Abstract
(R,S)-Ketamine has rapid and sustained antidepressant effects in depressed patients. Although the metabolism of (R,S)-ketamine to (2 R,6 R)-hydroxynorketamine (HNK), a metabolite of (R)-ketamine, has been reported to be essential for its antidepressant effects, recent evidence suggests otherwise. The present study investigated the role of the metabolism of (R)-ketamine to (2 R,6 R)-HNK in the antidepressant actions of (R)-ketamine. Antidepressant effects were evaluated using the forced swimming test in the lipopolysaccharide (LPS)-induced inflammation model of mice and the tail suspension test in naive mice. To prevent the metabolism of (R)-ketamine to (2 R,6 R)-HNK, mice were pretreated with cytochrome P450 (CYP) inhibitors. The concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK in plasma, brain, and cerebrospinal fluid (CSF) samples were determined using enantioselective liquid chromatography-tandem mass spectrometry. The concentrations of (R)-norketamine and (2 R,6 R)-HNK in plasma, brain, and CSF samples after administration of (R)-norketamine (10 mg/kg) and (2 R,6 R)-HNK (10 mg/kg), respectively, were higher than those generated after administration of (R)-ketamine (10 mg/kg). Nonetheless, while (R)-ketamine attenuated, neither (R)-norketamine nor (2 R,6 R)-HNK significantly altered immobility times of LPS-treated mice. Treatment with CYP inhibitors prior to administration of (R)-ketamine increased the plasma levels of (R)-ketamine, while generation of (2 R,6 R)-HNK was almost completely blocked. (R)-Ketamine exerted the antidepressant effects at a lower dose in the presence of CYP inhibitors than in their absence, which is consistent with exposure levels of (R)-ketamine but not (2 R,6 R)-HNK. These results indicate that metabolism to (2 R,6 R)-HNK is not necessary for the antidepressant effects of (R)-ketamine and that unmetabolized (R)-ketamine itself may be responsible for its antidepressant actions.
Introduction
The rapid and long-lasting antidepressant effects of (R,S)-ketamine in patients with major depressive disorder, including those with treatment-resistant depression, have recently gained much attention [1–4]. Despite these remarkable effects, a number of profound adverse effects such as psychotomimetic/dissociative symptoms, abuse potential, and neurotoxicity preclude the routine use of (R,S)-ketamine in daily clinical practice. (R,S)-Ketamine contains equal parts of (R)-ketamine and (S)-ketamine; based on its affinity for the N-methyl-D-aspartate (NMDA) receptor and its anesthetic potency, (S)-ketamine has been regarded as the active enantiomer. Recently, however, (R)-ketamine has been reported to exhibit more potent and longer-lasting antidepressant effects in rodents than (S)-ketamine [5–9]. Moreover, (R)-ketamine does not induce the unwanted effects observed with (R,S)-ketamine [6], suggesting that (R)-ketamine might be a safer antidepressant than (R,S)-ketamine.
Both (R)-ketamine and (S)-ketamine are extensively and stereoselectively metabolized by multiple cytochrome P450 (CYP) enzymes to several metabolites including the major metabolites (R)-norketamine and (2 R,6 R)-hydroxynorketamine (HNK) [10–12]. Recently, Zanos et al. [7] reported that biotransformation from (R,S)-ketamine to (2 S,6 S;2 R,6 R)-HNK is required for the antidepressant actions of (R,S)-ketamine. Particularly, (2 R,6 R)-HNK, which is biotransformed from (R)-ketamine, was proposed to have a critical role in the actions of (R,S)-ketamine, since (2 R,6 R)-HNK exhibits much more potent antidepressant effects than (2 S,6 S)-HNK in rodent models [7]. In contrast, Hashimoto’s group recently reported that (R)-ketamine, but not (2 R,6 R)-HNK, showed rapid and long-lasting antidepressant effects in three different rodent models [13, 14] and that the injection of (R)-ketamine into the brain regions exerted antidepressant effects in a learned helplessness model [15]. Likewise, (R)-norketamine, another major metabolite of (R)-ketamine, has been reported not to exert antidepressant effects in a learned helplessness model [13]. All these findings suggest that—in so far as where the antidepressant effects of (R)-ketamine are concerned—(R)-ketamine itself, and not its major metabolites (R)-norketamine and (2 R,6 R)-HNK, is responsible for the antidepressant effects in rodents. However, although the antidepressant actions of (R)-ketamine need to be compared with those of its metabolites according to exposure levels rather than dosage, no such studies have been reported to date. Furthermore, although Zanos et al. [7] used deuterated (R,S)-ketamine to prevent its metabolism to (2 S,6 S;2 R,6 R)-HNK, this method only partially prevented its metabolism, and levels of (R,S)-ketamine were essentially unchanged. In the present study, we validated the conditions in which the metabolism of (R)-ketamine to (2 R,6 R)-HNK is completely ablated, and consequently plasma levels of (R)-ketamine are increased by using CYP inhibitors. Therefore, we conclude that the present experimental conditions can be utilized to investigate the role of metabolism to (2 R,6 R)-HNK in the antidepressant effects of (R)-ketamine in relevant animal models.
This study was designed to elucidate the roles of the (R)-ketamine metabolites (R)-norketamine and (2 R,6 R)-HNK in the antidepressant actions of (R)-ketamine. We performed behavioral tests using (R)-ketamine and its two major metabolites, (R)-norketamine and (2 R,6 R)-HNK, in a mouse model of depression. Furthermore, we examined the antidepressant effects of (R)-ketamine in a condition that prevents the metabolism to (2 R,6 R)-HNK by using CYP inhibitors. The concentrations of (R)-ketamine and its metabolites in plasma, brain, and cerebrospinal fluid (CSF) samples were also measured.
