Abstract
Telomeres are repetitive nucleotide sequences located at the ends of chromosomes that protect genetic material. We use data from the Fragile Families and Child Wellbeing Study to analyze the relationship between exposure to spatially concentrated disadvantage and telomere length for white and black mothers. We find that neighborhood disadvantage is associated with shorter telomere length for mothers of both races. This finding highlights a potential mechanism through which the unique spatially concentrated disadvantage faced by African Americans contributes to racial health disparities. We conclude that equalizing the health and socioeconomic status of black and white Americans will be very difficult without reducing levels of residential segregation in the United States.
Keywords: telomere, segregation, neighborhood disadvantage, concentrated poverty
In his seminal book, The Truly Disadvantaged, William Julius Wilson (1987) notes the growing concentration of poverty within black inner city neighborhoods and hypothesized that long-term exposure to spatially concentrated disadvantage was central to the perpetuation of poverty among African Americans. Since that time, a large body of research has sought to establish the existence of “neighborhood effects” on individual social, economic, and health outcomes. Although analyses of multilevel survey data and nonexperimental results derived from static group comparisons generally produced results consistent with Wilson’s hypothesis (see Rubinowitz and Rosenbaum 2000; Sampson, Morenoff, and Gannon-Rowley 2002; Massey and Clampet-Lundquist 2008), experimental findings from the Moving to Opportunity (MTO) Study were initially less supportive (Kling, Lieberman, and Katz 2007).
The MTO study randomly assigned poor residents of public housing projects in five metropolitan areas to experimental and control groups. Members of the former group were offered housing vouchers that required subjects to move into a low-poverty neighborhood and the latter received no offer of vouchers but continued to receive project-based assistance. Statistical comparisons of the two groups five to seven years after random assignment revealed that members of the experimental group did experience lower levels of neighborhood poverty and improved mental and physical health (Ludwig et al. 2011) but that the intervention offered “no convincing evidence of effects on educational performance; employment and earnings; or household income, food security, and self-sufficiency” (Orr et al. 2003, xv). These disappointing conclusions were generally sustained when the evaluation was repeated ten to fifteen years after random assignment (Sanbonmatsu et al. 2011).
More recently, however, the tide of evidence has begun to turn in favor of Wilson’s “neighborhood effects” hypothesis. A quasi-experimental evaluation of a housing mobility project in New Jersey recently demonstrated that, when compared to members of a matched control group, adults who moved from a high-to low-poverty residential environment experienced significantly lower exposure to disorder and violence, a lower frequency of negative life events, better mental health, higher employment rates, more earned income, and lower rates of welfare receipt (Massey et al. 2013). At the same time, adults who moved also became more involved in their children’s academic development, and the children themselves evinced a dramatic increase in hours spent studying while gaining greater access to a quiet study space, higher quality schools, and lower levels of disorder and violence within schools, all of which allowed them to maintain strong grades despite attending more demanding schools (and hence receiving a much better education).
Using data from the Panel Study of Income Dynamics, Jeffrey Wodtke, David Harding, and Felix Elwert followed children from age one to seventeen and find that long-term exposure to concentrated neighborhood disadvantage sharply reduced the likelihood of high school graduation (2011), especially for children from low-income families (2016). Drawing on the same data source, Jonathan Rothwell and Douglas Massey show that, after adjusting for regional differences in purchasing power, lifetime household income would have been $910,000 greater if people born into bottom-quartile of neighborhoods had instead been raised within a top-quartile neighborhood, indicating a powerful neighborhood income effect that was two-thirds of the parental income effect (2014).
Finally, a recent reanalysis of the MTO subjects drawing on tax and census data from 2012 finds that children whose families moved into a low-poverty neighborhood before the age of thirteen by their mid-twenties earned annual incomes that were nearly $3,500 greater than their counterparts in the control group (Chetty, Hendren, and Katz 2016). In addition, they displayed marriage rates that were two percentage points higher and attended college at rates that were 2.5 points greater. Children in the experimental group also attended higher quality colleges and universities.
At this point, a consensus seems to be emerging that neighborhoods do indeed matter across a variety of dimensions of human well-being (Massey 2013). Social scientists are consequently moving away from simple demonstrations of the existence of neighborhood effects and attempting to identify and model the specific mechanisms by which exposure to spatially concentrated disadvantage affects critical human outcomes (compare Sampson 2012; Sharkey 2013). In the current analysis, we focus on the relationship between neighborhood disadvantage and health, one of the earliest associations to emerge experimentally from the MTO study (Ludwig 2012). Rather than offering additional evidence simply to confirm the existence of such a relationship, however, we explore a potentially important pathway by which concentrated neighborhood disadvantage may get “under the skin” of people growing up and living in poor neighborhoods to create a potential biological precursor of elevated morbidity and mortality in later life.
SOCIAL STRUCTURE, STRESS, AND HEALTH
We argue that one position within the social structure of society produces a high degree of exposure to spatially concentrated disadvantage. The social-structural position in question is defined by the intersection of high poverty and high residential segregation. The systematic residential segregation of any high-poverty group inevitably concentrates poverty spatially within neighborhoods inhabited by members of that group. Massey first identified this interaction using a simulation to show how rising rates of black poverty mechanically produced higher concentrations of black poverty as racial segregation increased, a relationship established empirically in subsequent research (Massey 1990; Massey and Eggers 1992; Massey and Fischer 2000). Although Massey’s empirical confirmation of the segregation-poverty interaction was questioned on statistical grounds (Jargowsky 1997), the underlying mathematics of the interaction were later worked out and confirmed: “racial segregation and income segregation within race contribute importantly to poverty concentration, as Massey argued” (Quillian 2012, 354).
The group most subject to this interaction is African Americans, who in many metropolitan areas are simultaneously the poorest and most segregated minority group. These circumstances expose them to uniquely high concentrations of neighborhood disadvantage compared with other racial-ethnic groups (Massey and Rugh forthcoming). This fact is important because, as Robert Sampson points out, when it comes to urban ecology “things go together” (2012). Areas of spatially concentrated disadvantage also tend to be areas of high crime, elevated violence, excessive mortality, low collective efficacy, fragmented social ties, and limited capacity for collective action. It is hardly surprising, therefore, that neighborhood disadvantage has been identified as the critical nexus for the intergenerational transmission of poverty among African Americans (Sharkey 2013).
