Abstract
Key points
Protective reflexes in the throat area (upper airway) are crucial for breathing.
Impairment of these reflexes can cause breathing problems during sleep such as obstructive sleep apnoea (OSA).
OSA is very common in people with spinal cord injury for unknown reasons.
This study shows major changes in protective reflexes that serve to keep the upper airway open in response to suction pressures in people with tetraplegia and OSA.
These results help us understand why OSA is so common in people with tetraplegia and provide new insight into how protective upper airway reflexes work more broadly.
Abstract
More than 60% of people with tetraplegia have obstructive sleep apnoea (OSA). However, the specific causes are unknown. Genioglossus, the largest upper‐airway dilator muscle, is important in maintaining upper‐airway patency. Impaired genioglossus muscle function following spinal cord injury may contribute to OSA. This study aimed to determine if genioglossus reflex responses to negative upper‐airway pressure are altered in people with OSA and tetraplegia compared to non‐neurologically impaired able‐bodied individuals with OSA. Genioglossus reflex responses measured via intramuscular electrodes to ∼60 brief (250 ms) pulses of negative upper‐airway pressure (∼−15 cmH2O at the mask) were compared between 13 participants (2 females) with tetraplegia plus OSA and 9 able‐bodied controls (2 females) matched for age and OSA severity. The initial short‐latency excitatory reflex response was absent in 6/13 people with tetraplegia and 1/9 controls. Genioglossus reflex inhibition in the absence of excitation was observed in three people with tetraplegia and none of the controls. When the excitatory response was present, it was significantly delayed in the tetraplegia group compared to able‐bodied controls: excitation onset latency (mean ± SD) was 32 ± 16 vs. 18 ± 9 ms, P = 0.045; peak excitation latency was 48 ± 17 vs. 33 ± 8 ms, P = 0.038. However, when present, amplitude of the excitation response was not different between groups, 195 ± 26 vs. 219 ± 98% at baseline, P = 0.55. There are major differences in genioglossus reflex morphology and timing in response to rapid changes in airway pressure in people with tetraplegia and OSA. Altered genioglossus function may contribute to the increased risk of OSA in people with tetraplegia. The precise mechanisms mediating these differences are unknown.
Keywords: spinal cord injury, sleep‐disordered breathing, upper airway physiology
Key points
Protective reflexes in the throat area (upper airway) are crucial for breathing.
Impairment of these reflexes can cause breathing problems during sleep such as obstructive sleep apnoea (OSA).
OSA is very common in people with spinal cord injury for unknown reasons.
This study shows major changes in protective reflexes that serve to keep the upper airway open in response to suction pressures in people with tetraplegia and OSA.
These results help us understand why OSA is so common in people with tetraplegia and provide new insight into how protective upper airway reflexes work more broadly.
Introduction
Obstructive sleep apnoea (OSA) is highly prevalent following spinal cord injury. Indeed, more than 60% of people with tetraplegia have OSA (Burns et al. 2000; Berlowitz et al. 2005; Chiodo et al. 2016). Increases in adipose tissue and neck circumference, altered neuromuscular and respiratory function, autonomic dysfunction, high nasal resistance and certain medications (e.g. CNS depressants) that are commonly used in people with spinal cord injury may increase the risk of OSA in people with tetraplegia (Fuller et al. 2013). However, while recent studies indicate a potential role for increased nasal resistance as a contributor to OSA for certain people with tetraplegia (Gainche et al. 2016; Wijesuriya et al. 2017), the specific pathophysiological factors that place people with tetraplegia at such a high risk of OSA are poorly understood.
OSA is characterised by recurrent upper airway collapse that leads to complete or partial cessation of breathing. Upper airway dilator muscles, including the largest airway dilator, genioglossus, are pivotal in the maintenance of upper airway patency (Horner et al. 1991a, 2014; White, 2005). Negative pressure during inspiration caused by diaphragm contraction can cause the upper airway to narrow. Both central and reflex activation of pharyngeal dilator muscles help to keep the upper airway open.
Able‐bodied people with OSA have increased genioglossus EMG activity awake compared to those without OSA (Mezzanotte et al. 1992; Fogel et al. 2001). Increased genioglossus muscle activity in people with OSA may be, at least in part, a neuromuscular compensatory response to having a narrow upper airway (Mezzanotte et al. 1992). Genioglossus activity is reduced during sleep onset in non‐neurologically impaired able‐bodied individuals (Worsnop et al. 2000). The reduction in activity at sleep onset is greater in people with OSA compared to those without OSA (Fogel et al. 2005). Thus, sleep‐related reductions in genioglossus muscle activity contribute to OSA pathogenesis. One mechanism that contributes to genioglossus activity is reflex activation from pressure‐sensitive mechanoreceptors in the upper airway (Horner et al. 1991a, b ). Other lung and chest wall stretch receptors may also be involved (Eckert et al. 2007; Carberry et al. 2015). Rapid changes in pharyngeal pressure cause an initial short‐latency activation followed by a sleep stage‐dependent inhibition phase (Eckert et al. 2007; Carberry et al. 2015). Poor genioglossus reflex function can impair airway dilatation and contribute to OSA (Eckert et al. 2013).
The genioglossus muscle and its sensory inputs are innervated by the cranial nerves. Thus, reflex function to changes in upper airway pressure should remain intact in people with spinal cord injury downstream from these local mechanisms. However, if the input from these afferents is altered after spinal cord injury or if other afferents below the level of the injury are also involved, genioglossus reflex responses may be altered in people with spinal cord injury and contribute, at least in part, to the high rates of OSA in this population. There are no data on genioglossus reflex responses to changes in upper airway pressure in people with spinal cord injury. Accordingly, this study aimed to carefully quantify genioglossus reflex responses (i.e. peak amplitude, latency and morphology) to rapid pulses of negative pressure in people with tetraplegia and determine potential differences in genioglossus reflex responses and airway collapsibility in people with tetraplegia and OSA compared to able‐bodied (non‐neurologically impaired) people with OSA.