Materials and Methods
Animals
Eight-week-old male C57BL/6 mice (Japan SLC, Shizuoka, Japan) and five-week-old male ICR mice (Charles River Laboratories, Kanagawa, Japan) were used for this study. The animals were housed under controlled temperatures and humidity conditions under a 12-h light/dark cycle (lights on at 7:00 a.m.). Food and water were provided ad libitum. The protocol was approved by the Animal Care and Experimentation Committee of each institution to which the authors who performed the study belonged. The protocols for the pharmacological studies, which used a lipopolysaccharide (LPS)-induced inflammation model of depression were approved by the Chiba University Institutional Animal Care and Use Committee. The protocols for the pharmacological studies that used the tail suspension test (TST) and all of the animal experimental procedures involving pharmacokinetic studies were approved by the Institutional Animal Care and Use Committee of Taisho Pharmaceutical Co., Ltd.
Compounds
(R)-Ketamine hydrochloride was prepared by the recrystallization of (R,S)-ketamine (Veterinary Ketalar® 50; Sanyo Yell Pharmaceutical Co., Ltd., Tokyo, Japan or Ketalar®; Daiichi Sankyo Pharmaceutical Ltd., Tokyo, Japan) and D-(−)-tartaric acid, as described previously [8]. (R)-Norketamine and (2 R,6 R)-HNK were prepared according to a previous report [7]. LPS (L-4130, serotype 0111:B4) was purchased from Sigma-Aldrich (St Louis, MO). (R)-Ketamine, (R)-norketamine, and (2 R,6 R)-HNK were dissolved in saline. CYP inhibitors, ticlopidine hydrochloride and 1-aminobenzotriazole (1-ABT), were obtained from Sigma-Aldrich (St. Louis, MO), and dissolved in saline to prepare a cocktail solution containing ticlopidine (4 mg/mL) and 1-ABT (10 mg/mL). As analytical standard substances, (R,S)-ketamine hydrochloride solution (50 mg/mL as a free base; Fujita Pharmaceutical Co., Ltd., Tokyo, Japan), (R,S)-norketamine hydrochloride (Funakoshi Co., Ltd., Tokyo, Japan), and the above-mentioned (2 R,6 R)-HNK were used. The analytical internal standard, 3,4,5,6-tetradeuterophenyl-norketamine hydrochloride (2H4-norketamine), in methanol (100 µg/ml as a free base) was purchased from Sigma-Aldrich (St Louis, MO). All other reagents were purchased commercially.
Behavioral Tests
Forced swimming test (FST) using the LPS-induced inflammatory model
Drug administration
The drug administration schedules are presented in Figs. 1a and 2g. Male C57BL/6 mice were used. Saline or LPS (0.5 mg/kg) was injected intraperitoneally (i.p.) as previously reported [14, 16, 17]. Twenty-three hours later, the mice were treated i.p. with saline, (R)-ketamine (10 mg/kg), (R)-norketamine (10 mg/kg), or (2 R,6 R)-HNK (10 mg/kg). A locomotion test (LMT) and the FST were performed at 1 and 3 h after the administration of each compound, respectively. In the CYP inhibition studies, mice were treated i.p. with saline or a CYP inhibitor cocktail (5 mL/kg) containing ticlopidine (4 mg/mL) and 1-ABT (10 mg/mL) at 22 h after LPS injection; (R)-ketamine (3 or 10 mg/kg) or saline was then administered i.p. at 1 h after the administration of the CYP inhibitors.
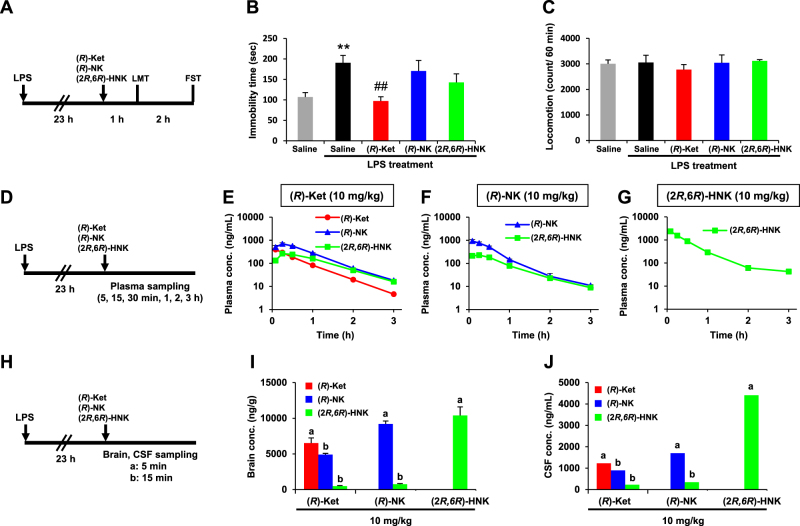
Fig. 1.
