Abstract
Key points
Chronic inflammation underlies many of the health decrements associated with obesity.
Circulating progenitor cells can sense and respond to inflammatory stimuli, increasing the local inflammatory response within tissues.
Here we show that 6 weeks of endurance exercise training significantly decreases inflammatory circulating progenitor cells in obese adults.
These findings provide novel cellular mechanisms for the beneficial effects of exercise in obese adults.
Abstract
Circulating progenitor cells (CPCs) and subpopulations are normally found in the bone marrow, but can migrate to peripheral tissues to participate in local inflammation and/or remodelling. The purpose of this study was to compare the CPC response, particularly the inflammatory‐primed haematopoietic stem and progenitor (HSPC) subpopulation, to a 6 week endurance exercise training (EET) intervention between lean and obese adults. Seventeen healthy weight (age: 23.9 ± 5.4 years, body mass index (BMI): 22.0 ± 2.6 kg m−2) and 10 obese (age: 29.0 ± 8.0 years, BMI: 33.1 ± 6.0 kg m−2) previously sedentary adults participated in an EET. Blood was collected before and after EET for quantification of CPCs and subpopulations via flow cytometry, colony forming unit assays and plasma concentrations of C‐X‐C motif chemokine 12 (CXCL12), granulocyte‐colony stimulating factor (G‐CSF), and chemokine (C‐C motif) ligand 2 (CCL2). Exercise training reduced the number of circulating HSPCs and adipose tissue‐derived mesenchymal stem cells (AT‐MSCs). EET increased the colony forming potential of granulocytes and macrophages irrespective of BMI. EET reduced the number of HSPCs expressing the chemokine receptor CCR2 and the pro‐inflammatory marker TLR4. EET‐induced changes in adipose tissue‐derived MSCs and bone marrow‐derived MSCs were negatively related to changes in absolute fitness. Our results indicate that EET, regardless of BMI status, decreases CPCs and subpopulations, particularly those primed for contribution to tissue inflammation.
Keywords: Hematopoietic stem cells, Inflammation, Obesity, Mesenchymal stem cell, chemokine c receptor 2
Key points
Chronic inflammation underlies many of the health decrements associated with obesity.
Circulating progenitor cells can sense and respond to inflammatory stimuli, increasing the local inflammatory response within tissues.
Here we show that 6 weeks of endurance exercise training significantly decreases inflammatory circulating progenitor cells in obese adults.
These findings provide novel cellular mechanisms for the beneficial effects of exercise in obese adults.
Introduction
Obesity is a growing concern in western countries, with approximately one‐third of adults affected (Ogden et al. 2014). Obesity can contribute to cardio‐metabolic comorbidities such as heart disease and/or failure, respiratory disease, type 2 diabetes and metabolic syndrome (Bastien et al. 2014). In adults, the increased adipose tissue as a result of obesity is connected with chronic low‐grade inflammation, characterized by overproduction of pro‐inflammatory cytokines and inflammatory cells (Park et al. 2005; Hotamisligil, 2006). Monocyte and macrophage infiltration into adipose tissue contributes to local inflammation and subsequent metabolic dysfunction (Considine, 2014). These inflammatory cell subtypes are derived from haematopoietic stem/progenitor cells (HSPCs) in the bone marrow, which differentiate into either common myeloid or lymphoid progenitors. Common myeloid progenitors further differentiate along the myeloid lineage towards mixed granulocyte/macrophage, and more differentiated pure macrophage, or pure granulocyte colonies until ultimately forming mature myeloid cells, including inflammatory monocytes. HSPCs, along with more primitive haematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs) and mesenchymal stem/stromal cells (MSCs) (Niemiro et al. 2017) are collectively referred to as circulating progenitor cells (CPCs) (Niemiro et al. 2017, 2018). All CPCs circulate in peripheral blood in low quantities, and home to extramedullary tissues to participate in tissue repair and inflammation (Emmons et al. 2017).
A variety of factors can influence the differentiation of HSPCs and their subpopulations towards a more myeloid or lymphoid bias, thereby affecting immune/inflammatory system function. Individuals with obesity have been shown to have higher CPCs (Bellows et al. 2011), higher circulating inflammatory monocytes (Devêvre et al. 2015; Niemiro et al. 2016) and increased HSPCs expressing toll‐like receptor 4 (TLR4) and C‐C chemokine receptor type 2 (CCR2) compared to healthy weight adults (Hardy et al. 2013; Niemiro et al. 2016). TLR4 stimulation has been shown to increase HSPC proliferation and differentiation towards myeloid lineages (Liu et al. 2015), and CCR2 is involved in HSPC homing towards sites of inflammation (Si et al. 2010). In humans, the effects of obesity on CPC populations remains largely unclear; however, alterations to CPC populations in adults with obesity may have clinical importance due to their proposed role in contributing to inflammatory processes in peripheral tissues.
In obese adults, endurance exercise training (EET) contributes to improved body composition, metabolism and inflammatory profiles (You et al. 2013). Much of the work examining the effects of exercise on CPCs has focused on acute exercise bouts, and the consensus from these studies is that acute exercise transiently increases CPCs in the peripheral blood in an intensity‐dependent manner (De Lisio & Parise, 2013; Boppart et al. 2015; Baker et al. 2017; Niemiro et al. 2017). However, the effects of EET or training status on CPC content are equivocal (Rakobowchuk et al. 2012; Emmons et al. 2017). Many studies that focused on EET and CPCs have relied on self‐reported training status and not individuals who participated in a structured exercise training (De Lisio & Parise, 2013), or EET that resulted in moderate increases in cardiorespiratory fitness (Rakobowchuk et al. 2012). Despite the growing body of evidence investigating the effects of EET on CPCs and their subpopulations, to our knowledge no work has examined the effects of EET on CPC subpopulations in obese participants. Furthermore, the effects of EET on markers of inflammatory bias on HSPCs, precursors of mature inflammatory cells, are unknown. Investigating the potential anti‐inflammatory effects of EET on HSPCs expressing inflammatory markers in individuals who are obese may identify novel mechanisms that explain the relationship between inflammation, EET and obesity.
Thus, the purpose of this study was to compare the inflammatory CPC response to a 6 week EET between lean and obese adults. We focused on a variety of CPC populations previously shown to be influenced by acute exercise (Niemiro et al. 2017), and also examined the expression of CCR2 and TLR4 specifically on HSPCs to determine if obesity/EET altered phenotypic markers associated with inflammatory cell production. We hypothesized that EET would decrease the quantity of HSPCs expressing inflammatory markers, as well as the number of myeloid‐biased progenitors in individuals with obesity compared to lean individuals.
