Abstract
Key points
This study examined the effect of different ‘doses’ of lifelong (>25 years) exercise on arterial stiffening (a hallmark of vascular ageing) in older adults.
There are clear dose‐dependent effects of lifelong exercise training on human arterial stiffness that vary according to the site and size of the arteries.
Similar to what we have observed previously with ventricular stiffening, 4–5 days week−1 of committed exercise over a lifetime are necessary to preserve ‘youthful’ vascular compliance, especially of the large central arteries.
Casual exercise training of two to three times per week may be sufficient for middle‐sized arteries like the carotid to minimize arterial stiffening with ageing.
However, there is little effect of exercise training on the small‐sized peripheral arteries at any dose.
Abstract
Central arterial stiffness increases with sedentary ageing. While near‐daily, vigorous lifelong (>25 years) endurance exercise training prevents arterial stiffening with ageing, this rigorous routine of exercise training over a lifetime is impractical for most individuals. The aim was to examine whether a less frequent ‘dose’ of lifelong exercise training (four to five sessions per week for > 30 min) that is consistent with current physical activity recommendations elicits similar benefits on central arterial stiffening with ageing. A cross‐sectional examination of 102 seniors (>60 years old) who had a consistent lifelong exercise history was performed. Subjects were stratified into four groups based on exercise frequency as an index of exercise ‘dose’: sedentary: fewer than two sessions per week; casual exercisers: two to three sessions per week; committed exercisers: four to five sessions per week; and Masters athletes: six to seven sessions per week plus regular competitions. Detailed measurements of arterial stiffness and left ventricular afterload were collected. Biological aortic age and central pulse wave velocity were younger in committed exercisers and Masters athletes compared to sedentary seniors. Total arterial compliance index (TACi) was lower, while carotid β‐stiffness index and effective arterial elastance were higher in sedentary seniors compared to the other groups. There appeared to be a dose–response threshold for carotid β‐stiffness index and TACi. Peripheral arterial stiffness was not significantly different among the groups. These data suggest that four to five weekly exercise sessions over a lifetime is associated with reduced central arterial stiffness in the elderly. A less frequent dose of lifelong exercise (two to three sessions per week) is associated with decreased ventricular afterload and peripheral resistance, while peripheral arterial stiffness is unaffected by any dose of exercise.
Keywords: arterial stiffness, exercise training, aging
Key points
This study examined the effect of different ‘doses’ of lifelong (>25 years) exercise on arterial stiffening (a hallmark of vascular ageing) in older adults.
There are clear dose‐dependent effects of lifelong exercise training on human arterial stiffness that vary according to the site and size of the arteries.
Similar to what we have observed previously with ventricular stiffening, 4–5 days week−1 of committed exercise over a lifetime are necessary to preserve ‘youthful’ vascular compliance, especially of the large central arteries.
Casual exercise training of two to three times per week may be sufficient for middle‐sized arteries like the carotid to minimize arterial stiffening with ageing.
However, there is little effect of exercise training on the small‐sized peripheral arteries at any dose.
Introduction
A notable consequence of sedentary ageing is large‐vessel arterial stiffening. This pathophysiological process is characterized by the development of fibrosis and collagen cross‐linked products in the arterial wall. Central arterial stiffening increases the risk of cardiovascular‐related morbidity and mortality in older adults (Vlachopoulos et al. 2010); thus, the development of strategies to forestall age‐associated cardiovascular (CV) diseases has important clinical implications.
Sustained, regular endurance exercise training is one such favourable strategy. We and others have previously shown that Masters athletes who have performed near‐daily (6–7 sessions per week) vigorous endurance exercise training plus competition for the majority of their adult lives have more compliant central arteries compared to their sedentary peers (Vaitkevicius et al. 1993; Gates et al. 2003; Shibata & Levine, 2011, 2012). While these findings support others (Arbab‐Zadeh et al. 2004; Bhella et al. 2014) from our laboratory that underscore the critical role of sustained, lifelong physical activity in mitigating ventricular and arterial stiffening with ageing, the rigorous training and competition routine of Masters athletes is not feasible or practical for most individuals.
Previously, we showed in these same subjects that a less frequent ‘dose’ of lifelong exercise training (4–5 sessions per week for >30 min) that is consistent with current physical activity recommendations (∼150 min week−1) prevents cardiac atrophy and stiffening associated with sedentary ageing (Bhella et al. 2014). Moreover, this volume of exercise training was associated with higher maximal oxygen uptake (), stroke index and effective arterial elastance index (E ai) during exercise (Carrick‐Ranson et al. 2014). Since resting E a increases with sedentary ageing as a result of ventricular and central arterial stiffening (Redfield et al. 2005), four to five sessions per week of dynamic exercise over a lifetime may also be an effective and practical exercise frequency to prevent both ventricular and central arterial stiffening with ageing. However, the effects of different doses of life‐long exercise training on arterial compliance with ageing is still unclear.
Accordingly, to test the hypothesis that a threshold dose of four to five weekly exercise sessions over a lifetime would be associated with a reduction in central arterial stiffness, we performed a cross‐sectional examination of detailed measurements of central arterial stiffness in seniors (>60 years) who had performed a consistent frequency of exercise training for >25 years, focusing on weekly exercise frequency as an index of exercise dose.
Methods
Ethical approval
All subjects signed an informed consent approved by the institutional review boards of the University of Texas Southwestern and Texas Health Resources Presbyterian Hospital of Dallas and performed in accordance with the Declaration of Helsinki.
Subject recruitment
Details of the subject recruitment process and study design were reported previously (Bhella et al. 2014; Carrick‐Ranson et al. 2014). One hundred and two healthy seniors were recruited and stratified into four groups based on lifelong frequency of exercise training. Sedentary subjects (n = 27) exercised no more than once per week during the previous 25 years, ‘casual’ exercisers (n = 25) engaged in two to three sessions per week, ‘committed’ exercisers (n = 25) performed four to five sessions per week and competitive Masters athletes (n = 25) trained six to seven times per week and participated in regular competitions. Exercise sessions were defined as periods of aerobic exercise of at least 30 min.
