Abstract
Escherichia coli is responsible for a wide variety of community and hospital acquired extraintestinal infections, and the emergence of ESBL resistant isolates is a major clinical concern. In this study, we characterized the genomic attributes of an OXA-48 and CTX-M-3 producing E. coli EC-IMP153. Whole-genome initial assembly produced 146 contigs with a combined 5,504,170 bp in size and a G+C content of 50.5%. wgSNPs-based phylogenetic comparison with 36 publically available genomes was also performed. Comprehensive genomic analysis showed that EC-IMP153 belonged to sequence type ST-405 and harbored several resistance determinants including the β-lactam resistance genes blaOXA-48, blaCTX-M-3, blaTEM-1B, blaOXA-1, and blaCMY-70, aminoglycoside fyuA and aac(3)IId, tetracycline tet(A) and tet(R), and fluoroquinolone gyrA, parC, and mfd resistance determinants. Plasmids with the following incompatibility groups were detected in silico and confirmed using PBRT: IncI1-α, IncL, IncW, Col (BS512), and IncF. To our knowledge this is the first in-depth genomic analysis of an OXA-48 producing E. coli ST-405 isolated from a patient in Lebanon and linked to a blood stream infection. Continuous monitoring is necessary to better understand the continued diffusion of such pathogens, especially in view of the population movements triggered by unrest in the Middle East.
1. Introduction
Escherichia coli is responsible for a wide variety of community and hospital acquired extraintestinal infections. Extraintestinal pathogenic E. coli (ExPEC) is widely known to cause bloodstream, urinary and respiratory tract, cerebrospinal fluid, and peritoneum infections [1, 2]. Successful treatment of infections caused by pathogenic E. coli could be achieved through the use of β-lactams. In recent years, there has been an evident increase in β-lactamases production, including extended spectrum β-lactamase (ESBL), plasmid-mediated AmpC β-lactamase (e.g., CMY), and carbapenemases produced by ExPEC [3]. Three most significant classes of carbapenemases are the class A (e.g., KPC), class B also known as metallo β-lactamases (e.g., NDM), and class D OXA-types (e.g., OXA-48) [3].
The OXA-48 β-lactamase was first identified in Enterobacteriaceae in Turkey in 2001, with OXA-48 positive Enterobacteriaceae belonging to ESBL producers and nonproducers [4]. Subsequently E. coli producing OXA-48-like variants were reported in different countries around the world [5] including Turkey, Belgium, France, and Lebanon [6]. Generally, OXA-48 producing isolates are multidrug-resistant (MDR) and are able to hydrolyze antimicrobial agents at different levels, exhibiting high hydrolyzing activity towards penicillins, low activity against carbapenems, and sparing broad-spectrum cephalosporins, and are not susceptible to β-lactamase inhibitors [7].
Genes encoding for β-lactamases are generally located on mobile genetic elements such as transposons, plasmids, and integrons [8]. The spread of the blaOXA-48 gene is regularly linked to the dissemination of the 62-kb IncL/M-type plasmid, with most of the OXA-48-positive Enterobacteriaceae harboring this specific type of plasmid [9]. Previously Matar et al. (2008) [6] reported the emergence of carbapenemase OXA-48 in Lebanon in the periods 2008-2010 with Klebsiella pneumoniae being the major OXA-48 producing Enterobacteriaceae. In 2012, E. coli strains isolated from Lebanon represented 73% of clinical producers of OXA-48 [10].
In this study, using genome sequencing we aimed at investigating and characterizing the genetic background and horizontally transferable MDR resistance determinants in E. coli EC-IMP153. Particular interest was given to the IncL/M-type plasmid carrying blaOXA-48 and to IncFII plasmid having blaTEM-1B, blaCTXM-3, blaOXA-1, tet(A), and tet(R). The genetic environment of the plasmids carried by EC-IMP153 revealed the coexistence of multiple resistance genes on the same plasmid. Additionally, MLST, wgSNPs, and comparative genome analysis were considered to investigate the phylogeny of the isolate.
2. Materials and Methods
2.1. Sample Collection
The isolate was recovered from the blood samples of a patient admitted to the American University of Beirut Medical Center (AUBMC) in 2010.