Methods
Ethical approval
This study was approved by the University of Illinois Institution Review Board and conformed to the standards of use of human participants in research as outlined in the sixth Declaration of Helsinki, except for registration in a database. Each participant provided written informed consent prior to participating in this study. This study was an additional analysis of some participants previously described (Allen et al. 2018).
Participants and study design
Twenty‐seven previously sedentary (≤30 min of moderate or high intensity exercise per week and ≤10 aggregate Godin–Shepard Leisure Time Physical Activity Questionnaire score) females and males between the ages of 20 and 45 years were recruited and divided into lean or obese groups based on body mass index (BMI); lean (BMI < 25 kg m−2; n = 17) and obese (BMI > 30 kg m−2; n = 10). Participant characteristics are shown in Table 1. Participants that qualified for the study were free of metabolic and gastrointestinal disease, were not pregnant or lactating, not taking medications that would impact bowel function, and not taking antibiotics for at least 3 months prior to or during the study. After 2 weeks of baseline testing, including a dual X‐ray absorptiometry (DXA) scan, blood draw, maximal exercise test and dietary analysis, all subjects completed a 6 week EET intervention for 30–60 min per day, three times per week, in which exercise intensity and duration progressed to 60 min at 75% heart rate reserve (HRR) (Allen et al. 2018). Participants were instructed by a registered dietician to maintain their current diet, which included maintenance of overall dietary patterns, maintenance of alcoholic/caffeinated beverage consumption and continuation of any dietary supplement usages that occurred prior to the beginning of the study, which is explained in more detail by Allen and colleagues (Allen et al. 2018).
Table 1.
Participant demographics
| Lean | Obese | ||
|---|---|---|---|
| n = 17 (8 female) | n = 10 (7 female) | ||
| Age (years) | 23.9 ± 5.4 | 29.0 ± 8 | |
| Height (cm) | 171.5 ± 10.4 | 171.5 ± 11.2 | |
| Weight (kg) | Pre | 65.3 ± 12.9 | 98.6 ± 27.9b |
| Post | 65.3 ± 13.7 | 98.0 ± 28.1b | |
| Body fat (%) | Pre | 25.2 ± 7.1 | 36.6 ± 4.6b |
| Post | 24.5 ± 6.4a | 35.3 ± 4.6a , b | |
| BMI (kg m−2) | Pre | 22.0 ± 2.6 | 33.1 ± 6.0b |
| Post | 22.0 ± 2.7 | 32.9 ± 6.0b | |
| Absolute (L min−1)c | Pre | 2.6 ± 0.6 | 3.1 ± 1.0 |
| Post | 2.9 ± 0.7a | 3.3 ± 1.0a | |
| Relative (mL kg−1 min−1)c | Pre | 40.3 ± 5.3 | 31.3 ± 5.0b |
| Post | 44.7 ± 6.7a | 34.3 ± 5.2a , b |
Participant information is presented as mean ± SD.
Main effect of exercise, P < 0.05;
main effect of weight class, P < 0.05;
Lean: n = 15, Obese: n = 9.
Body composition assessments
Height, weight and adiposity were completed before and after the 6 week EET. Standing height and weight measurements were completed with participants wearing lightweight clothing and no shoes using a stadiometer (Seca, model 240, Chino, CA, USA) and a Tanita WB‐300 Plus digital scale (Tanita, Tokyo, Japan), respectively. Whole body composition was measured by DXA (Hologic Discovery A Bone Densitometer; software version 12.7.3; Hologic, Bedford, MA, USA).
Maximal oxygen uptake testing
Prior to and after the exercise intervention, participants completed a maximal oxygen uptake test to assess cardiorespiratory fitness (; Parvo Medics Tru Max 2400, Sandy, UT, USA). This test involved walking or running to maximal exertion on a treadmill using the Bruce Testing protocol as described previously (Allen et al. 2018). Heart rate (HR) was continuously recorded via a wireless heart rate monitor (Polar Electro, Lake Success, NY, USA) and the test ended upon volitional fatigue. was verified if two or more of the following criteria were met: (1) a rate of perceived exertion (RPE) scale > 18, (2) HR within 10 beats min−1 of age‐predicted maximum HR, (3) a plateau in HR (<3 beats min−1 change) over the last two intensity stages, and/or (4) respiratory exchange ratio > 1.10. testing was completed after blood collection at both time points to avoid any effects of acute exercise on blood parameters.
EET protocol
The EET intervention consisted of three supervised 30–60 min, moderate to vigorous intensity (60–75% of HRR) EET sessions per week (Allen et al. 2018). Participants chose from a cycle ergometer or treadmill during each exercise session. Percentage of HRR was calculated as:
HRmax was determined during the baseline test. During week 1 of training, sessions were 30 min at 60% HRR. Training sessions for week 2 lasted 45 min at a similar intensity. Training sessions at week 3 were increased to 60 min at 60% HRR. During weeks 4–6 of training, there was an increase in intensity of 5% HRR per week, progressing up to 75% of HRR for 60 min per session during week 6. All participants were 100% compliant in completing necessary EET sessions.
Blood draw
Participants reported to the lab 3–4 days before starting the exercise regimen and 3–4 days after the last exercise session having fasted for at least 8–10 h. Participants were seated in an upright position and blood was drawn into EDTA tubes and sodium heparin tubes.
Granulocyte and macrophage colony forming unit assay
The sodium heparin tubes were used for granulocyte and macrophage colony forming unit (CFU) assay (MethoCult; StemCell Technologies, Vancouver, BC, Canada) as per manufacturer's instructions. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated using sterile Ficoll‐Paque Plus (GE Life Sciences, Pittsburgh, PA, USA) density gradient, per manufacturer's instructions (400 g, 22°C, 40 min with the brake off) with modifications. Instead of using PBS, blood was mixed with Iscove's modified Dulbecco's medium mixed with 2% fetal bovine serum. Cells were diluted to approximately 2.0 × 106 cells mL−1 in each tube, and 400 μL was added to MethoCult methylcellulose medium (H4534, without erythropoietin (EPO); STEMCELL Technologies, Vancouver, BC, Canada), and plated per manufacturer's instructions. Colonies were counted 12 days after plating. To prevent variability in colony counting, the same researcher, who was blinded to each condition, counted all colonies in the study. Participants were plated in triplicate, with the average amount of granulocyte (CFU‐G), macrophage (CFU‐M), granulocyte/macrophage (CFU‐GM) and total (CFU‐T) colonies per person reported.