Subjects were recruited primarily from the Cooper Center Longitudinal Study (CCLS; Wei et al. 1999), a cohort of more than 80,000 individuals in whom physical activity and CV risk factors have been quantified and followed for >40 years. Using the CCLS database, investigators identified healthy subjects who had consistently reported the same level of regular exercise on clinic questionnaires over multiple visits spanning at least 20 years. Interested subjects underwent a comprehensive exercise history examination conducted by an experienced exercise physiologist and assisted by family members when possible. If exercise histories could be corroborated, subjects were invited to participate in the next phase of screening. The sedentary population was enriched with subjects recruited from local senior groups such as bingo, gardening, volunteer groups and health fairs (most subjects in this group came from non‐Cooper Clinic sources). The Masters athlete population was enriched by direct recruitment from the top performers (10–15%) at regional and national endurance events (Arbab‐Zadeh et al. 2004) with most selected from race results. Regardless of the source of referral, however, all subjects were equally well vetted and rigorously screened in terms of medical history, physical examination and detailed exercise training history.
All recruited subjects underwent the following screening protocol. First, a medical history and physical exam were recorded by a study physician and/or nurse. Obesity (BMI > 30 kg m−2), regular tobacco use within the past 10 years, hypertension (24 h ambulatory blood pressure > 140/90 mmHg), diabetes, chronic obstructive pulmonary disease, atrial fibrillation, obstructive coronary artery disease or significant valvular disease were exclusion criteria. Second, an exercise stress test was performed on all subjects, with ECG or echocardiography changes suggestive of ischaemia or abnormal wall motion criteria for exclusion.
Assessment of arterial stiffness
Biological aortic age
Biological aortic age was determined from the central aortic arterial pressure waveform using the Modelflow algorithm as previously described (Shibata & Levine, 2011). First, using input ages from 20 to 90 years, Modelflow stroke volume (SV) was generated from a central blood pressure waveform reconstructed from a finger blood pressure waveform (Beatscope 1.1a; Finapres Medical Systems, Enschede, the Netherlands; Wesseling et al. 1993). Biological aortic age was then determined by an inverse function of the linear regression between input age and generated Modelflow SV equation by using SV from the acetylene rebreathing method (Jarvis et al. 2007) as an input signal. This index has been previously validated in our laboratory, demonstrating a high age specificity in sedentary adults, and high reproducibility in response to changes in haemodynamic loading conditions (Shibata & Levine, 2012).
Pulse wave velocity
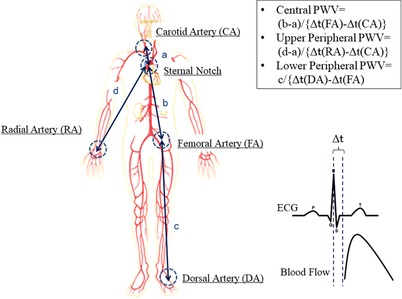
Central, and upper and lower limb peripheral pulse wave velocity (PWV) was measured with Doppler ultrasound (iE 33, Phillips, Amsterdam, the Netherlands) and calculated as the distance between measurement sites divided by the time delay between the two waveforms (Laurent et al. 2006). Pulse transit time was calculated by subtracting the time between the peak of the R‐wave and the foot of the carotid flow profile from the time between the peak of the R‐wave and the foot of the femoral flow for central PWV, and carotid from radial flow profiles and femoral from dorsal flow profiles for upper limb and lower limb peripheral PWV, respectively. The peak of the R‐wave and the foot of the flow profile were visually determined by the same researcher. The distance between arterial measurement sites was calculated by subtracting the distance between the carotid site and the sternal notch from the distance between the sternal notch and the femoral site, and between the sternal notch and the radial site for central and upper limb peripheral PWV, and between femoral and dorsal for lower limb peripheral PWV, respectively. Central PWV (carotid–femoral) and upper limb peripheral PWV (carotid–radial) were also measured with SphygmoCor Mx device (AtCor Medical, Itasca, IL, USA). Blood pressure waveforms were measured just before Doppler flow measurements and these pressure waveforms were used for analysing augmentation indices and carotid artery stiffness indices. Intraclass correlation coefficient (ICC) of central PWV between the Doppler method and SphygmoCor was relatively high (ICC: 0.742, 95% confidence interval: 0.576–0.843), while that of upper limb peripheral PWV was low (ICC: 0.168, 95% confidence interval: −0.350 to 0.488).
Local arterial stiffness
β‐Stiffness index and distensibility coefficient of the common carotid artery were calculated from systolic and diastolic carotid dimensions and pressures (Hirai et al. 1989; Laurent et al. 2006). Sequential measurement of right common carotid and brachial pressure waveforms with applanation tonometry (SphygmoCor, Mx) was immediately followed by brachial arm cuff blood pressure measurement (Korotkoff sounds detected using electrosphygmomanometry; SunTech Medical, Morrisville, NC, USA). Systemic diastolic and mean blood pressures were estimated from the brachial blood pressure waveform calibrated with arm‐cuff systolic and diastolic blood pressures. These mean and diastolic blood pressures were used to calibrate a right common carotid blood pressure waveform to obtain carotid systolic (P s) and diastolic blood pressures (P d) (Kelly & Fitchett, 1992; Laurent et al. 2006). The cross‐sectional area of the right common carotid artery was measured from the images acquired with a high‐resolution (Sono‐CT) linear‐array ultrasound (Phillips iE33) transducer (∼ 9 MHz). The measurements were made 1–2 cm proximal to the carotid bulb, with the transducer placed at a 90° angle to the vessel so that near and far wall interfaces were clearly discernible. Acoustic quantification was applied for the edge detection of the internal arterial wall (Q‐Lab, Phillips), and maximum and minimum areas were to be considered systolic (A s) and diastolic (A d) areas.
