Abstract
Key points
Experimental pain or its anticipation influence motor preparation processes as well as upcoming movement execution, but the underlying physiological mechanisms remain unknown.
Our results showed that movement‐related pain modulates corticospinal excitability during motor preparation.
In accordance with the pain adaptation theory, corticospinal excitability was higher when the muscle has an antagonist (vs. an agonist) role for the upcoming movement associated with pain.
Anticipation of movement‐related pain also affects motor initiation and execution, with slower movement initiation (longer reaction times) and faster movement execution compared to movements that do not evoke pain.
These results confirm the implementation of protective strategies during motor preparation known to be relevant for acute pain, but which may potentially have detrimental long‐term consequences and lead to the development of chronic pain.
Abstract
When a movement repeatedly generates pain, we anticipate movement‐related pain and establish self‐protective strategies during motor preparation, but the underlying mechanisms remains poorly understood. The current study investigated the effect of movement‐related pain anticipation on the modulation of behaviour and corticospinal excitability during the preparation of arm movements. Participants completed an instructed‐delay reaction‐time (RT) task consisting of elbow flexions and extensions instructed by visual cues. Nociceptive laser stimulations (unconditioned stimuli) were applied to the lateral epicondyle during movement execution in a specific direction (CS+) but not in the other (CS−), depending on experimental group. During motor preparation, transcranial magnetic stimulation was used to measure corticospinal excitability in the biceps brachii (BB). RT and peak end‐point velocity were also measured. Neurophysiological results revealed an opposite modulation of corticospinal excitability in BB depending on whether it plays an agonist (i.e. flexion) or antagonist (i.e. extension) role for the CS+ movements (P < 0.001). Moreover, behavioural results showed that for the CS+ movements RT did not change relative to baseline, whereas the CS− movements were initiated more quickly (P = 0.023) and the CS+ flexion movements were faster relative to the CS− flexion movements (P < 0.001). This is consistent with the pain adaptation theory which proposes that in order to protect the body from further pain, agonist muscle activity is reduced and antagonist muscle activity is increased. If these strategies are initially relevant and lead to short‐term pain alleviation, they may potentially have detrimental long‐term consequences and lead to the development of chronic pain.
Keywords: action preparation, pain anticipation, transcranial magnetic stimulation
Key points
Experimental pain or its anticipation influence motor preparation processes as well as upcoming movement execution, but the underlying physiological mechanisms remain unknown.
Our results showed that movement‐related pain modulates corticospinal excitability during motor preparation.
In accordance with the pain adaptation theory, corticospinal excitability was higher when the muscle has an antagonist (vs. an agonist) role for the upcoming movement associated with pain.
Anticipation of movement‐related pain also affects motor initiation and execution, with slower movement initiation (longer reaction times) and faster movement execution compared to movements that do not evoke pain.
These results confirm the implementation of protective strategies during motor preparation known to be relevant for acute pain, but which may potentially have detrimental long‐term consequences and lead to the development of chronic pain.
Introduction
There is growing evidence that the nociceptive and motor systems are extensively connected. Neuroimaging studies report activation of movement‐related brain areas in response to nociceptive stimuli (Peyron et al. 2000; Koyama et al. 2005; Perini & Bergstrand, 2013; Coombes & Misra, 2015; Misra & Coombes, 2015) and transcranial magnetic stimulation (TMS) studies reveal that phasic experimental pain induces an inhibition of the corticospinal excitability measured at rest (Valeriani et al. 1999, 2001; Dubé & Mercier, 2011; Mercier et al. 2016). As pointed out by recent reviews (Hodges & Tucker, 2011; Bank et al. 2013), a large number of studies report an impact of experimental pain on motor execution during various motor tasks, but less is known about the effect of pain on motor preparation. This is surprising given that a major role of pain is to protect the organism from potentially threatening events. As such, when a movement repeatedly generates pain, the central nervous system (CNS) should eventually be able to anticipate movement‐related pain, and establish self‐protective strategies during motor preparation in order to avoid pain or to minimize its harmful consequences (Wiech & Tracey, 2013; Zaman et al. 2015). Importantly, such protective strategies, while initially adaptive, could in the long term lead to maladaptive motor behaviours known to be involved in the transition from acute to chronic pain and its associated disability (Asmundson et al. 1999; Leeuw et al. 2007; Hodges & Tucker, 2011; Vlaeyen, 2015).
Two recent studies have investigated whether pain occurring during motor preparation influences the forthcoming motor response, when compared to a context without pain. The first study demonstrated that self‐initiated movements performed to stop a painful stimulation are preceded by a lower amplitude of the movement‐preparatory brain activity (i.e. readiness potential) (Postorino et al. 2017). The second study reported that in the presence of pain, externally cued movements are initiated faster, and this is associated with a suppression of premotor cortex beta oscillations in the EEG (Misra et al. 2017). Parallel to this, another recent study explored the effect of pain anticipation on behavioural motor changes, using a pain‐related fear conditioning paradigm (Karos et al. 2017). Results revealed that movements associated with pain are performed faster, more forcefully and more accurately than movements without pain. Taken together, these findings provide evidence that experimental pain or its anticipation influence motor preparation processes as well as upcoming movement execution. However, the mechanisms underlying these effects of pain anticipation during motor preparation remain largely unknown. Because previous studies provide evidence that pain or the expectation of pain modulates activity in the motor cortex, the main objective of the present study was to investigate the effect of movement‐related pain anticipation on the modulation of corticospinal excitability during preparation of arm movements. Based on previous studies showing that during motor preparation the agonist/antagonist role of the muscle and the direction of movement both influence the effect of pain on muscle activity (Lund et al. 1991; Graven‐Nielsen et al. 1997; Falla et al. 2006) and the modulation of corticospinal output (Neige et al. 2017), the second objective was to determine the effect of pain‐eliciting movement direction on the modulation of corticospinal excitability. Finally, the third objective was to confirm that movement‐related pain anticipation affects movement initiation (i.e. reaction time) and execution (i.e. peak velocity) in a direction‐specific manner (Karos et al. 2017).
To address these objectives, participants completed an instructed delay reaction‐time (RT) task with two movement directions (elbow flexion or extension, specified using a visual cue (conditioned stimulus, CS)]. Nociceptive laser stimulations were used as the unconditioned stimuli (pain‐US) and were applied during movement execution in a specific direction (CS+) but not in the other (CS−) (Meulders et al. 2011).