Flow cytometry
The EDTA‐anticoagulant tubes were used for flow cytometry analysis as previously described (Niemiro et al. 2017, 2018). Briefly, PBMCs were isolated using Ficoll‐Paque Plus (GE Life Sciences) density gradient, per manufacturer's instructions. Plasma was collected from the Ficoll‐Paque density gradient spin and stored in 500 μL aliquots at −80°C until further analysis. PBMCs were split into three different samples: CPC, MSC and common myeloid progenitor (CMP)/common lymphoid progenitor (CLP) samples. CPCs were quantified using previously defined protocols (Niemiro et al. 2017, 2018). Antibodies for CPC (CD34+), HSPC (CD34+/CD45dim) and HSC (CD34+/CD45dim/CD38−) enumeration were phycoerythrin (PE)‐conjugated CD34 (1:100, Invitrogen, Grand Island, NY, USA), PE‐Cy 5‐conjugated CD38 antibody (1:50, BD Biosciences, San Jose, CA, USA), and FITC‐conjugated CD45 (1:200, Invitrogen). Antibodies for EPC (CD45−/CD34+/CD31+), EC (CD45−/CD31+) and MSC (bone marrow derived (BM‐MSC): CD45−/CD34−/CD31−/CD105+, and adipose derived (AT‐MSC): CD45−/CD34+/CD31−/CD105+) enumeration were PE conjugated CD34 (1:100, Invitrogen), FITC‐conjugated CD45 (1:200, Invitrogen), Vio‐blue conjugated CD105 (1:200, Miltenyi Biotec, San Diego, CA, USA) and PE‐Cy7 CD31 (1:200; BioLegend, San Diego, CA, USA). Antibodies for CMP (Lin−/CD34+/CD38+/IL3Rα−(CD123)/CD45RA−) and CLP (Lin−/CD34+/CD38+/IL3Rαlow(CD123)/CD10+) enumeration were FITC conjugated lineage panel cocktail (1:100; BD Pharmingen, San Jose, CA, USA), PE‐conjugated CD34 (1:100, Invitrogen), PE‐Cy 5‐conjugated CD38 antibody (1:50, BD Biosciences), Brilliant violet (BV)‐510‐congugated CD123 (1:100; BD Pharmingen), BV‐605 conjugated CD45RA (1:100; BD Biosciences) and BV‐421‐conjugated CD10 (1:50; BD Pharmingen) and were based on previous studies (Choi et al. 2015). All antibodies were diluted in 5% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA, USA)/phosphate buffered saline.
Flow quantification was performed on an Attune Focusing Flow Cytometer (Life Technologies Carlsbad, CA, USA) on at least 200,000 PBMCs. Doublet discrimination was applied, and gates were derived from isotype controls. Concentrations of cell populations were calculated from gated PBMCs. Gates were established based on previously described protocols (Bellows et al. 2011; Niemiro et al. 2017, 2018). The differential expression of CD34 has been shown to suggest differences in origin of either AT‐MSCs or BM‐MSCs, and quantification was performed as previously described (Traktuev et al. 2008; Niemiro et al. 2017). Briefly, CD45− singlet cells were selected, and then CD31− cells were selected. Next, cells were gated on CD105+, then based on differential expression of CD34. Proportions of cellular populations of interest were calculated using the following equation:
Enzyme‐linked immunosorbent assay
Human CXCL12/stromal derived factor‐1α, granulocyte colony stimulating factor (G‐CSF) and chemokine (C‐C motif) ligand 2 (CCL2) were quantified in stored plasma using the commercially available Quantikine enzyme‐linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) at Pre (before EET) and Post (after EET) time points. ELISAs were performed according to the manufacturer's instructions with samples run in duplicate. Thawed samples were centrifuged for 5 min at 10,000 g before analysis to ensure platelets were eliminated from the plasma sample.
Statistical analysis
Demographics, cell populations, CFU assays and plasma protein concentrations were analysed by a 2 × 2 mixed‐model ANOVA (between‐subjects factor: weight status (Lean/Obese); within‐subjects factor: time points (Pre/Post) in SPSS (IBM, version 25; Armonk, NY, USA). Data were log transformed prior to analyses if normality was not met by Shapiro–Wilks. Changes in cell populations and changes in fitness were calculated by subtracting the Pre values from the Post values. Spearman rho (ρ) analyses were implemented to determine relationships between progenitor cell populations (% of PBCMs, change in AT‐ and BM‐MSCs) to cardiorespiratory fitness ( or change in ). Data are presented as means ± standard deviation (SD) with P < 0.05 considered statistically significant.
Results
Participant demographic data are presented in Table 1. The obese group had a higher BMI than the lean group in the Pre and Post condition (33.1 ± 6.0 vs. 22.0 ± 2.6 kg m−2; 32.9 ± 6.0 vs. 22.0 ± 2.7 kg m−2, respectively; P < 0.001); however, no weight changes were observed as a result of EET. The obese group also had a higher percentage body fat compared to the lean group before and after EET (36.6 ± 4.6 vs. 25.2 ± 6.4%; 35.3 ± 4.6 vs. 24.5 ± 6.4%, respectively; P < 0.001) and EET decreased body fat percentage in both groups after the exercise intervention (P = 0.03). Additionally, the obese group had a lower relative compared to the lean group before and after EET (31.3 ± 5.0 vs. 40.3 ± 5.3 kg mL−1 min−1; 34.3 ± 5.2 vs. 44.7 ± 6.7 kg mL−1 min−1), respectively; P < 0.001), and both groups increased their relative and absolute compared to before the EET (P < 0.0001 and P < 0.0001, respectively).
Circulating proportion and concentrations of PBMCs that are CPCs, HSPCs and CLPs are decreased following EET
The concentration of CPCs was reduced after EET with a specific reduction in lean adults (P < 0.05; Table 2). The proportion of CPCs was not affected by EET (P > 0.05, Table 2). Both the concentration and proportion of HSPC content was reduced following EET (P < 0.001; Table 2). The concentration or proportion of EPCs did not change after the exercise intervention (P > 0.05, Table 2). Following EET, the concentration of AT‐MSCs was significantly decreased (P < 0.05, Table 2), and the proportion of AT‐MSCs showed a trend to be reduced (P = 0.08, Table 2). The concentration and proportion of BM‐MSCs showed a trend to decrease after EET (P = 0.06 and P = 0.09, Table 2). The proportion of BM‐MSCs showed a trend to have a BMI × time interaction, driven by a decrease in the obese group after EET (P = 0.068; Table 2). Neither CMP concentration nor proportion was changed as a result of EET (P > 0.05; Table 2). Exercise training decreased the concentration and proportion of CLPs irrespective of BMI (both P values <0.05; Table 2). The ratio of CMP:CLP cells was also unaltered by EET (P > 0.05; Table 2).
Table 2.