Ascending and descending aortic β‐stiffness indices were calculated from simultaneous cardiac MRI aortic cross‐sectional area and brachial cuff blood pressure measurements. Moreover, ascending and descending aortic strain were also calculated only from the aortic cross‐sectional area since central blood pressures were not available during the MRI measurement. MRI of the aortic arch was obtained in the transverse plane at the level of the right pulmonary artery using a 1.5 T clinical magnetic resonance scanner (NT model; Philips Corp., Amsterdam, the Netherlands) to assess aortic pulsative dimensions (Redheuil et al. 2011). MRI data were analysed using commercially available software (Q‐flow, NEXA Group Pty Ltd, New South Wales, Australia) and maximum and minimum areas were considered systolic (A s) and diastolic (A d) areas. The β‐stiffness index of the carotid artery and the ascending and descending aortas, the distensibility coefficient of the carotid artery and the strain of the ascending and descending aortas were calculated by the following equations, respectively: β‐stiffness index=ln(Ps/Pd)/{(As‐Ad)/Ad}, distensibility coefficient={(As‐Ad)/Ad}/(Ps‐Pd), and Strain=(As‐Ad)/Ad.
Augmentation index
Carotid augmentation blood pressure was quantified as the difference between the first and second systolic peaks (Pauca et al. 2001; Laurent et al. 2006). The carotid blood pressure augmentation index was calculated as augmentation pressure expressed as a percentage of the pulse pressure determined with SphygmoCor Mx (Pauca et al. 2001; Laurent et al. 2006). The central augmentation pressure and index were calculated with a central pressure waveform reconstructed from radial blood pressure waveform by the inverse transfer function method with SphygmoCor Mx (Chen et al. 1997; Pauca et al. 2001). To reduce the confounding effect of heart rate, augmentation indices were additionally normalized to a heart rate of 75 bpm.
Total arterial compliance, effective arterial elastance and total peripheral resistance
Total arterial compliance (TAC) was calculated from stroke volume (SV) using the acetylene rebreathing method (Jarvis et al. 2007) divided by central arterial pulse pressure reconstructed from the radial blood pressure waveform (SV/central pulse pressure). E a and total peripheral resistance (TPR) were calculated as central systolic pressure/SV and 80 × cardiac output/central mean pressure, respectively (Sunagawa et al. 1983; Kelly et al. 1992; Chemla et al. 2003). To reduce the confounding effect of body size, stroke index (SVi) was used for the calculation of TACi, E ai and TPRi.
Statistics
A one‐way analysis of variance (ANOVA) was used to determine differences in variables among the four groups. Post‐hoc analysis (Student–Newman–Keuls method) was performed when a significant main effect was found. The partial η2 of the one‐way ANOVA was used to estimate effect size for primary outcome variables. A chi‐square test was used to determine gender differences between groups. Commercially available software was used to perform all analyses (SigmaStat 3.5, Systat Software, Inc., San Jose, CA, USA; SPSS 22.0, IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant for ANOVA and post hoc analysis. Data are presented as the mean ± standard deviation in tables and figures.
Results
Subjects’ characteristics
Age and male/female ratio were not significantly different among groups (Table 1). The subjects were predominantly white (one black for casual and one Asian for committed). Body mass index, total body mass and body fat were significantly lower in Masters athletes, while maximal oxygen uptake () increased in a dose‐dependent manner as reported previously (Bhella et al. 2014; Carrick‐Ranson et al. 2014; Table 1).
Table 1.
Basic subjects characteristics
| Sedentary Subjects (Q1) | Casual exercisers (Q2) | Committed exercisers (Q3) | Competitive exercisers (Q4) | ANOVA/χ2 | Post hoc analysis | Effect size, partial η2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 vs. Q2 | Q1 vs. Q3 | Q1 vs. Q4 | Q2 vs. Q3 | Q2 vs. Q4 | Q3 vs. Q4 | |||||||
| Subjects (sex, m/f) | 27 (15/12) | 25 (18/7) | 25 (20/5) | 25 (17/8) | 0.286 | |||||||
| Age (years) | 70 ± 6 | 70 ± 6 | 68 ± 6 | 70 ± 4 | 0.486 | 0.024 | ||||||
| Height (cm) | 169 ± 10 | 174 ± 10 | 173 ± 8 | 171 ± 10 | 0.327 | 0.034 | ||||||
| Weight (kg) | 75 ± 11 | 76 ± 14 | 73 ± 11 | 66 ± 12 | 0.015 | 0.741 | 0.72 | 0.023 | 0.777 | 0.019 | 0.024 | 0.100 |
| Body fat (%) | 32 ± 8 | 30 ± 7 | 29 ± 5 | 22 ± 7 | <0.001 | 0.23 | 0.209 | <0.001 | 0.61 | <0.001 | 0.002 | 0.225 |
| Body mass index (kg m−2) | 25.9 ± 2.5 | 25.0 ± 2.9 | 24.3 ± 2.9 | 22.2 ± 2.4 | <0.001 | 0.183 | 0.084 | <0.001 | 0.424 | 0.002 | 0.006 | 0.214 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.2 | 0.058 | 0.073 | ||||||
| (ml kg−1 min−1) | 23.7 ± 4.9 | 25.8 ± 4.8 | 32.0 ± 5.8 | 39.5 ± 5.3 | <0.001 | 0.145 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.594 |
| Ambulatory SBP (mmHg) | 125 ± 8 | 124 ± 7 | 125 ± 8 | 122 ± 11 | 0.639 | 0.019 | ||||||
| Ambulatory DBP (mmHg) | 73 ± 6 | 71 ± 6 | 74 ± 7 | 74 ± 6 | 0.406 | 0.033 | ||||||
, maximal oxygen consumption; ambulatory SBP, average systolic blood pressure of 24 h ambulatory blood pressures; ambulatory DBP, average diastolic blood pressure of 24 h ambulatory blood pressures; ANOVA, analysis of variance; χ2, chi‐square test. Post hoc analysis was performed by the Student–Newman–Keuls test.
Blood pressure, stroke volume and related indices
Resting SV and SVi increased with a greater frequency of lifelong exercise training (Table 2). Arm cuff measures of systolic blood pressure tended to be higher, and central systolic and mean blood pressures were significantly higher in sedentary seniors (P ≤ 0.016 versus casual and committed exercisers) (Table 2). TACi was lower, while E ai was higher in sedentary seniors compared to the other groups (Table 2). Augmentation indices were not different among the groups (Table 3).