Methods
Ethical approval
The experiment was approved by the Ethics Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux de la Capitale‐Nationale ‐ Institut de Réadaptation en Déficience Physique de Québec (project number 2016‐072) and conformed to the latest revision of the Declaration of Helsinki, except for registration in a database.
Participants
Thirty healthy volunteers participated in the study after providing written informed consent. All participants were right handed as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971) (mean score = 93.8%, SD = 9). None of them reported neurological or psychiatric conditions, pain or musculoskeletal disorders in the upper limb, or TMS contraindications. As catastrophizing thoughts associated with pain have been shown to influence how pain is experienced (Sullivan et al. 2001), all participants completed a French version of the Pain Catastrophizing Scale (PCS) (McCracken et al. 1992; Sullivan et al. 1995). Individual scores were all below 30, indicating absence of a clinically relevant level of catastrophizing (Sullivan et al. 1995).
Experimental design and task
Participants came to the laboratory for a single 2‐h session. Before starting the experiment, participants completed a practice bout consisting of 10 arm flexion and extension movements (five for each direction) without TMS, to familiarize themselves with the task and the KINARM system, a robotized exoskeleton (BKIN technologies Ltd, Kingston, ON, Canada).
They then followed the experimental protocol illustrated in Fig. 1. As changes in corticospinal excitability associated with motor preparation (without pain) can vary according to the direction of the upcoming movement (flexion or extension) (Neige et al. 2017), participants first completed a No‐Pain Testing phase during which TMS was applied during motor preparation. They were then pseudo‐randomly assigned to one of two experimental groups (Pain Flexion or Pain Extension) and completed the Pain Conditioning phase. The latter consisted in an associative learning paradigm in which movements made in a given direction (i.e. flexion for the Pain Flexion group/extension for the Pain Extension group) systematically elicited a painful stimulation (named CS+). The opposite movement direction never elicited a painful stimulation (named CS−) (Meulders et al. 2011; Meulders & Vlaeyen, 2013). No TMS was applied during this Pain Conditioning phase. Finally, participants completed a third phase, Pain Testing, that was similar to the No‐Pain Testing phase (i.e. with TMS), but in the presence of pain in the conditioned direction. Behavioural data were also recorded during this phase. At the end of the experiment, participants rated their mean pain level using a numerical rating scale (NRS) separately for both the CS+ and the CS−.
Figure 1. Schematic illustration of the experimental protocol.
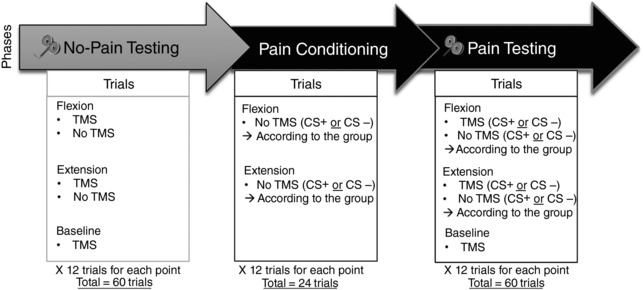
The grey arrow represents the No‐Pain Testing phase completed without pain for all participants. The first black arrow represents the Pain Conditioning phase which was similar to the No‐Pain Testing phase except that the pain was applied during CS+ movement execution, and that no TMS was applied. The second black arrow corresponds to the Pain Testing phase, similar to the Pain Conditioning phase but with TMS applied during preparation of the CS+ and CS– movements. Note that the order of the conditions within each phase was randomized across participants. TMS = transcranial magnetic stimulation; US = unconditioned stimulus; CS = conditioned stimulus.
Figure 2 illustrates the instructed‐delay RT task used in this study for the No‐Pain Testing, the Pain Conditioning and the Pain Testing phases. Movements consisted of monoarticular elbow flexion or extension, performed within the KINARM system. The KINARM consists of a robotized exoskeleton interfaced with a 2D virtual environment projected on a semi‐transparent screen. This system enables recording of arm kinematics, presentation of visual targets, application of forces/passive displacements and laser/TMS triggering. The participant's right arm was fully supported by the exoskeleton, and the shoulder joint was immobilized. The position of the index fingertip was presented on the screen, but the arm was occluded from view. The experimenter manually started each trial and the robot passively moved the arm to the Start position indicated by a white circle (radius = 1 cm): the shoulder was abducted at 90°; the upper arm was at 30° relative to the frontal plane; and elbow flexed at 90°. After 500 ms, two potential targets were simultaneously presented at a distance equivalent to 15° of elbow flexion and extension, respectively (white circles, 2 cm radius). After 1000 ms, only one target remained illuminated and turned red (Informative Signal) to inform the participant on the direction of movement to be performed. After a fixed motor preparation period of 1500 ms, the target turned green. This was the signal (Response Signal) to reach and pass through the target as quickly as possible (without stopping in it). The exoskeleton produced a dampening force field to break the movement after the target was reached. During this instructed‐delay RT task, TMS was delivered at 1250 ms (i.e. 250 ms prior to the Response Signal appearance, during motor preparation). On some trials, TMS was instead delivered 500 ms after the robot has passively moved the arm to the Starting position (i.e. at the moment at which the two potential targets would normally appear), to establish a baseline condition for corticospinal excitability in the task context, but before movement preparation. Therefore, baseline trials were intermingled with experimental trials rather than performed prior to the experiment (note, however, that no movements were performed during these trials). On other trials, no TMS was applied, in order to measure behavioural outcomes (reaction time and peak velocity) without movement interference from TMS‐induced muscle contraction (Terao et al. 1997; Burle et al. 2002). For movements associated with pain (CS+ in the Pain Conditioning and Pain Testing phases), the pain‐US laser nociceptive stimulation was applied as soon as the elbow angle rotation exceeded 5° relative to the starting position (i.e. beginning of movement execution).
Figure 2. Schematic representation of the instructed‐delay reaction time task.
