± 6.13a | 634.18 ± 15.39b | 651.32 ± 14.58b |
| PI | 12.89 ± 0.51c | 12.56 ± 0.23c | 15.59 ± 0.37a | 13.98 ± 0.67b | 14.42 ± 0.79b |
SFA saturated fatty acids, UFA unsaturated fatty acids, AI atherogenic index, TI thrombogenic index, Cox calculated oxidizability, DBI double bound index, OS oxidative susceptibility, IV iodine value, PI peroxidisability index
*Different superscript within row are significantly different at p < 0.05;
The positive effects of temperature treatment on the release of lipids from different matrices have been evidenced in previous studies. In this context, Zheng et al. (2011) reported that elevated temperature (100 °C) with simultaneous application of cellulase greatly enhanced the recovery of total lipids from the microalgae Chlorella vulgaris. These antecedents could, at least, in part explain the high lipid content obtained in this study, and confirm that enzymatic treatment followed by hydrodistillation enhanced cell disruption, washing and diffusion process (key processes for lipid extraction from biological materials), improving thereby the extraction efficiency.
Analytical data of the fatty acid methyl esters (FAMEs) and their nutritional quality indexes are displayed in Table 2. A total of 12 FAMEs were identified with the most abundant being monounsaturated fatty acids (MUFAs) (77.78–81.87%) in all samples. Irrespective to enzyme-treatment, the main fatty acids were petroselinic (71.18–78.82%) followed by linoleic (9.07–10.54%) and palmitic acids (2.02–5.48%). Considering the two former fatty acids, enzymatic treatment did not induce significant (p > 0.05) changes in their amounts, however, a small improvement in the amount of α-linolenic and arachidic acid was observed in ETH with respect to the NTNH samples. The reciprocal trend was observed for palmitic, palmitoleic and oleic acids. The obtained profile (C18:1 (n-12) > C18:2 (n-6) > C16:0) is consistent with that obtained in intact coriander seeds (Marichali et al. 2014).
Interestingly, the hydrodistilled residues of coriander seeds might represent a consolidated source of petroselinic acid, an active ingredient frequently used in cosmetic formulations as a moisturizing, anti-ageing, and reducing agents.
Considering that fatty acid profile and lipid content in oil seed crops were key determinants of their nutritional value, and any compositional changes could influence its nutritional relevance, hence it is of particular significance to evaluate the lipid quality in all sample oils. At this point, the high UFA/SFA ratio and the lower (n-3)/(n-6) ratio (lower than 0.2), atherogenic index (AI), thrombogenic index (TI), peroxidisability index (PI), calculated oxidizability (Cox) and the oxidative susceptibility (OS) are indicative to the high nutritional quality of the oil (Sinanoglou et al. 2011).
In the present study, data comparison showed that the values of UFA/SFA, (n-3)/(n-6) ratios, DBI and IV values were higher in ETH (11.17–12.37) with respect to the NTNH (7.24) and the NTH (10.78) samples (Table 2). This was mostly due to the increase in the polyunsaturated α-linolenic and the monounsaturated petroselinic acid contents, versus a decrease of the SFA palmitic and stearic acids contents. As a consequence, the oil from ETH samples exhibited the highest Cox, SO and PI values, suggesting their high vulnerability to oxidation in comparison with those of NTNH and NTH residual seeds. In contrast, the oil samples from ETH samples showed very low AI (0.08–0.1) and TI (0.12–0.13) values which indicate their healthy properties.
In addition to the well recognized nutritional and therapeutic properties of lipids of C. sativum seeds, other secondary metabolites namely polyphenols, have been reported to exhibit a wide array of food-related biological activities including antioxidant, antimicrobial, anti-diabetic, and anticancer activities, among others (Sahib et al. 2013). The content of these valuable metabolites is, however, prone to the extraction procedure (Putnik et al. 2017; Barba et al. 2016). Given their importance, we also analyzed the total phenol contents (TPC) and total flavonoid contents (TFC) and evaluated the antioxidant activities of different extracts, a task which to our knowledge has not been previously investigated. This analysis could provide insight into the possible valorisation of the residue by-products of coriander seeds as source of natural antioxidants.