Table 2.
Blood pressures, stroke volume, and their related indexes
| Sedentary subjects (Q1) | Casual exercisers (Q2) | Committed exercisers (Q3) | Competitive exercisers (Q4) | ANOVA | Post hoc analysis | Effect size, partial η2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 vs. Q2 | Q1 vs. Q3 | Q1 vs. Q4 | Q2 vs. Q3 | Q2 vs. Q4 | Q3 vs. Q4 | |||||||
| Cuff SBP (mmHg) | 126 ± 16 | 117 ± 12 | 119 ± 13 | 121 ± 15 | 0.083 | 0.066 | ||||||
| Cuff DBP (mmHg) | 74 ± 8 | 71 ± 7 | 69 ± 8 | 71 ± 9 | 0.172 | 0.049 | ||||||
| HR (bpm) | 66 ± 11 | 62 ± 7 | 58 ± 9 | 56 ± 7 | <0.001 | 0.088 | 0.004 | <0.001 | 0.129 | 0.063 | 0.452 | 0.165 |
| SV (ml) | 76 ± 24 | 80 ± 17 | 85 ± 18 | 89 ± 20 | 0.083 | 0.066 | ||||||
| SVi (ml m−2) | 40 ± 10 | 42 ± 7 | 45 ± 9 | 50 ± 8 | <0.001 | 0.454 | 0.08 | <0.001 | 0.165 | 0.002 | 0.037 | 0.180 |
| Central SP (mmHg) | 115 ± 12 | 106 ± 10 | 106 ± 13 | 110 ± 11 | 0.011 | 0.015 | 0.015 | 0.082 | 0.822 | 0.408 | 0.293 | 0.108 |
| Central DP (mmHg) | 71 ± 10 | 66 ± 6 | 67 ± 9 | 69 ± 8 | 0.077 | 0.067 | ||||||
| Central MP (mmHg) | 90 ± 11 | 82 ± 7 | 83 ± 9 | 86 ± 8 | 0.009 | 0.013 | 0.016 | 0.124 | 0.784 | 0.287 | 0.217 | 0.110 |
| Central TACi (ml m−2 mmHg−1) | 0.88 ± 0.33 | 1.01 ± 0.23 | 1.11 ± 0.33 | 1.09 ± 0.22 | 0.014 | 0.089 | 0.018 | 0.022 | 0.42 | 0.336 | 0.769 | 0.102 |
| Central E ai (mmHg ml−1 m−2) | 3.06 ± 0.83 | 2.58 ± 0.43 | 2.44 ± 0.60 | 2.23 ± 0.44 | <0.001 | 0.005 | <0.001 | <0.001 | 0.406 | 0.108 | 0.23 | 0.217 |
| Central TPRi (dynes s cm−5) | 2869 ± 614 | 2606 ± 472 | 2625 ± 569 | 2525 ± 623 | 0.159 | 0.051 | ||||||
Cuff SBP, systolic blood pressure measured by arm cuff; cuff DBP, diastolic blood pressure measured by arm cuff; HR, heart rate; SV, stroke volume; SVi, stroke volume index; central SP, central systolic pressure reconstructed from radial pressure waveform; central DP, central diastolic pressure reconstructed from radial pressure waveform; central MP, central diastolic pressure reconstructed from radial pressure waveform; central TACi, total arterial compliance index calculated from reconstructed central blood pressure; central E ai, effective arterial elastance index calculated from reconstructed central blood pressure; central TPRi, total peripheral resistance index calculated from reconstructed central blood pressure.
Table 3.
Arterial compliance indexes
| Sedentary subjects (Q1) | Casual exercisers (Q2) | Committed exercisers (Q3) | Competitive exercisers (Q4) | ANOVA | Post hoc analysis | Effect size, partial η2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 vs. Q2 | Q1 vs. Q3 | Q1 vs. Q4 | Q2 vs. Q3 | Q2 vs. Q4 | Q3 vs. Q4 | |||||||
| The Modelflow aortic age (years) | 67 ± 14 | 66 ± 15 | 56 ± 20 | 43 ± 14 | <0.001 | 0.854 | 0.066 | <0.001 | 0.044 | <0.001 | 0.005 | 0.258 |
| Central PWV (m s−1) | 10.6 ± 3.6 | 9.8 ± 1.9 | 8.4 ± 1.8 | 7.8 ± 1.1 | <0.001 | 0.219 | 0.004 | <0.001 | 0.038 | 0.009 | 0.354 | 0.191 |
| U‐Peripheral PWV (m s−1) | 9.2 ± 1.8 | 9.6 ± 1.5 | 9.5 ± 1.9 | 9.0 ± 1.5 | 0.575 | 0.021 | ||||||
| L‐Peripheral PWV (m s−1) | 10.8 ± 1.3 | 10.5 ± 1.6 | 10.3 ± 1.3 | 10.5 ± 1.4 | 0.82 | 0.018 | ||||||
| Central PWV (Sphy) (m s−1) | 8.7 ± 1.6 | 8.9 ± 1.7 | 8.1 ± 1.2 | 7.9 ± 1.1 | 0.171 | 0.076 | ||||||
| Peripheral PWV (Sphy) (m s−1) | 7.7 ± 1.4 | 7.9 ± 1.2 | 7.8 ± 1.5 | 8.2 ± 0.9 | 0.73 | 0.020 | ||||||
| PWA‐P (central) (mmHg) | 12 ± 6 | 9 ± 4 | 12 ± 7 | 13 ± 4 | 0.265 | 0.061 | ||||||
| PWA‐I (central) (unit) | 26 ± 9 | 23 ± 10 | 27 ± 11 | 29 ± 9 | 0.387 | 0.047 | ||||||
| PWI @ HR75 (central) (unit) | 21 ± 9 | 17 ± 9 | 18 ± 11 | 21 ± 9 | 0.443 | 0.041 | ||||||
| PWA‐P (carotid) (mmHg) | 9 ± 6 | 5 ± 8 | 8 ± 7 | 9 ± 3 | 0.194 | 0.072 | ||||||
| PWA‐I (carotid) (unit) | 21 ± 13 | 13 ± 17 | 19 ± 16 | 21 ± 6 | 0.277 | 0.059 | ||||||
| PWI @ HR75 (carotid) (unit) | 15 ± 11 | 6 ± 16 | 9 ± 16 | 11 ± 7 | 0.232 | 0.065 | ||||||
| Carotid systolic area (mm2) | 49 ± 11 | 45 ± 7 | 48 ± 8 | 42 ± 7 | 0.017 | 0.142 | 0.745 | 0.03 | 0.113 | 0.363 | 0.036 | 0.101 |
| Carotid diastolic area (mm2) | 45 ± 9 | 40 ± 7 | 44 ± 8 | 38 ± 6 | 0.005 | 0.062 | 0.582 | 0.009 | 0.082 | 0.344 | 0.022 | 0.126 |