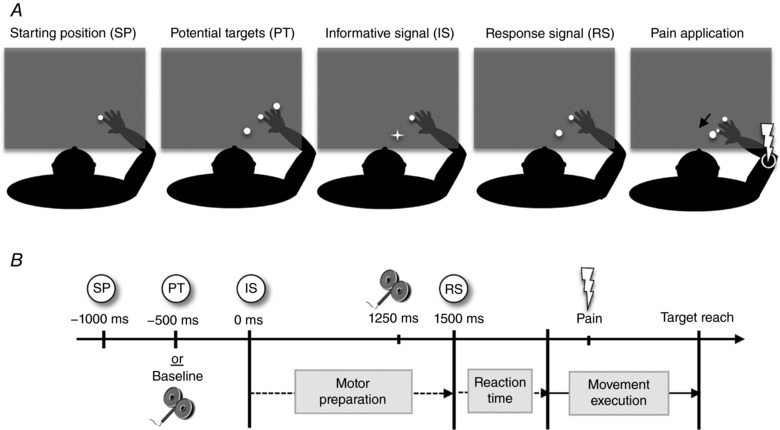
A, illustration of the different targets displayed on the screen during the instructed‐delay RT task. B, schematic representation of the time course of a trial (example of a CS+ pain flexion movement). The participant's arm is passively brought to the Starting position (SP) by the exoskeleton; after 500 ms TMS is applied (Baseline condition). For other trials and after 1000 ms delay, the Informative signal (IS) indicates the direction of movement to be performed; after a 1250 ms delay, TMS is applied [during the motor preparation period, before the Response signal (RS) at 1500 ms]. During the Pain Conditioning and the Pain Testing phases, the IS served as the conditioned stimulus (CS) and pain‐US was applied on the right epicondyle during movement execution for the CS+.
Nociceptive laser stimulation
Experimental pain was delivered to the participant's right lateral epicondyle by using a neodymium:doped‐yttrium‐aluminium‐perovskite (Nd:YAP) laser stimulator (Deka: Stimul 1340, Electronic Engineering, Florence, Italy), with a wavelength of 1.34 μm, a spot diameter of 5 mm and a pulse duration of 6 ms. This allows the excitation of free nerve endings in superficial skin layers, selectively activating Aδ and C fibres (Bromm & Treede, 1991). Laser intensity was individually set for each participant (at rest) during a calibration phase performed at the beginning of the experiment. Briefly, stimuli were delivered with a progressively increasing intensity and participants were asked to rate their pain using an 11‐point NRS ranging from 0 (no pain) to 10 (worst pain imaginable). The lowest intensity that was rated ≥3/10 was selected, to prevent any skin damage (Madden et al. 2016). To avoid habituation, nociceptor sensitization or skin damage, the laser beam was slightly displaced after each stimulation (Iannetti et al. 2004; Madden et al. 2016) on a grid drawn on the skin, during both calibration and the actual experiment.
Transcranial magnetic stimulation
Single‐pulse stimulation was delivered over the left M1 (contralateral to the dominant right arm) using a 70‐mm figure‐of‐eight coil connected to a monophasic Magstim BiStim² stimulator (The Magstim Co., Whitland, UK). The coil was placed tangentially to the scalp with the handle pointing toward the back and laterally at 45° away from the midsagittal line, resulting in a posterior–anterior current flow. Coil orientation and position was guided throughout the experiment using a neuronavigation system (Brainsight, Rogue research, Montreal, QC, Canada). Stimulation parameters were defined with the participant's arm positioned at the Starting position, to avoid any effect of arm position changes on corticospinal excitability (Mitsuhashi et al. 2007; Mogk et al. 2014; Nuzzo et al. 2016). The optimal stimulation site on the scalp (hotspot) was defined as the location eliciting the largest motor evoked potential (MEP) amplitude in biceps brachii (BB). Resting motor threshold (rMT) was then determined as the lowest stimulation intensity required to evoke at least five MEPs of 50 μV out of 10 stimulations (Rossini et al. 1999). During the experiment, TMS stimulation intensity was fixed at 120% rMT.
EMG and kinematic recordings
EMG activity was recorded from the middle portion of the right BB for MEP and RT measures and lateral head of the right triceps brachii (TB) for RT only, MEPs being difficult to evoke in this muscle in this arm posture (Neige et al. 2017). Pairs of surface Ag/AgCl electrodes (Kendall Medi‐trace 200, Covidien, Dublin, Ireland) were placed in bipolar configuration over the muscle belly. The ground electrode was positioned over the right acromion. EMG signals were amplified (×1000), band‐pass filtered (10–500 Hz), digitized at a sampling rate of 1000 Hz with the KINARM data acquisition card (National Instruments PCI‐6229 DAQ card, Austin, TX, USA) and stored on a computer for offline analysis. EMG background was visually monitored throughout the experiment to ensure a complete muscle relaxation at the beginning of each trial.
Arm kinematics recorded from the KINARM motor encoders were sampled at 1000 Hz.
Data analysis
Data analysis was performed using custom‐made Matlab scripts (The Mathworks Inc., Natick, MA, USA). EMG signals were pre‐processed using a second‐order Butterworth notch filter (bandstop of 58 and 62 Hz).
Neurophysiological data
To quantify corticospinal excitability, peak‐to‐peak amplitude of MEPs was measured. The average MEP amplitude for each condition (12 MEPs for each movement direction in each phase) during the instructed‐delay RT task was normalized against the average MEP amplitude for the Baseline condition (taken during both the No‐Pain Testing and Pain Testing phases, total of 24 MEPs; see Fig. 1), using the following equation:
Positive values correspond to an increase in corticospinal excitability during the instructed‐delay RT task, and negative values to a decrease.
Behavioural data
Reaction time
The EMG signal was used to precisely quantify RT for each condition in the no‐TMS trials, as the time difference between the appearance of the Response Signal and the onset of the EMG burst in the agonist muscle (i.e. BB for flexion, TB for extension) using a threshold method (Di Fabio, 1987; Hodges & Bui, 1996). Briefly, the mean and standard deviation (SD) of EMG baseline were calculated in a 250 ms window starting when the two white targets were presented to the participant. EMG onset was automatically detected (and visually confirmed) when 75% of EMG data points exceeded 3 SD above baseline for a period of 50 ms. When the EMG burst was initiated less than 100 ms after the Response signal appeared, it was considered as a false start and the trial was removed from further analyses.
Peak velocity
During movement execution, the peak velocity was defined as the greatest velocity of the index fingertip achieved between the Starting Position and the final target.
To assess if movement‐related pain anticipation affected movement initiation (i.e. reaction time) and execution (i.e. peak movement velocity) in a direction‐specific manner, data were normalized and expressed as a percentage of change from the mean No‐Pain Testing behavioural performance. This was done after verifying that there was no difference between movement directions during the No‐Pain Testing phase.