Effects of (R)-ketamine ((R)-Ket), (R)-norketamine ((R)-NK), and (2 R,6 R)-hydroxynorketamine ((2 R,6 R)-HNK) in the lipopolysaccharide (LPS)-induced inflammation model and the pharmacokinetic profiles of these compounds. a Experimental schedules for the evaluation of the pharmacological effects of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK. The compounds were administered intraperitoneally (i.p.). b Effects of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK on the LPS-induced increase in the immobility time during the forced swimming test (FST; one-way analysis of variance, F4,35 = 5.553, P = 0.001). c Effects of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK in the locomotion test (LMT; F4,35 = 0.356, P = 0.838). b, c Data are represented as the mean ± SEM (n = 8/group). **P < 0.01 compared with Saline, ##P < 0.01 compared with saline–LPS treatment. d, h Experimental schedules for the pharmacokinetic studies following the intraperitoneal administration of (R)-Ket, (R)-NK, or (2 R,6 R)-HNK. e–g Plasma concentration–time profiles after the administration of (R)-Ket (E), (R)-NK (F), or (2 R,6 R)-HNK (g). Blood was collected sequentially at 5, 15, and 30 min and at 1, 2, and 3 h from the tail vein of each individual mouse. i, j Concentrations of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK in the brain (i) and cerebrospinal fluid (CSF; j) at the time points ((a) 5 min, (b) 15 min) corresponding to their plasma tmax (time at the plasma maximum concentration). e–g, i The plasma and brain levels are represented as the mean ± SEM (n = 3/group). j Each CSF level represents a value derived from a pooled CSF specimen collected from three mice
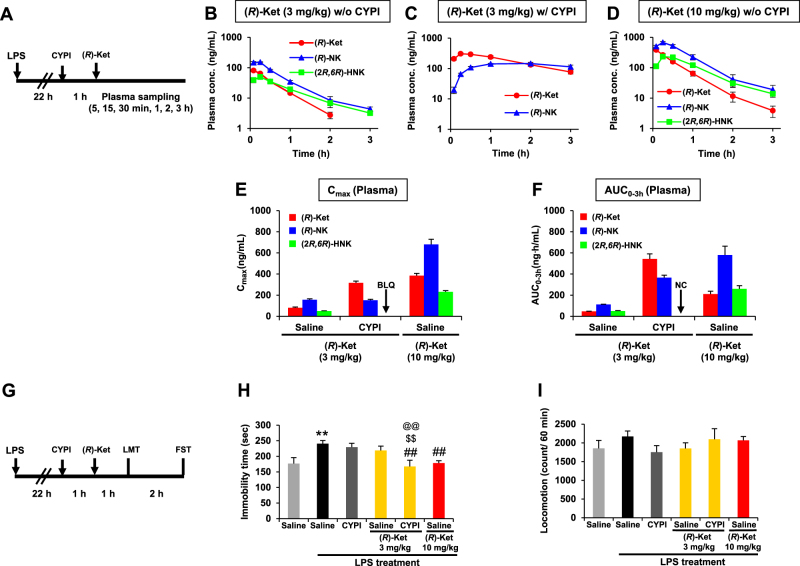
Fig. 2.
Effects of cytochrome P450 inhibitors (CYPI) on the antidepressant effects and pharmacokinetic profiles of (R)-ketamine ((R)-Ket) in the lipopolysaccharide (LPS)-induced inflammation model. a Experimental schedules for the pharmacokinetic studies following the intraperitoneal administration of (R)-Ket. b–d Plasma concentration–time profiles of (R)-Ket, (R)-norketamine ((R)-NK), and (2 R,6 R)-hydroxynorketamine ((2 R,6 R)-HNK) after the administration of (R)-Ket (3 mg/kg) without (b) and with (c) CYPI pretreatment, and (R)-Ket (10 mg/kg) without CYPI pretreatment (d). Following the administration of (R)-Ket, blood was collected sequentially at 5, 15, and 30 min and at 1, 2, and 3 h from the tail vein of each individual mouse. e, f Effect of CYPI pretreatment on the plasma maximum concentration (Cmax; e) and the area under the concentration–time curve from time 0 to 3 h (AUC0-3h; F) of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK. Pretreatment with CYPI completely blocked (2 R,6 R)-HNK formation to below the lower limit of quantification (BLQ; < 3 ng/mL); therefore, the AUC0–3h was not calculable (NC) because of the BLQ of the analyte. b–f Data are represented as the mean ± SEM (n = 3/group). g Experimental schedules for the evaluation of the pharmacological effects of (R)-Ket. h Effects of (R)-Ket on the LPS-increased immobility in the forced swimming test (FST) in the presence or absence of CYPI (one-way analysis of variance, F5,42 = 4.707, P = 0.002). i Effects of (R)-Ket on the locomotion test in the presence or absence of CYPI (LMT; F5,42 = 0.804, P = 0.553). h, i Data are represented as the mean ± SEM (n = 8/group). **P < 0.01 compared with saline, ##P < 0.01 compared with saline–LPS treatment, @@P < 0.01 compared with CYPI/(R)-ketamine (3 mg/kg)-LPS treatment, $$P < 0.01 compared with saline/(R)-ketamine (3 mg/kg)-LPS treatment
FST
The FST was conducted using an automated forced-swim apparatus (SCANET MV-40; MELQUEST Co., Ltd., Toyama, Japan). Mice were placed individually in a cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water maintained at a temperature of 23 ± 1 °C. The immobility time was calculated using the activity time as (total) − (active) time by the apparatus analysis software. The immobility time of each mouse was recorded for a period of 6 min. It should be noted that we did not conduct pre-swim session, which has been widely used for the FST [18, 19], because depressive-like behavior (increased immobility) is induced by LPS treatment.
LMT
The LMT was performed using an animal movement analysis system (SCANET MV-40; MELQUEST Co., Ltd.). Mice were placed in experimental cages (length × width × height: 560 × 560 × 330 mm). The cumulative exercise was then recorded for 60 min. The cages were cleaned between the testing sessions.
Pharmacokinetic studies
The administration schedules are shown in Figs. 1d,h, 2a. (R)-Ketamine (10 mg/kg), (R)-norketamine (10 mg/kg), or (2 R,6 R)-HNK (10 mg/kg) was administered i.p. at 23 h after the LPS injection. To determine the plasma concentration–time profiles, blood samples (40 μL) were collected sequentially at 5, 15, and 30 min and at 1, 2, and 3 h from the tail vein of each individual mouse into a tube containing ethylenediamine-N,N,N’,N’-tetraacetic acid potassium salt dehydrate (EDTA-2K). Plasma was obtained by centrifugation of the blood samples (preset value, 5600 × g, 6 min). To determine the concentrations of (R)-ketamine and its metabolites in the brain and CSF, the animals were killed immediately after the collection of jugular vein blood into a tube containing EDTA-2K at 5 and 15 min after the administration of each compound; the CSF and brain were then obtained immediately. In addition, the effects of the CYP inhibitors on the pharmacokinetic profiles of (R)-ketamine were investigated in LPS-treated mice. After (R)-ketamine was administered i.p., blood samples were collected sequentially at 5, 15, and 30 min and at 1, 2, and 3 h from the tail vein of each individual mouse. To investigate the effects of CYP inhibition on the brain and CSF penetration of each compound, jugular vein blood, CSF, and the brain were also collected at 30 min post dose as mentioned above. The concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK were determined as described in the “Bioanalysis” section (Supplementary Materials and Methods).