Previous work has found that neighborhood disadvantage is associated with biological markers linked to stress or health, including cortisol levels and C-protein reactivity (Rudolph et al. 2014; Hackman et al. 2012; Karb et al. 2012), blood pressure (Cathorall et al. 2015), DNA methylation (King et al. 2016), and summative measures such as allostatic load (Gustafsson et al. 2014; Finch et al. 2010). In this article, we conceptualize the attenuation of telomere length as a potential mechanism by which exposure to neighborhood disadvantage undermines health in later life. Telomeres are repetitive nucleotide sequences located at the ends of human chromosomes, which act as buffers to protect genetic material from deterioration and errant recombination during cell division (Blackburn 2006; Kipling 1995).
Telomeres naturally shorten in the course of human aging (Wilhide 2014). The normal process of shortening over time can be accelerated by exposure to environmental stressors, however (Sapolsky 2004). Despite some evidence of positive publication bias, meta-analyses provide support for the association between perceived stress and telomere length (Schutte and Malouf 2014; Mathur et al. 2016). These results support the conclusion of Elissa Epel and her colleagues that “stress … is significantly associated with higher oxidative stress, lower telomerase activity, and shorter telomere length” (2004, 17312).
We hypothesize that one critical source of environmental stress for African Americans is their elevated exposure to spatially concentrated disadvantage, thus yielding a potential biosocial pathway connecting their position in the U.S. social structure to health. Specifically, the combination of high poverty and high segregation uniquely expose African Americans to spatially concentrated disadvantage, yielding a prolonged exposure to stress, which functions over time to shorten telomeres of African Americans prematurely, with potential adverse health consequences later in life.
Admittedly, the nature of the relationship between shortened telomeres and poor health is not settled. Some scholars have argued that the poor prediction of mortality risk (Glei et al. 2016) and physical decline (Harris et al. 2016) by telomere length, particularly in the oldest-old (Yu et al. 2015; Martin-Ruiz et al. 2005), implies that telomere length is a “weak biomarker [of human aging] with poor predictive accuracy compared with many traditional covariates” (Sanders and Newman 2013). However, numerous other studies document associations between telomere length and a variety of subsequent health outcomes, including all-cause mortality risk, infectious disease mortality, coronary heart disease, stroke, myocardial infarction, diabetes, cancer incidence, and cancer mortality (Glei et al. 2016; see also Marioni et al. 2016; Fitzpatrick et al. 2011; Haycock et al. 2014; Goglin et al. 2016; D’Mello et al. 2014; Willeit, Willeit, and Mayr 2010).
Though the exact mechanisms that link telomere length to subsequent health is unclear, a recent review highlights a variety of potential mechanisms by which telomere length might influence health, concluding that “telomere attrition can lead to potentially maladaptive cellular changes, block cell division, and interfere with tissue replacement,” and that “greater overall telomere attrition predicts mortality and age-related diseases” (Blackburn, Epel, and Lin 2015, 1193). Such a link between stressful social environments and compromised health has long been hypothesized, that persistent exposure to disadvantaged circumstances contributes to a process of human “weathering” in which disadvantaged populations age prematurely from high levels of stress and consequently experience poorer health as the life course proceeds (Geronimus 1992; see also DiPrete and Eirich 2006; Geronimus et al. 2006; Walsemann, Gee, and Geronimus 2009).
We argue here that a key source of stress for African Americans, beyond whatever instances of exclusion and discrimination they may experience while navigating U.S. society, is their long-term exposure to high spatial concentrations of disadvantage, which potentially contribute to weathering at the cellular level in the form of shortened telomeres (Epel et al. 2004; Sapolsky 2004). Because racial gaps in health and mortality typically are not eliminated by controlling for socioeconomic status and demographic factors, we argue that racial differences in exposure to concentrated disadvantage carry considerable potential to account more fully for black shortfalls in health (see Kitagawa and Hauser 1973; Geruso 2012).
One potential mechanism is the pathway hypothesized here, in which high levels of segregation and poverty interact to concentrate poverty within black neighborhoods, which in turn exposes African Americans to high concentrations of neighborhood disadvantage, which ultimately shortens black telomere lengths to foretell an elevated risk of health problems over the life course. The connection between segregation, poverty, and neighborhood disadvantage is well established (Quillian 2012) and evidence of a link between telomere length and poor health is rapidly accumulating (see Blackburn, Epel, and Lin 2015). Here we seek to demonstrate an association between concentrated neighborhood disadvantage and telomere length in a large nationally representative sample.
Prior work offers suggestive evidence of such a link. Katherine Theall and her colleagues, for example, gathered data from children in New Orleans and show that exposure to neighborhood disorder and poverty was associated with shorter telomeres (2013). Using a sample of adults from locations around the United States, Belinda Needham and her colleagues also find a strong negative relationship between telomere length and the quality of the neighborhood environment, as measured by aesthetics, safety, and social cohesion (2015). Likewise, Minjung Park and her colleagues used data from a longitudinal survey of Dutch adults to demonstrate that telomere length varied inversely with neighborhood quality, as measured by self-reported disorder, crime, and noise (2015).
Recently Arline Geronimus and her colleagues compiled a sample of respondents from three Detroit neighborhoods and find that respondents who were most satisfied with their neighborhood circumstances displayed significantly longer telomeres than others. Using data from the Fragile Families and Child Wellbeing Study, Colter Mitchell and his colleagues demonstrate that black boys who experienced disadvantaged home environments displayed significantly shorter telomeres by age nine than statistically similar boys who grew up in advantaged environments (2014). Likewise, Stacy Drury and her colleagues find that telomeres were significantly shorter among children who reported greater exposure to family violence and disruption (2014). Irdan Shalev and his colleagues discovered that children exposed to multiple sources of violence and mistreatment while growing up displayed significantly more telomere shrinkage between ages five and ten than other children (2013).
DATA AND METHODS
Our data come from the Fragile Families and Child Wellbeing Study, which is based on a stratified, multistage, probability sample of children born in large U.S. cities between 1998 and 2000. Around three-quarters of the births were to unmarried mothers (hence yielding “fragile” families). Baseline interviews were conducted with mothers in the hospital soon after the child’s birth, and fathers were interviewed in the hospital or by phone. Follow-up interviews were conducted with both parents when the child was one, three, five, and nine years old. About nine years after the birth of their child, 2,667 mothers provided saliva samples to enable biological assays of telomere length.
Telomere length was assessed using a quantitative real-time polymerase chain reaction (PCR) assay that yielded absolute measurements in numbers of kilobases. To guard against overly influential outlier cases, we eliminated respondents with telomere lengths below the 1st percentile or above the 99th percentile of the distribution and to facilitate analysis and we took the natural log of telomere length as our dependent variable in a simple linear model (see Mitchell et al. 2014). Most research on telomeres to date has relied on peripheral blood mononuclear cells from whole blood as a source of DNA. To consider the relationship between DNA from blood and that derived from saliva, Mitchell and colleagues asked sixteen healthy adult volunteers (ten females and six males) to contribute both blood and saliva samples (2014).