Statistical analysis
Statistical analyses were performed using Statistical Program for the Social Sciences (SPSS) version 24 software (SPSS Inc., Chicago, IL, USA). Normality of data distributions was verified using the Shapiro–Wilk test. Homogeneity of variances was assessed by Mauchly's test and a Huynh–Feldt correction was applied if the sphericity assumption was violated. Pre‐planned post hoc analyses were performed on significant interactions after applying a Bonferroni correction for multiple comparisons. Corrected P values for multiple comparisons are reported in the Results section. The α level for all analyses was fixed at 0.05. Partial eta squared (ηp2) values are reported when results are statistically significant to express the portion of the total variance attributable to the tested factor or interaction. Values in parentheses in the text represent mean ± SD. ANOVA was performed on normalized MEPs amplitude with Phase (No‐Pain Testing vs. Pain Testing phases) and Movement direction (CS+ vs. CS−) as within‐subject factors and Group (Pain Flexion vs. Pain Extension) as the between‐subject factor. Other ANOVAs were performed on normalized RT and peak velocity data, with Movement direction (CS+ vs. CS−) as the within‐subject factor, and Group (Pain Flexion vs. Pain Extension) as the between‐subject factor.
Additional analyses were performed to control for potential methodological biases. First, Student two‐tailed unpaired t tests were used to compare demographic data, pain catastrophizing score, laser intensity, rMT and pain ratings between groups. Second, an ANOVA was performed to compare raw MEP amplitude during the Baseline condition between Phases (No‐Pain Testing vs. Pain Testing) and Group (Pain Flexion vs. Pain Extension). No difference on corticospinal excitability was expected either between Phases or between Groups. Finally, a two‐tailed paired sample t test was used to compare RT and peak velocity across directions.
Results
Group characteristics
As illustrated in Table 1, the characteristics of the two groups of participants did not differ significantly.
Table 1.
Comparison of baseline characteristics, TMS and laser parameters across groups (Pain Extension and Pain Flexion); data represent mean ± SD
| Pain Extension (n = 15) | Pain Flexion (n = 15) | P value (t tests) | |
|---|---|---|---|
| Age (years) | 22.8 ± 2.3 | 24 ± 2.8 | 0.089 |
| Sex | 7 F/8 M | 7 F/8 M | – |
| PCS score | 8.4 ± 7.2 | 10.3 ± 5.6 | 0.437 |
| Mean laser intensity (J) | 3.7 ± 0.5 | 3.7 ± 0.8 | 0.978 |
| Pain rating for the CS+ movement (NRS/10) | 3.7 ± 0.5 | 3.6 ± 0.9 | 0.622 |
| Pain rating for the CS− movement (NRS/10) | 0.12 ± 0.3 | 0.35 ± 0.3 | 0.413 |
| rMT (%MSO) | 49.3 ± 9.1 | 49.1 ± 10 | 0.970 |
F = females; M = males; PCS = Pain Catastrophizing Scale; NRS = numeric rating scale; rMT = resting motor threshold; MSO = maximum output stimulator.
Methodological considerations
The ANOVA on MEP amplitude during Baseline conditions revealed no significant difference between Phases [No‐Pain Testing: 0.55 (0.45) mV vs. Pain Testing: 0.59 (0.54) mV; F 1,28 < 1; P = 0.600] or Group [Pain Flexion: 0.51 (0.53) mV vs. Pain Extension: 0.63 (0.46) mV; F 1,28 < 1; P = 0.495], or Phase × Group interaction (F 2,56 < 1; P = 0.772). This indicates that corticospinal excitability measured during the baseline did not differ despite the presence of pain during the Pain Testing phase. Finally, as previously observed in a study using a similar motor preparation paradigm (without pain) (Neige et al. 2017), no difference in RT and peak velocity was observed between Flexion and Extension movements during the No‐Pain Testing phase (all P > 0.354).
Neurophysiological data
Figure 3 illustrates the normalized amplitude of MEPs recorded in BB during the No‐Pain and Pain Testing phases, according to movement direction for the two experimental groups (Pain Flexion and Pain Extension). ANOVAs performed on normalized MEP amplitude recorded during motor preparation revealed a main effect only of Phase (F 1,28 = 5.4; P = 0.028; ƞp2 = 0.16) indicating that MEP amplitudes were globally lower during the No‐Pain Testing than during the Pain Testing phase. However, there was a significant triple interaction (Phase × Movement Direction × Group; F 1,28 = 4.4; P = 0.044; ƞp2 = 0.14). Table 2 presents the results of the pre‐planned post hoc analyses, revealing that the only difference between groups was observed in the Pain Testing phase during the preparation of the CS+ movement. This indicates that for the CS+ movement, corticospinal excitability was higher when BB played an antagonist role (i.e. extension movement) than when it played an agonist role (i.e. flexion movement) (see Fig. 4).
Figure 3. Mean (± SEM) change in the normalized MEP amplitude during the No‐Pain Testing (left panel) and Pain Testing (right panel) phase for the two groups.
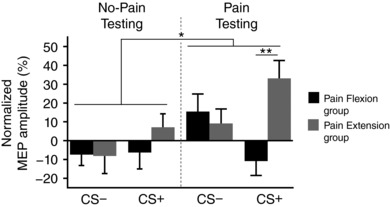
The Movement direction associated with pain corresponds to the CS+ whereas the Movement direction not associated with pain corresponds to the CS−. * P < 0.05; ** P < 0.005.
Table 2.
Post hoc analyses for the significant Phase × Movement direction × Group interaction
| Phase | Movement direction | Group | P value (corrected) | |
|---|---|---|---|---|
| No‐Pain Testing | CS− | Pain Flexion vs. | Pain Extension | 0.947 |
| CS+ | Pain Flexion vs. | Pain Extension | 0.242 | |
| Pain Testing | CS− | Pain Flexion vs. | Pain Extension | 0.606 |
| CS+ | Pain Flexion vs. | Pain Extension | 0.001a | |
P < 0.005.
Figure 4. Average raw MEP data from a representative participant from each experimental group.
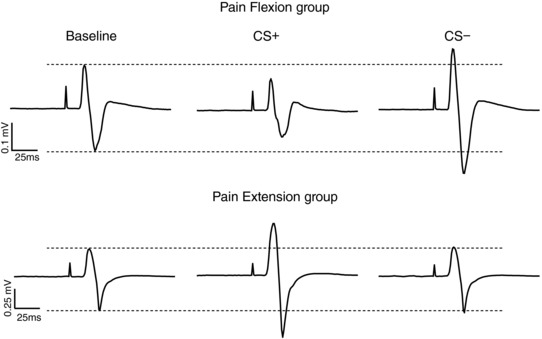
Pain Flexion group (upper panel) and Pain Extension group (lower panel) during the baseline condition and during the Pain Testing Phase for the Movement direction associated with pain (CS+) and the Movement direction not associated with pain (CS−).