Effects of enzyme treatment on TPC, TFC and antioxidant activity of coriander extracts from intact and hydrodistilled residual seeds
Data presented in Table 3, showed that hydrodistilled residual seeds contain appreciable amounts of TPC (15.55-64.99 mg GAE/g extract) and TFC (4.61–13.89 mg QE/g extract), and their contents are somewhat variables depending on solvent used and enzyme-treatment. In general, these metabolites were better extracted with polar solvents with 80% methanol being the most effective in extracting TPC (54.62–93.51 GAE/g extract), while water was more effective in extracting TFC (8.56–17.39 mg QE/g extract) from the hydrodistilled residual materials. The lowest recoveries of TPC and TFC were observed for the less polar solvent ethyl acetate and the apolar solvent petroleum ether. Such solvent-dependant difference in TPC and TFC suggests that phenolics and flavonoids in coriander seeds (both intact or residue by-products) were mainly of high and medium polarity based on “like dissolve like” concept (Thoo et al. 2010).
Table 3.
Total phenol contents (TPC) and total flavonoid contents (TFC) in intact (NTNH), untreated (NTH) and enzyme-treated samples of coriander seeds
| NTNH | NTH | Cellulase | Hemicellulase | Binary mixture | |
|---|---|---|---|---|---|
| TPC (mg GAE/g extract) | |||||
| Water | 56.29 ± 3,20aB | 46.66 ± 1.47bB | 42.77 ± 2.54cC | 34.07 ± 1.78dC | 32.59 ± 3.06dC |
| Methanol | 46.11 ± 3.47dC | 49.62 ± 3.24dB | 54.25 ± 1.28cB | 62.96 ± 6.44aA | 56.66 ± 2.00bB |
| 80% methanol | 89.81 ± 7.05aA | 93.51 ± 7.98aA | 64.99 ± 2.00bA | 54.62 ± 2.85cB | 62.40 ± 6.31bA |
| Ethyl acetate | 39.62 ± 1.7aD | 29.07 ± 3.90bC | 22.59 ± 0.32cD | 22.96 ± 1.95cD | 20.74 ± 0.32dD |
| Petroleum ether | 14.81 ± 0.32cE | 19.25 ± 1.40aD | 15.92 ± 0.32bE | 15.55 ± 1.11bcE | 16.11 ± 1.11bE |
| TFC (mg QE/g extract) | |||||
| Water | 17.39 ± 0.09aB | 8.83 ± 0.10dA | 8.56 ± 0.10dA | 13.89 ± 0.09bA | 11 ± 0.09cA |
| Methanol | 9.89 ± 0.10aC | 4.83 ± 0.09eC | 5.17 ± 0.25cE | 5.72 ± 0.09bC | 4.61 ± 0.16dC |
| 80% methanol | 19.11 ± 0.76aA | 9.05 ± 0.10bA | 7.22 ± 0.25cB | 4.89 ± 0.09eD | 6.67 ± 0.10dB |
| Ethyl acetate | 6.50 ± 0.09bD | 5.61 ± 0.00cB | 5.50 ± 0.10cD | 8.06 ± 0.51aB | 6.67 ± 0.19bB |
| Petroleum ether | 4.89 ± 0.34cE | 5.45 ± 0.33bB | 6.17 ± 0.10aC | 5.67 ± 0.19bC | 4.89 ± 0.19cC |
*Data represent mean values ± SD (n = 3). Means followed by different capital letters within columns indicate differences between solvents. Means followed by different lowercase letters within rows indicate differences between control and enzymatically treated samples