Methods
Ethical approval
The study was performed in accordance with the ethical principles outlined in the Declaration of Helsinki (2013) for medical research involving humans, except for the registration in a database. All participants provided informed written or verbal consent as appropriate. The protocol was approved by the South Eastern Sydney Local Health District and Austin Health's Human Research Ethics Committees 14/032 (HREC/14/POWH/133).
Participants
Sixteen people with tetraplegia plus OSA and nine able‐bodied individuals with OSA were recruited from outpatient clinics from two spinal cord centres (Austin Health, Melbourne and Prince of Wales Hospital, Sydney) and from people living with spinal cord injury in the community via advertisement. OSA was defined as an apnoea/hypopnea index (AHI) of 10 events per hour of sleep or above (Berry et al. 2012). Tetraplegia was defined as an injury to the spinal cord at or above the level of T1 vertebra. All participants with tetraplegia were defined as motor complete injuries [American Spinal Injury Association Impairment Scale (AIS) A or B, Table 1]. Prior to the study, standard overnight polysomnography was conducted to assess OSA severity in each participant.
Table 1.
Individual characteristics for the participants with tetraplegia
| Subject number | Age (years) | Sex | AHI (no. h–1) | Injury level | AIS | Medications | Reflex activity (Yes/No) |
|---|---|---|---|---|---|---|---|
| 1 | 44 | M | 41 | C4 | B | Antispasmodic | Yes |
| 2 | 65 | M | 30 | C4 | A | Antispasmodic | No |
| 3 | 57 | M | 36 | C5–C6 | B | Yes | |
| 4 | 29 | M | 45 | C5–C6 | B | Yes | |
| 5 | 62 | M | 66 | C6 | B | Yes | |
| 6 | 46 | M | 36 | C6 | B | Antispasmodic | Yes |
| 7 | 47 | M | 16 | C5 | A | Suppression only | |
| 8 | 44 | F | 16 | C5 | A | Antispasmodic/benzodiazepam | Suppression only |
| 9 | 37 | M | 35 | C4 | A | Antidepressant | No |
| 10 | 65 | M | 12 | C7 | A | Antidepressant | Yes |
| 11 | 69 | M | 47 | C6 | B | Antidepressant | Suppression only |
| 12 | 36 | M | 76 | C5 | A | Antispasmodic | Yes |
| 13 | 40 | F | 31 | C5 | A | No |
AHI = apnoea/hypopnea index, AIS = ASIA (American Spinal Injury Association) impairment scale, F = female, M = male.
Equipment and measurements
Genioglossus EMG was measured using two intramuscular bipolar electrodes. The electrodes were stainless steel Teflon‐coated wires (0.33 mm in diameter), with approximately 1.5 mm length of the coating removed at the tip to create the recording site. The wires were inserted into the genioglossus muscle per‐orally (approximately 4 mm either side of the frenulum to a depth of 1–1.5 cm) (Eckert et al. 2007) or per‐cutaneously (Eastwood et al. 2003). The site was anaesthetised prior to insertion with 1–2% topical lignocaine. Wires were inserted using a 25‐gauge needle and secured to the skin using Tegaderm tape.
Two pressure transducers (pressure‐tipped catheters, Millar, Houston, TX, USA) were introduced via the nostril to measure upper airway pressures as described previously (Carberry et al. 2015; Gainche et al. 2016). One catheter was advanced to the level of the epiglottis with the tip positioned 1–2 cm below the base of the tongue. The other was placed to the back of the nose with the tip positioned 0.5–1 cm distal to the nasopharyngeal wall to record pressure at the choanae. Each catheter was secured to the nose with tape. Participants were instrumented with a non‐vented nasal mask (modified ResMed Mirage, Sydney, Australia, or a modified Philips Respironics Profile Lite, Murrysville, PA, USA) connected to a two‐way breathing valve (Hans Rudolph, Series 1410, Kansas City, MO, USA) with the inspiratory valve placed upstream (Fig. 1). A heated pneumotachograph (Hans Rudolph Inc., model 3700 or Fleisch No.3, Phipps & Bird, Richmond, VA, USA) attached to the nasal mask and a differential pressure transducer (Validyne Corporation, MP45, Northbridge, CA, USA) was used to measure airflow. An additional differential pressure transducer referenced to atmosphere was attached to measure mask pressure.
Figure 1. Schematic of the experimental set‐up.
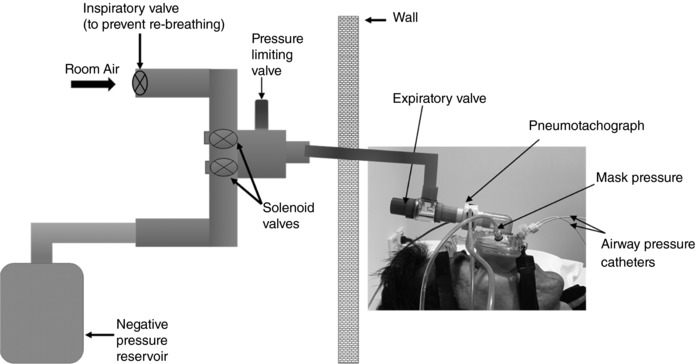
Participants lay supine connected to a solenoid valve system on the other side of a soundproof wall while breathing through a nasal mask attached to a pneumotachograph and an expiratory valve. A differential pressure sensor (mask pressure) and two nasal pressure catheters (choanal pressure and epiglottic pressure sensors) were used to measure stimulus characteristics and airway collapsibility. One arm of the solenoid was connected to room air (atmospheric pressure) and the other was connected to a negative pressure reservoir evacuated to approximately −100 cmH2O. Brief pulses (250 ms) were delivered every 2–10 breaths during early inspiration when airflow reached 2 l min−1. An adjustable valve limited the pressure delivered to the mask to approximately −15 cmH2O.