Behavioural data
Figure 5 A shows RT results during Pain Testing (normalized to the No‐Pain Testing) according to Movement direction and Group. The ANOVA revealed a main effect of Movement direction (F 1,28 = 5.8; P = 0.023; ƞp2 = 0.17), RT being shorter for the CS− movement (i.e. extension for the Pain Flexion group and flexion for the Pain Extension group). No significant main effect of Group (F1,28 < 1; P = 0.354) or Group × Movement direction interaction was found (F1,28 < 1; P = 0.456).
Figure 5. Mean (± SEM) change in reaction times and peak velocity for the Pain Testing phase normalized to the No‐Pain Testing phase for the two groups.
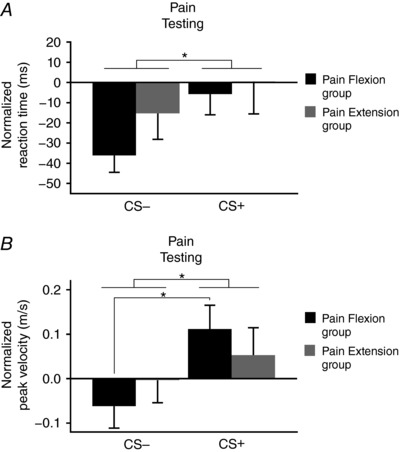
A, reaction time; B, peak velocity. CS+ = movement direction associated with pain; CS− = movement direction not associated with pain. * P < 0.05 for the comparisons indicated.
Figure 5 B presents peak velocity results during Pain Testing (normalized to the No‐Pain Testing) according to Movement direction and Group. The ANOVA revealed a main effect of Movement direction (F 1,28 = 16.8; P < .001; ƞp2 = 0.38), peak velocity being higher for the CS+ (i.e. flexion for the Pain flexion group and extension for the Pain extension group). No significant main effect of Group was observed (F1,28 < 1; P = 0.490). However, a significant Group × Movement direction interaction was found (F 1,28 = 4.3; P = 0.046; ƞp2 = 0.14). The post hoc analyses indicated that participants from the Pain Flexion group executed CS+ movements faster (F 1,28 = 1911; P < .001; ƞp2 = 0.41), while no such difference was observed for the Pain Extension group (F 1,28 = 2; P = 0.166).
Discussion
The main objective of this study was to investigate how anticipation of motor‐related pain influences motor preparation processes as well as upcoming movement initiation and execution.
Overall, results revealed that movement‐related pain was associated with slower movement initiation (longer RTs) compared to movements that do not evoke pain, and to faster movement execution (especially for flexion movements). These behavioural changes are paralleled by changes in the modulation of corticospinal excitability during motor preparation, depending on the agonist/antagonist role of the muscle relative to the upcoming painful movement: corticospinal excitability of the BB was much higher during preparation for painful extension movements (i.e. when the biceps is an antagonist) than for painful flexion movements (i.e. when the biceps is an agonist).
Anticipation of movement‐related pain affects motor initiation and execution
RTs were found to be reduced during the Pain Testing phase for the movement direction not associated with pain (CS−), but not changed for the direction associated with pain (CS+).
RT is an outcome measure used to assess a range of processes that occur during motor preparation (Jahanshahi, 2003; Neige et al. 2018) and has been reported to decrease over time, reflecting a practice/learning effect (Norrie, 1967). The lack of reduction in RT for the CS+ movements could thus be a reflection of additional protective strategies implemented during motor preparation in order to avoid movement‐related pain. Alternatively, the lack of reduction in RT could reflect the fact that pain interfered with motor learning (Boudreau et al. 2010; Bouffard et al. 2014). Indeed, longer RTs have been reported during motor acquisition in the presence of experimental tonic pain when compared to a no‐pain condition (Boudreau et al. 2010). Moreover, an impact of pain on anticipatory aspects of motor strategies while learning to compensate for a force‐field have been observed in previous studies (Lamothe et al. 2014; Bouffard et al. 2016).
Peak velocity results showed that during the Pain Testing phase, CS+ movements were executed faster than CS− movements. This result is compatible with a recent study that used a fear of pain conditioning paradigm and tested circular arm movements (Karos et al. 2017), who suggest that this result reflects a ‘get it over and done with’ motor strategy when movement‐related pain cannot be avoided.
Overall, the behavioural results of the current study indicate that participants had a longer movement initiation phase followed by a quicker movement execution phase for the movement direction associated with pain. Regarding the existing literature, this appears to be a specific consequence of the anticipation of movement‐related pain and associated motor strategies implemented during motor preparation. Indeed, it has been shown that pain applied during motor preparation leads to shorter RT, without affecting movement execution (Misra et al. 2017). Moreover, experimental tonic pain (i.e. unrelated to movements) induced in BB leads to a decreased movement acceleration, amplitude and peak velocity (Ervilha et al. 2004).
The modulation of corticospinal excitability during motor preparation is specific to the functional role of the muscle involved in the painful movement
Neurophysiological results revealed that corticospinal excitability of BB is much higher during preparation for painful extension than for painful flexion movements. This indicated an opposite modulation of corticospinal excitability according to the agonist or antagonist role of the muscle (i.e. flexion vs. extension) for the movement direction associated with pain (CS+), mainly driven by higher MEPs for the CS+ movements in the Pain Extension Group.
According to the pain adaptation theory (Lund et al. 1991), in order to protect the body from further pain, the agonist muscle activity that produces a painful movement should be reduced and the antagonist muscle activity increased, and this effect would be mediated by ascending nociceptive input onto the spinal motoneuron pool (Lund et al. 1991). Thus, the specific changes observed in the current study could be interpreted as the implementation of protective strategies during anticipation of the impending nociceptive stimulation related to movement execution (Lund et al. 1991; Hodges & Tucker, 2011). While previous studies provided support for such predictions of the pain adaptation theory (Graven‐Nielsen et al. 1997, 2002; but see Martin et al. 2008 and Hodges & Tucker, 2011 for contrasting results), they mostly focused on the movement execution phase (Graven‐Nielsen et al. 1997; Ciubotariu et al. 2004). The results of the current study expand these findings by highlighting specific changes in corticospinal excitability in arm muscle during motor preparation, depending of the functional role of the muscle for the CS+ movements. As these effects are observed prior to the application of pain, they are probably attributable to neurocognitive modulatory mechanisms mediated by supraspinal areas involved in pain anticipation. Indeed, neuroimaging studies provide evidence that pain anticipation alone (i.e. when the motor system is at rest) activates pain‐related brain areas such as the primary somatosensory cortex, the anterior cingulate cortex, the insula, the anterior medial frontal cortex and the periaqueductal grey (Sawamoto et al. 2000; Porro et al. 2002; Apkarian et al. 2005; Koyama et al. 2005; Fairhurst et al. 2007; Brown et al. 2014). However, pain anticipation alone does not modulate corticospinal excitability (Dubé & Mercier, 2011), suggesting that additional brain areas are involved during anticipation of pain‐related movement.