From the hydrodistilled residual materials, an improved release of phenolics and flavonoids was observed in cellulase-treated samples as compared to the NTH. The results could be explained by an increased degradation of cell-wall structures as a result of the hydrolysis of cell wall components (Barba et al. 2016), especially glycosidic/bond linkage between phenolic compounds and cell wall polysaccharides (Albouchi et al. 2013). The release of phenolics and flavonoids was exacerbated by heat treatment (during distillation) which was associated with increased pressure and acidification of the bulk medium, leading ultimately to breakage and destructuration of cell walls (Rosellό-Soto et al. 2015; Ennigrou et al. 2017). In contrast, application of the binary mixture resulted in lower phenolic and flavonoid recoveries from the hydrodistilled residues. This fact could be attributed to the competitive adsorption to the cell wall polysaccharides, leading to steric hindrance of enzymes binding positions to substrate, which negatively influence the breakdown of cell wall components (Boulila et al. 2015). Other possible causes of the lower phenolic and flavonoid recoveries in the presence of the binary mixture were (ii) the presence of lignin (14.3% in coriander seeds) which limits accessibility of cellulase and hemicellulase to their substrate (Boulila et al. 2015), and (ii) cellulase inhibition by xylan and xylooligomers (released from hemicellulose during enzymatic catalysis by hemicellulase) which have an affinity to cellulose and consequently, may physically block the access of enzyme to its substrate (Boulila et al. 2015).
Regardless that change in TPC and TFC of different extracts can influence their antioxidant activity through their effects on the performance of hydrogen atom transfer- or single electron transfer-based antioxidant reaction; it is of interest to evaluate the antioxidant activity of the studied extracts.
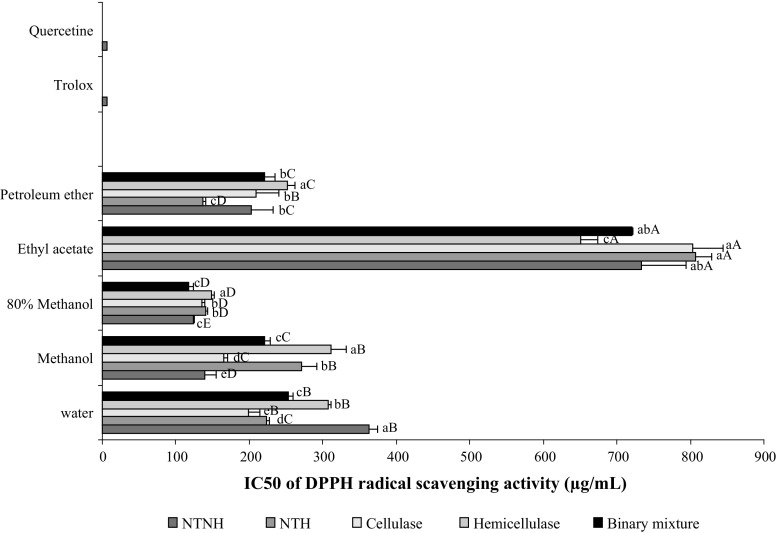
Figure 1 showed that all the assessed extracts are able to reduce the stable, purple-coloured DPPH· into yellow coloured DPPH, although less pronounced than the standard antioxidants trolox and quercetin. Among five solvents, 80% methanol exhibited the highest RSA with EC50 values ranging from 118 to 149 µg/mL. The most active extracts were those issued from hydrodistilled residual seeds treated with the binary enzymes mixture. Additionally, the polar extracts (water and methanol) from hydrodilled residues of cellulase-treated seeds showed better activities with respect to those issued from hemicellulase and the binary mixture treatments. Another point to be considered is that the petroleum ether showed appreciable RSA with EC50 ranging from 136.3 to 220.4 µg/mL. This observation suggests that petroleum ether extract could contain noticeable amount of molecules with RSA such as tocopherols, carotenoids and a smaller amount of apolar flavonoids, and that the RSA of coriander seeds was partially related to their TPC or TFC. Collectively these results clearly showed that coriander by-products from the distillation process could represent an attractive source of natural scavenger agents.