Experimental protocol
Experiments were conducted during wakefulness confirmed via electroencephalogram. Participants were studied supine. Brief (250 ms), rapid negative‐pressure pulses were delivered to the mask during early inspiration using a computer‐controlled solenoid (Sydney) or balloon (Melbourne) valve systems. One arm of the valve was connected to room air (atmospheric pressure) while the other was connected to a large negative pressure reservoir evacuated to approximately −100 cmH2O. A spring adjustable valve was placed in series to limit the pressure delivered to the mask to approximately −15 cmH2O (Fig. 1). Negative pressure pulses were triggered by custom‐written software that monitored the inspiratory flow rate. Pulses were delivered randomly every 2–10 breaths during early inspiration when airflow reached 2 l min−1.
Data were acquired on a personal computer using a 16‐bit analog to digital converter (CED 1401; Cambridge Electronic Design Ltd, Cambridge, UK) and Spike2 software (version 2.7; Cambridge Electronic Design). CED 1902 amplifiers were used to record EMG data at a sample rate of 2000 Hz with a band pass filter of 30–1000 Hz. Pressure channels were sampled at 1000 Hz. Remaining channels were sampled at 250 Hz.
Data analyses
Pulses were analysed using customised software (Spike 2, CED) which detected the steepest slope in mask pressure during the negative pressure pulses. This point was used to time‐lock pressure and genioglossus EMG signals for ensemble averaging. Pulses were excluded from analysis if they were delivered during a swallow, or if movement or signal artefacts were present. All other pulses in which mask pressure was ≤−10 cmH2O were included in the analysis. The point immediately before the sudden decline in choanal pressure was defined as stimulus onset (time zero) as described previously (Carberry et al. 2015).
EMG signals were rectified and ensemble‐averaged for 200 ms before and 1 s after the sudden drop in mask pressure for each participant to quantify reflex responses to negative pressure pulses. Similarly, pressure signals (mask, choanal and epiglottic) were averaged to assess upper airway collapsibility and negative pressure pulse stimulus characteristics. Upper airway collapsibility was calculated as the pressure difference between nadir mean choanal and epiglottic pressures during negative pressure pulses as described previously (Hilditch et al. 2008; Carberry et al. 2015). The collapsibility index was calculated as: (choanal pressure nadir – epiglottic pressure at the point of choanal pressure nadir)/choanal pressure)×100.
Reflex amplitude was expressed as a percentage of the mean pre‐stimulus (100 ms) EMG and in absolute units (μV). To be classified as reflex excitation or suppression of genioglossus EMG, the EMG response to the negative pressure pulses had to increase/decrease by at least two standard deviations above or below the mean pre‐stimulus EMG for greater than 10 ms (see Fig. 2). When present, excitation onset, excitation peak amplitude, suppression onset and suppression nadir of the genioglossus EMG were quantified as described previously (Eckert et al. 2007). In cases where there was no short‐latency excitation peak according to the established criteria outlined above, the maximal value on the averaged rectified EMG within the 20–60 ms post‐stimulus window was used as a conservative estimate of excitation amplitude to enable data from all participants in each group to be compared (‘alternate criteria’).
Figure 2. Example of a genioglossus reflex response in an able‐bodied control.
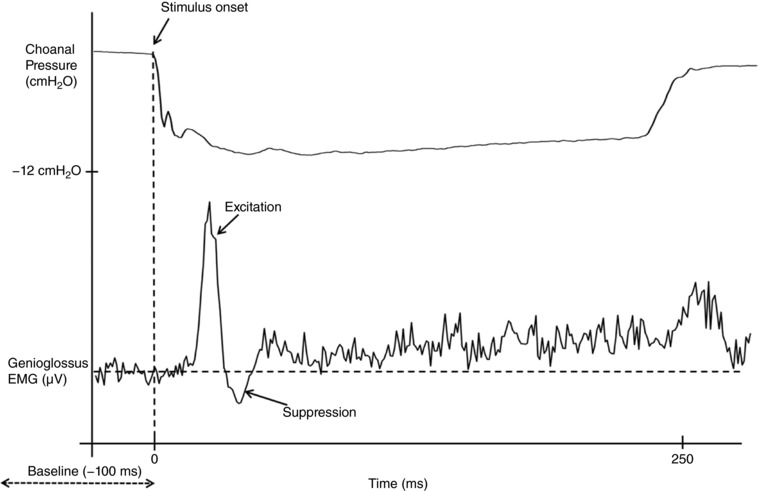
The lower trace indicates an ensemble average of the rectified genioglossus EMG of 75 negative pressure pules in a 31‐year‐old male with obstructive sleep apnoea (apnoea/hypopnoea index = 15 events per hour of sleep). The top trace shows the ensemble averaged choanal pressure tracing.
Statistical analyses
Key study outcomes were compared between groups using either two‐tailed, Student's t tests or Mann–Whitney U tests as appropriate. Data are presented as either means ± standard deviation for normally distributed variables or medians, 25th and 75th centiles for non‐normally distributed variables. The incidence of the short‐latency excitatory reflex was compared between groups with a chi‐squared test. P < 0.05 was considered statistically significant without correction for multiple comparisons. Analyses were performed in SPSS statistical analysis software, version 23 (IBM, Armonk, NY, USA).
Results
Participant characteristics
Sixteen participants with tetraplegia and nine able‐bodied participants with no neurological injury completed the study. In total, 3/16 participants with tetraplegia had an AHI below 10 events h–1 sleep and they were excluded from group (tetraplegia vs. able‐bodied) comparisons. The remaining 13 participants with tetraplegia (2 females) and 9 able‐bodied controls (2 females) were well matched for age (49 ± 13 vs. 48 ± 12 years, P = 0.8), body mass index (27 ± 5 vs. 30 ± 6 kg m−2, P = 0.3) and AHI (37 ± 19 vs. 33 ± 25 events h–1 sleep, P = 0.6). Equal numbers of participants were recruited from the Sydney and Melbourne sites. Injury characteristics, medication use, demographic, anthropometric and reflex summary data for the participants with tetraplegia are displayed in Table 1. Two of the participants with tetraplegia were prescribed continuous positive airway pressure therapy but were non‐complaint (<2 h night objective compliance). All of the other participants were untreated for their OSA at the time of the study.