TST using naive mice
Drug administration
The drug administration schedules are shown in Fig. 3g. Male ICR mice were used. Mice were treated i.p. with saline or a CYP inhibitor cocktail (5 mL/kg) containing ticlopidine (4 mg/mL) and 1-ABT (10 mg/mL). One hour later, (R)-ketamine (10 or 30 mg/kg) or saline was administered i.p. The TST was performed at 24 h after the (R)-ketamine administration. Notably, higher doses of (R)-ketamine than those used in the LPS-induced inflammation model were used in native mice, because we previously obtained the results that higher doses of (R)-ketamine were needed in naive mice [9] than in depressed mice [14] to exert its antidepressant effects.
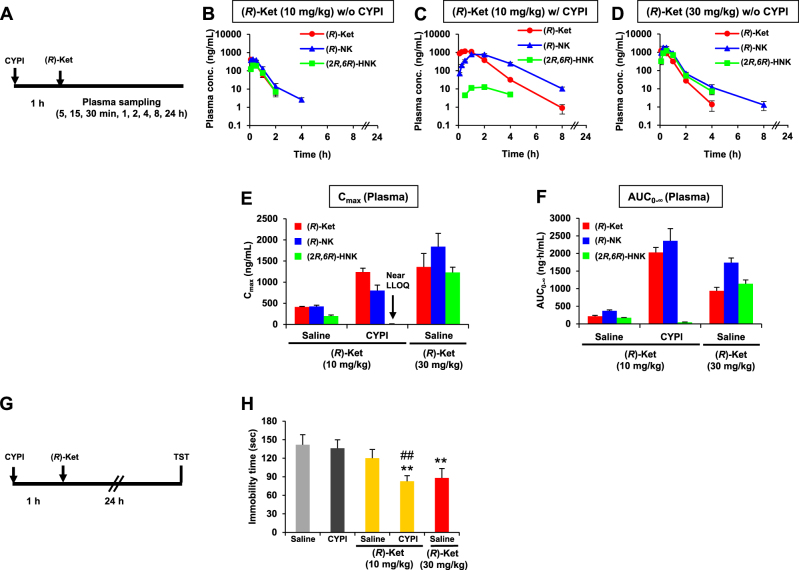
Fig. 3.
Effects of cytochrome P450 inhibitors (CYPI) on the antidepressant effects and pharmacokinetic profiles of (R)-ketamine ((R)-Ket) in naive ICR mice. a Experimental schedules for the pharmacokinetic studies following the intraperitoneal administration of (R)-Ket. b–d Plasma concentration–time profiles of (R)-Ket, (R)-norketamine ((R)-NK), and (2 R,6 R)-hydroxynorketamine ((2 R,6 R)-HNK) after the administration of (R)-Ket (10 mg/kg, i.p.) without (b) and with (c) CYPI pretreatment and (R)-Ket (30 mg/kg, i.p.) without CYPI pretreatment (d). Following the (R)-Ket administration, blood was collected sequentially at 5, 15, and 30 min and at 1, 2, 4, 8, and 24 h from the tail vein of each individual mouse. e, f Effect of the CYPI pretreatment on the plasma maximum concentration (Cmax; e) and the area under the concentration–time curve from time 0 extrapolated to infinite time (AUC0-∞; f) of (R)-Ket, (R)-NK, and (2 R,6 R)-HNK. Pretreatment with CYPI markedly decreased the (2 R,6 R)-HNK levels to near the lower limit of quantification (LLOQ; 3 ng/mL). b–f Data are represented as the mean ± SEM (n = 3/group). g Experimental schedule for the evaluation of the pharmacological effects of (R)-Ket. h Effects of (R)-Ket on the immobility in the tail suspension test (TST) in the presence or absence of CYPI (one-way analysis of variance, F4,75 = 3.89, P = 0.006). Data are represented as the mean ± SEM (n = 16/group). **P < 0.01 compared with CYPI, ##P < 0.01 compared with saline/(R)-ketamine (10 mg/kg) treatment
TST
The TST was performed according to a previously described method [9]. Mice were suspended by the tail from a metal rod using adhesive tape. The rod was fixed 47 cm above the surface of a table in a sound-isolated room. The test session was recorded for 6 min, and the immobility time was determined by an observer who was blinded to the treatment conditions. The mice were considered immobile only when they hung passively and were completely motionless.
Pharmacokinetic studies
The schedules are shown in Fig. 3a. Blood samples (35 μL) were collected sequentially from the tail vein of each individual mouse at 5, 15, and 30 min and at 1, 2, 4, 8, and 24 h after the administration of (R)-ketamine, and plasma was collected as described above. The concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK were determined as described in the “Bioanalysis” section (Supplementary Materials and Methods).
Data Analysis
All data are shown as the mean ± SEM. The statistical analysis was performed using PASW Statistics 20 (formerly SPSS Statistics; SPSS; IBM Corp., Tokyo, Japan) for the LPS-induced inflammation model and the SAS System, version 9.2 (SAS Institute Japan Ltd., Tokyo, Japan), for the TST using naive mice. Comparisons between groups were performed using a one-way ANOVA, followed by post hoc Fisher’s least significant difference tests. P values of less than 0.05 were considered statistically significant. The pharmacokinetic parameters were calculated using a non-compartmental model with WinNonlin 7.0 software (Certara, Princeton, NJ).