Fig. 1.
DPPH radical scavenging activities in term of IC50 (µg/mL) of the different extracts from intact (NTNH: neither enzymatically treated nor hydrodistilled), untreated (NTH not enzymatically treated but hydrodistilled) and enzyme-treated samples of coriander seeds. Bars not sharing a common letter are significantly different (p < 0.05). Capital letters indicate differences between solvents and lowercase letters indicate differences between treatments
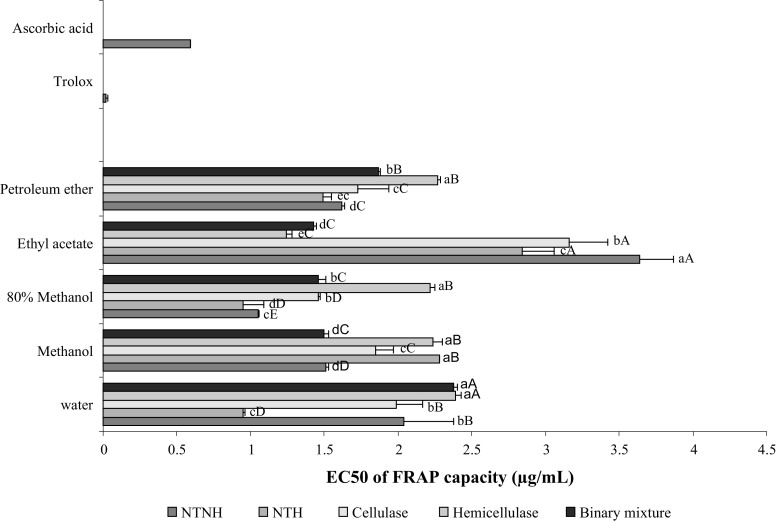
As for the DPPH assay, the 80% methanol extract showed the strongest ferric reducing ability with EC50 values ranging from 1.053 to 2.221 mg/mL (Fig. 2). Among the different matrices analyzed, the NTH samples exhibited the greatest antioxidant activity (EC50 = 0.951 mg/mL) measured in the FRAP assay. In contrast, residual hydrodistilled seeds from the hemicellulase-treated samples showed the lowest reducing ability (2.221–2.385 mg/mL). In general, the ability of all extracts to donate electron appears to be lower to moderate. These results were not in accordance with those observed by Gallo et al. (2010) who found strong reducing power of the 50% ethanol extracts of coriander seeds obtained by microwaves (78.765 mmol trolox/100 g) and ultrasounds (1.198 mmol trolox/100 g). In that work, the results are expressed in different units to our work; the comparison must be qualitative since small variations in experimental conditions can largely affect the results.
Fig. 2.
FRAP capacities in term of EC50 (mg/mL) of different extracts from intact (NTNH neither enzymatically treated nor hydrodistilled), untreated (NTH: not enzymatically treated but hydrodistilled) and enzyme-treated samples of coriander seeds. Bars not sharing a common letter are significantly different (p < 0.05). Capital letters indicate differences between solvents and lowercase letters indicate differences between treatments
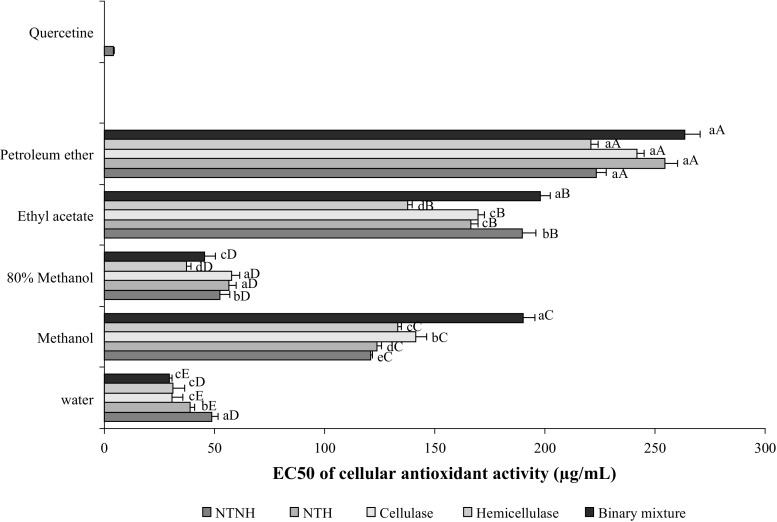
However, although their wide usage in the early stage of the screening of antioxidant activities of extracts/components, the ability of the both in vitro assays (DPPH and FRAP) to predict in vivo activity is questionable because they did not reflect the physiological conditions and none of them take into account the bioavailability, uptake, membrane permeability and metabolism of the antioxidant agent in the cellular environment (Wolfe and Liu 2007). To overcome this gap and to get more realistic picture, cellular models that mimic the physiological conditions has been recently developed and successfully tested for the screening of antioxidant activity. In the present study, the cellular antioxidant activity (CAA) of the different extracts was evaluated in mice splenocytes.