Genioglossus reflex responses to negative pressure pulses
Stimulus characteristics
The magnitude of the negative pressure pulses delivered to the mask and choanae was similar between groups (Table 2). Similarly, the latency from the onset of the first sudden dip in choanal pressure (stimulus onset, Fig. 2) to choanal pressure nadir was not systematically different between people with tetraplegia and able‐bodied participants (P = 0.85, Table 2), nor was the rate of change in choanal pressure from stimulus onset to choanal pressure nadir (P = 0.96, Table 2). Stimulus magnitude was also not different between the Sydney and Melbourne sites (e.g. nadir choanal pressure = −14.3 ± 2.6 vs. −12.6 ± 2.2 cmH2O, P = 0.09).
Table 2.
Reflex morphology and stimulus characteristics
| Able‐bodied (n = 9) | Tetraplegia (n = 13) | |
|---|---|---|
| Mask pressure nadir (cmH2O) | −14.4 ± 1.9 | −15.0 ± 2.2 |
| Choanal pressure nadir (cmH2O) | −14.0 ± 2.9 | −13.0 ± 1.9 |
| Stimulus onset–choanal pressure nadir latency (ms) | 107 ± 91 | 110 ± 74 |
| Rate of change in choanal pressure (cmH2O ms–1) | −0.25 ± 0.21 | −0.26 ± 0.22 |
| Epiglottic pressure nadir (cmH2O) | −9.2 ± 2.9 | −8.6 ± 4.4 |
| No reflex | n = 1 | n = 3 |
| Excitation | n = 8 | n = 7 |
| a. Excitation only | n = 7 | n = 7 |
| b. Excitation followed by suppression | n = 1 | n = 0 |
| Suppression only | n = 0 | n = 3 |
Reflex morphology and EMG responses
Genioglossus reflex excitatory responses to negative pressure pulses were present in only 7 of the 13 (54%) participants with tetraplegia and OSA. Phasic genioglossus EMG was clearly present in all but one participant during quiet breathing (Fig. 3). However, the participant who did not display clear phasic EMG during quiet breathing had a robust excitatory reflex response to negative pressure pulses. Five of the six participants with tetraplegia who had no excitatory response were classified as having complete motor and sensory impairment (AIS A). Most able‐bodied participants with OSA (8/9 or 89%) had a short‐latency reflex excitatory response. However, the difference in incidence for the two groups did not reach statistical significance (P = 0.083).
Figure 3. Examples of phasic genioglossus EMG activity during quiet breathing in two people with tetraplegia (top two tracings) and two able‐bodied controls (lower two tracings).
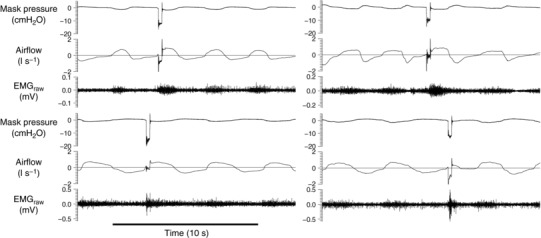
Each example shows 15 s of data (four breaths) including the mask pressure tracing, airflow signal and raw genioglossus electromyogram (EMGraw). An example of a transient negative pressure pulse delivered during early inspiration in each example is also displayed. The horizontal line indicates zero flow.
Baseline pre‐stimulus genioglossus EMG was not different between tetraplegia and able‐bodied participants (20 ± 11 vs. 17 ± 14 μV, P = 0.5). When present, reflex excitation onset was significantly delayed in the tetraplegia group compared to able‐bodied controls (32 ± 16 vs. 18 ± 9 ms, P = 0.045). Similarly, peak excitation latency was delayed by ∼15 s in the tetraplegia group (Fig. 4 A). This delayed excitatory response was also observed in the two participants with tetraplegia without OSA (43 and 48 ms). However, when present, the amplitude of the genioglossus excitation peak was not different between the tetraplegia and able‐bodied groups (Fig. 4 B) or between Sydney and Melbourne sites [200 (169, 234) vs. 165 (150, 228)% baseline, P = 0.58 for the site comparison]. However, when peak values from the averaged rectified EMG within the 20–60 s post‐stimulus period were included for participants who did not have a short‐latency reflex excitatory response (‘alternate criteria’), peak amplitude was less in people with tetraplegia compared to able‐bodied participants (161 ± 43 vs. 231 ± 91% baseline, P = 0.035).
Figure 4. Scatter plots of excitation peak latency (A) and amplitude (B) in people with spinal cord injury and able‐bodied participants.
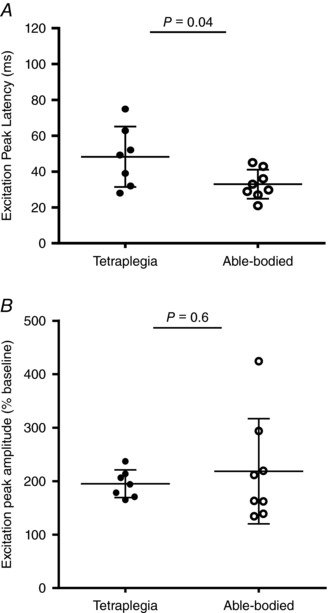
Horizontal lines indicate mean ± SD.
One able‐bodied participant had a subsequent suppression of genioglossus EMG in addition to the initial excitation (Fig. 2), while no one from the tetraplegia group had this response pattern. Three of the participants with tetraplegia and OSA had reflex suppression of the genioglossus EMG without any preceding initial excitation phase (Fig. 5, see also Table 3). Two of the three participants with tetraplegia but without OSA (AHI below 10 events h–1) had an excitatory response, while the other did not have any detectable reflex response.
Figure 5. Example of a suppression‐only genioglossus reflex response in an individual with tetraplegia and obstructive sleep apnoea.