Motor preparation, initiation and execution are three independent processes affected differently by movement‐related pain
Although both neurophysiological and behavioural variables were found to be modulated by the expectation of movement‐related pain, the specific pattern of modulation varies across variables. Indeed, an opposite pattern of modulation was observed for MEPs prior to the CS+ according to the experimental group (i.e. corticospinal excitability was higher for the Extension than for the Flexion group), while a similar pattern of modulation was observed across groups for behavioural data (RT and peak velocity). These different patterns of modulation suggest that these phenomena are reflecting independent processes. Several distinct cortical/subcortical regions are involved in the preparation, initiation and execution of the motor response, and it seems likely that not all of them are modulated and impacted by pain in a similar way. For example, a recent systematic review investigating changes in RT induced by non‐invasive brain stimulation in healthy participants (Neige et al. 2018) revealed that excitatory stimulation over M1 does not consistently induce an effect on RT whereas excitatory stimulation over supplementary motor area, a cortical area more closely involved in movement initiation (Cunnington et al. 2002), does affect RT. Moreover, many TMS studies reported a non‐specific inhibition of corticospinal excitability during the motor preparation period (Hasbroucq et al. 1997; Touge et al. 1998; Massé‐Alarie et al. 2018) whereas a progressive increase of corticospinal excitability in the agonist muscle is generally observed during the motor initiation period (Chen et al. 1998; Leocani et al. 2000; Duque et al. 2017; Massé‐Alarie et al. 2018), suggesting the existence of different mechanisms between these two processes. Altogether, these results highlight the complexity of the mechanisms that are involved in action preparation and the need for more studies looking simultaneously at neurophysiological and behavioural measures to understand the functional relevance of the observed neurophysiological mechanisms.
Another important aspect to keep in mind is that in the present study, changes in corticospinal excitability were only recorded in BB muscle, whereas RT and peak velocity are affected by the various muscles acting at the elbow. Finally, it is possible that spinally mediated mechanisms related to the nociceptive input took place and influenced the different measures, specifically during motor execution. It is important to note that the peak velocity occurred on average 65.2 ± 23 ms after the nociceptive stimulation while reflex force responses at the upper limb were reported to occur with an average latency of ∼100 ms (Peterson et al. 2014). Therefore, it appears unlikely that the higher velocity for the CS+ Pain Flexion Group than for the Pain Extension Group would be explained by the fact that the laser stimuli triggered nociceptive withdrawal reflexes. Furthermore, if the stimulation triggered a withdrawal reflex, the latter would always be towards flexion, and would therefore reduce elbow extension velocity in the CS+ pain extension condition. Our findings do not exclude the possibility that other spinal mechanisms than the withdrawal reflex may have contributed to the observed effect, however.
Limitations
Several limitations need to be taken into consideration when interpreting the results of this study. First, an alternative explanation for our results could be that the presence of pain, an unpleasant sensory and emotional experience, induces a negative emotional state, and that the emotional state rather than a pain–motor system interaction is responsible for the reported results. Literature on the fear‐conditioning paradigm with other US stimuli (e.g. fearful images) provides evidence that negative emotional state or anxiety increase MEP amplitude measured at rest (Oathes et al. 2008; Schutter et al. 2008; Baumert et al. 2011) or during motor preparation (Coombes et al. 2009; Tanaka et al. 2014; Gökdemir et al. 2018). However, this hypothesis would be difficult to reconcile with our observation of an opposite modulation of corticospinal excitability during preparation for painful extension vs. flexion as well as with the absence of a significant difference between the No‐Pain and Pain Testing phases on the baseline measures. Secondly, the instructed delay‐RT task used in the current study involved monoarticular elbow flexion or extension, while movements of the shoulder were prevented. This may have prevented the implementation of pain avoidance strategies during movement execution (Meulders et al. 2016), thereby limiting the ecological validity of the task.
Conclusion
Our results suggest that movement‐related pain specifically influences motor behaviour and modulates corticospinal excitability during the preparation phase of arm movements. This is consistent with the pain adaptation theory, which proposes that in order to protect the body from further pain, agonist muscle activity that produces a painful movement must be reduced, and antagonist activity increased (Lund et al. 1991). Interestingly, a recent theory on the effect of pain on motor control states that while such protective strategies may be initially relevant and lead to short‐term pain alleviation, they may potentially have detrimental long‐term consequences and lead to the development of chronic pain (Hodges & Tucker, 2011). Thus, the current paradigm could be useful to evaluate corticospinal excitability changes during motor preparation in clinical populations in which pain is evoked by movements (such as musculoskeletal disorders) and detect the development of potential maladaptive motor strategies.
Additional information
Competing interests
None declared.
Author contributions
CM, CN and LB and designed the study; CN, MG and NM performed data collection; CM, CN, LB, MG and NM analysed the data; CM, CN and LB drafted the paper. All authors commented on the paper, approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgments
We thank Nicolas Robitaille, PhD, for his help in the development of the task.
Funding
This work was supported by the Canadian Institute for Health Research (CIHR) [grant number MOP‐125869]. CN was supported by a studentship from the Centre for Interdisciplinary Research in Rehabilitation and Social Integration (CIRRIS). CM was supported by salary award from the Fonds de Recherche Quebec‐Santé (FRQS).
Biography
Cécilia Neige completed a Master degree at the Grenoble‐Alpes University (Grenoble, France). She is currently a PhD student at the Centre for Interdisciplinary Research in Rehabilitation and Social Integration (CIRRIS) affiliated to Laval University (Quebec City, Canada). She is working under the supervision of Professor Catherine Mercier and Professor Laurent Bouyer. One of the goals of the team is to better understand the interaction between pain and motor control.

Edited by: Janet Taylor & Richard Carson
Linked articles This article is highlighted by a Perspective by Coombes et al. To read this article, visit https://doi.org/10.1113/JP276325.