Six weeks of EET decreases the concentration and proportion of circulating HSPCs, AT‐MSCs and CLPs
| Main effects | |||||||
|---|---|---|---|---|---|---|---|
| P value | |||||||
| BMI | Exercise | Interaction | |||||
| Cell subsets | Lean | Obese | (Lean and obese) | (Pre and post) | P value | ||
| CPCs | Content (cells μL−1)b | Pre | 3.98 ± 4.58 | 1.99 ± 1.63 | 0.57 | 0.02 a | 0.04 a |
| Post | 1.31 ± 1.43 | 2.01 ± 2.8 | |||||
| Proportion (%) | Pre | 0.89 ± 0.62 | 0.93 ± 0.59 | 0.33 | 0.51 | 0.16 | |
| Post | 0.59 ± 0.53 | 1.06 ± 1.16 | |||||
| HSPCs | Content (cells μL−1) | Pre | 0.32 ± 0.25 | 0.26 ± 0.19 | 0.6 | <0.001 a | 0.53 |
| Post | 0.07 ± 0.08 | 0.06 ± 0.07 | |||||
| Proportion (%) | Pre | 0.09 ± 0.08 | 0.12 ± 0.08 | 0.4 | <0.001 a | 0.39 | |
| Post | 0.03 ± 0.02 | 0.03 ± 0.02 | |||||
| EPCs | Content (cells μL−1) | Pre | 0.01 ± 0.02 | 0.05 ± 0.012 | 0.21 | 0.14 | 0.29 |
| Post | 0.005 ± 0.005 | 0.008 ± 0.01 | |||||
| Proportion (%) | Pre | 0.008 ± 0.02 | 0.02 ± 0.06 | 0.33 | 0.18 | 0.5 | |
| Post | 0.002 ± 0.002 | 0.005 ± 0.004 | |||||
| AT‐MSCs | Content (cells μL−1) | Pre | 0.07 ± 0.12 | 0.04 ± 0.07 | 0.4 | 0.04 a | 0.43 |
| Post | 0.01 ± 0.01 | 0.009 ± 0.007 | |||||
| Proportion (%) | Pre | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.86 | 0.08a | 0.97 | |
| Post | 0.006 ± 0.004 | 0.007 ± 0.005 | |||||
| BM‐MSCs | Content (cells μL−1) | Pre | 0.09 ± 0.026 | 0.2 ± 0.36 | 0.53 | 0.06a | 0.28 |
| Post | 0.04 ± 0.08 | 0.01 ± 0.01 | |||||
| Proportion (%) | Pre | 0.02 ± 0.05 | 0.11 ± 0.2 | 0.193 | 0.09 | 0.068 | |
| Post | 0.03 ± 0.03 | 0.01 ± 0.01 | |||||
| CMPs | Content (cells μL−1) | Pre | 0.006 ± 0.01 | 0.002 ± 0.002 | 0.32 | 0.18 | 0.28 |
| Post | 0.001 ± 0.001 | 0.001 ± 0.001 | |||||
| Proportion (%) | Pre | 0.002 ± 0.002 | 0.0008 ± 0.001 | 0.23 | 0.89 | 1 | |
| Post | 0.001 ± 0.003 | 0.0008 ± 0.007 | |||||
| CLPs | Content (cells μL−1) | Pre | 0.009 ± 0.01 | 0.001 ± 0.002 | 0.93 | 0.009 a | 0.94 |
| Post | 0.001 ± 0.001 | 0.001 ± 0.001 | |||||
| Proportion (%) | Pre | 0.003 ± 0.005 | 0.005 ± 0.008 | 0.75 | 0.045 a | 0.48 | |
| Post | 0.001 ± 0.001 | 0.0007 ± 0.0009 | |||||
| CMP:CLPc | Ratio (content) | Pre | 0.61 ± 0.57 | 0.6 ± 0.9 | 0.49 | 0.15 | 0.44 |
| Post | 1.03 ± 1.71 | 1.39 ± 1.14 | |||||
P < 0.05; n = 17 Lean, n = 10 Obese;
Concentration of cells per microlitre of isolated PBMCs;
Lean Pre: n = 16, Obese Pre: n = 10, Lean Post: n = 14, Obese Post: n = 9; data are presented as means ± SD.
Colony forming units of innate immune cells are higher in obesity with more primitive colonies responding to EET
The proliferation potential of CPCs was assessed using the CFU assay. Visually, the size of all of the CFU colonies were larger following EET (data not shown). The number of CFU‐Gs was higher in the obese group compared to the lean group (P = 0.01; Fig. 1 A), whereas CFU‐Ms trended higher in the obese group (P = 0.1, Fig. 1 B). Neither CFU‐Gs nor CFU‐Ms were altered by EET. The number of CFU‐GMs was higher in the obese group before and after the EET (main effect of weight class, P = 0.03) and was increased following the EET irrespective of BMI status (main effect of time, P = 0.05; Fig. 1 C). CFU‐T trended higher in the obese group (P = 0.07; Fig. 1 D) and increased as a result of the EET (P = 0.02; Fig. 1 D).
Figure 1. Colony forming unit potential of innate immune populations are elevated during obesity and responsive to EET.
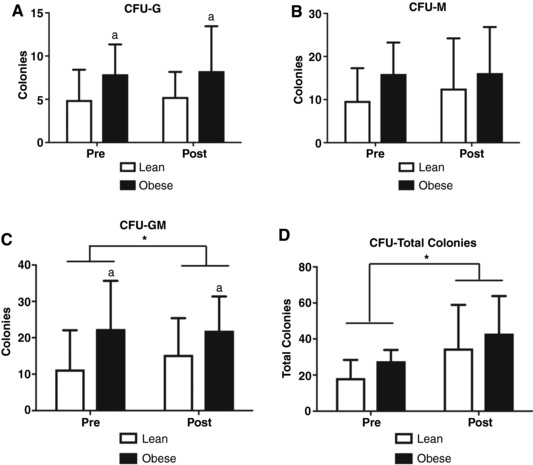
A, CFU‐Gs are elevated in in obese adults but unaffected by EET (amain effect of group). B, CFU‐Ms are not changed by exercise nor different between BMI classes. C, CFU‐GMs are elevated in obese participants (main effect of group) and increase as a result of EET in both BMI classes (main effect of exercise). D, total CFU colonies trended higher in obese adults and are increased by EET irrespective of BMI status (main effect of exercise). * P < 0.05; a P < 0.05; n = 17 Lean, n = 9 Obese; data are presented as means ± SD.
Inflammatory progenitor cells are decreased after 6 weeks of EET
The number of CCR2+ HSPCs and level of CCR2 expression on HSPCs was decreased after EET in both groups (P = 0.006, Fig. 2 A and P < 0.001, Fig. 3 B, respectively). The percentage of HSPCs expressing CCR2 was decreased after EET in the obese group (P = 0.02, Fig. 2 C). The number of TLR4+ HSPCs was decreased after EET in both the lean and obese groups (P = 0.006, Fig. 2 D), and the level of expression of TLR4 on HSPCs was increased in the lean and obese groups after the EET (P = 0.03, Fig. 2 E). The percentage of TLR4‐expressing HSPCs did not change with EET in the lean and obese groups (Fig. 2 F).