Results
Effects of (R)-ketamine, (R)-norketamine, and (2R,6R)-HNK on depressive-like behavior in the LPS-induced inflammation model
The effects of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK at 3 h after administration were investigated in the LPS-induced inflammation model. The LPS treatment induced an increase in the immobility time for mice in the FST, indicating depressive-like behavior (Fig. 1b). (R)-Ketamine (10 mg/kg) significantly attenuated the LPS-induced depressive-like behavior in the FST, while both (R)-norketamine (10 mg/kg) and (2 R,6 R)-HNK (10 mg/kg) did not significantly attenuate the increased immobility time (Fig. 1b). There were no differences in locomotor activity among the five groups (Fig. 1c).
Pharmacokinetic studies were conducted in separate cohorts of LPS-treated mice. (R)-Ketamine was rapidly absorbed after administration (10 mg/kg), and the plasma concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK reached maximum concentration (Cmax) levels at 5, 15, and 15 min, respectively, after the administration of (R)-ketamine (Fig. 1e; Table S1). The plasma concentrations of (R)-norketamine and (2 R,6 R)-HNK declined rapidly at a rate similar to that of (R)-ketamine, leaving concentrations of less than 6% of their Cmax levels at 3 h post dose (Fig. 1e). Concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK in the brain at each plasma tmax (time at the plasma Cmax) after the administration of (R)-ketamine were 6520, 4910, and 483 ng/g, respectively (Fig. 1i; Table S2), and those in the CSF were 1230, 895, and 229 ng/mL, respectively (Fig. 1j; Table S2). Like (R)-ketamine, both (R)-norketamine and (2 R,6 R)-HNK were rapidly absorbed after the administration of each compound (10 mg/kg; Fig. 1f,g). The plasma (Fig. 1f), brain (Fig. 1i), and CSF (Fig. 1j) levels (at the plasma tmax) of (R)-norketamine and (2 R,6 R)-HNK after the administration of (R)-norketamine were higher than those obtained after the administration of (R)-ketamine (Table S2). Likewise, the plasma (Fig. 1g), brain (Fig. 1i) and CSF (Fig. 1j) levels (at the plasma tmax) of (2 R,6 R)-HNK after the administration of (2 R,6 R)-HNK were higher than those obtained after the administration of (R)-ketamine (Table S2). In addition, the plasma area under the concentration–time curve from 0 to 3 h (AUC0–3h) of (2 R,6 R)-HNK after the administration of (2 R,6 R)-HNK was higher than that after the administration of (R)-ketamine (Table S1). Of note, we observed differences in the concentrations of (R)-ketamine and its major metabolites at early time points between jugular vein blood (Table S2) and tail vein blood (Table 1 and S1), as described in the Supplementary Results section.
Table 1.
Effect of cytochrome P450 inhibitors (CYPI) on pharmacokinetic parameters of (R)-ketamine ((R)-Ket), (R)-norketamine ((R)-NK), and (2 R,6 R)-hydroxynorketamine ((2 R,6 R)-HNK) after the intraperitoneal administration of (R)-Ket in lipopolysaccharide-treated mice
| Dose (mg/kg) | CYPI | Analyte | Cmaxa(ng/mL) | AUC0–3hb(ng·h/mL) |
|---|---|---|---|---|
| 3 | − | (R)-Ket | 81.1 ± 6.99 | 47.1 ± 1.44 |
| (R)-NK | 157 ± 8.37 | 112 ± 2.33 | ||
| (2 R,6 R)-HNK | 49.7 ± 2.61 | 49.4 ± 4.66 | ||
| 3 | + | (R)-Ket | 317 ± 16.0 | 543 ± 48.3 |
| (R)-NK | 152 ± 9.24 | 366 ± 23.7 | ||
| (2 R,6 R)-HNK | BLQc | NCd | ||
| 10 | − | (R)-Ket | 385 ± 21.5 | 211 ± 26.2 |
| (R)-NK | 681 ± 47.9 | 580 ± 83.7 | ||
| (2 R,6 R)-HNK | 232 ± 11.5 | 260 ± 29.2 |
Blood was collected sequentially at 5, 15, and 30 min and at 1, 2, and 3 h from the tail vein of each individual mouse. Data are represented as the mean ± SEM (n = 3)
a Maximum concentration
b Area under the concentration–time curve from time 0 to 3 h
c Below the lower limit of quantification (<3 ng/mL)
d Not calculable due to BLQ of the analyte
Effect of (R)-ketamine on depressive-like behavior in the LPS-induced inflammation model in the presence or absence of CYP inhibitors
Pharmacokinetic studies were conducted in LPS-treated mice. The plasma concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK increased in a dose-dependent manner, and reached maximum concentration (Cmax) levels at 5, 15, and 15 min, respectively, after the administration of (R)-ketamine in the absence of CYP inhibitors (Fig. 2b,d). Pretreatment with the CYP inhibitors increased both the plasma Cmax and the AUC0–3h of (R)-ketamine after the administration of (R)-ketamine (3 mg/kg), and the plasma Cmax and AUC0–3h values in the presence of CYP inhibitors were equivalent and 2.6-fold, respectively, compared with those obtained after the administration of (R)-ketamine (10 mg/kg) alone (Fig. 2e,f; Table 1). Importantly, the formation of (2 R,6 R)-HNK was completely blocked to below the lower limit of quantification (LLOQ; < 3 ng/mL) level by pretreatment with the CYP inhibitors (Fig. 2c,e and f; Table 1). In contrast, the CYP inhibitors did not affect the plasma Cmax of (R)-norketamine (Fig. 2e; Table 1), while the same treatment increased the plasma AUC0–3h of (R)-norketamine (Fig. 2f; Table 1). Pretreatment with the CYP inhibitors did not change the brain (and CSF) to plasma concentration ratios of (R)-ketamine or (R)-norketamine (Table S3), indicating that the CYP inhibitors did not affect the brain and CSF penetration of (R)-ketamine and its metabolite.