2.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility profile of EC-IMP153 isolate was screened through the disk diffusion method on Muller-Hinton agar for the following antibiotics: ampicillin, gentamicin, piperacillin, piperacillin-tazobactam, amoxicillin-clavulanic acid, cefepime, ceftriaxone, cefuroxime, cefotaxime, ceftazidime, aztreonam, imipenem, meropenem, ertapenem, colistin, trimethoprim-sulfamethoxazole, tetracycline, and ticaracillin. E. coli ATCC 25922 was used for routine quality control. Results were interpreted according to the Clinical Laboratory Standards Institutes (CLSI) criteria [11]. Screening tests for ESBL production were performed through double-disk synergy test (cefotaxime and ceftazidime disks with and without clavulanic acid) following the CLSI criteria [11]. K. pneumoniae ATCC 700603 was used as positive control for ESBL production.
2.3. DNA Isolation
Extraction of bacterial DNA was performed, after growing on tryptone soy broth overnight at 37°C, using the NucleoSpin® Tissue Kit (Macherey-Nagel, Germany) according to the manufacturer's protocol.
2.4. Genome Sequencing
Library preparation was done using genomic DNA (gDNA). Bioruptor® NGS was used to sonicate 1 μg of sample DNA. The resulting sheared DNA was used as input for library preparation using the Illumina TruSeq DNA library preparation kit (Illumina). The gDNA was further subjected to end-repair, A-tailing, ligation of adaptors as recommended by the manufacturer. Fragments between 500 and 1000 bp were selected using the Pippin Prep™ DNA size selection system (Sage Science). qPCR was used to quantify the resulting library in triplicate at 1:1000 and using the Kapa library quantification kit (Kapa Biosystems, Woburn, MA, USA), following the manufacturer's instructions. The resultant library size was assessed using an Agilent Bioanalyzer with the High Sensitivity DNA Kit. The library was multiplexed, clustered, and sequenced on an Illumina MiSeq with paired-end 500 cycles protocol to read a length of 250 bp.
2.5. Genome Assembly
Genome assembly was performed de novo using A5 with default parameters [14]. This pipeline automates the processes through several steps: read quality filtering and error correction, contig assembly, permissive draft scaffolding, misassembly detection, and conservative scaffolding.
2.6. Genome Annotation and Analysis
The assembled draft genome was annotated using RAST. The RAST server identifies protein-encoding genes, rRNA and tRNA, and predicts the different subsystems within the genome [15]. The Antibiotic Resistance Database (ARDB) [16] and ResFinder server v2.1 were used to identify resistance genes using a threshold of 90% identity (ID) [17]. The multilocus sequence type (MLST) was determined using two different MLST schemes available on CGE server, i.e., MLST1; the Achtman scheme and MLST2; the Pasteur scheme. The presence of plasmids and corresponding sequence types (STs) were determined using both in silico PlasmidFinder 1.3 server [18] and pMLST available on CGE. IS-finder was used to identify insertion sequences (ISs) and identify IS-families [19]. PLACNETw separated chromosomal genome from accessory plasmid genomes, based on paired-end reads assembly [20].
2.7. Plasmid DNA Extraction
For plasmid extraction the isolate was inoculated on Luria Bertani broth and incubated overnight at 37°C. The QIAprep Spin Miniprep Kit (Qiagen, Hilden, Germany) was used according to the manufacturer's instructions.
2.8. Identification of Resistance Genes
The β-lactamase gene blaOXA-48 was traced by PCR amplification, on both crude genomic DNA and bacterial plasmid DNA, using the following set of primers: OXA-48A (5′-TTGGTGGCATCGATTATCGG-3′) and OXA-48B (5′-GAGCACTTCTTTTGTGATGGC-3′) as described by Aktas et al. (2008) [12]. PCR amplicons were electrophoresed on 1.5% agarose gels. Automated sequencing was performed on ABI 3500 DNA analyzer using the Big Dye system (Applied Biosystems Foster City, CA, USA). blaOXA-48 gene sequence was compared with online sequences using the BLASTn. Similarly, blaAmpC was amplified through PCR on both crude genomic DNA and plasmid DNA using the following set of primers: AmpC-F: (5′-ATGATGAAAAAATCGTTATGC-3′) and AmpC-R: (5′-TTGCAGCTTTTCAAGAATGCGC-3′) [13] (Table 1).
Table 1.
Primers sequences of blaOXA-48 and blaAmpC and PCR conditions used for the amplification along with target size in (bp).
The locations and genetic environment of blaOXA-48 and blaCTXM-3 were determined through sequence alignment on BioNumerics v7.6.1 beta software (Applied Maths, Sint-Martens-Latem, Belgium). The sequence of IncFII plasmid was extracted and the genetic environment of resistance genes blaTEM-1B, blaCTXM-3, and blaOXA-1 was annotated using RAST and IS-finder.