Figure 2. EET modifies CCR2 and TLR4 expression on HSPCs.
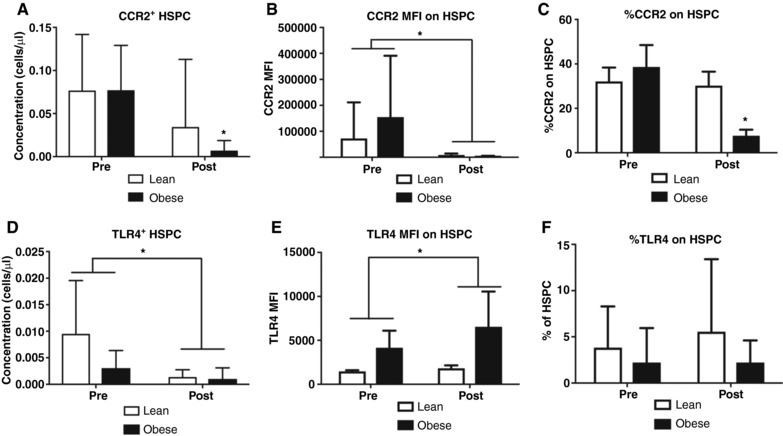
A, the concentration CCR2+ HSPCs in isolated PBMCs was significantly decreased in both groups as a result of 6 weeks of EET (main effects of exercise). B, CCR2 content (expressed as median fluorescence intensity: MFI) was significantly decreased after 6 weeks of EET in both groups (main effect of exercise). C, the percentage of HSPCs expressing CCR2 was significantly decreased in the obese (group × time interaction), but not lean, group as a result of 6 weeks of EET. D, the concentration of HSPCs expressing TLR4 in isolated PBMCs was decreased after 6 weeks of EET in both lean and obese adults (main effect of exercise). E, TLR4 content (expressed as median fluorescence intensity: MFI) was significantly increased in both lean and obese adults after 6 weeks of EET. F, the percentage of HSPCs expressing TLR4 was not changed with 6 weeks of EET in lean or obese adults. * P < 0.05; n = 17 Lean, n = 10 Obese; data are presented as means ± SD.
Figure 3. EET attenuates circulating CPC mobilization factors.
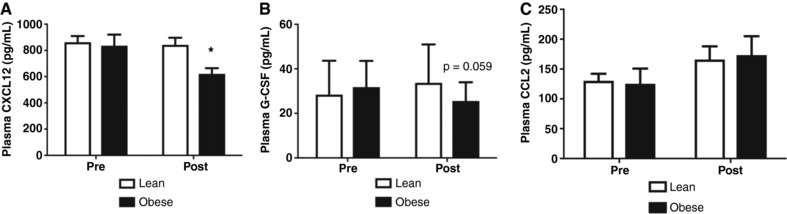
A, CXCL12 levels declined in lean, but not obese, participants as a result of EET training (group × time interaction). B, G‐CSF showed a trend to be decreased in the obese group as a result of the EET (group × time interaction). C, CCL2 was not altered after 6 weeks of EET. * P < 0.05; n = 17 Lean, n = 10 Obese; data are presented as means ± SD.
CXCL12 is decreased after EET
CXCL12 levels decreased following EET in the obese, but not lean, participants (interaction effect P = 0.01; Fig. 3 A). G‐CSF did not significantly change as a result of EET but showed a trend to decrease in adults with obesity (P = 0.06; Fig. 3 B). CCL2 was not altered after EET (P > 0.05, Fig. 3 C).
The baseline proportion of HSPCs is positively related to cardiorespiratory fitness, and changes in AT‐MSCs and BM‐MSCs are negatively related to changes in fitness.
The proportion of HSPCs was positively related to baseline absolute fitness (Absolute ; ρ = 0.432, P = 0.03, Fig. 4 A). No relationships were seen between other progenitor cell populations and fitness. The change in AT‐MSC content from Pre to Post was negatively related to changes in absolute fitness in both groups (ρ = −0.51, P < 0.05; Fig. 4 B). The change in BM‐MSC content from Pre to Post was negatively related to changes in absolute fitness in both groups (ρ = −0.41, P < 0.05; Fig. 4 C).
Figure 4. Baseline fitness is positively related to baseline proportions of HSPCs, and changes in AT‐MSCs and BM‐MSCs are negatively related to changes in absolute fitness.
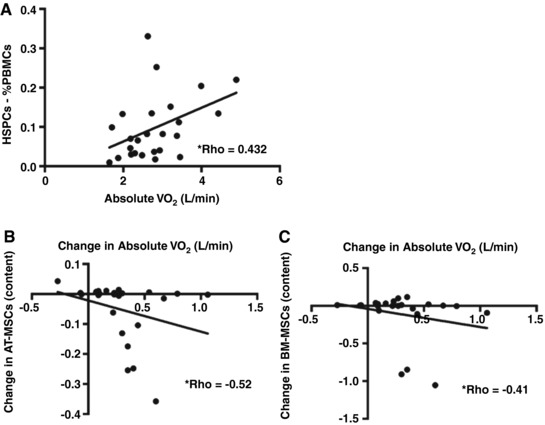
A, the proportion of HSPCs is positively related to baseline absolute (ρ = 0.432). * P < 0.05; n = 25. B, the change in AT‐MSC content in isolated PBMCs from EET was negatively related to changes in absolute (ρ = −0.52; P < 0.05); n = 25. C, the change in BM‐MSC content in isolated PBMCs from EET was negatively related to changes in absolute (ρ = −0.41; P < 0.05). n = 25.
Discussion
The aim of the present study was to compare the effects of 6 weeks of EET on CPC populations, particularly HSPCs expressing pro‐inflammatory markers, between lean and obese adults. The main findings of this study were: (1) the concentration of HSPCs, AT‐MSCs and CLPs, and the proportion of HSPCs and CLPs are decreased in both lean adults and obese adults as a result of 6 weeks of EET; (2) EET increases the number of more primitive CFU‐GM colonies, while adults with obesity have a higher number of CFU‐G and CFU‐GM colonies, irrespective of exercise; (3) EET decreases inflammatory markers CCR2 and TLR4 on HSPCs, particularly in adults with obesity. These results identify a potential novel mechanism for the anti‐inflammatory effects of EET in obesity.