The effects of (R)-ketamine at 3 h after administration were investigated in the LPS-induced inflammation model. In the absence of CYP inhibitors, (R)-ketamine (3 mg/kg) did not attenuate the increased immobility time of LPS-treated mice in the FST, while a higher dose of (R)-ketamine (10 mg/kg) significantly attenuated the increased immobility time (Fig. 2h). In contrast, in the presence of CYP inhibitors, 3 mg/kg of (R)-ketamine significantly attenuated the increased immobility time of LPS-treated mice (Fig. 2h), which is consistent with pharmacokinetic results that pretreatment with the CYP inhibitors increased plasma levels of (R)-ketamine. The CYP inhibitors themselves did not alter the increased immobility time of LPS-treated mice (Fig. 2h). There were no differences in locomotor activity among the six groups (Fig. 2i).
Effect of (R)-ketamine in naive mice in the presence or absence of CYP inhibitors
Pharmacokinetic studies were conducted in naive mice. (R)-Ketamine was rapidly absorbed and metabolized to form (R)-norketamine and (2 R,6 R)-HNK. The plasma concentrations of (R)-ketamine, (R)-norketamine, and (2 R,6 R)-HNK increased in a dose-dependent manner, and reached Cmax levels at 5, 15, and 15 min, respectively, after the administration of (R)-ketamine in the absence of CYP inhibitors (Fig. 3b,d). The plasma levels of (R)-ketamine and its metabolites decreased rapidly and in parallel to below the LLOQ levels at 24 h post dose (Fig. 3b-d). Pretreatment with the CYP inhibitors increased the plasma levels of both (R)-ketamine and (R)-norketamine, while the same treatment markedly decreased the plasma levels of (2 R,6 R)-HNK, which was barely detectable near the LLOQ levels (Fig. 3c,e and f; Table 2). The CYP inhibitors increased both the plasma Cmax and the AUC from time 0 extrapolated to infinite time (AUC0–∞) of (R)-ketamine after the administration of (R)-ketamine (10 mg/kg), and the plasma Cmax and AUC0–∞ values in the presence of CYP inhibitors were equivalent and 2.2-fold, respectively, compared with those obtained after the administration of (R)-ketamine (30 mg/kg) alone (Fig. 3e,f; Table 2). The plasma Cmax and AUC0–∞ values of (R)-norketamine after (R)-ketamine administration (10 mg/kg) in the presence of the CYP inhibitors were 0.4- and 1.4-fold, respectively, compared with those after the administration of (R)-ketamine (30 mg/kg) alone (Fig. 3e,f; Table 2).
Table 2.
Effect of cytochrome P450 inhibitors (CYPI) on pharmacokinetic parameters of (R)-ketamine ((R)-Ket), (R)-norketamine ((R)-NK), and (2 R,6 R)-hydroxynorketamine ((2 R,6 R)-HNK) after the intraperitoneal administration of (R)-Ket in naive mice
| Dose (mg/kg) | CYPI | Analyte | Cmaxa(ng/mL) | AUC0–∞b(ng·h/mL) |
|---|---|---|---|---|
| 10 | − | (R)-Ket | 414 ± 12.8 | 216 ± 22.5 |
| (R)-NK | 427 ± 26.6 | 369 ± 27.2 | ||
| (2 R,6 R)-HNK | 199 ± 24.4 | 174 ± 4.66 | ||
| 10 | + | (R)-Ket | 1240 ± 90.1 | 2030 ± 139 |
| (R)-NK | 802 ± 130 | 2360 ± 46 | ||
| (2 R,6 R)-HNK | 12.6 ± 2.57 | 43.5 ± 8.49 | ||
| 30 | − | (R)-Ket | 1360 ± 318 | 938 ± 99.3 |
| (R)-NK | 1840 ± 314 | 1740 ± 132 | ||
| (2 R,6 R)-HNK | 1230 ± 124 | 1140 ± 107 |
Blood was collected sequentially at 5, 15, and 30 min and at 1, 2, 4, 8, and 24 h from the tail vein of each individual mouse. Data are represented as the mean ± SEM (n = 3)
a Maximum concentration
b Area under the concentration–time curve from time 0 extrapolated to infinite time
The effects of (R)-ketamine at 24 h after administration were investigated in naive mice. To investigate the sustained antidepressant effect of (R)-ketamine, we employed the TST of naive mice in which we previously demonstrated the sustained antidepressant effect of (R)-ketamine [9]. In the absence of the CYP inhibitors, (R)-ketamine (10 mg/kg) did not significantly alter the immobility time of mice in the TST, while a higher dose of (R)-ketamine (30 mg/kg) significantly decreased the immobility time (Fig. 3h). In contrast, in the presence of the CYP inhibitors, 10 mg/kg of (R)-ketamine significantly decreased immobility time of naive mice (Fig. 3h), which is consistent with pharmacokinetic results that pretreatment with the CYP inhibitors increased plasma levels of (R)-ketamine. The CYP inhibitors themselves did not alter the immobility time (Fig. 3h).
DISCUSSION
Recently, (2 R,6 R)-HNK, which is derived from the metabolism of (R)-ketamine, has been proposed to mediate the antidepressant effects of (R,S)-ketamine [7]. In the present study, we investigated whether the generation of (2 R,6 R)-HNK is necessary for (R)-ketamine to exert its antidepressant effects by examining the pharmacokinetic/pharmacodynamic relationship and by blocking metabolism to (2 R,6 R)-HNK using CYP inhibitors. In addition, we investigated the role of (R)-norketamine, another major metabolite of (R)-ketamine, in the antidepressant actions.