As illustrated in Fig. 3, all extracts were effectively absorbed into splenocytes cells, but at varying degrees, with the polar extracts being the most effectives. Water extracts exhibited the greatest CAA with the lowest EC50 values of 13.43 and 14.42 µg/mL for the hydrodistilled residues issued from seeds treated with the binary enzymes mixture and cellulase, respectively. The ethyl acetate and petroleum ether extracts were the least actives.
Fig. 3.
EC50 (µg/mL) values of the CAA of different extracts from intact (NTNH: neither enzymatically treated nor hydrodistilled), untreated (NTH not enzymatically treated but hydrodistilled) and enzyme-treated samples of coriander seeds. Bars not sharing a common letter are significantly different (p < 0.05). Capital letters indicate differences between solvents and lowercase letters indicate differences between treatments
The ability of polar extracts to prevent the intracellular oxidation of DCFH could be attributed to the presence of some putative antioxidant agents like quercetin, kaempferol, luteolin, isorhamnetin, rutin, gallic; rosmarinic and o-coumaric acids, among others (Wolfe et al. 2008; Zhao et al. 2015) that directly quenched the peroxyl radicals and inhibits the generation of DCF. The latter authors also mentioned that the CAA of phenolic compounds is tightly associated with the number and position of hydroxyl groups, and that flavonoids with the structure of 3′,4′-o-dihydroxyl group in the B-ring, 2,3-double bond combined with a 4-keto group in the C-ring, and a 3-hydroxyl group showed the strongest CAA (Wolfe et al. 2008).
The antioxidant activity of coriander seeds in the CAA assay was first investigated here. However, when compared with other earlier reports, our results fit well with the CAA values described for fruits, vegetables and legumes (Wolfe et al. 2008). In addition, the intriguing CAA activity of different extracts could, at least in part, confirms the traditional uses of coriander in folk medicine and supports its use for the treatment of oxidant-related diseases such as diabetes, obesity, cardiovascular diseases, cancer, and liver injuries, among others.
Conclusion
Results of the current study clearly showed that enzyme-treatment of coriander seeds resulted in an improved essential oil yield and its main component linalool. Additionally, a better recovery of lipids with improved nutritional quality was obtained from the hydrodistilled residues of enzyme-treated seeds. The positive effect of enzyme-treatment on the release of phenolic compounds with enhanced antioxidant activity was evidenced in hydrodistilled residual seeds of enzyme-treated samples. These results suggest that coriander by-products from the distillation process could represent an attractive source of lipids and natural antioxidants. This is of interest from practical stand point, where the integral exploitation (as foods, feeds, cosmetics and pharmaceutics) of the raw material is envisaged.