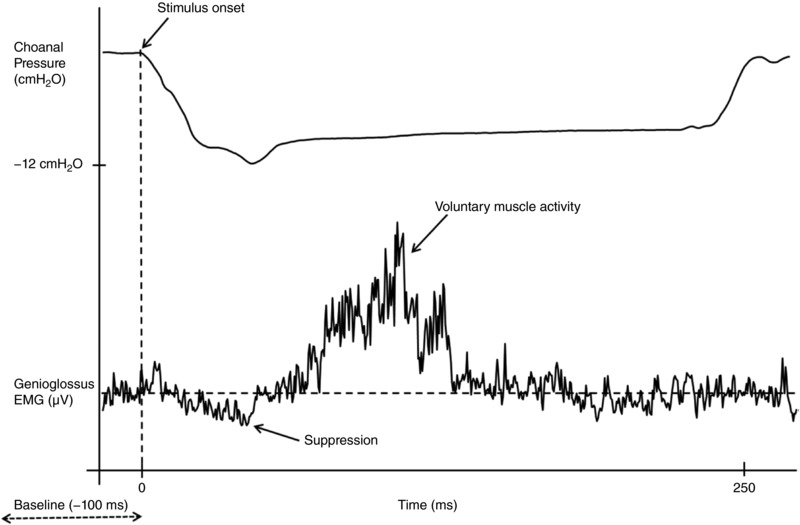
The lower trace shows the ensemble average of the rectified genioglossus EMG in response to 44 negative pressure pulses in a 69‐year‐old male with an apnoea/hypopnoea index of 47 events per hour of sleep. The top trace shows the ensemble average of the choanal pressure tracing.
Table 3.
Characteristics of the reflex suppression of genioglossus electromyography
| Able‐bodied (n = 1) | Tetraplegia (n = 3) | |
|---|---|---|
| Suppression onset latency (ms) | 35 | 24 ± 4 |
| Suppression nadir amplitude (% baseline) | 44 | 50 ± 10 |
| Suppression duration (ms) | 12 | 53 ± 26 |
Upper airway collapsibility in response to negative pressure pulses
There was no systematic difference in upper airway collapsibility between people with OSA and tetraplegia versus able‐bodied controls [34 (3, 81) vs. 39 (22, 67)%, P = 0.95].
Discussion
The main findings of this study are that genioglossus reflex responses to brief pulses of negative airway pressure are fundamentally different in people with tetraplegia and OSA compared to well‐matched able‐bodied controls with OSA. Firstly, almost half of the tetraplegia cohort did not have any detectable genioglossus reflex excitatory response despite receiving pulses of upper airway suction in excess of −10 cmH2O. Secondly, in participants with tetraplegia who had a genioglossus reflex excitatory response (54%), reflex onset and peak genioglossus excitatory activity (peak amplitude) were markedly delayed (almost double the control group). Thirdly, three of the participants with tetraplegia had reflex suppression of the genioglossus EMG in the absence of an excitatory response. Genioglossus EMG suppression with no accompanying excitation was not observed in any able‐bodied participants with OSA and has not been reported in previous studies of healthy people without OSA (Eckert et al. 2005, 2007; Carberry et al. 2015). These findings provide new insight into the potential mechanisms mediating reflex responses to changes in airway pressure in humans and have implications for people with tetraplegia and OSA as described below.
Delayed genioglossus excitatory reflex response to negative pressure in people with tetraplegia
Transient pressure changes in the upper airway stimulate pressure‐sensitive receptors. Afferent information is relayed to the nucleus of the solitary tract via the superior laryngeal nerve. The motor output to genioglossus originates in the hypoglossal motor nucleus and arrives at the muscle via the hypoglossal nerve (Mathew et al. 1984; Chamberlin et al. 2007; Eckert et al. 2007). This pressure‐sensitive reflex arc also receives input from hypoglossal premotor neurons in the medullary reticular formation that project to genioglossus motor neurons (Chamberlin et al. 2007). These components of the reflex should remain intact in people with sustained injuries to the spinal cord in the region of C4–C7. Thus, in the current study, delayed excitatory reflex response (when present) in participants with tetraplegia, despite a similar amplitude of excitation, is consistent with delayed sensory processing or demyelination.
Inflammation and upper airway oedema is common in untreated people with OSA and contributes to pharyngeal sensory impairment (Kimoff et al. 2001; Boyd et al. 2004). However, chronic inflammation may occur to a greater extent after tetraplegia (Wang et al. 2007). Spinal cord injury induces reorganisational changes within cortical and subcortical (i.e. brain stem and spinal cord) structures (Raineteau & Schwab, 2001; Forster, 2003). These changes, or unopposed parasympathetic drive, may have contributed to the delay in reflex activation detected in the current study. For example, autonomic dysfunction following spinal cord injury increases upper airway oedema (Karlsson, 1999; Weaver et al. 2006) and airway resistance (Gainche et al. 2016; Wijesuriya et al. 2017), both of which are associated with altered sensory/afferent information processing in able‐bodied people (Butler et al. 1996; Banzett et al. 2000; Tun et al. 2000; Eckert et al. 2004; Jeffery et al. 2006; Chou & Davenport, 2007; Saboisky et al. 2007; Ruehland et al. 2017). Consistent with impaired respiratory sensation, we have recently shown poor perception of increased nasal resistance in people with tetraplegia compared to able‐bodied controls (Wijesuriya et al. 2017).
Medication use in the people with tetraplegia that we studied may have also contributed to changes in genioglossus reflex function. A majority of participants with tetraplegia were taking multiple medications at the time of the study, including antidepressants and antispasmodics, both of which can affect respiratory/muscle function (Berlowitz et al. 2005). For instance, some antidepressants increase genioglossus muscle activity in able‐bodied participants during sleep (Taranto‐Montemurro et al. 2016). Recent data indicate that common benzodiazepine and non‐benzodiazepine hypnotics do not impair genioglossus reflex activity during sleep (Carter et al. 2016; Carberry et al. 2017). Baclofen, an anti‐spasmodic muscle relaxant commonly used in people with spinal cord injury, has a dose‐dependent respiratory effect (Finnimore et al. 1995; Bensmail et al. 2006) which may reduce genioglossus muscle activity. However, baseline genioglossus activity (pre‐stimulus) during quiet breathing was not different between groups. Baclofen also reduces reflex activity through presynaptic mechanisms involving the primary afferents (Ono et al. 1979; Li et al. 2004). However, there were no clear differences in genioglossus activity or reflex responses between those on versus not on anti‐spasmodic medications. Thus, it is unlikely that the observed delay in reflex latency to negative pressure pulses in people with tetraplegia was related to medication use.