Fluoroquinolone resistance-determining regions (QRDR) of gyrA and parC were compared to the amino acid sequence of that in E. coli K-12 (GenBank accession no. NC_000913). Protein alignment was performed on EMBOSS Needle tool available on EMBL-EBI website (http://www.ebi.ac.uk/Tools/).
2.9. Plasmid Typing
EC-IMP153 isolate genomic DNA was subjected to PCR-based replicon typing analysis (PBRT) to determine plasmid incompatibility groups as described by Carattoli et al. (2011) [21]. Eight multiplex PCRs were performed for the amplification of 28 replicons: L/M, N, FIA, FIB, FIC, FII, FIIS, FIIK, FIB-M, W, Y, P, A/C, T, K, U, R, B/O, HI1, HI2, I1, I2, X1, X2, and HIB-M representative of major plasmid incompatibility groups and replicase genes that are typically found on resistance plasmids circulating among Enterobacteriaceae [21, 22].
2.10. Genome Comparative Analysis
E.coli EC-IMP153 was compared with the following genomes of ST-405 E. coli isolates: 50579417 (Accession #: NZ_LNHL00000000) OXA-48 producing E. coli isolated from a patient in Norway with a travel history to Thailand and used as a reference strain [23], E.coli LAU-EC4 (Accession #: AYOP0100000000), and E.coli LAU-EC5 (Accession #: AYOG0100000000) isolated from Lebanon [24] and circular visualization was constructed using CGViewer [25].
2.11. wgSNPs Phylogenetic Analysis
The genome was compared against the GenBank Nucleotide database using BLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to identify the closest relative genomes available on the database (Supplementary Table 1: reference genomes accession numbers). BioNumerics v7.6.1 beta software (Applied Maths, Sint-Martens-Latem, Belgium) was used to align E. coli genomes against reference genome E. coli 50579417. SNP-calling was performed by mapping the paired-end reads of isolate EC-IMP153 and 34 assembled E. coli genomes obtained from NCBI to the reference genome of 50579417 E. coli strain. K. pneumoniae genome was used as an outgroup. SNPs were deduced through strict SNP filtering for each genome sequence using BioNumerics Chromosome Comparisons module. A neighbor-joining (NJ) tree was generated in BioNumerics by using mutation filtering module, filtered from wgSNP data input.
2.12. Nucleotide Sequence Accession Number
The whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession number . The versions described here is LJOJ01000000.
3. Results
3.1. Antimicrobial Resistance Patterns
E. coli EC-IMP153 was found to be resistant to tetracycline, ticaracillin, gentamycin, ampicillin, piperacillin, piperacillin/tazobactam, amoxicillin/clavulanic acid, cefepime, ceftriaxone, cefuroxime, azetronam, ceftazidime, and cefotaxime. It additionally showed intermediate resistance to trimethoprime/sulfamethoxazole but was sensitive to ertapenem, colistin, meropenem, and imipenem.
3.2. Genome Characterization
E. coli EC-IMP153 genome consisted of 5,504,170 bp and a G+C content of 50.5% in 146 contigs, with 5,384 coding sequences (CDS) and a total of 116 predicted RNAs. RAST also distinguished genes encoding carbohydrate metabolism (777), amino acids and derivatives (410), cofactors, vitamins, prosthetic groups, pigments (286), cell wall and capsule (284), and virulence disease and defense (116) (Figure 1)
Figure 1.
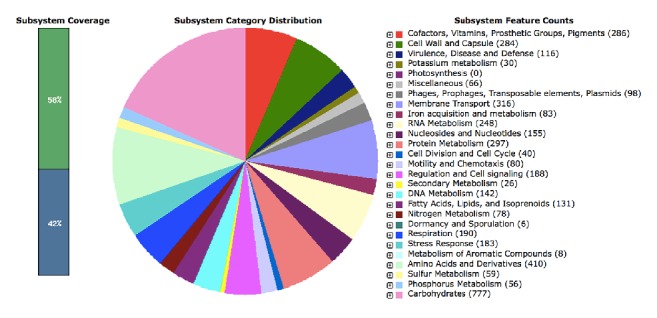
Subsystem categorical distribution in E. coli EC-IMP153.
3.3. Isolate Typing
In silico (MLST1) based analysis using seven house-keeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA) classified EC-IMP153 as belonging to ST-405 based on Achtman scheme and ST-44 based on the Pasteur scheme. The serotype was predicted to be O102:H6.