| Carotid beta stiffness | 7.0 ± 2.7 | 5.6 ± 2.1 | 5.9 ± 2.0 | 5.1 ± 1.3 | 0.014 | 0.047 | 0.052 | 0.009 | 0.657 | 0.41 | 0.414 | 0.106 |
| Carotid distensibility coefficient | 13.5 ± 5.0 | 18.4 ± 7.2 | 16.7 ± 4.4 | 17.8 ± 4.0 | 0.008 | 0.008 | 0.034 | 0.015 | 0.495 | 0.663 | 0.487 | 0.117 |
| Ascending systolic area (mm2) | 957 ± 213 | 977 ± 192 | 1022 ± 179 | 928 ± 219 | 0.449 | 0.030 | ||||||
| Ascending diastolic area (mm2) | 911 ± 211 | 927 ± 197 | 959 ± 179 | 863 ± 215 | 0.443 | 0.030 | ||||||
| Ascending beta stiffness (unit) | 13.5 ± 8.1 | 13.7 ± 8.8 | 10.6 ± 7.5 | 9.2 ± 5.7 | 0.125 | 0.063 | ||||||
| Ascending strain (%) | 5.2 ± 2.9 | 5.9 ± 4.6 | 6.9 ± 4.4 | 7.9 ± 4.4 | 0.17 | 0.056 | ||||||
| Descending systolic area (mm2) | 579 ± 104 | 577 ± 97 | 616 ± 130 | 584 ± 115 | 0.608 | 0.021 | ||||||
| Descending diastolic area (mm2) | 538 ± 114 | 534 ± 94 | 570 ± 130 | 537 ± 116 | 0.69 | 0.017 | ||||||
| Descending beta stiffness (unit) | 10.4 ± 8.2 | 7.4 ± 3.5 | 8.0 ± 5.2 | 7.5 ± 4.8 | 0.28 | 0.043 | ||||||
| Descending strain (%) | 8.5 ± 5.7 | 8.2 ± 3.6 | 8.7 ± 4.7 | 9.3 ± 4.4 | 0.866 | 0.008 | ||||||
PWV, pulse wave velocity; U‐Peripheral, upper limb peripheral; L‐Peripheral; lower limb peripheral; Sphy, estimated by SphygmoCor; PWA‐P, pulse wave analysis augmentation pressure; PWA‐I, pulse wave analysis augmentation index; PWI@HR75, augmenation index at heart rate of 75 beats min−1; ANOVA, analysis of variance. Post hoc analysis was performed by the Student–Newman–Keuls test.
Arterial stiffness
Biological aortic age was younger, while central PWV was lower in committed and Masters athletes compared to a lower frequency of lifelong exercise training (P ≤ 0.066 versus casual exercisers and sedentary seniors) (Figs. 1 and 2). In contrast, carotid artery β‐stiffness index was significantly higher in sedentary seniors compared to the other groups (P ≤ 0.047), but not between the three exercise‐trained groups (Fig. 3). Similarly, the carotid distensibility coefficient was significantly lower in sedentary seniors compared to the other groups. Upper and lower limb peripheral PWVs were not significantly different among groups (Table 3).
Figure 1. The Modelflow aortic age for sedentary subjects (Q1, n = 27), casual exercisers (Q2, n = 25), committed exercisers (Q3, n = 25) and competitive exercisers (Q4, n = 25).
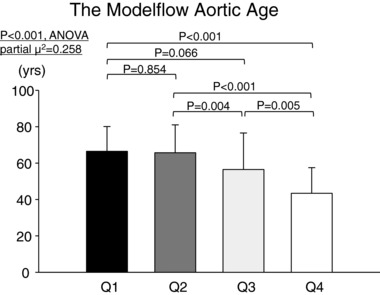
P‐values are derived from post hoc analysis (Student–Newman–Keuls method).
Figure 2. Central pulse wave velocity for sedentary subjects (Q1, n = 27), casual exercisers (Q2, n = 25), committed exercisers (Q3, n = 25) and competitive exercisers (Q4, n = 25).
P‐values are derived from post hoc analysis (Student–Newman–Keuls method).
Figure 3. Carotid β‐stiffness index for sedentary subjects (Q1, n = 27), casual exercisers (Q2, n = 25), committed exercisers (Q3, n = 25) and competitive exercisers (Q4, n = 25).
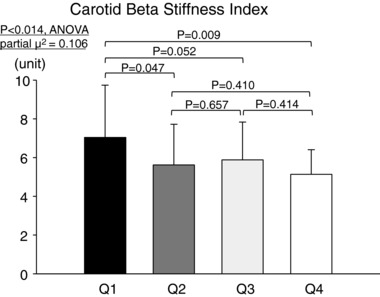
P‐values are derived from post hoc analysis (Student–Newman–Keuls method).
Discussion
The major findings from the present study were as follows: (1) four to five weekly sessions of exercise over a lifetime was associated with a reduction in central arterial stiffness in seniors, similar to what we have previously observed regarding myocardial stiffness; (2) a lifelong casual exercise frequency (2–3 sessions per week) was associated with lower carotid artery stiffness, left ventricular afterload (E ai) and central blood pressures in the seniors, while this dose of exercise training did not affect the central arterial stiffness; and (3) peripheral arterial stiffness was unaffected by lifelong exercise training, irrespective of ‘dose’. These current findings extend previous observations from our laboratory (Bhella et al. 2014; Carrick‐Ranson et al. 2014) and others (Vaitkevicius et al. 1993; Gates et al. 2003) underscoring the favourable effects of aerobic exercise for > 30 min, four to five times per week throughout a lifetime on the adverse consequences of ageing on CV stiffening.