References
- Apkarian AV, Bushnell MC, Treede RD & Zubieta JK (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9, 463–484. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Norton PJ & Norton GR (1999). Beyond pain: the role of fear and avoidance in chronicity. Clin Psychol Rev 19, 97–119. [DOI] [PubMed] [Google Scholar]
- Bank PJM, Peper CE, Marinus J, Beek PJ & van Hilten JJ (2013). Motor consequences of experimentally induced limb pain: a systematic review. Eur J Pain 17, 145–157. [DOI] [PubMed] [Google Scholar]
- Baumert A, Sinclair C, MacLeod C & Hammond G (2011). Negative emotional processing induced by spoken scenarios modulates corticospinal excitability. Cogn Affect Behav Neurosci 11, 404–412. [DOI] [PubMed] [Google Scholar]
- Boudreau SA, Hennings K, Svensson P, Sessle BJ & Arendt‐Nielsen L (2010). The effects of training time, sensory loss and pain on human motor learning. J Oral Rehabil 37, 704–718. [DOI] [PubMed] [Google Scholar]
- Bouffard J, Bouyer LJ, Roy J‐S & Mercier C (2014). Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. J Neurosci 34, 9190–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard J, Bouyer LJ, Roy JS & Mercier C (2016). Pain induced during both the acquisition and retention phases of locomotor adaptation does not interfere with improvements in motor performance. Neural Plast 2016, 8539096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromm B & Treede RD (1991). Laser‐evoked cerebral potentials in the assessment of cutaneous pain sensitivity in normal subjects and patients. Rev Neurol (Paris) 147, 625–643. [PubMed] [Google Scholar]
- Brown CA, El‐Deredy W & Jones AKP (2014). When the brain expects pain: common neural responses to pain anticipation are related to clinical pain and distress in fibromyalgia and osteoarthritis. Eur J Neurosci 39, 663–672. [DOI] [PubMed] [Google Scholar]
- Burle B, Bonnet M, Vidal F & Possamaï C (2002). A transcranial magnetic stimulation study of information processing in the motor cortex: relationship between the silent period and the reaction time delay. Psychophysiology 39, 207–217. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG & Hallett M (1998). Time course of corticospinal excitability in reaction time and self‐paced movements. Ann Neurol 44, 317–325. [DOI] [PubMed] [Google Scholar]
- Ciubotariu A, Arendt‐Nielsen L & Graven‐Nielsen T (2004). The influence of muscle pain and fatigue on the activity of synergistic muscles of the leg. Eur J Appl Physiol 91, 604–614. [DOI] [PubMed] [Google Scholar]
- Coombes SA & Misra G (2015). Pain and motor processing in the human cerebellum. Pain 157, 1–38. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Tandonnet C, Fujiyama H, Janelle CM, Cauraugh JH & Summers JJ (2009). Emotion and motor preparation: a transcranial magnetic stimulation study of corticospinal motor tract excitability. Cogn Affect Behav Neurosci 9, 380–388. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L & Moser E (2002). The preparation and execution of self‐initiated and externally‐triggered movement: a study of event‐related fMRI. Neuroimage 15, 373–385. [DOI] [PubMed] [Google Scholar]
- Dubé JA & Mercier C (2011). Effect of pain and pain expectation on primary motor cortex excitability. Clin Neurophysiol 122, 2318–2323. [DOI] [PubMed] [Google Scholar]
- Duque J, Greenhouse I, Labruna L & Ivry RB (2017). Physiological markers of motor inhibition during human behavior. Trends Neurosci 40, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervilha UF, Arendt‐Nielsen L, Duarte M & Graven‐Nielsen T (2004). Effect of load level and muscle pain intensity on the motor control of elbow‐flexion movements. Eur J Appl Physiol 92, 168–175. [DOI] [PubMed] [Google Scholar]
- Di Fabio R (1987). Reliability of computerized surface electromyography for determining the onsent of muscle activity. Phys Ther 67, 43–48. [DOI] [PubMed] [Google Scholar]
- Fairhurst M, Wiech K, Dunckley P & Tracey I (2007). Anticipatory brainstem activity predicts neural processing of pain in humans. Pain 128, 101–110. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D, Dahl MK & Graven‐Nielsen T (2006). Muscle pain induces task‐dependent changes in cervical agonist/antagonist activity. J Appl Physiol 102, 601–609. [DOI] [PubMed] [Google Scholar]
- Gökdemir S, Gündüz A, Özkara Ç & Kızıltan ME (2018). Fear‐conditioned alterations of motor cortex excitability: the role of amygdala. Neurosci Lett 662, 346–350. [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen T, Lund H, Arendt‐Nielsen L, Danneskiold‐Samsøe B & Bliddal H (2002). Inhibition of maximal voluntary contraction force by experimental muscle pain: a centrally mediated mechanism. Muscle Nerve 26, 708–712. [DOI] [PubMed] [Google Scholar]
- Graven‐Nielsen T, Svensson P & Arendt‐Nielsen L (1997). Effects of experimental muscle pain on muscle activity and co‐ordination during static and dynamic motor function. Electroencephalogr Clin Neurophysiol 105, 156–164. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M & Possamaï CA (1997). Preparatory inhibition of cortico‐spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res 5, 185–192. [DOI] [PubMed] [Google Scholar]
- Hodges PW & Bui BH (1996). A comparision of computer‐based methodes for the determination of onset of muscle contration using electromyography. Electroencephalogr Clin Neurophysiol 101, 511–519. [DOI] [PubMed] [Google Scholar]
- Hodges PW & Tucker K (2011). Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152, S90–S98. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Leandri M, Truini A, Zambreanu L, Cruccu G & Tracey I (2004). Aδ nociceptor response to laser stimuli: selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin Neurophysiol 115, 2629–2637. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M (2003). Reaction time as an index of motor preparation/programming and speed of response initiation. Handb Clin Neurophysiol 1, 203–229. [Google Scholar]
- Karos K, Meulders A, Gatzounis R, Seelen HAM, Geers RPG & Vlaeyen JWS (2017). Fear of pain changes movement: motor behaviour following the acquisition of pain‐related fear. Eur J Pain (United Kingdom) 21, 1432–1442. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ & Coghill RC (2005). The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A 102, 12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe M, Roy J‐S, Bouffard J, Gagné M, Bouyer LJ & Mercier C (2014). Effect of tonic pain on motor acquisition and retention while learning to reach in a force field. PLoS One 9, e99159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K & Vlaeyen JWS (2007). The fear‐avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 30, 77–94. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K & Hallett M (2000). Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123, 1161–1173. [DOI] [PubMed] [Google Scholar]
- Lund JP, Donga R, Widmer CG & Stohler CS (1991). The pain‐adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 69, 683–694. [DOI] [PubMed] [Google Scholar]