After discarding a single outlier (by four standard deviations) from one of the blood samples, they find that telomere length was greater in the saliva samples but nonetheless highly correlated with telomere length in the blood samples. This difference is not surprising since different cell types have different rates of division and thus different rates of telomere attrition. Because the saliva- and blood-based telomere lengths were highly correlated with one another, Mitchell and colleagues conclude there was no a priori reason to prefer one source over the other and proceeded with their analysis of telomere lengths derived from the Fragile Families data.
Our principal independent variable is an index of neighborhood disadvantage developed by Wodtke, Harding, and Elwert (2011). This measure is created from a principal component analysis of tract-level items that included rates of poverty, unemployment, female headedness, and welfare receipt, along with the percentages of persons age twenty-five and older who lacked a high school diploma, held a college degree, and occupied a managerial or professional occupation. We measured neighborhood disadvantage cumulatively from the first to the fifth wave of the survey using census tract records from the 2000 Census and the 2005–2009 American Community Survey (ACS), linking them to geocoded individual records for mothers and children. For the 2000 values and the few cases dating to 1998 or 1999, we used 2000 census estimates; for the 2007 and 2008 values, we used the ACS 2005–2009 estimates; and for years between, we interpolated.
To make sure that we were not capturing overall changes in tract values (stemming from nationwide events such as the Great Recession), we standardized all values within years across the national sample of census tracts. To incorporate the effects of moves between neighborhoods during the period of observation, we merged tract data from the census and the ACS; for individuals who changed tracts between waves, we set the midpoint between the two data collection dates as the year in which they moved. All models were estimated in Stata 14 and controlled as appropriate for indicators of demographic characteristics, educational attainment, living arrangements, and socioeconomic status.
Table 1 presents means for each independent variable by race-ethnicity for mothers at the time of the age-nine survey. The huge differential in exposure to cumulative neighborhood disadvantage between whites and blacks is immediately apparent in the first line of the table. Whereas the Wodtke index of neighborhood disadvantage stood at −0.252 for white mothers, the factor score for black mothers was 1.198. The mean age of mothers at the time of telomere collection was 33.4 years for blacks, and 36.4 years for whites, and the percentage foreign born was around 3 percent for whites and blacks. Black mothers displayed higher body mass indices (ratio of weight to height squared) than their white counterparts, with BMI Z-scores of 0.161 and −0.261 respectively. The average number of moves made before the child reached age nine differed slightly by race, with the number being 3.1 for blacks and 2.7 for whites.
Table 1.
Means of Variables in Analysis of Neighborhood Disadvantage and Telomere Length
| Variable | Total | Whites | Blacks |
|---|---|---|---|
| Neighborhood disadvantage | |||
| Wodtke index | 0.760 | −0.252 | 1.198 |
| Telomere length | |||
| Logged kilobases | 1.839 | 1.801 | 1.855 |
| Mother’s characteristics | |||
| Age at TL collection | 34.326 | 36.407 | 33.424 |
| Foreign born | 0.030 | 0.028 | 0.031 |
| Body mass (Z-score) | 0.034 | −0.261 | 0.161 |
| Moves during study | 2.997 | 2.679 | 3.136 |
| Education at birth of child | |||
| Less than high school | 0.279 | 0.168 | 0.328 |
| High school | 0.329 | 0.249 | 0.363 |
| Some college | 0.271 | 0.281 | 0.266 |
| College or more | 0.121 | 0.302 | 0.043 |
| Mother-father relationship at birth | |||
| Married | 0.238 | 0.509 | 0.120 |
| Cohabiting | 0.335 | 0.301 | 0.349 |
| Other | 0.427 | 0.189 | 0.531 |
| Household SES at birth | |||
| Household poverty ratio | 2.431 | 4.060 | 1.726 |
| Household welfare | 0.379 | 0.213 | 0.450 |
| Number of cases | 1,661 | 502 | 1,159 |
Source: Authors' compilation of data from the Fragile Families and Child Wellbeing Survey.
As one might expect, education levels differed by race. Whereas 33 percent of black respondents had less than a high school education, only 17 percent of white respondents did. Likewise, 30 percent of whites but only 4 percent of blacks were college graduates. A similar contrast was observed for the mothers’ family situation at the time of the birth. Just 12 percent of black mothers were married and 35 percent were cohabiting, versus 50 percent and 31 percent of whites. In other words, nearly 80 percent of white mothers but only 47 percent of black mothers were married or cohabiting at the time of birth. As with mother’s education, household income was greater for whites than for blacks. The income-to-poverty ratio was 4.1 for white and 1.7 for blacks. As one would expect given these income figures, the percentage on welfare for black mothers was double that of white mothers, with figures of 45 percent and 21 percent respectively.
TELOMERES, SOCIOECONOMIC STATUS, AND NEIGHBORHOOD DISADVANTAGE
To set the stage for our multivariate analysis, we offer a simple description of intergroup differences in telomere length (TL), socioeconomic status, and neighborhood disadvantage. As table 1 shows, we observe clear differences in TL between whites and blacks. White mothers clearly stand out for their low values, averaging 1.801 kilobases to 1.855 for blacks, a statistically significant difference. To understand the distribution of telomere length, figure 1 plots mothers’ telomere length (in logged kilobases) by race. Though they have a similar range of values, it is clear from this histogram that the distribution of telomere length is distributed more to the right for blacks relative to whites. It may seem surprising that black mothers have longer telomeres than white mothers given their greater exposure to disadvantage, but other researchers have noted similar black-white differentials over the life course (Needham et al. 2015; Brown et al. 2016; Hansen et al. 2016; Lynch et al. 2016; Drury et al. 2014; Hunt et al. 2008). Understanding the reasons for this racial differential in telomere length remains an important task for future research.
Figure 1.