In the LPS-induced inflammation model of mice, (R)-ketamine attenuated the increase in the immobility time of LPS-treated mice in the FST without affecting locomotor activity, indicating antidepressant effects. In contrast, both (R)-norketamine and (2 R,6 R)-HNK did not show significant antidepressant effects at the same dose. This result is consistent with previous findings that (2 R,6 R)-HNK (10 mg/kg) did not exhibit significant antidepressant effects in the LPS-induced inflammation model and a chronic social defeat stress model, while both (R)-ketamine (10 mg/kg) and (S)-ketamine (10 mg/kg) showed antidepressant activity [14]. Moreover, very recently, Shirayama and Hashimoto [13] have reported that both (R)-norketamine (20 mg/kg) and (2 R,6 R)-HNK (20 and 40 mg/kg) had no effect in a rat learned helplessness model, while (R)-ketamine (20 mg/kg) elicited antidepressant effects in the same model. In the present study, the pharmacokinetic data demonstrated that the plasma, brain, and CSF concentrations of (R)-norketamine and (2 R,6 R)-HNK following the administration of each compound (10 mg/kg) were higher than those generated after the administration of (R)-ketamine (10 mg/kg). Therefore, a sufficient exposure to (R)-norketamine and (2 R,6 R)-HNK was achieved at the dose used. On the basis of the pharmacokinetic/pharmacodynamic relationship, both (R)-norketamine and (2 R,6 R)-HNK might not contribute to the antidepressant actions of (R)-ketamine in the LPS-induced inflammation model, at least at 3 h after drug administration. Of note, Suzuki et al. [20] recently claimed that (2 R,6 R)-HNK may mediate the sustained antidepressant effects of (R,S)-ketamine via NMDA receptor inhibition. However, the present pharmacokinetic data indicated that the plasma concentrations of (2 R,6 R)-HNK declined at a rate similar to those of (R)-ketamine following (R)-ketamine administration. Therefore, from a pharmacokinetic point of view, it is unlikely that (2 R,6 R)-HNK mediates the sustained antidepressant effects of (R)-ketamine.
Next, we investigated the involvement of (2 R,6 R)-HNK in the actions of (R)-ketamine by inhibiting the metabolism of (R)-ketamine to (2 R,6 R)-HNK. In humans, (R,S)-ketamine has been proposed to be metabolized to (2 S,6 S;2 R,6 R)-HNK by N-demethylation with multiple CYP isoforms including CYP2B6, followed by hydroxylation with CYP2A6, CYP3A5, and CYP2B6 [12]. On the basis of this information, we successfully validated this condition, demonstrating that a combination of 1-ABT, a multiple CYP inhibitor [21–23], and ticlopidine, a CYP2B6 inhibitor [24, 25], prevented the generation of (2 R,6 R)-HNK from (R)-ketamine. Indeed, plasma levels of (2 R,6 R)-HNK after the administration of (R)-ketamine were not detected (in the LPS-treated mice) or were barely detected (in naive mice) by pretreatment with the CYP inhibitors. In contrast, in the presence of the CYP inhibitors, the plasma levels of (R)-ketamine after the administration of ineffective doses of (R)-ketamine (3 mg/kg for the LPS-treated mice and 10 mg/kg for the naive mice) were increased to approximately the same levels obtained after the administration of effective doses of (R)-ketamine (10 mg/kg for the LPS-treated mice and 30 mg/kg for the naive mice). Therefore, if (R)-ketamine exerts the antidepressant effects, at the ineffective doses, in the presence of the CYP inhibitors, it indicates that (R)-ketamine itself, and not (2 R,6 R)-HNK, is responsible for its antidepressant effects. In the present study, we obtained the results that in the presence of the CYP inhibitors, (R)-ketamine, at an ineffective dose (3 mg/kg), exerted the antidepressant effects in the FST of LPS-treated mice at 3 h after the administration. Likewise, (R)-ketamine, at an ineffective dose (10 mg/kg), exerted the antidepressant effects in the TST of naive mice at 24 h after the administration in the presence of the CYP inhibitors. Therefore, the antidepressant effects of (R)-ketamine paralleled the plasma levels of (R)-ketamine, but not (2 R,6 R)-HNK. These results provide direct evidence that the generation of (2 R,6 R)-HNK is not essential for (R)-ketamine’s acute and sustained antidepressant effects, at least in two different mouse models.
Although (R)-norketamine may not be involved in the antidepressant actions of (R)-ketamine, based on the results of the pharmacokinetic/pharmacodynamic relationship, we did not provide direct evidence for the role of (R)-norketamine in the antidepressant actions of (R)-ketamine in the present study. Therefore, the involvement of (R)-norketamine in the antidepressant effects of (R)-ketamine needs to be further investigated under conditions in which the generation of (R)-norketamine is prevented.
The present results contradict previously reported results [7]. Zanos et al. [7] claimed that the formation of (2 S,6 S;2 R,6 R)-HNK is essential and sufficient to exert the antidepressant effects of (R,S)-ketamine and that (2 R,6 R)-HNK, in particular, has a critical role. In their study, they demonstrated that deuterated (R,S)-ketamine, which reduced the formation of (2 S,6 S;2 R,6 R)-HNK, prevented the sustained (24 h) antidepressant activity of (R,S)-ketamine. The discrepancy between these previously reported results and the present results requires some discussion. In their study, although the exposure levels of (2 S,6 S;2 R,6 R)-HNK in the brain were reduced when the deuterated (R,S)-ketamine was administered, the levels of (R,S)-ketamine and (R,S)-norketamine were not changed. In addition, metabolism to (2 S,6 S;2 R,6 R)-HNK was only partially, and not completely, prevented. In contrast, the presently reported experimental conditions almost completely prevented the formation of (2 R,6 R)-HNK, resulting in reasonable increases in the plasma levels of both (R)-ketamine and (R)-norketamine. Thus, the present condition can investigate the roles of (2 R,6 R)-HNK in the antidepressant effects of (R)-ketamine more clearly and adequately. Although they demonstrated that (2 R,6 R)-HNK induced the sustained antidepressant effects in several animal models, these results do not necessarily mean that the formation of (2 R,6 R)-HNK is essential for the antidepressant effects of (R)-ketamine. The antidepressant effects of (2 R,6 R)-HNK may occur at exposure levels higher than those obtained after the effective doses of (R)-ketamine administration. Indeed, we observed in the present study that (2 R,6 R)-HNK tended to reduce the increased immobility time in LPS-treated mice. (2 R,6 R)-HNK and (R,S)-ketamine, at the same dose, have recently been reported to show similar effects on serotonin release in the medial prefrontal cortex [26] as well as on AMPA receptor functions in the nucleus accumbens and dopamine neurons in the ventral tegmental area [27]. These findings are underpinned by the recent report that both (R,S)-ketamine and (2 R,6 R)-HNK inhibit NMDA receptor at rest and eukaryotic elongation factor 2 phosphorylation, while higher concentration is required for (2 R,6 R)-HNK than (R,S)-ketamine [20]. However, Pham et al. [26] also reported that local injection of (R,S)-ketamine (2 nmol) and (2 R,6 R)-HNK (2 nmol) into the medial prefrontal cortex exerted the antidepressant effects at 24 h after injection in the FST, indicating that both (R,S)-ketamine and (2 R,6 R)-HNK show the sustained antidepressant effects on their own, presumably through different mechanisms. Moreover, this result also indicates that biotransformation to (2 R,6 R)-HNK is not necessary for (R,S)-ketamine’s antidepressant effect.