Acknowledgements
The authors are thankful to the Direction Générale de la Recherche Scientifique (DGRS, Tunisia) and the Centre National de la Recherche Scientifique (CNRS, France) for financial support, Research project “Laboratoire International de Recherche Analytique (LIRA-Tunisia)”.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- Adams R. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Carol Stream: Allured; 2001. [Google Scholar]
- Albouchi F, Hassen I, Casabianca H, Hosni K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. South Afr J Bot. 2013;87:164–174. doi: 10.1016/j.sajb.2013.04.003. [DOI] [Google Scholar]
- Barba FJ, Zhu Z, Koubaa M, Sant’Ana AS, Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trend Food Sci Technol. 2016;49:96–109. doi: 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Boulila A, Hassen I, Haouari L, Mejri F, Ben Amor I, Casabianca C, Hosni K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.) Ind Crop Prod. 2015;74:485–493. doi: 10.1016/j.indcrop.2015.05.050. [DOI] [Google Scholar]
- Burdock GA, Carabin IG. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem Toxicol. 2009;47:22–34. doi: 10.1016/j.fct.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Cecchi G, Biasini S, Castano J. Méthanolyse rapide des huiles en solvent. Note de laboratoire. Rev Fr Corps Gras. 1985;4:163–164. [Google Scholar]
- Cecchi T, Passamonti P, Alfei B, Cecchi P. Monovarietal extra virgin olive oils from the Marche region, Italy: analytical and sensory characterization. Int J Food Prop. 2011;14:483–495. doi: 10.1080/10942910903254811. [DOI] [Google Scholar]
- Chandran J, Amma KPP, Menon N, Purushothaman J, Nisha P. Effect of enzyme assisted extraction on quality and yield of volatile oil from black pepper and cardamom. Food Sci Biotechnol. 2012;21:1611–1617. doi: 10.1007/s10068-012-0214-y. [DOI] [Google Scholar]
- Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Ennigrou A, Casabianca H, Laarif A, Hanchi B, Hosni K. Maturation-related changes in phytochemicals and biological activities of the Brazilian pepper tree (Schinus terebinthifolius Raddi) fruits. South Afr J Bot. 2017;108:407–415. doi: 10.1016/j.sajb.2016.09.005. [DOI] [Google Scholar]
- Fatemi SH, Hammond EG. Analysis of oleate, linoleate and linolenate hydroperoxyde in oxidized ester mixtures. Lipids. 1980;15:379–385. doi: 10.1007/BF02533555. [DOI] [Google Scholar]
- Gallo M, Ferracane R, Graziani G, Ritieni A, Fogliano V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules. 2010;15:6365–6374. doi: 10.3390/molecules15096365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gignon A, Matos A-R, Aferay D, Zuily-Foudil Y, Pham-Thi A-T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (Ecotype Columbia) Ann Bot. 2004;94:345–351. doi: 10.1093/aob/mch150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosni K, Hassen I, Chaâbane H, Jemli M, Dallali S, Sebei H, Casabianca H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): impact on yield chemical composition and antimicrobial activity. Ind Crop Prod. 2013;47:291–299. doi: 10.1016/j.indcrop.2013.03.023. [DOI] [Google Scholar]
- Kang MJ, Shin MS, Park JN, Lee SS. The effects of polyunsaturated:saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br J Nutr. 2005;94:526–532. doi: 10.1079/BJN20051523. [DOI] [PubMed] [Google Scholar]
- Laribi B, Kouki K, M’Hamdi M, Bettaieb T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015;103:9–26. doi: 10.1016/j.fitote.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Marichali A, Dallali S, Ouerghemmi S, Sebei H, Hosni K. Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind Crop Prod. 2014;55:248–257. doi: 10.1016/j.indcrop.2014.02.033. [DOI] [Google Scholar]