Changes in reflex morphology to negative pressure pulses in people with tetraplegia
Negative pressure pulses delivered to a breathing mask stimulate multiple afferents throughout the respiratory system beyond the upper airway receptors, including subglottic intra‐ and extra‐thoracic receptors (i.e. tracheal, lung receptors) and receptors in the muscles of the chest wall (Davis & Sears, 1970; Horner et al. 1991b; Butler et al. 1995). For instance, genioglossus reflex responses to negative pressure pulses are greater when pulses are delivered with an open glottis (in the presence of afferent input from both upper airway and subglottic receptors), compared to when the glottis is closed (Horner et al. 1991b). Genioglossus reflex morphology is also maintained following dense upper airway anaesthesia with lignocaine (Carberry et al. 2015). Thus, the absence of an excitatory reflex response in genioglossus in a substantial proportion of people with tetraplegia may suggest a disruption of afferent pathways below the level of injury.
The hypoglossal motor neurons which innervate the genioglossus muscle receive both excitatory and inhibitory inputs (Chamberlin et al. 2007; Eckert et al. 2007). A state‐dependent suppression of the genioglossus EMG, subsequent to the initial excitatory response, has been reported in able‐bodied people (Eckert et al. 2007, 2008, 2010) with similar latency to other respiratory pump muscles in response to brief pulses of negative pressure (e.g. Davis & Sears, 1970; Butler et al. 1995). The inhibitory component was particularly dominant during rapid eye movement sleep (Shea et al. 1999; Eckert et al. 2007). The presence of reflex suppression of genioglossus EMG without preceding excitation to negative pressure pulses that occurred in almost one in three of the people with tetraplegia during wakefulness in the current study indicates a predominance of inhibition to transient negative pressure pulses in many people with tetraplegia. This reflex morphology has not been reported in awake able‐bodied individuals (Eckert et al. 2007, 2008, 2010; Carberry et al. 2015). A similar inhibitory predominant reflex response occurs in response to transient loads to breathing in several other inspiratory muscles, including the scalene, parasternal intercostal and diaphragm muscles. These respiratory reflexes are believed to be mediated by intramuscular receptors within the inspiratory muscles via intersegmental neural pathways (Davis & Sears, 1970; Butler et al. 1995, 1997; De Troyer et al. 1999; Murray et al. 2012; McBain et al. 2016).
The short‐latency inhibitory response to respiratory loading in the scalene muscles and diaphragm is absent in most people with tetraplegia, probably due to lack of intercostal muscle afferent input (McBain et al. 2015, 2016). The lack of/reduced afferent input from intercostal muscle receptors in people with tetraplegia may impair genioglossus muscle responses to negative pressure. In addition, the contrasting findings of genioglossus reflex suppression in tetraplegia in the current study compared to absence reflex inhibition to the pump muscles to respiratory loading in previous studies suggests that the mechanism for inhibition in the genioglossus may be different to that in other respiratory muscles. The current study consisted of people both with complete motor‐sensory tetraplegia (AIS A) and incomplete sensory tetraplegia (AIS B). The large variation in the extent of injury in the present group may have contributed to the variation in genioglossus reflex morphology observed in our study. Nonetheless, the source of the genioglossus reflex dysfunction in people with tetraplegia in wakefulness is unclear.
Altered genioglossus reflex function in people with tetraplegia: implications for sleep disordered breathing
Delayed or absent genioglossus reflex excitation as well as more pronounced reflex inhibition in response to brief pulses of negative upper airway pressure in people following tetraplegia may contribute to a discoordination of tongue muscles with other pharyngeal and respiratory pump muscles, which subsequently may affect airway patency. Indeed, genioglossus reflex inhibition to suction pressure in the airway without excitation, as observed in some of the tetraplegia participants during wakefulness, has previously only been observed during rapid eye movement sleep (Eckert et al. 2007), a time at which OSA severity tends to be most severe (Okabe et al. 1994; Shea et al. 1999). Discoordination of neural drive to genioglossus may be an important contributor to airway narrowing during sleep in OSA (Dotan et al. 2013, 2015). This may contribute to the counterproductive motion of genioglossus that has recently been reported in some able‐bodied people with OSA using a magnetic resonance imaging tagging technique (Brown et al. 2013). It remains unknown if counterproductive motion of genioglossus is more common in people with tetraplegia.
The upper airway collapsibility index was similar between the able‐bodied participants with OSA and people with spinal cord injury and OSA in the current study. This is not surprising given that the two groups were well‐matched for OSA severity, baseline EMG was similar and airway collapse under the current experimental conditions occurs prior to the genioglossus reflex and serves as the trigger for the reflex rather than a modifier of airway collapsibility. Recent pharyngeal critical closing pressure (P crit) findings measured during sleep also showed increased upper airway collapsibility in people with cervical and thoracic spinal cord injury and OSA compared to healthy controls without OSA (Sankari et al. 2014).
Conclusions
The present study has provided novel insights into abnormal genioglossus reflex responses in people with tetraplegia and OSA in response to rapid changes in negative airway pressure. These findings identify potential mechanisms for the high rates of OSA in people with tetraplegia. Further research is required to understand how features of short latency reflexes manifest during sleep.
Additional information
Competing interests
DJE has a Commonwealth Government of Australia Cooperative Research Centre grant (industry partner: Oventus Medical) and serves as a consultant for Bayer. These industry partnerships do not conflict with the current physiology study. The other authors do not have any competing interests to disclose.