Data regarding the relationship between physical activity level, cardiorespiratory fitness and exercise training on CPC content are equivocal. Cross‐sectional studies have shown that endurance trained athletes have elevated levels of CPCs compared to sedentary controls (Bonsignore et al. 2002), while others have shown no difference in CPC content when individuals were stratified into high fitness and low fitness groups (Baker et al. 2017). Our baseline cross‐sectional analysis aligns with these previous data, as well as data from elite wheelchair athletes (Niemiro et al. 2018), showing that cardiorespiratory fitness is positively associated with HSPCs. Conversely, results from longitudinal studies indicate that EET does not alter CPC content (Wardyn et al. 2008; Rakobowchuk et al. 2012). However, these studies are limited in that they used interval training rather than steady state training (Rakobowchuk et al. 2012). Conversely, EET under hypoxic conditions (Wang et al. 2014), or in patients with lower limb ischaemia (Sandri et al. 2005) significantly increased CPC content. To our knowledge, this is the first study to describe a reduction in the proportion and concentration of HSPCs following EET in both lean and obese adults. Discrepancies in the literature could be due to the lack of a consistent endurance exercise intervention (Emmons et al. 2016), variability in time of CPC/HSPC quantification following the most recent exercise bout (with some not specified) (Bonsignore et al. 2002; Wardyn et al. 2008; Niño et al. 2015), and lack of reported dietary controls. We extend the findings of previous studies by further characterizing more committed haematopoietic progenitors. Similar to HSPCs, the proportion and concentration of common lymphoid progenitors decreased following EET, while CFU‐GM and CFU‐T colonies increased. We are not the first to report discrepancies between phenotypic CPCs and CFUs (Shaw et al. 2011), and this is not surprising given that CFUs are a population of more mature progenitors (Choi et al. 2015). Together, these findings suggest that EET is related to a decrease in the concentration and proportion of more primitive haematopoietic progenitor cell populations and an increase in more committed haematopoietic progenitors in circulation. Interestingly, animal studies have shown an increase in HSPC content in the bone marrow following exercise training (Baker et al. 2011), and that EET remodels the HSPC niche in the bone marrow in rodent models of obesity (Adler et al. 2014). As such, we speculate that EET may create a more favourable bone marrow environment for HSPCs resulting in increased HSPC maintenance and preservation within the bone marrow and an expansion of more differentiated populations in circulation. Previous studies have suggested that HSPCs may not originate directly from the bone marrow and may be mobilized from marginal pools within the vasculature (Agha et al. 2018). As such, EET may increase HSPC content in the marginal pools which could also explain their decrease in circulation.
Cell surface markers have been identified that regulate HSPC recruitment and differentiation. Rodent studies have shown that HSPCs are recruited towards inflamed adipose tissue in a CCR2‐dependent manner (Si et al. 2010). Furthermore, once HSPCs are recruited to inflamed tissues, they can exacerbate the local inflammatory response through differentiation towards myeloid lineages, a process driven by TLR4 stimulation (Liu et al. 2015). Since individuals with obesity are in a chronic state of low‐grade inflammation, we investigated if EET could modulate CCR2 and TLR4 expression on HSPCs. Exercise training significantly decreased the number of circulating HSPCs expressing CCR2. This decrease could have been due to a decrease in overall HSPC content, thus we further evaluated if the proportion of HSPCs expressing CCR2 and the level of CCR2 expression on HSPCs were influenced by exercise training. Interestingly, we observed a significant decrease in the proportion of CCR2‐expressing HSPCs specifically in individuals with obesity and a decrease in overall CCR2 expression, which was independent of weight status. Our research extends recent research that has shown that exercise can reduce the level of expression of CCR2 on inflammatory monocytes in individuals with obesity (Barry et al. 2017) to show that CCR2 is also reduced in HSPCs, inflammatory monocyte precursors. The decreased proportion of CCR2+ HSPCs in obese adults suggests that EET influences the CCR2 regulation in HSPCs in obesity. Furthermore, these results suggest that circulating HSPCs have decreased homing capacity following EET, perhaps reflecting an increased propensity to remain within the bone marrow niche. Similar to our CCR2 findings, EET decreased the concentration of HSPCs expressing TLR4; however, TLR4 expression on HSPCs was increased by EET. These results suggest that the overall content of HSPCs with myeloid differentiation potential was decreased; however, the capacity of those remaining HSPCs in circulation to respond to inflammatory stimuli was increased. This aligns with our CFU findings. It will be interesting to investigate if these exercise‐induced alterations in circulating HSPCs result in decreased local tissue inflammation in obesity due to decreased HSPC homing and subsequent myeloid cell production. Alternatively, this could reflect an increased potential of individual HSPCs to directly respond to pathogens following EET.
To evaluate the mechanisms responsible for alterations in CPC content, we quantified CXCL12 and G‐CSF, two chemokines involved in CPC mobilization (Bendall & Bradstock, 2014; Niemiro et al. 2017). Specifically, G‐CSF is the most common CPC mobilization agent used in a bone marrow transplant and disrupts the CXCR4/CXCL12 axis of CPCs anchored within the bone marrow (Mohty & Ho, 2011). Similarly, HSPCs migrate towards an increasing CXCL12 gradient (Wright et al. 2002; Sugiyama et al. 2006; Massberg et al. 2007). Thus, we reasoned that changes in these cytokines could explain mechanisms responsible for the observed alterations in CPC subpopulations. Our results revealed EET significantly decreased CXCL12 concentration and tended to decrease G‐CSF concentration, specifically in adults with obesity. Interestingly, the change in the concentrations of these chemokines was not related to the change in CPC content (data not shown). These results support previous findings from acute exercise studies that show that exercise‐induced changes in G‐CSF and CXCL12 are only temporally related to alterations in CPC content (Niemiro et al. 2017; Agha et al. 2018). We also evaluated circulating CCL2 levels because it has been shown that CCR2 is required for stem and progenitor cell homing to inflamed tissues (Si et al. 2010; Dutta et al. 2015). Since CCL2 is the primary ligand for CCR2, we reasoned that changes in CCL2 could explain specific decreases in CCR2+ HSPCs in adults with obesity. We did not find any changes in plasma levels of CCL2 after EET in either the lean or obese groups, which suggests that other mechanisms may be responsible for the decreased proportion of CCR2‐expressing HSPCs. It is possible that peripheral tissues have higher levels of expression of CCL2, particularly in obese adults, which promotes removal of CCR2+ HSPCs from the circulation. Unfortunately, we were not able to collect adipose or muscle biopsies in the present study to further evaluate this hypothesis. Further, CCR2 is known to bind other CCLs (Shi & Pamer, 2011); however, lack of sample precluded us from evaluating a broader panel of CCLs.