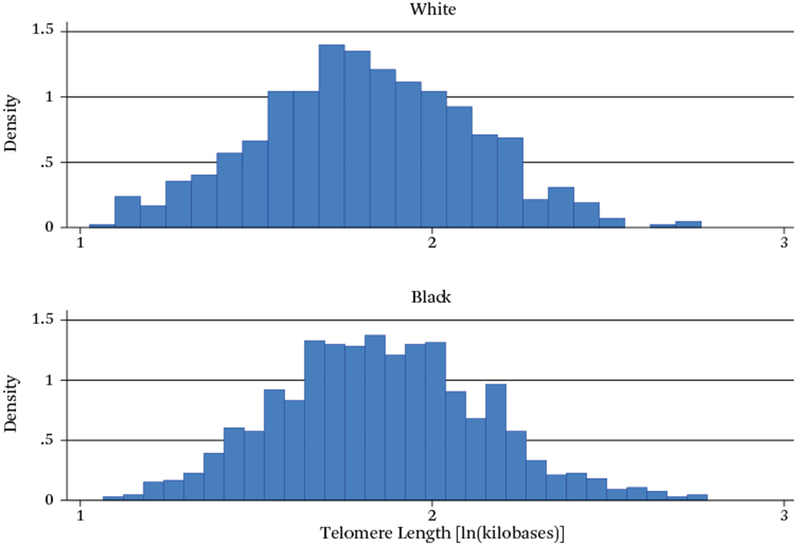
Density Distribution, Telomere Length
Source: Authors’ compilation of data from the Fragile Families and Child Wellbeing Survey.
Whatever the baseline telomere length, what we are trying to test here is whether exposure to a stressor “speeds up” the rate at which telomeres attrite—that is, whether it affects the net difference between rates of telomere loss and synthesis. Thus we set aside any investigation of racial differences in baseline TL and focus simply on whether exposure to neighborhood disadvantage is indeed associated with shorter telomeres and whether the strength of this association differs between blacks and whites. Though the Fragile Families data limit us to a single measurement of telomere length and cannot sustain attributions of causality, observed point-in-time differences by neighborhood disadvantage nonetheless provide suggestive evidence of telomere attrition.
Besides neighborhood disadvantage, family deprivation might also influence telomere length and thus is important also to consider. Figure 2 calibrates the potential for intergroup differences in household income to shorten telomeres by showing the distribution of income-to-poverty ratios for respondents by race. In both cases, these ratios concentrate at values below 5.0; and as one might expect, values for blacks are skewed much more toward the low end of the scale and whites more toward the upper end. In addition, whereas black mothers display virtually no income-to-poverty ratios above 5.0, such values are frequent among white mothers. Thus, income differences are also likely to be associated with shorter TL and need to be controlled in statistical models.
Figure 2.
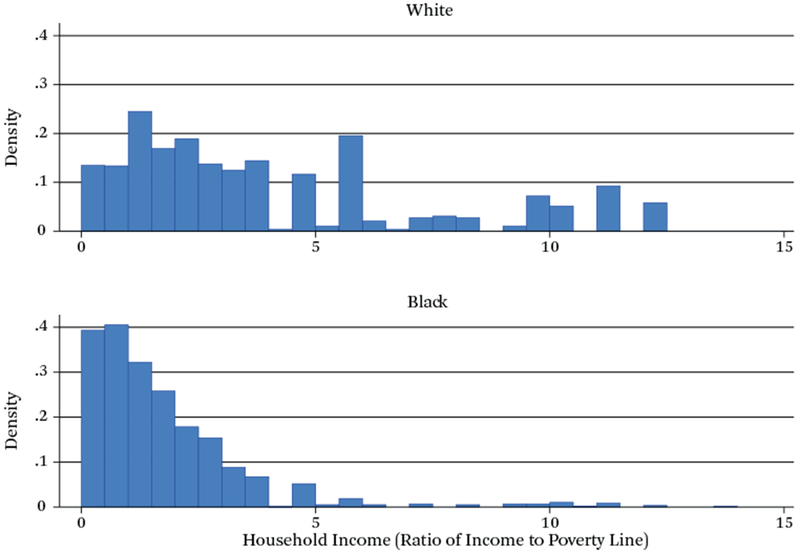
Density Distribution, Income to Poverty Ratio
Source: Authors’ compilation of data from the Fragile Families and Child Wellbeing Survey.
However, the black-white differential in household income is not nearly as extreme as the racial differential in neighborhood income, as shown in figure 3, which presents distributions of the Wodtke neighborhood disadvantage index for white and black mothers. Whereas neighborhood disadvantage indices for the vast majority of white mothers lie between −1.0 and +1.0, indicating low to moderate levels of neighborhood disadvantage, the vast majority of black mothers display indices that are above 1.0, most falling in the range from 1.0 to 4.0 but with some values of 5.0 or greater, index values that are almost never observed for whites. Thus the racial contrast is much greater with respect to neighborhood socioeconomic status than household socioeconomic status.
Figure 3.
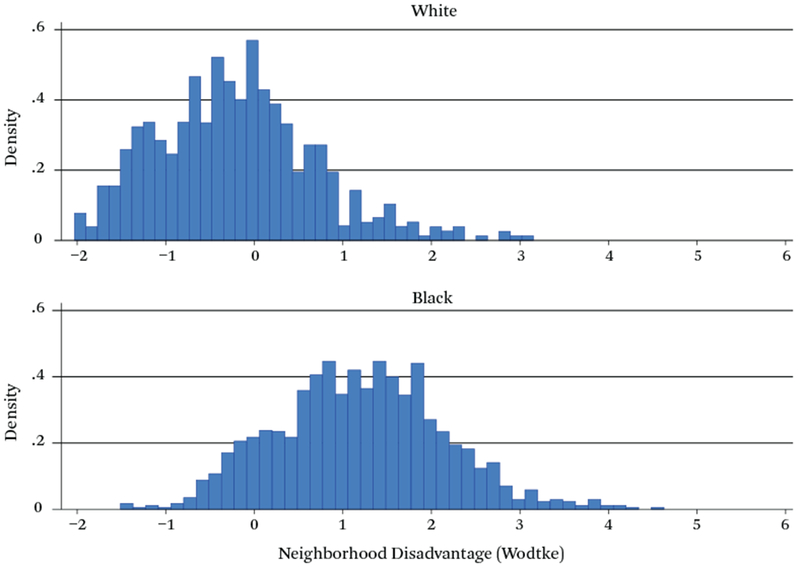
Density Distribution, Wodtke Index
Source: Authors’ compilation of data from the Fragile Families and Child Wellbeing Survey.
EFFECTS OF NEIGHBORHOOD DISADVANTAGE ON TELOMERE LENGTH
Table 2 presents two ordinary least squares (OLS) regression models estimated to predict the natural log of telomere length for black and white mothers in the Fragile Families dataset. The left-hand model includes dummy variables for group membership and indicators of the mother’s demographics, education, relationship with the father, and socioeconomic status at the time of the child’s birth. The right-hand equation contains the same independent variables with the addition of the Wodtke Index of neighborhood disadvantage. Looking at the left-hand columns we see that in the absence of a control for neighborhood disadvantage the average TL for black mothers remains significantly greater than that of white mothers (p < .01), consistent with the data shown in figure 1. In addition, with the notable exception of having some college education, socioeconomic status does not seem to play a significant role in determining TL among respondents, a finding that was robust across different model specifications we considered.