Central artery stiffness
In the present study, central arterial stiffness was comprehensively examined using established approaches such as central PWV and the aortic β‐stiffness index, as well as a newer index estimating the biological (as opposed to the chronological) age of the aorta. Previous work from our laboratory demonstrates that this latter approach, which conceptually reflects structural changes of the aortic wall, accurately identifies sedentary ageing and lifelong exercise training‐related changes in aortic stiffness (Shibata & Levine, 2011). It is especially compelling that all indices studied in the current work, which used completely different sensors and analytic approaches to quantify large vessel stiffening, found similar patterns in large vessel arterial stiffening in response to graded lifelong exercise frequency; specifically, engageing in more than three sessions per week over a lifetime resulted in more ‘youthful’ measures compared to sedentary seniors, which could be estimated to be approximately 10 and 25 years younger in committed exercisers and competitive Masters athletes, respectively. These findings are important as large epidemiological studies have shown that central arterial stiffness assessed by PWV is a strong independent predictor of CV‐related and all‐cause mortality in older adults (Vlachopoulos et al. 2010). Moreover, although the clinical impact of biological aortic age for predicting future CV events has not been confirmed by large epidemiological studies, this index provides strong physiological confirmation that changes in central arterial stiffness with exercise training measured in this and other studies (Vaitkevicius et al. 1993; Tanaka et al. 1998; Tanaka et al. 2000; Gates et al. 2003; Pierce et al. 2013) are not secondary to central blood pressure changes.
In contrast, the central aortic β‐stiffness index was not significantly improved with lifelong exercise training, irrespective of weekly exercise frequency. The apparent absence of a training effect in our findings may be related to study methodology including the use of cuff pressure rather than central blood pressure, due to the technical difficulties in using the tonometry instrumentation in the MRI due to the strong magnetic field. The simultaneous collection of aortic dimensions and central blood pressure would provide the most precise assessment of aortic β‐stiffness. Thus, given that we found divergent effects on cuff and central blood pressures in relation to lifelong exercise frequency, some caution should be exercised regarding the interpretation of the current aortic β‐stiffness findings.
Carotid artery stiffness
We found that the carotid β‐stiffness index was higher in sedentary seniors, but similar among the exercise‐trained groups, suggesting that only small beneficial effects are obtained above a low frequency of lifelong exercise training (2–3 sessions per week). Thus, a large beneficial effect was obtained with relatively low levels of exercise, which is consistent with what has been previously reported in several epidemiological studies of exercise and CV risk. However, Tanaka et al. (2000) found that carotid β‐stiffness index was significantly improved only in highly endurance trained (≥ 5 sessions per week) but not in recreationally active (3–4 sessions per week) senior men compared to their sedentary peers. We speculate that the discrepancy between study findings is related to methodological differences in the exercise histories of the exercise trained subjects, as Tanaka et al. examined senior men with a consistent exercise history of > 2 years, while we examined senior men and women with a consistent exercise training history > 25 years. This point is important, as the age when exercise training is begun may determine the effectiveness of exercise training to improve CV structure and function, particularly in properties like ventricular or large blood vessel compliance (Fujimoto et al. 2010; Shibata & Levine, 2012). Therefore, the initiation of exercise training earlier in life coupled with a longer exercise training history may explain why we observed significant improvements in carotid stiffening with a less frequent dose of lifelong exercise training.
Similar to carotid β‐stiffness, systolic blood pressures were increased in sedentary seniors. Since the carotid sinus is the site of the carotid baroreflex, it is possible that improved baroreflex function due to a compliant carotid artery lowers systolic blood pressure. In addition, central systolic blood pressure is influenced by alterations in the interaction between forward traveling and reflected waves, mostly by reductions in timing and amplitude of reflected waves. Although augmentation indices did not show significant differences, changes in these factors may be another underlying mechanism of lower central blood pressure. These findings suggest that even a modest frequency of lifelong exercise training provokes favourable effects in central blood pressure regulatory mechanisms.
Peripheral artery stiffness
In contrast to central arterial stiffness, we were unable to show any clear effect on peripheral blood vessels. Previous studies have reported conflicting findings regarding the effect of aerobic exercise training on peripheral arterial stiffness, with significant improvements reported in young, healthy middle‐aged (Rakobowchuk et al. 2008; Currie et al. 2009) or pre‐hypertensive individuals (Collier et al. 2008), while either no effect or a very small favourable effect has been observed in healthy adults of varying ages and fitness levels (Tanaka et al. 1998; Hayashi et al. 2005; Cook et al. 2006).
Differences among previous findings appear to be influenced by study design, as the majority of longitudinal training studies have shown positive findings (Collier et al. 2008; Rakobowchuk et al. 2008; Currie et al. 2009), while cross‐sectional examinations including the current study report no effect (Tanaka et al. 1998; Cook et al. 2006). A likely explanation for these findings is that the inter‐individual difference in peripheral arterial compliance is larger than that of intra‐individual difference resulting from exercise training. The smaller effect size of peripheral PWV (partial η2 = 0.021 for upper limb and 0.018 for lower limb) compared to central PWV (partial η2 = 0.191) observed in the present study supports this contention.
In addition, we visually detect the foot of flow waveform to calculate time differences. More sophisticated approaches such as the intersecting tangents or second derivative method may have revealed positive findings by reducing variability.