MSCs have gained popularity as a potential source for cell therapy due to their multi‐lineage differentiation potential, and their role in regulating tissue repair via paracrine mechanisms (Bartczak et al. 2017). Following acute exercise, MSC content has been shown to decrease (Marycz et al. 2016), or remain unchanged (Niemiro et al. 2017); however, the effects of exercise training on circulating MSC content has not previously been evaluated. Our results reveal a trending decrease in the proportion of, and a decrease in the content of, reportedly adipose tissue derived fraction of CD34+ MSCs (Dominici et al. 2006; Traktuev et al. 2008), and a trend for a decrease in the reportedly bone marrow‐derived CD34− fraction of MSCs in individuals with obesity (Lin et al. 2013). Interestingly, we found that the change in BM‐MSC and AT‐MSC concentration was negatively related to the change in absolute . These results indicate that increases in cardiorespiratory fitness are associated with decreases in circulating MSC content. However, it is important to note that while participants were encouraged to maintain their normal diet, there were still reductions in body fat percentage after the EET. Therefore, it should be noted that changes in AT‐MSCs may also be influenced by changes in body fat percentage. Future studies should investigate the precise nature and physiological relevance of this relationship and investigate if these changes are due to changes in body fat or exercise, or a combination of both.
A limitation of the study is that we were not able to include non‐exercised control groups because this study was a sub‐analysis of a larger parent study (Allen et al. 2018). The aim of the present study was to compare inflammatory CPC subpopulations at baseline and their responses to training between lean and obese adults. Thus, the addition of non‐exercise trained control groups would have required the addition of two extra groups of participants, which was not feasible. Lacking these groups creates the possibility that our observed changes in cell populations could be due to a factor other than the exercise training intervention. Indeed, it has been shown that sympathetic nervous system activation participates in CPC mobilization in response to exercise (Agha et al. 2018). As such, participants may have been more anxious during the baseline blood collection than the post‐training blood collection, which could have accounted for the decrease in CPC content post‐training. Importantly, the study by Agha and colleagues, as well as a separate study by Kröpfl and colleagues (Kröpfl et al. 2014), examined the role of catecholamines in CPC mobilization using a randomized cross‐over design; thus, examining baseline data on separate trial days in these studies can inform how participants would respond to repeated blood collection in the present study. Both previous studies demonstrated similar values for adrenaline (epinephrine), noradrenaline (norepinephrine) and CPCs at baseline between visits. Although we were not able to measure catecholamine levels in the present study, findings from previous studies suggest that levels would have been similar between our Pre/Post time points and would not have contributed to differences observed at rest in our participants. We included several protocols to ensure differences were due to our intervention and not artifacts. For example, strict dietary controls were in place for 3 days prior to blood cell analyses, analyses were conducted at the same time of day to control for circadian variations, and participants were monitored during each exercise session to ensure successful completion. These data will support future studies in the lab that will have the primary aim of examining the effects of training on various CPC populations in lean and obese adults, which will necessitate the inclusion of a non‐exercise trained control.
Together, our results indicate that EET decreases CPC subpopulations, particularly inflammatory‐primed HSPCs in lean and obese adults. Our findings provide a better understanding of the factors regulating CPCs and suggest that EET may be beneficial for adults with obesity through decreases in the content of inflammatory‐primed HSPCs. Future studies should evaluate the physiological relevance of reduced circulating HSPCs as a result of EET, particularly if such changes parallel exercise‐induced improvements in peripheral tissue inflammation and metabolic function in obese individuals.***
Additional information
Competing interests
The authors declare no competing interests. The results of the study are presented clearly, honestly and without fabrication, falsification or inappropriate data manipulation.
Author contributions
This study was conducted in the Exercise Immunology Laboratory and the Exercise and Stem Cell Laboratory at the University of Illinois at Urbana‐Champaign. G.M.N., M.DeL., J.M.A., H.D.H. and J.A.W. designed the research; G.M.N., J.M.A., L.J.M. collected data and performed research; G.M.N. and M.DeL. analysed data; G.M.N., N.A.K., and M.DeL were involved in interpretation of data; N.A.K., H.D.H., J.A.W. and M.DeL. supervised research and provided funding/reagents. G.M.N. and M.DeL. wrote the manuscript and all authors provided critical revisions important for intellectual content of the finished manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Funding was provided by an American College of Sports Medicine (ACSM) Foundation Grant and a Natural Sciences and Engineering Research Council (NSERC, Canada) Discovery Grant to M.DeL. G.M.N. was supported by an Egg Nutrition Center Fellowship.
Acknowledgements
The authors would like to thank the participants and undergraduate staff for their time and dedication to the study. We would also like to thank Dr Anne M. Walk for her help with statistical analyses and SPSS methodology.
Biography
Grace Niemiro is currently at the University of Illinois at Urbana‐Champaign. She received her Bachelor's degree in Chemistry at the University of Illinois in 2014, where she then started her PhD in Kinesiology. Her research focuses on the exercise and lifestyle effects on circulating progenitor cells in both children and adults. In her future career, Grace hopes to become a tenure‐track professor at a research‐intensive university investigating the effects of obesity on the immune system, and how this may negatively affect cognitive functioning.

Edited by: Scott Powers & Bettina Mittendorfer
This is an Editor's Choice article from the 15 July 2018 issue.