Table 2.
OLS Regression, Effect of Neighborhood Disadvantage
| Controlling for Social Background |
Controlling for Social Background and Neighborhood Disadvantage |
|||
|---|---|---|---|---|
| Independent Variables | B | SE | B | SE |
| Mother’s race-ethnicity | ||||
| White | – | – | – | – |
| Black | 0.052** | 0.018 | 0.074*** | 0.020 |
| Neighborhood disadvantage | ||||
| Wodtke index | – | – | −0.023* | 0.009 |
| Mother’s characteristics | ||||
| Age at telomere collection | −0.003+ | 0.001 | −0.003+ | 0.001 |
| Foreign born | 0.041 | 0.043 | 0.032 | 0.043 |
| Body mass index | −0.001 | 0.008 | 0.004 | 0.008 |
| Moves during study | 0.000 | 0.003 | −0.001 | 0.003 |
| Mother’s education at birth | ||||
| Less than high school | – | – | – | – |
| High school | 0.015 | 0.019 | 0.009 | 0.019 |
| Some college | 0.043* | 0.021 | 0.032 | 0.021 |
| College or more | 0.024 | 0.034 | 0.006 | 0.035 |
| Mother-father relationship at birth | ||||
| Married | – | – | – | – |
| Cohabiting | 0.028 | 0.024 | 0.031 | 0.024 |
| Other | 0.025 | 0.025 | 0.028 | 0.025 |
| Mother’s SES at birth | ||||
| Household poverty ratio | 0.003 | 0.004 | 0.001 | 0.004 |
| Household welfare use | −0.008 | 0.017 | −0.005 | 0.017 |
| Constant | 1.847*** | 0.061 | 1.859*** | 0.061 |
| R2 | 0.008 | 0.010 | ||
| Number of cases | 1,661 | 1,661 | ||
Source: Authors’ compilation of data from the Fragile Families and Child Wellbeing Survey.
p < .1;
p < .05;
p < .01;
p < .001
The right-hand equation adds in neighborhood disadvantage, which is significantly associated with shorter TL (p < .05). According to the estimated model, each point increase in the index of neighborhood disadvantage is associated with a decline of 0.023 logarithmic points of TL. Including neighborhood disadvantage in the model slightly increases the black-white gap in TL, though the shift is not significant. Although this model estimates a single coefficient for neighborhood disadvantage, we might anticipate that whites and blacks experience neighborhood disadvantage differently. To test this possibility, we estimated an additional model with an interaction term that allowed the effect of neighborhood disadvantage to vary between races but we did not find a significant interaction. We came to the same conclusion when we estimated the entire model separately for black and white mothers and found the coefficients for neighborhood disadvantage not to be statistically different in the black and white models.
Thus neighborhood disadvantage appears to operate similarly with regard to telomere length across the races. The Fragile Families data, however, only contain information on neighborhood disadvantage for the nine years of women’s lives subsequent to the baseline interview. Our analysis thus ignores whatever neighborhood circumstances women experienced before the survey date, around two-thirds of their lifetimes. Although we do not have information on the specific tract of residence for this period, in additional models not shown we use fixed effects to account for the city of residence at the time of the baseline survey and found that findings were robust to the inclusion of these city-level controls.
CONCLUSION AND IMPLICATIONS
Research clearly establishes that segregation and poverty interact to concentrate poverty spatially. The residential segregation of any group with a high rate of poverty inevitably concentrates poverty at high levels within neighborhoods inhabited by that group. Studies also identify telomeres (nucleotide sequences located at the ends of chromosomes) as critical buffers that protect genetic material from deterioration during cell division and that telomeres naturally shorten with age to foretell senescence. However, research also reveals that long-term exposure to high levels of stress can shorten human telomeres prematurely, potentially increasing later risks of morbidity and mortality.
In the current analysis, we hypothesized that prolonged exposure to spatially concentrated disadvantage constitutes a key source of stress for African Americans and thus may help to explain persistent racial differentials in health and life expectancy that do not disappear when socioeconomic status is controlled. To support this hypothesis we used multilevel data from the Fragile Families and Child Wellbeing Study and, using an index developed by Wodtke and colleagues (2011), we documented the distinctively high concentrations of neighborhood disadvantage experienced by African Americans relative to whites and confirmed that black inequality with respect to neighborhood disadvantage far exceeds that with respect to household income.
We went on to estimate regression models that predicted telomere length while controlling for other socioeconomic and demographic characteristics. We find the Wodtke index to be a significant predictor of telomere length among both blacks and whites. Subsequent investigations reveal no significant difference between blacks and whites in the extent to which exposure to neighborhood disadvantage was associated with shorter TL. Blacks are simply exposed to far more neighborhood disadvantage than whites, thus predicting greater shortening from their baseline TL. The difference in average neighborhood disadvantage among white and black respondents (Wodtke index = −0.252 and 1.198, respectively) implies a predicted difference in telomere length of 0.04 logarithmic points. Thus an African American respondent living in an average black neighborhood would be expected to have telomeres 0.04 logarithmic points shorter than those of an identical respondent living in an average white neighborhood. Conversely, an average white respondent living in an average white neighborhood would be expected to have telomeres 0.04 logarithmic points longer than those of an identical respondent living in an average black neighborhood. Though our results rely on cross-sectional comparisons of telomere length and cannot be taken as causal, they do provide suggestive evidence that the prolonged exposure to spatially concentrated disadvantaged experienced by African Americans is associated with greater telomere attrition.
In relying on between-person comparisons of telomere length, this study faces two major challenges future work should seek to address. First, as with all cross-sectional data analyses, correlations with omitted variables threaten between-person comparisons of telomere length. Though we included a variety of potentially relevant controls, the use of cross-sectional comparisons precludes us from asserting a causal relationship between neighborhood disadvantage and telomere length. Second, the proposed theoretical model with telomeres as a mechanism linking neighborhood disadvantage to later-life health outcomes is primarily motivated by work on the health consequences of telomere attrition (that is, change in telomere length over time). However, we are unable to measure the actual process of telomere attrition with a single time point of data available. Though differences in length could imply differences in telomere attrition, they also capture mean differences between groups. The measurement of telomeres at multiple time points is becoming more common in datasets, including future waves of the Fragile Families Study itself. As these data become available, researchers should seek to determine whether differences in exposure to neighborhood disadvantage are diachronically associated with actual telomere attrition and not just associated with cross-sectional TL disparities at a point in time.