Potential underlying mechanisms
Arteriosclerosis with sedentary ageing is characterized by profound structural remodelling of the arterial wall including the accumulation of connective tissue and extracellular collagen cross‐linked products (advanced glycation end‐products), and the degeneration of elastin (Lakatta, 2003; Lakatta & Levy, 2003). Previously, we have reported that biological aortic age is younger, indicative of more compliant central blood vessels in Masters athletes compared to age‐matched sedentary controls (Shibata & Levine, 2011). This finding provides evidence that sustained, vigorous lifelong endurance exercise training may inhibit the adverse vascular remodelling associated with human ageing. Conversely, 1 year of exercise training, encompassing over 200 min per week of moderate and high‐intensity exercise, failed to substantially improve biological aortic age in previously sedentary seniors (Shibata & Levine, 2012). This latter finding suggests that the age‐related changes in large elastic blood vessel structure are not reversible by exercise training alone when initiated later in life.
Vascular functional adaptations, which are influenced by smooth muscle tone and endothelial function, also characterize the improved arterial compliance with exercise training. For example, endothelial function is improved with lifelong exercise training and with several months of exercise training in previously sedentary seniors (Luk et al. 2012; Shibata & Levine, 2012; Cornelissen et al. 2014; Kim et al. 2014). It is likely that the peripheral vasculature, particularly the arterioles where the majority of vascular resistance is produced, is strongly influenced by these training‐related effects. In the present study, total arterial compliance, blood pressures and cardiac afterload, which are all influenced by smooth muscle tone, were improved with even a modest (2–3 sessions per week) amount of lifelong exercise. These current findings are similar to that reported with 1 year of endurance training in previously sedentary seniors (Shibata & Levine, 2012) suggesting that the functional components of arterial compliance appears to be more readily influenced by exercise training compared to structural components, particularly if exercise is initiated later in life.
Perspective
Given the importance of vascular stiffening to health and clinical outcomes with human ageing, it is important to develop strategies to forestall age‐related CV diseases. Exercise training is one approach; however, as noted in a recent review (Seals, 2014), the minimal and/or optimal dose of exercise training to preserve or improve vascular structure and function with human ageing has yet to be clearly established. The present findings constitute an important step in this process by demonstrating the minimal frequency of lifelong exercise required to preserve compliant central arteries in older age. Importantly, this minimum exercise frequency is consistent with and strengthens current recommendations for weekly physical activity (∼ 150 min week−1).
Moreover, several indices from the present study showed significant differences between sedentary and casual exercisers (carotid β‐stiffness index, central SP, central MP and E ai); however, there appears to be a dose–response threshold for carotid β‐stiffness index and TACi (i.e. no significant difference among the exercise‐trained groups). This finding emphasizes the clinical importance of a lesser amount (2–3 times per week) of weekly aerobic exercise on vascular stiffness and blood pressures.
According to previous large epidemiological studies, the reference values of PWV, carotid distensibility coefficient and central blood pressure in a healthy 70 year old were approximately 10.4 m s−1, 13.8 and 114 mmHg, respectively (Herbert et al. 2014; Cunha et al. 2015; Engelen et al. 2015). Thus, the sedentary subjects in this current study are comparable in terms of arterial stiffness and blood pressures to what has been previously reported in the general population, supporting the generalizability of the present findings.
Study limitations
First, this study did not invasively examine the structural and functional adaptations associated with lifelong exercise training frequency; only non‐invasive techniques were employed. While invasive assessment of vascular tissue by biopsy would provide the most compelling evidence of structural remodelling, this is not practical in healthy individuals. Second, factors (dietary intake, non‐exercise physical activity levels, social background, educational levels and economic status) not assessed in the current study may influence adherence to exercise training over a lifetime, and consequently arterial compliance. Moreover, small group differences in BP and BMI may have influenced the current findings even though these effects were not statistically significant and these variables were within normal ranges; therefore, we cannot exclude the possibility that the improved arterial compliance demonstrated in our trained subjects is achieved by more than exercise training alone. Accordingly, we also performed ANCOVA including BMI and central blood pressure as covariates and obtained similar results, indicating that effects of modifying these risk factors per se are likely to be small. Third, group allocation was based on lifelong exercise frequency, thus limiting any conclusions based on other components of an exercise training programme including intensity, duration or mode, all of which may have a profound impact on the vascular adaptations to exercise training. Fourth, this study was not designed to focus on sex difference of exercise effects. Thus, future studies are required to properly address sex differences. Our preliminary analysis did not show any significant interactions for sex and exercise frequency, while borderline significance was observed in the carotid artery β‐stiffness index and distensibility coefficient (P = 0.077 and P = 0.079, two‐way ANOVA). Fifth, our subjects were non‐obese and normotensive, and were carefully screened for CV disease; therefore, it is unclear whether these current results are applicable to a broader population of patients with greater amounts of co‐morbidities and CV disease risk factors. Sixth, we must acknowledge that there were some small differences in probability observed in quantifying the changes in central PWV among groups between the Doppler and SphygmoCor devices. Considering that both methods show similar directionality and magnitude, albeit it with different statistical probabilities, we are reasonably confident that the conclusion is correct.
Lastly, the Student–Newman–Keuls multiple comparison test was used for post hoc analysis in the present study. Since this test does not limit the chance of a Type I error at 5%, some positive results need to be carefully interpreted given the subject number in the present study.
Conclusions
In summary, these current findings suggest that > 4–5 weekly sessions of committed lifelong exercise is associated with a more ‘youthful’ levels of central artery compliance in the elderly. A lower frequency of lifelong exercise (2–3 sessions per week) is associated with improved carotid artery compliance and decreased left ventricular afterload. Irrespective of frequency, lifelong exercise training does not significantly influence peripheral arterial stiffness.
Additional information
Competing interests
There are no competing interests.
Author contributions
The experiments were performed in the Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas & the University of Texas Southwestern Medical Center at Dallas, TX, USA. S.S. and B.D.L. contributed to conception or design of the work, acquisition, analysis or interpretation of data for the work, and drafting the work or revising it critically for important intellectual content. N.F., J.L.H., G.C.R. and P.S.B. contributed to acquisition, analysis or interpretation of data for the work and drafting the work or revising it critically for important intellectual content. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by National Institutes of Health Grant R01 AG17479.