References
- Adler BJ, Kaushansky K & Rubin CT (2014). Obesity‐driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol 10, 737–748. [DOI] [PubMed] [Google Scholar]
- Agha NH, Baker FL, Kunz HE, Graff R, Azadan R, Dolan C, Laughlin MS, Hosing C, Markofski MM, Bond RA, Bollard CM & Simpson RJ (2018). Vigorous exercise mobilizes CD34+ hematopoietic stem cells to peripheral blood via the β2‐adrenergic receptor. Brain Behav Immun 68, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD & Woods JA (2018). Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50, 747–757. [DOI] [PubMed] [Google Scholar]
- Baker JM, De Lisio M & Parise G (2011). Endurance exercise training promotes medullary hematopoiesis. FASEB J 25, 4348–4357. [DOI] [PubMed] [Google Scholar]
- Baker JM, Nederveen JP & Parise G (2017). Aerobic exercise in humans mobilizes HSCs in an intensity‐dependent manner. J Appl Physiol (1985) 122, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JC, Simtchouk S, Durrer C, Jung ME & Little JP (2017). Short‐term exercise training alters leukocyte chemokine receptors in obese adults. Med Sci Sports Exerc 49, 1631–1640. [DOI] [PubMed] [Google Scholar]
- Bartczak A, McGilvray I & Keating A (2017). Mesenchymal stromal cell therapy to promote cardiac tissue regeneration and repair. Curr Opin Organ Transplant 22, 86–96. [DOI] [PubMed] [Google Scholar]
- Bastien M, Poirier P, Lemieux I & Després J‐P (2014). Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56, 369–381. [DOI] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS & Kolonin MG (2011). Influence of BMI on level of circulating progenitor cells. Obesity 19, 1722–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall LJ & Bradstock KF (2014). G‐CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev 25, 355–367. [DOI] [PubMed] [Google Scholar]
- Bonsignore MR, Morici G, Santoro A, Pagano M, Cascio L, Bonanno A, Abate P, Mirabella F, Profita M, Insalaco G, Gioia M, Vignola AM, Majolino I, Testa U & Hogg JC (2002). Circulating hematopoietic progenitor cells in runners. J Appl Physiol (1985) 93, 1691–1697. [DOI] [PubMed] [Google Scholar]
- Boppart MD, De Lisio M & Witkowski S (2015). Exercise and stem cells. Prog Mol Biol Transl Sci 135, 423–456. [DOI] [PubMed] [Google Scholar]
- Choi JS, Mahadik BP & Harley BAC (2015). Engineering the hematopoietic stem cell niche: frontiers in biomaterial science. Biotechnol J 10, 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV (2014). Activated monocytes: yet another link between systemic inflammation and obesity. J Clin Endocrinol Metab 99, 2347–2349. [DOI] [PubMed] [Google Scholar]
- De Lisio M & Parise G (2013). Exercise and hematopoietic stem and progenitor cells: protection, quantity, and function. Exerc Sport Sci Rev 41, 116–122. [DOI] [PubMed] [Google Scholar]
- Devêvre EF, Renovato‐Martins M, Clément K, Sautès‐Fridman C, Cremer I & Poitou C (2015). Profiling of the three circulating monocyte subpopulations in human obesity. J Immunol 194, 3917–3923. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D & Horwitz E (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R & Nahrendorf M (2015). Myocardial infarction activates CCR2+ hematopoietic stem and progenitor cells. Cell Stem Cell 16, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R, Niemiro GM & De Lisio M (2016). Exercise as an adjuvant therapy for hematopoietic stem cell mobilization. Stem Cells Int 2016, 7131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R, Niemiro GM & De Lisio M (2017). Hematopoiesis with obesity and exercise: role of the bone marrow niche. Exerc Immunol Rev 23, 82–95. [PubMed] [Google Scholar]
- Hardy OT, Kim A, Ciccarelli C, Hayman LL & Wiecha J (2013). Increased Toll‐like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes 8, e19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- Kröpfl JM, Stelzer I, Mangge H, Pekovits K, Fuchs R, Allard N, Schinagl L, Hofmann P, Dohr G, Wallner‐Liebmann S, Domej W & Müller W (2014). Exercise‐induced norepinephrine decreases circulating hematopoietic stem and progenitor cell colony‐forming capacity. PLoS One 9, e106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C‐S, Xin Z‐C, Dai J & Lue TF (2013). Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histol Histopathol 28, 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang Y, Ding Y, Baez I, Payne KJ & Borghesi L (2015). Cutting edge: hematopoietic stem cell expansion and common lymphoid progenitor depletion require hematopoietic‐derived, cell‐autonomous TLR4 in a model of chronic endotoxin. J Immunol 195, 2524–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marycz K, Mierzejewska K, Śmieszek A, Suszynska E, Malicka I, Kucia M & Ratajczak MZ (2016). Endurance exercise mobilizes developmentally early stem cells into peripheral blood and increases their number in bone marrow: implications for tissue regeneration. Stem Cells Int 2016, 5756901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic‐Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB & von Andrian UH (2007). Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M & Ho AD (2011). In and out of the niche: perspectives in mobilization of hematopoietic stem cells. Exp Hematol 39, 723–729. [DOI] [PubMed] [Google Scholar]
- Niemiro GM, Edwards T, Barfield JP, Beals JW, Broad EM, Motl RW, Burd NA, Pilutti LA & Lisio MD (2018). Circulating progenitor cell response to exercise in wheelchair racing athletes. Med Sci Sports Exerc 50, 88–97. [DOI] [PubMed] [Google Scholar]
- Niemiro GM, Parel J, Beals J, van Vliet S, Paluska SA, Moore DR, Burd NA & De Lisio M (2017). Kinetics of circulating progenitor cell mobilization during submaximal exercise. J Appl Physiol (1985) 122, 675–682. [DOI] [PubMed] [Google Scholar]
- Niemiro GM, Raine LB, Khan NA, Emmons R, Little J, Kramer AF, Hillman CH & De Lisio M (2016). Circulating progenitor cells are positively associated with cognitive function among overweight/obese children. Brain Behav Immun 57, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niño O, Balague N, Aragones D, Blasi J, Alamo JM, Corral L, Javierre C, Miguel M, Viscor G & Ventura JL (2015). CD34+ circulating progenitor cells after different training programs. Int J Sports Med 36, 292–296. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK & Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Park JY & Yu R (2005). Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF‐α and IL‐6. Diabetes Res Clin Pract 69, 29–35. [DOI] [PubMed] [Google Scholar]
- Rakobowchuk M, Harris E, Taylor A, Baliga V, Cubbon RM, Rossiter HB & Birch KM (2012). Heavy and moderate interval exercise training alters low‐flow‐mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol 97, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G & Hambrecht R (2005). Effects of exercise and ischemia on mobilization and functional activation of blood‐derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation 111, 3391–3399. [DOI] [PubMed] [Google Scholar]
- Shaw SY, Cheng S, Cupples LA, Larson MG, McCabe EL, Ngwa JS, Wang YA, Martin RP, Klein RJ, Hashmi B, Ajijola OA, Lau E, O'Donnell CJ, Vasan RS, Cohen KS & Wang TJ (2011). Genetic and clinical correlates of early‐outgrowth colony‐forming units. Circ Cardiovasc Genet 4, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C & Pamer EG (2011). Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Tsou C‐L, Croft K & Charo IF (2010). CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 120, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M & Nagasawa T (2006). Maintenance of the hematopoietic stem cell pool by CXCL12‐CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld‐Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH & March KL (2008). A population of multipotent CD34‐positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102, 77–85. [DOI] [PubMed] [Google Scholar]
- Wang J‐S, Lee M‐Y, Lien H‐Y & Weng T‐P (2014). Hypoxic exercise training improves cardiac/muscular hemodynamics and is associated with modulated circulating progenitor cells in sedentary men. Int J Cardiol 170, 315–323. [DOI] [PubMed] [Google Scholar]
- Wardyn GG, Rennard SI, Brusnahan SK, McGuire TR, Carlson ML, Smith LM, McGranaghan S & Sharp JG (2008). Effects of exercise on hematological parameters, circulating side population cells, and cytokines. Exp Hematol 36, 216–223. [DOI] [PubMed] [Google Scholar]
- Wright DE, Bowman EP, Wagers AJ, Butcher EC & Weissman IL (2002). Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med 195, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You T, Arsenis NC, Disanzo BL & LaMonte MJ (2013). Effects of exercise training on chronic inflammation in obesity. Sports Med 43, 243–256. [DOI] [PubMed] [Google Scholar]