- Patel DK, Desai SN, Gandhi HP, Devkar RV, Ramachandran AV. Cardio protective effect of Coriandrum sativum L. on isoproterenol induced myocardial necrosis in rats. Food Chem Toxicol. 2012;50:3120–3125. doi: 10.1016/j.fct.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Puri M, Sharma D, Barrow CJ. Enzyme-assisted extraction of bioactives from plants. Trend Biotechnol. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Putnik P, Kovačević DB, Jambrak AR, Barba FJ, Cravotto G, Binello A, Lorenzo JM, Shpigelman A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes: A review. Molecules. 2017;22(5):680. doi: 10.3390/molecules22050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosellό-Soto E, Parniakov O, Deng Q, Patras A, Koubaa M, Grimi N, Bousetta N, Tiwari BK, Vorobiev E, Lebovka N, Barba FJ. Application of non-conventional extraction methods: toward a sustainable and green production of valuable compounds from mushrooms. Food Eng Rev. 2015;8:214–234. doi: 10.1007/s12393-015-9131-1. [DOI] [Google Scholar]
- Sahib NG, Anwar F, Gilani AH, Hamid AA, Saari N, Alkharfy KM. Coriander (Coriandrum sativum L.): a potential source of high-value components for functional foods and nutraceuticals—a review. Phytother Res. 2013;27:1439–1456. doi: 10.1002/ptr.4897. [DOI] [PubMed] [Google Scholar]
- Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- Sinanoglou VJ, Strati IF, Miniadis-Meimaroglou S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: a comparative study. Food Chem. 2011;124:971–977. doi: 10.1016/j.foodchem.2010.07.037. [DOI] [Google Scholar]
- Singleton VL, Rossi JR. Colorimetric of total phenolics withphosphomolybdic–phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Sowbhagya HB, Chitra VN. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit Rev Food Sci Nutr. 2010;50:146–161. doi: 10.1080/10408390802248775. [DOI] [PubMed] [Google Scholar]
- Sowbhagya HB, Purnima KT, Florence SP, Rao AGA, Srinivas P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009;113:1234–1238. doi: 10.1016/j.foodchem.2008.08.011. [DOI] [Google Scholar]
- Sowbhagya HB, Srinivas P, Krishnamurthy N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010;120:230–234. doi: 10.1016/j.foodchem.2009.10.013. [DOI] [Google Scholar]
- Sowbhagya HB, Srinivas P, Purnima KT, Krishnamurthy N. Enzyme-assisted extraction of volatiles from cumin (Cuminum cyminum L.) seeds. Food Chem. 2011;127:1856–1861. doi: 10.1016/j.foodchem.2011.02.001. [DOI] [Google Scholar]
- Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia) Food Chem. 2010;120:290–295. doi: 10.1016/j.foodchem.2009.09.064. [DOI] [Google Scholar]
- Ulbrich TLV, Southgate DAT. Coronary heart disease seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidant, foods, and dietary supplements. J Agric Food Chem. 2007;55:8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- Wolfe KL, Kang X, He X, Dong M, Zhang Q, Liu RH. Cellular antioxidant activity of common fruits. J Agric Food Chem. 2008;56:8418–8426. doi: 10.1021/jf801381y. [DOI] [PubMed] [Google Scholar]
- Xu C, Yagiz Y, Borejsza-Wysocki W, Lu J, Gu L, Ramírez-Rodrigues MM, Marshall MR. Enzyme release of phenolics from muscadine grape (Vitis rotundifolia Michx.) skins and seeds. Food Chem. 2014;157:20–29. doi: 10.1016/j.foodchem.2014.01.128. [DOI] [PubMed] [Google Scholar]
- Zhao C-F, Lei DJ, Song GH, Zhang H, Xu H, Yu L-J. Characterisation of water-soluble proanthocyanidins of Pyracantha fortuneana fruit and their improvement in cell bioavailable antioxidant activity of quercetin. Food Chem. 2015;169:484–491. doi: 10.1016/j.foodchem.2014.07.091. [DOI] [PubMed] [Google Scholar]
- Zheng H, Yin J, Gao Z, Huang H, Ji X, Dou C. Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl Biochem Biotechnol. 2011;164:1215–1224. doi: 10.1007/s12010-011-9207-1. [DOI] [PubMed] [Google Scholar]