Biography
Shigeki Shibata had a great interest in the field of exercise physiology and ageing when he graduated from the School of Medicine, Niigata University (1998). After completing an initial clinical practice of anaesthesiology in Japan, he worked on a PhD in Anaesthesiology and Environmental Physiology focusing on cardiovascular physiology, exercise training and anaesthesia in Nihon University (2002–2006). In order to extend his skills and knowledge, he worked in the Institute for Exercise and Environmental Medicine (2005–2011). Currently, he is working in the School of Medicine, Kyorin University to further explore his original research interest.

Edited by Laura Bennet & Philip Ainslie
Linked articles This article is highlighted by a Perspective by Pierce. To read this article, visit https://doi.org/10.1113/JP276253.
This is an Editor's Choice article from the 15 July 2018 issue.
References
- Arbab‐Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D & Levine BD (2004). Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick‐Ranson G, Palmer MD, Boyd KN, Adams‐Huet B & Levine BD (2014). Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol 64, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick‐Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E & Levine BD (2014). The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol 116, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemla D, Antony I, Lecarpentier Y & Nitenberg A (2003). Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 285, H614–H620. [DOI] [PubMed] [Google Scholar]
- Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL & Kass DA (1997). Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 95, 1827–1836. [DOI] [PubMed] [Google Scholar]
- Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN & Fernhall B (2008). Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre‐ and stage‐1 hypertensives. J Hum Hypertens 22, 678–686. [DOI] [PubMed] [Google Scholar]
- Cook JN, DeVan AE, Schleifer JL, Anton MM, Cortez‐Cooper MY & Tanaka H (2006). Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol 290, H1596–H1600. [DOI] [PubMed] [Google Scholar]
- Cornelissen VA, Onkelinx S, Goetschalckx K, Thomaes T, Janssens S, Fagard R, Verhamme P & Vanhees L (2014). Exercise‐based cardiac rehabilitation improves endothelial function assessed by flow‐mediated dilation but not by pulse amplitude tonometry. Eur J Prev Cardiol 21, 39–48. [DOI] [PubMed] [Google Scholar]
- Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, Nilsson PM, Scuteri A & Sousa N (2015). Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hypertens 33, 1438–1445. [DOI] [PubMed] [Google Scholar]
- Currie KD, Thomas SG & Goodman JM (2009). Effects of short‐term endurance exercise training on vascular function in young males. Eur J Appl Physiol 107, 211–218. [DOI] [PubMed] [Google Scholar]
- Engelen L, Bossuyt J, Ferreira I, van Bortel LM, Reesink KD, Segers P, Stehouwer CD, Laurent S, Boutouyrie P; Reference Values for Arterial Measurements Collaboration (2015). Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens 33, 1981–1996. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Prasad A, Hastings JL, Arbab‐Zadeh A, Bhella PS, Shibata S, Palmer D & Levine BD (2010). Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation 122, 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Graves J & Seals DR (2003). Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J 24, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Sugawara J, Komine H, Maeda S & Yokoi T (2005). Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle‐aged sedentary men. Jpn J Physiol 55, 235–239. [DOI] [PubMed] [Google Scholar]
- Herbert A, Cruickshank JK, Laurent S, Boutouyrie P, Reference Values for Arterial Measurements Collaboration (2014). Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J 35, 3122–3133. [DOI] [PubMed] [Google Scholar]
- Hirai T, Sasayama S, Kawasaki T & Yagi S (1989). Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80, 78–86. [DOI] [PubMed] [Google Scholar]
- Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG & Pawelczyk JA (2007). Simultaneous determination of the accuracy and precision of closed‐circuit cardiac output rebreathing techniques. J Appl Physiol 103, 867–874. [DOI] [PubMed] [Google Scholar]
- Kelly R & Fitchett D (1992). Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol 20, 952–963. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS & Kass DA (1992). Effective arterial elastance as index of arterial vascular load in humans. Circulation 86, 513–521. [DOI] [PubMed] [Google Scholar]
- Kim C, Choi HE, Jung H, Kang SH, Kim JH & Byun YS (2014). Impact of aerobic exercise training on endothelial function in acute coronary syndrome. Ann Rehabil Med 38, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG ( 2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I & Struijker‐Boudier H (2006). Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27, 2588–2605. [DOI] [PubMed] [Google Scholar]
- Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Lee SW, Li SW, Fong B, Wong WK, Tam S, Lau CP & Tse HF (2012). Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol 19, 830–839. [DOI] [PubMed] [Google Scholar]
- Pauca AL, O'Rourke MF & Kon ND (2001). Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38, 932–937. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Casey DP, Fiedorowicz JG, Seals DR, Curry TB, Barnes JN, Wilson DR & Stauss HM (2013). Aortic pulse wave velocity and reflecting distance estimation from peripheral waveforms in humans: detection of age‐ and exercise training‐related differences. Am J Physiol Heart Circ Physiol 305, H135–H142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ & MacDonald MJ (2008). Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow‐mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol 295, R236–R242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ & Kass DA (2005). Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation 112, 2254–2262. [DOI] [PubMed] [Google Scholar]
- Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO, Bluemke D & Lima JA (2011). Age‐related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol 58, 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR ( 2014). Edward F. Adolph Distinguished Lecture: The remarkable anti‐aging effects of aerobic exercise on systemic arteries. J Appl Physiol 117, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S & Levine BD (2011). Biological aortic age derived from the arterial pressure waveform. J Appl Physiol 110, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S & Levine BD (2012). Effect of exercise training on biologic vascular age in healthy seniors. Am J Physiol Heart Circ Physiol 302, H1340–H1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa K, Maughan WL, Burkhoff D & Sagawa K (1983). Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 245, H773–H780. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA & Seals DR (1998). Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18, 127–132. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA & Seals DR (2000). Aging, habitual exercise, and dynamic arterial compliance. Circulation 102, 1270–1275. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC & Lakatta EG (1993). Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K & Stefanadis C (2010). Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol 55, 1318–1327. [DOI] [PubMed] [Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr & Blair SN (1999). Relationship between low cardiorespiratory fitness and mortality in normal‐weight, overweight, and obese men. JAMA 282, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ & Schreuder JJ (1993). Computation of aortic flow from pressure in humans using a nonlinear, three‐element model. J Appl Physiol 74, 2566–2573. [DOI] [PubMed] [Google Scholar]