Author contributions
NSW contributed to data acquisition, analysis, interpretation and drafted the manuscript. LG contributed to data acquisition and analysis. DJE, ASJ and DJB contributed to the study conception and design, data interpretation, provided important intellectual content and assisted with data collection. DJE also helped draft the manuscript. JEB contributed to data analysis and interpretation and provided important intellectual input. JCC contributed to data acquisition and analysis. ML, PR, FOD, WR and JCC provided technical expertise during data collection and feedback on the manuscript. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (1065913) and proudly supported by the Transport Accident Commission. DJE is supported by an NHMRC Senior Research Fellowship (1116942). ASJ is supported by an ARC Future Fellowship (FT100100203). JEB is supported by an NHMRC Senior Research Fellowship (1042646). JCC is supported by an NHMRC CRE NeuroSleep Fellowship (1060992).
Acknowledgement
The authors would like to thank the study participants for volunteering their time to participate in the study.
Biographies
Nirupama Wijesuriya received her PhD from The University of Sydney in 2012 which focused on altered neurophysiological and cognitive function with spinal cord injury (SCI). In 2014 she joined the sleep and breathing laboratory at Neuroscience Research Australia as a postdoctoral researcher where she performed several detailed physiological studies to investigate the causes of increased risk of obstructive sleep apnoea in people with SCI. Dr Wijesuriya is currently a senior clinical trial officer at Sydney University.
Laura Gainche is a geneticist/physiologist who acquired her Masters at Paris 7 University. She has neuroscience‐related experience from Europe, USA and Japan. Her PhD work into the pathogenesis of obstructive sleep apnoea in quadriplegia was developed and conducted at the University of Melbourne, Australia. She did some postdoctoral work at the University of Lausanne, Switzerland. After becoming a mother, she is now refocusing her passion for science to conquer physiological questions in women's biology.
Edited by: Michael Hogan & Gregory Funk
Linked articles This article is highlighted by a Perspective by Fuller. To read this article, visit https://doi.org/10.1113/JP276162.
References
- Banzett RB, Dempsey JA, O'Donnell DE & Wamboldt MZ (2000). Symptom perception and respiratory sensation in asthma. Am J Resp Crit Care Med 162, 1178–1182. [DOI] [PubMed] [Google Scholar]
- Bensmail D, Salva MAQ, Roche N, Benyahia S, Bohic M, Denys P, Bussel B & Lofaso F (2006). Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology 67, 1432–1436. [DOI] [PubMed] [Google Scholar]
- Berlowitz DJ, Brown DJ, Campbell DA & Pierce RJ (2005). A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil 86, 1193–1199. [DOI] [PubMed] [Google Scholar]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM & American Academy of Sleep Medicine (2012). Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8, 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JH, Petrof BJ, Hamid Q, Fraser R & Kimoff RJ (2004). Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 170, 541–546. [DOI] [PubMed] [Google Scholar]
- Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC & Bilston LE (2013). Respiratory movement of upper airway tissue in obstructive sleep apnea. Sleep 36, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Little JW, Hussey JD, Lyman P & Lakshminarayanan S (2000). Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil 81, 1334–1339. [DOI] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Crawford MR & Gandevia SC (1995). Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. J Physiol 487, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK & Gandevia SC (1996). Impaired reflex responses to airway occlusion in the inspiratory muscles of asthmatic subjects. Thorax 51, 490‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Glanville AR & Gandevia SC (1997). Pulmonary afferents are not necessary for the reflex inhibition of human inspiratory muscles produced by airway occlusion. J Neurophysiol 78, 170–176. [DOI] [PubMed] [Google Scholar]
- Carberry JC, Fisher LP, Grunstein RR, Gandevia SC, McKenzie DK, Butler JE & Eckert DJ (2017). Role of common hypnotics on the phenotypic causes of OSA: paradoxical effects of zolpidem. Eur Respir J 50, pii: 1701344. [DOI] [PubMed] [Google Scholar]
- Carberry JC, Hensen H, Fisher LP, Saboisky JP, Butler JE, Gandevia SC & Eckert DJ (2015). Mechanisms contributing to the response of upper‐airway muscles to changes in airway pressure. J Appl Physiol 118, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Carter SG, Berger MS, Carberry JC, Bilston LE, Butler JE, Tong BK, Martins RT, Fisher LP, McKenzie DK, Grunstein RR & Eckert DJ (2016). Zopiclone increases the arousal threshold without impairing genioglossus activity in obstructive sleep apnea. Sleep 39, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP & Malhotra A (2007). Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo AE, Sitrin RG & Bauman KA (2016). Sleep disordered breathing in spinal cord injury: a systematic review. J Spinal Cord Med 39, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YL & Davenport PW (2007). The effect of increased background resistance on the resistive load threshold for eliciting the respiratory‐related evoked potential. J Appl Physiol 103, 2012–2017. [DOI] [PubMed] [Google Scholar]
- Davis JN & Sears TA (1970). The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol 209, 711–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Brunko E, Leduc D & Jammes Y (1999). Reflex inhibition of canine inspiratory intercostals by diaphragmatic tension receptors. J Physiol 514, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan Y, Pillar G, Schwartz AR & Oliven A (2015). Asynchrony of lingual muscle recruitment during sleep in obstructive sleep apnea. J Appl Physiol 118, 1516–1524. [DOI] [PubMed] [Google Scholar]