Although our results are necessarily preliminary and await replication by other researchers using other datasets, they nonetheless add to a growing body of work demonstrating how inequality can be perpetuated through the nexus of neighborhood disadvantage, and potentially through biological as well as social mechanisms. Given the salient role of neighborhoods in perpetuating poverty over the life course and across generations, the research presented here suggests that improvements in black education and income alone may not be enough to eliminate racial differentials in health and socioeconomic status as long as residential segregation remains a characteristic structural feature of American society. Moving toward a more just and equal society requires not simply reducing discrimination in the social and economic spheres, but equalizing opportunities in the residential sphere as well. As of 2010, more than half of blacks inhabiting U.S. metropolitan areas remained highly segregated and one-third were hypersegregated (Massey and Tannen 2015). As long as such conditions continue to prevail, segregation will continue to serve as the linchpin of racial stratification in the United States (Pettigrew 1979).
Contributor Information
DOUGLAS S. MASSEY, Princeton University.
BRANDON WAGNER, Texas Tech University.
LOUIS DONNELLY, Princeton University.
SARA MCLANAHAN, Princeton University.
JEANNE BROOKS-GUNN, Columbia University.
IRWIN GARFINKEL, Columbia University.
COLTER MITCHELL, University of Michigan.
DANIEL A. NOTTERMAN, Princeton University.
REFERENCES
- Blackburn Elizabeth H, ed. 2006. Telomeres. Cold Spring Harbor, N.Y.: Cold Spring Harbor Monograph Series. [Google Scholar]
- Blackburn Elizabeth H., Epel Elissa S., and Lin Jue. 2015. “Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection.” Science 350(6265): 1103–98. [DOI] [PubMed] [Google Scholar]
- Brown Lauren, Belinda Needham, and Jennifer Ailshire. 2016. “Telomere Length Among Older U.S. Adults: Differences by Race/Ethnicity, Gender, and Age.” Journal of Aging and Health (July 27): 1–7. DOI: 10.1177/089826431666139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathorall Michelle L., Xin Huaibo, Peachy Andrew, Bibeau Daniel L., Schulz Mark, and Aronson Robert. 2015. “Neighborhood Disadvantage and Variations in Blood Pressure.” American Journal of Health Education 46(5): 266–73. [Google Scholar]
- Raj Chetty, Hendren Nathaniel, and Katz Lawrence F.. 2016. “The Effects of Exposure to Better Neighborhoods on Children: New Evidence from the Moving to Opportunity Experiment.” American Economic Review 106(4): 855–902. [DOI] [PubMed] [Google Scholar]
- D’Mello Matthew J. J., Ross Stephanie A., Briel Matthias, Anand Sonia S., Gerstein Hertzel, and Pare Guillaume. 2014. “The Association Between Shortened Leukocyte Telomere Length and Cardio-Metabolic Outcomes: A Systematic Review and Meta-Analysis.” Cardiovascular Genetics (November 18). DOI: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- DiPrete Thomas A., and Eirich Gregory A.. 2006. “Cumulative Advantage as a Mechanism for Inequality: A Review of Theoretical and Empirical Developments.” Annual Review of Sociology 32(1): 271–97. [Google Scholar]
- Drury Stacy S., Mabile Emily, Brett Zoë H., Esteves Kyle, Jones Edward, Shirtcliff Elizabeth A., and Theall Katherine P.. 2014. “The Association of Telomere Length with Family Violence and Disruption.” Pediatrics 134(1): e128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel Elissa S., Blackburn Elizabeth H., Lin Jue, Dhabhar Firdaus S., Adler Nancy E., Morrow Jason D., and Cawthon Richard M.. 2004. “Accelerated Telomere Shortening in Response to Life Stress.” Proceedings of the National Academy of Sciences 101(49): 17312–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch Brian K., Do D. Phuong, Heron Melonie, Bird Chloe, Seeman Teresa, and Lurie Nicole. 2010. “Neighborhood Effects on Health: Concentrated Advantage and Disadvantage.” Health and Place 16(5): 1058–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick Annette L., Kronmal Richard A., Kimura Masayuki, Gardner Jeffrey P., Psaty Bruce M., Jenny Nancy S., Tracy Russell P., Hardikar Sheetal, and Aviv Abraham. 2011. “Leukocyte Telomere Length and Mortality in the Cardiovascular Health Study.” Journals of Gerontology: Series A 66A(4): 421–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus Arline T. 1992. “The Weathering Hypothesis and the Health of African American Women and Infants.” Ethnicity and Disease 2(3): 207–21. [PubMed] [Google Scholar]
- Geronimus Arline T., Hicken Margaret, Keene Danya, and Bound John. 2006. “‘Weathering’ and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States.” American Journal of Public Health 96(5): 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus Arline T., Pearson Jay A., Linnenbringer Erin, Schulz Amy J., Reyes Angela G., Epel Elissa S., Lin Jue, and Blackburn Elizabeth H.. 2015. “Race-Ethnicity, Poverty, Urban Stressors, and Telomere Length in a Detroit Community-Based Sample.” Journal of Health and Social Behavior (April 30). DOI: 10.1177/0022146515582100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geruso Michael. 2012. “Black-White Disparities in Life Expectancy: How Much Can the Standard SES Variables Explain?” Demography 49(2): 553–74. [DOI] [PubMed] [Google Scholar]
- Glei Dana A., Goldman Noreen, Risques Rosa Ana, Rehkopf David H., Dow William H., Rosero-Bixby Luis, and Weinstein Maxine. 2016. “Predicting Survival from Telomere Length Versus Conventional Predictors: A Multinational Population-Based Cohort Study.” PloS One 11(4): e0152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goglin Sarah E., Farzaneh-Far Ramin, Epel Elissa S., Lin Jue, Blackburn Elizabeth H., and Whooley Mary A.. 2016. “Change in Leukocyte Telomere Length Predicts Mortality in Patients with Stable Coronary Heart Disease from the Heart and Soul Study.” PLoS One 11(12): e0168868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson Per E., Sebastian Miguel San, Janlert Urban, Theorell Töres, Westerlund Hugo, and Hammarstrom Anne. 2014. “Life-Course Accumulation of Neighborhood Disadvantage and Allostatic Load: Empirical Integration of Three Social Determinants of Health Frameworks.” American Journal of Public Health 104(5): 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman Daniel A., Betancourt Laura M., Brodsky Nancy L., Hurt Hallam, and Farah Martha J.. 2012. “Neighborhood Disadvantage and Stress Reactivity.” Frontiers in Human Neuroscience 6(277): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson Matthew E. B., Hunt Steven C., Stone Rivka C., Horvath Kent, Herbig Utz, Ranciaro Alessia, Hirbo Jibril, Beggs William, Reiner Alexander P., Wilson James G., Kimura Masayuki, De Vivo Immaculata, Chen Maxine M., Kark Jeremy D., Levy Daniel, Nyambo Thomas, Tishkoff Sarah A., and Aviv Abraham. 