- Madden VJ, Catley MJ, Grabherr L, Mazzola F, Shohag M & Moseley GL (2016). The effect of repeated laser stimuli to ink‐marked skin on skin temperature—recommendations for a safe experimental protocol in humans. PeerJ 4, e1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC & Taylor JL (2008). Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586, 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé‐Alarie H, Neige C, Bouyer LJ & Mercier C (2018). Modulation of corticospinal excitability of trunk muscles in preparation of rapid arm movement. Neuroscience 369, 231–241. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Zayfert C & Gross RT (1992). The pain anxiety symptoms scale: development and validation of a scale to measure fear of pain. Pain 50, 67–73. [DOI] [PubMed] [Google Scholar]
- Mercier C, Gagné M, Reilly KT & Bouyer LJ (2016). Effect of experimental cutaneous hand pain on corticospinal excitability and short afferent inhibition. Brain Sci 29, pii: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulders A, Franssen M, Fonteyne R & Vlaeyen JWS (2016). Acquisition and extinction of operant pain‐related avoidance behavior using a 3 degrees‐of‐freedom robotic arm. Pain 157, 1094–1104. [DOI] [PubMed] [Google Scholar]
- Meulders A, Vansteenwegen D & Vlaeyen JWS (2011). The acquisition of fear of movement‐related pain and associative learning: a novel pain‐relevant human fear conditioning paradigm. Pain 152, 2460–2469. [DOI] [PubMed] [Google Scholar]
- Meulders A & Vlaeyen JWS (2013). Mere intention to perform painful movements elicits fear of movement‐related pain: an experimental study on fear acquisition beyond actual movements. J Pain 14, 412–423. [DOI] [PubMed] [Google Scholar]
- Misra G & Coombes SA (2015). Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb Cortex 25, 1906–1919. [DOI] [PubMed] [Google Scholar]
- Misra G, Ofori E, Chung JW & Coombes SA (2017). Pain‐related suppression of beta oscillations facilitates voluntary movement. Cereb Cortex 27, 2592–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi K, Seki K, Akamatsu C & Handa Y (2007). Modulation of excitability in the cerebral cortex projecting to upper extremity muscles by rotational positioning of the forearm. Tohoku J Exp Med 212, 221–228. [DOI] [PubMed] [Google Scholar]
- Mogk JPM, Rogers LM, Murray WM, Perreault EJ & Stinear JW (2014). Corticomotor excitability of arm muscles modulates according to static position and orientation of the upper limb. Clin Neurophysiol 125, 2046–2054. [DOI] [PubMed] [Google Scholar]
- Neige C, Massé‐Alarie H, Gagné M, Bouyer LJ & Mercier C (2017). Modulation of corticospinal output in agonist and antagonist proximal arm muscles during motor preparation. PLoS One 12, e0188801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neige C, Massé‐Alarie H & Mercier C (2018). Stimulating the healthy brain to investigate neural correlates of motor preparation: a systematic review. Neural Plast 2018, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrie ML (1967). Practice effects on reaction latency for simple and complex movements. Res Quarterly Am Assoc Heal Phys Educ Recreat 38, 79–85. [PubMed] [Google Scholar]
- Nuzzo J, Trajano GS, Barry BK, Gandevia SC & Taylor JL (2016). Arm‐posture‐dependent changes in corticospinal excitability are largely spinal in origin. J Neurophysiol 115, 2076–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes DJ, Bruce JM & Nitschke JB (2008). Worry facilitates corticospinal motor response to transcranial magnetic stimulation. Depress Anxiety 25, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Perini I & Bergstrand S (2013). Where pain meets action in the human brain. J Neurosci 33, 15930–15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Riley ZA, Krepkovich ET, Murray WM & Perreault EJ (2014). Withdrawal reflexes in the upper limb adapt to arm posture and stimulus location. Muscle Nerve 49, 716–723. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B & García‐Larrea L (2000). Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30, 263–288. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M & Nichelli P (2002). Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22, 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postorino M, May ES, Nickel MM, Tiemann L & Ploner M (2017). The influence of pain on motor preparation in the human brain. J Neurophysiol 118, 2267–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Berardelli A, Deuschl G, Hallett M, MaertensdeNoordhout AM, Paulus W & Pauri F (1999). Applications of magnetic cortical stimulation. Int Fed Clin Neurophysiol 52, 171–185. [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J & Shibasaki H (2000). Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event‐related functional magnetic resonance imaging study. J Neurosci 20, 7438–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, Hofman D & Van Honk J (2008). Fearful faces selectively increase corticospinal motor tract excitability: a transcranial magnetic stimulation study. Psychophysiology 45, 345–348. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR & Pivik J (1995). The pain catastrophizing scale: development and validation. Psychol Assess 7, 524–532. [Google Scholar]
- Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA & Lefebvre JC (2001). Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17, 52–64. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Funase K, Sekiya H, Sasaki J & Tanaka YM (2014). Psychological pressure facilitates corticospinal excitability: motor preparation processes and EMG activity in a choice reaction task. Int J Sport Exerc Psychol 12, 287–301. [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba‐Shimizu K & Kanazawa I (1997). Shortening of simple reaction time by peripheral electrical and submotor‐threshold magnetic cortical stimulation. Exp Brain Res 115, 541–545. [DOI] [PubMed] [Google Scholar]
- Touge T, Taylor JL & Rothwell JC (1998). Reduced excitability of the cortico‐spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol 109, 489–495. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Le Pera D, Profice P, Saturno E & Tonali P (2001). Inhibition of biceps brachii muscle motor area by painful heat stimulation of the skin. Exp Brain Res 139, 168–172. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Profice P, Le PD, Saturno E & Tonali P (1999). Inhibition of the human primary motor area by painful heat stimulation of the skin. Clin Neurophysiol 110, 1475–1480. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JWS (2015). Learning to predict and control harmful events: chronic pain and conditioning. Pain 156(Suppl), S86–93. [DOI] [PubMed] [Google Scholar]
- Wiech K & Tracey I (2013). Pain, decisions, and actions: a motivational perspective. Front Neurosci 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman J, Vlaeyen JWS, Van Oudenhove L, Wiech K & Van Diest I (2015). Associative fear learning and perceptual discrimination: a perceptual pathway in the development of chronic pain. Neurosci Biobehav Rev 51, 118–125. [DOI] [PubMed] [Google Scholar]