It should be noted that there are some limitations in the present study. To fully elucidate the roles of (2 R,6 R)-HNK in the antidepressant effects of (R)-ketamine and to clarify discrepancy between results of other laboratories, studies in other behavioral models including chronic stress models are warranted. Moreover, although we investigated the antidepressant effects of (R)-ketamine at 24 h after the administration, the roles of metabolites of (R)-ketamine may differ at discrete time points. For example, the role of (2 R,6 R)-HNK in the long-lasting antidepressant effects of (R)-ketamine (e.g., at 7 days) needs to be clarified.
In conclusion, we demonstrated that (2 R,6 R)-HNK, and possibly (R)-norketamine, are not essential for the antidepressant actions of (R)-ketamine. Because (2 R,6 R)-HNK is formed from only (R)-ketamine, the present results also suggest that metabolism to (2 R,6 R)-HNK is not involved in the antidepressant effects of (R,S)-ketamine. The present study should provide an important insight into the active substance responsible for the antidepressant effects of (R)-ketamine, thereby facilitating further studies on this important issue in ketamine research.
Electronic supplementary material
Acknowledgements
We thank Drs. Takashi Hashihayata, Dai Nozawa, and Masahiro Abe of Taisho Pharmaceutical Co., Ltd. for providing authentic standards for (R)-ketamine and (2 R,6 R)-HNK. We also thank Dr. Rodney W. Stevens of Taisho Pharmaceutical Co., Ltd. for proofreading the manuscript.
Conflict of interest
C.Y. was supported by a Research Fellowship of the Japan Society for the Promotion of Science. K.H. is an inventor named on a filed patent application for “The use of R-ketamine in the treatment of psychiatric diseases” by Chiba University. K.H. has received research support from Dainippon Sumitomo, Mochida, Otsuka, and Taisho. J.Y., H.T., H.K., A.M.Y., and S.C. are employees of Taisho Pharmaceutical Co., Ltd. Y.Q. declares no biomedical financial interests or potential conflicts of interest.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0084-y).
References
- 1.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 2.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto K. Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin Ther Targets. 2016;20:1389–92. doi: 10.1080/14728222.2016.1238899. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28. doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JC, Li SX, Hashimoto K. R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361:9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 10.Adams JD, Jr, Baillie TA, Trevor AJ, Castagnoli N., Jr Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spectrom. 1981;8:527–38. doi: 10.1002/bms.1200081103. [DOI] [PubMed] [Google Scholar]
- 11.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–8. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–87. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. Int J Neuropsychopharmacol. 2018;21:84–8. doi: 10.1093/ijnp/pyx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. R)-Ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry. 2017;82:e83–84. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur Arch Psychiatry Clin Neurosci. 2017;267:177–82. doi: 10.1007/s00406-016-0718-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014b;18 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed]
- 17.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. 2017;39:134–44. doi: 10.1016/j.jnutbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Villard V, Meunier J, Chevallier N, Maurice T. Pharmacological interaction with thesigma1 (σ1)-receptor in the acute behavioral effects of antidepressants. J Pharmacol Sci. 2011;115:279–92. doi: 10.1254/jphs.10191FP. [DOI] [PubMed] [Google Scholar]
- 19.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe A, Mayumi K, Nishimura K, Osaki H. In vivo use of the CYP inhibitor 1-aminobenzotriazole to increase long-term exposure in mice. Biopharm Drug Dispos. 2016;37:373–8. doi: 10.1002/bdd.2020. [DOI] [PubMed] [Google Scholar]
- 22.Balani SK, Li P, Nguyen J, Cardoza K, Zeng H, Mu DX, et al. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in guinea pigs and mice using serial sampling. Drug Metab Dispos. 2004;32:1092–5. doi: 10.1124/dmd.104.000349. [DOI] [PubMed] [Google Scholar]
- 23.Balani SK, Zhu T, Yang TJ, Liu Z, He B, Lee FW. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in rats, dogs, and monkeys. Drug Metab Dispos. 2002;30:1059–62. doi: 10.1124/dmd.30.10.1059. [DOI] [PubMed] [Google Scholar]
- 24.Molnari JC, Hassan HE, Moeller BM, Myers AL. Drug interaction study between bupropion and ticlopidine in male CF-1 mice. Biol Pharm Bull. 2011;34:447–51. doi: 10.1248/bpb.34.447. [DOI] [PubMed] [Google Scholar]
- 25.Peltoniemi MA, Saari TI, Hagelberg NM, Reponen P, Turpeinen M, Laine K, et al. Exposure to oral S-ketamine is unaffected by itraconazole but greatly increased by ticlopidine. Clin Pharmacol Ther. 2011;90:296–302. doi: 10.1038/clpt.2011.140. [DOI] [PubMed] [Google Scholar]
- 26.Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC et al. Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry. 2018. 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed]
- 27.Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2018. 10.1038/mp.2017.239. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.