2016. “Shorter Telomere Length in Europeans Than in Africans Due to Polygenetic Adaptation.” Human Molecular Genetics 25(11): 2324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Sarah E., Marioni Riccardo E., Martin-Ruiz Carmen, Pattie Alison, Gow Alan J., Cox Simon R., Corley Janie, von Zglinicki Thomas, Starr John M., and Deary Ian J.. 2016. “Longitudinal Telomere Length Shortening and Cognitive and Physical Decline in Later Life: The Lothian Birth Cohorts 1936 and 1921.” Mechanisms of Ageing and Development 154 (March): 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock Philip C., Heydon Emma E., Kaptoge Stephen, Butterworth Adam S., Thompson Alex, and Willeit Peter. 2014. “Leucocyte Telomere Length and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis.” British Medical Journal 349 (July 8): g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt Steven C., Chen Wei, Gardner Jeffrey P., Kimura Masayuki, Srinivasan Sathanur R., Eckfeldt John H., Berenson Gerald S., and Aviv Abraham. 2008. “Leukocyte Telomeres Are Longer in African Americans Than in Whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study.” Aging Cell 7(4): 451–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jargowsky Paul A. 1997. Poverty and Place: Ghettos, Barrios, and the American City . New York: Russell Sage Foundation. [Google Scholar]
- Karb Rebecca A., Elliott Michael R., Dowd Jennifer B., and Morenoff Jeffrey D.. 2012. “Neighborhood-Level Stressors, Social Support, and Diurnal Patterns of Cortisol: The Chicago Community Adult Health Study.” Social Science and Medicine 75(6): 1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Katherine, Kane Jennifer Buher, Scarbrough Peter, Hoyo Cathrine, and Murphy Susan. 2016. “Neighborhood and Family Environment of Expectant Mothers May Influence Prenatal Programming of Adult Cancer Risk: Discussion and an Illustrative Biomarker Example.” Biodemography and Social Biology 62(1): 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipling David. 1995. The Telomere . New York: Oxford University Press. [Google Scholar]
- Kitagawa Evelyn M., and Hauser Phillip M.. 1973. Differential Mortality in the United States: A Study in Socioeconomic Epidemiology . Cambridge, Mass: Harvard University Press. [Google Scholar]
- Kling Jeffrey R., Liebman Jeffrey B., and Katz Lawrence F.. 2007. “Experimental Analysis of Neighborhood Effects.” Econometrica 75(1): 83–119. [Google Scholar]
- Ludwig Jens. 2012. “Guest Editor’s Introduction: Special Issue on MTO.” Cityscape 14(2): 1–28. [Google Scholar]
- Ludwig Jens, Sanbonmatsu Lisa, Gennetian Lisa, Adam Emma, Duncan Greg J., Katz Lawrence F., Kessler Ronald C., Kling Jeffrey R., Lindau Stacy Tessler, Whitaker Robert C., and McDade Thomas W.. 2011. “Neighborhoods, Obesity and Diabetes: A Randomized Social Experiment.” New England Journal of Medicine 365(16): 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch Shannon M., Peek MK, Mitra Nandita, Ravichandran Krithika, Branas Charles, Spangler Elaine, Zhou Wenting, Paskett Electra D., Gehlert Sarah, DeGraffinreid Cecilia, Rebbeck Timothy R., and Riethman Harold. 2016. “Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting.” PLoS One 11(1): e0146723 DOI: 10.1371/journal.pone.01146723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni Riccardo E., Sarah E. Harris, Shah Sonia, McRae Allan F., von Zglinicki Thomas, Martin-Ruiz Carmen, Wray Naomi R., Visscher Peter M., and Deary Ian J.. 2016. “The Epigenetic Clock and Telomere Length are Independently Associated with Chronological Age and Mortality.” International Journal of Epidemiology 45(2): 424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ruiz Carmen M., Gussekloo Jacobijn, van Heemst Diana, von Zglinicki Thomas, and Westendorp Rudi G. J.. 2005. “Telomere Length in White Blood Cells Is Not Associated with Morbidity or Mortality in the Oldest-Old: A Population-Based Study.” Aging Cell 4(6): 287–90. [DOI] [PubMed] [Google Scholar]
- Massey Douglas S. 1990. “American Apartheid: Segregation and the Making of the Underclass.” American Journal of Sociology 95(2): 1153–88. [Google Scholar]
- Massey Douglas S. 2013. “Inheritance of Poverty or Inheritance of Place? The Emerging Consensus on Neighborhoods and Stratification.” Contemporary Sociology 42(5): 690–97. [Google Scholar]
- Massey Douglas S., Albright Len, Casciano Rebecca, Derickson Elizabeth, and Kinsey David. 2013. Climbing Mount Laurel: The Struggle for Affordable Housing and Social Mobility in an American Suburb . Princeton, N.J.: Princeton University Press. [Google Scholar]
- Massey Douglas S., and Clampet-Lundquist Susan. 2008. “Neighborhood Effects on Economic Self-Sufficiency: A Reconsideration of the Moving to Opportunity Experiment.” American Journal of Sociology 114(1): 107–43. [Google Scholar]
- Massey Douglas S., and Eggers Mitchell E.. 1992. “A Longitudinal Analysis of Urban Poverty: Blacks in U.S. Metropolitan Areas Between 1970 and 1980.” Social Science Research 21(2): 175–203. [Google Scholar]
- Massey Douglas S., and Fischer Mary J.. 2000. “How Segregation Concentrates Poverty.” Ethnic and Racial Studies 23(4): 670–91. [Google Scholar]
- Massey Douglas S., and Rugh Jacob S.. forthcoming. “Zoning, Affordable Housing, and Segregation in U.S. Metropolitan Areas” In The Fight for Fair Housing: Causes, Consequences and Future Implications of the 1968 Federal Fair Housing Act , edited by Squires Gregory. New York: Taylor and Francis. [Google Scholar]