- Dotan Y, Pillar G, Tov N, Oliven R, Steinfeld U, Gaitini L, Odeh M, Schwartz AR & Oliven A (2013). Dissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesia. Eur Respir J 41, 74–84. [DOI] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I & Hillman DR (2003). Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine‐wire electrodes. J Appl Physiol 94, 1849–1858. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Catcheside PG, McDonald R, Adams AM, Webster KE, Hlavac MC & McEvoy RD (2005). Sustained hypoxia depresses sensory processing of respiratory resistive loads. Am J Resp Crit Care Med 172, 1047–1054. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Catcheside PG & McEvoy RD (2004). Blunted sensation of dyspnoea and near fatal asthma. Eur Resp J 24, 197–199. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, McEvoy RD, George KE, Thomson KJ & Catcheside PG (2007). Genioglossus reflex inhibition to upper‐airway negative‐pressure stimuli during wakefulness and sleep in healthy males. J Physiol 581, 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, McEvoy RD, George KE, Thomson KJ & Catcheside PG (2008). Effects of hypoxia on genioglossus and scalene reflex responses to brief pulses of negative upper‐airway pressure during wakefulness and sleep in healthy men. J Appl Physiol 104, 1426–1435. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Saboisky JP, Jordan AS, White DP & Malhotra A (2010). A secondary reflex suppression phase is present in genioglossus but not tensor palatini in response to negative upper airway pressure. J Appl Physiol 108, 1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DJ, White DP, Jordan AS, Malhotra A & Wellman A (2013). Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Resp Crit Care Med 188, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnimore A, Roebuck M, Sajkov D & McEvoy R (1995). The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Resp J 8, 230–234. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA & White DP (2001). Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Am J Resp Crit Care Med 164, 2025–2030. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D & Pierce RJ (2005). The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 564, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV (2003). Invited review: plasticity in the control of breathing following sensory denervation. J Appl Physiol 94, 784–794. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Lee K‐Z & Tester NJ (2013). The impact of spinal cord injury on breathing during sleep. Respir Physiol Neurobiol 188, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainche L, Berlowitz DJ, LeGuen M, Ruehland WR, O'Donoghue FJ, Trinder J, Graco M, Schembri R, Eckert DJ, Rochford PD & Jordan AS (2016). Nasal resistance is elevated in people with tetraplegia and is reduced by topical sympathomimetic administration. J Clin Sleep Med 12, 1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilditch CJ, McEvoy RD, George KE, Thompson CC, Ryan MK, Rischmueller M & Catcheside PG (2008). Upper airway surface tension but not upper airway collapsibility is elevated in primary Sjogren's syndrome. Sleep 31, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Hughes SW & Malhotra A (2014). State‐dependent and reflex drives to the upper airway: basic physiology with clinical implications. J Appl Physiol 116, 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB & Guz A (1991a). Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K & Guz A (1991b). Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery S, Butler JE, McKenzie DK, Wang L & Gandevia SC (2006). Brief airway occlusion produces prolonged reflex inhibition of inspiratory muscles in obstructive sleep apnea. Sleep 29, 321–328. [DOI] [PubMed] [Google Scholar]
- Karlsson AK (1999). Autonomic dysreflexia. Spinal Cord 37, 383–391. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ, Sforza E, Champagne V, Ofiara L & Gendron D (2001). Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med 164, 250–255. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ & Bennett DJ (2004). Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92, 2694–2703. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Sant'ambrogio G, Fisher JT & Sant'mbrogio FB (1984). Laryngeal pressure receptors. Respir Physiol 57, 113–122. [DOI] [PubMed] [Google Scholar]
- McBain RA, Hudson AL, Gandevia SC & Butler JE (2015). Short‐latency inhibitory reflex responses to inspiratory loading of the scalene muscles are impaired in spinal cord injury. Exp Physiol 100, 216–225. [DOI] [PubMed] [Google Scholar]
- McBain RA, Taylor JL, Gorman RB, Gandevia SC & Butler JE (2016). Human intersegmental reflexes from intercostal afferents to scalene muscles. Exp Physiol 101, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ & White DP (1992). Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NPS, McKenzie DK, Gandevia SC & Butler JE (2012). Effect of airway inflammation on short‐latency reflex inhibition to inspiratory loading in human scalene muscles. Respir Physiol Neurobiol 181, 148–153. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hida W, Kikuchi Y, Taguchi O, Takishima T & Shirato K (1994). Upper airway muscle activity during rem and non‐rem sleep of patients with obstructive apnea. Chest 106, 767–773. [DOI] [PubMed] [Google Scholar]
- Ono H, Fukuda H & Kudo Y (1979). Mechanisms of depressant action of baclofen on the spinal reflex in the rat. Neuropharmacology 18, 647–653. [DOI] [PubMed] [Google Scholar]
- Raineteau O & Schwab ME (2001). Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2, 263–273. [DOI] [PubMed] [Google Scholar]
- Ruehland WR, Rochford PD, Pierce RJ, Webster KE, Trinder JA, Jordan AS & O'Donoghue FJ (2017). Sensory detection of threshold intensity resistive loads in severe obstructive sleep apnoea. Respir Physiol Neurobiol 236, 29–41. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP & Gandevia SC (2007). Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol 585, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankari A, Bascom AT & Badr MS (2014). Upper airway mechanics in chronic spinal cord injury during sleep. J Appl Physiol 116, 1390–1395. [DOI] [PubMed] [Google Scholar]
- Shea SA, Edwards JK & White DP (1999). Effect of wake‐sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol 520, 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto‐Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP & Wellman A (2016). Desipramine increases genioglossus activity and reduces upper airway collapsibility during Non‐REM sleep in healthy subjects. Am J Respir Crit Care Med 194, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun Y, Hida W, Okabe S, Kikuchi Y, Kurosawa H, Tabata M & Shirato K (2000). Inspiratory effort sensation to added resistive loading in patients with obstructive sleep apnea. Chest 118, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Wang T‐D, Wang Y‐H, Huang T‐S, Su T‐C, Pan S‐L & Chen S‐Y (2007). Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc 106, 919–928. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Marsh DR, Gris D, Brown A & Dekaban GA (2006). Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention In Progress in Brain Research, ed. Lynne CW. & Canio P, pp. 245–263. Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- White DP (2005). Pathogenesis of obstructive and central sleep apnea. Am J Resp Crit Care Med 172, 1363–1370. [DOI] [PubMed] [Google Scholar]
- Wijesuriya NS, Lewis C, Butler JE, Lee BB, Jordan AS, Berlowitz DJ & Eckert DJ (2017). High nasal resistance is stable over time but poorly perceived in people with tetraplegia and obstructive sleep apnoea. Respir Physiol Neurobiol 235, 27–33. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Kim Y, Trinder J & Pierce R (2000). Effect of age on sleep onset‐related changes in respiratory pump and upper airway muscle function. J Appl Physiol 88, 1831–1839. [DOI] [PubMed] [Google Scholar]