- Massey Douglas S., and Tannen Jonathan. 2015. “A Research Note on Trends in Black Hypersegregation.” Demography 52(3): 1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur Maya B., Epel Elissa, Kind Shelley, Desai Manisha, Parks Christine G., Sandler Dale P., Khazeni Nayer. 2016. “Perceived Stress and Telomere Length: A Systematic Review, Meta-Analysis, and Methodologic Considerations for Advancing the Field.” Brain , Behavior, and Immunity 54 (May): 158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell Colter, Hobcraft John, McLanahan Sara S., Siegel Susan Rutherford, Berg Arthur, Brooks-Gunne Jeanne, Garfinkel Irwin, and Notterman Daniel. 2014. “Social Disadvantage, Genetic Sensitivity, and Children’s Telomere Length.” Proceedings of the National Academy of Sciences 111(16): 5944–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham Belinda L., Rehkopf David, Adler Nancy, Gregorich Steven, Lin Jue, Blackburn Elizabeth H., and Epel Elissa S.. 2015. “Leukocyte Telomere Length and Mortality in the National Health and Nutrition Examination Survey, 1999–2002.” Epidemiology 26(4): 528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr Larry, Feins Judith D., Jacob Robin, Beecroft Erik, Sanbonmatsu Lisa, Katz Lawrence F., Liebman Jeffrey B., and Kling Jeffrey R.. 2003. Moving to Opportunity: Interim Impacts Evaluation . Washington: U.S. Department of Housing and Urban Development. [Google Scholar]
- Park Minjung, Verhoeven Josine E., Cuijpers Pim, Reynolds Charles F. III, Penninx Brenda W. J. H.. 2015. “Where You Live May Make You Old: The Association Between Perceived Poor Neighborhood Quality and Leukocyte Telomere Length.” PLoS One 10(6): e0128460 DOI: 10.1371/journal.pone.0128460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew Thomas. 1979. “Racial Change and Social Policy.” Annals of the American Academy of Political and Social Science 441(1): 114–31. [Google Scholar]
- Quillian Lincoln. 2012. “Segregation and Poverty Concentration: The Role of Three Segregations.” American Sociological Review 77(3): 354–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell Jonathan, and Massey Douglas S.. 2014. “Geographic Effects on Intergenerational Income Mobility.” Economic Geography 91(1): 83–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinowitz Leonard S., and Rosenbaum James E.. 2000. Crossing the Class and Color Lines: From Public Housing to White Suburbia. Chicago: University of Chicago Press. [Google Scholar]
- Rudolph Kara E., Wand Gary S., Stuart Elizabeth A., Glass Thomas A., Marques Andrea H., Duncko Roman, and Merikangas Kathleen R.. 2014. “The Association Between Cortisol and Neighborhood Disadvantage in a U.S. Population-Based Sample of Adolescents.” Health and Place 25(1): 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson Robert J. 2012.Great American City: Chicago and the Enduring Neighborhood Effect .Chicago:University of Chicago Press. [Google Scholar]
- Sampson Robert J., Morenoff Jeffrey and Gannon-Rowley T. 2002. “Assessing Neighborhood Effects: Social Processes and New Directions in Research.” Annual Review of Sociology 28(1): 443–78. [Google Scholar]
- Sanbonmatsu Lisa, Ludwig Jens, Katz Lawrence F., Gennetian Lisa A., Duncan Greg J., Kessler Ronald C., Adam Emma, McDade Thomas W., Lindau Stacy Tessler, Sciandra Matthew Yang Fanghua, Lai Ijun, Congdon William, Amick Joe, Gillette Ryan, Zabek Michael A., Marvakov Jordan, Yusuf Sabrina, and Potter Nicholas A.. 2011. Moving to Opportunity for Fair Housing Demonstration Program: Final Impacts Evaluation . Washington: U.S. Department of Housing and Urban Development. [Google Scholar]
- Sanders Jason L., and Newman Anne B.. 2013. “Telomere Length in Epidemiology: A Biomarker of Aging, Age-Related Disease, Both, or Neither?” Epidemiological Reviews 35(1): 112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky Robert M. 2004. “Organismal Stress and Telomeric Aging: An Unexpected Connection.” Proceedings of the National Academy of Sciences 101(50): 17323–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte Nicola S., and Malouff John M.. 2014. “The Relationship Between Perceived Stress and Telomere Length: A Meta-Analysis.” Stress and Health 32(4): 313–19. [DOI] [PubMed] [Google Scholar]
- Shalev Idan, Moffitt Terrie E., Sugden Karen, Williams Brittany, Houts Renate M., Danese Andrea, Mill Jonathan, Aresneault Louise, and Caspi Avshalom. 2013. “Exposure to Violence During Childhood Is Associated with Telomere Erosion from 5 to 10 Years of Age: A Longitudinal Study.” Molecular Psychiatry 18(5): 576–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey Patrick .2013.Stuck in Place: Urban Neighborhoods and the End of Progress toward Racial Equality .Chicago:University of Chicago Press. [Google Scholar]
- Theall Katherine P., Brett Zoë H., Shirtcliff Elizabeth A., Dunn Erin C., and Drury Stacy S.. 2013. Neighborhood Disorder and Telomeres: Connecting Children’s Exposure to Community Level Stress and Cellular Response.” Social Science and Medicine 85(1): 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsemann Katrina M., Gee Gilbert C., and Geronimus Arline. 2009. “Ethnic Differences in Trajectories of Depressive Symptoms.” Journal of Health and Social Behavior 50(1): 82–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhide Eli. 2014. Understanding Telomeres: The Science of Aging Well . Seattle, Wash.: Amazon Digital Services. [Google Scholar]
- Willeit Peter, Willeit Johann, and Mayr Agnes. 2010. “Telomere Length and Risk of Incident Cancer and Cancer Mortality.” Journal of the American Medical Association 304(1): 69–75. [DOI] [PubMed] [Google Scholar]
- Wilson William J. 1987. The Truly Disadvantaged: The Inner City, the Underclass, and Public Policy. Chicago: University of Chicago Press. [Google Scholar]
- Wodtke Geoffrey T., Harding David J., and Elwert Felix. 2011. “Neighborhood Effects in Temporal Perspective: The Impact of Long-Term Exposure to Concentrated Disadvantage on High School Graduation.” American Sociological Review 76(5): 713–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodtke Geoffrey T., Harding David J., and Elwert Felix. 2016. “Neighborhood Effect Heterogeneity by Family Income and Developmental Period.” American Journal of Sociology 121(4): 1168–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Ruby, Tang Nelson, Leung Jason, and Woo Jean. 2015. “Telomere Length Is Not Associated with Frailty in Older Chinese Elderly: Cross-Sectional and Longitudinal Analysis.” Mechanisms of Ageing and Development 152 (December): 74–79. [DOI] [PubMed] [Google Scholar]