3.4. Mobile Genetic Elements
EC-IMP153 was positive for the plasmids with the following incompatibility groups: IncF (IncFII, IncFIA, and IncFIB), IncI1-α, and Col (BS512) in silico pMLST analysis using the FAB (FII:FIA:FIB) typing scheme for IncF plasmids, showing that the replicons belonged to the F31:A4:B1 type. Plasmid profiling by PBRT confirmed the presence of IncFIA, IncFII, IncI1-α, IncL, and IncW type plasmids. PLACNETw network (Figure 2) revealed the presence of fragmented, short read contigs corresponding to the IncL/M plasmid that specifically carried the blaOXA-48 gene.
Figure 2.
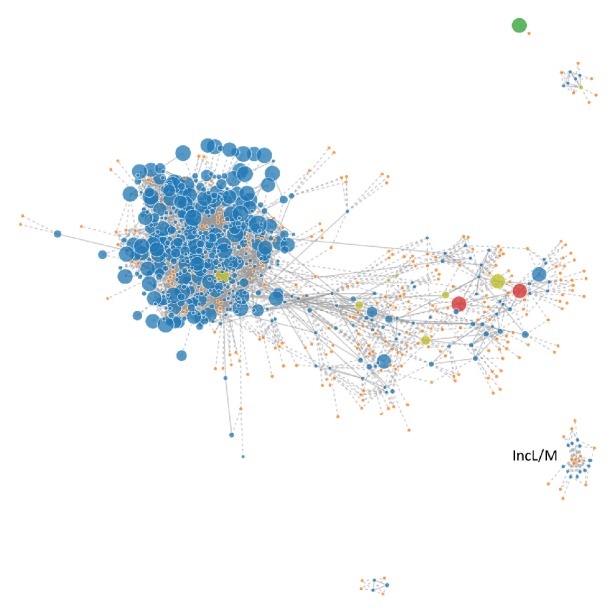
PLACNETw caption of plasmid IncL/M. Separate representation of chromosomal genome and IncL/M plasmid in E. coli EC-IMP153.
IS-Finder identified 158 insertion sequences (ISs) and 180 open-reading frames (ORFs) related to ISs. Important ISs families included IS1 family (IS1A, IS1B, IS1D, IS1G, IS1H, IS1R, IS1S, IS1SD, IS1X2, and IS1X4), IS1380 family (ISEcp1 and ISEc9) with ISEcp1 detected upstream of blaCTX-M-3 gene, IS3 family (IS103, IS1203, IS1397, IS150, IS2, IS911, ISEc27, ISEc52, ISKpn8, ISSd1, and ISSFl10), and IS5 family (IS5, IS5D, and ISKpn26).
3.5. Identification of Antibiotic Resistance Genes
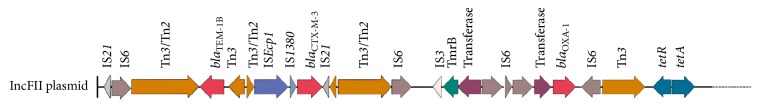
bla TEM-1B, blaCTXM-3, and blaOXA-1, in addition to the gene encoding a tetracycline efflux protein tet(A) and tet(R), coexisted on the same plasmid IncFII. blaCTX-M-3 was associated with a Tn2/Tn3 hybrid with an upstream ISEcp1. The downstream end of Tn2 was truncated by IS21. This multiresistance region (MRR) also included tet(A), tet(R) genes and blaOXA-1 (Figure 3).
Figure 3.
IncFII plasmid genomic environment. Structure of the blaTEM-1B, blaCTX-M-3, and blaOXA-1 genes on IncFII plasmid in E. coli EC-IMP153.
bla OXA-48 gene specific PCR assay was additionally used on a plasmid extract which confirmed that the isolate was blaOXA-48 carrier. Sequence analysis was done and the sequence matched with the publically available NCBI sequences for the blaOXA-48 gene. blaOXA-48 was the only resistance determinant found on IncL plasmid associated with Tn1999 transposon. Only one copy of IS1R was found upstream of the gene and lysR being located downstream.
Aminoglycoside (aac(3)-IId), macrolide (mphA), and tetracycline (tet(A) and tet(R)) resistance genes were detected. CARD Resistance Gene Identifier (RGI) further revealed the presence of other resistance determinants including blaCMY-70 (β-lactam resistance) and mfd, gyrA, and parC (fluroquinolone resistance). Mutations in gyrA and parC genes leading to a single amino acid substitution in parC (S80I) and double substitutions in gyrA (S83L and D87N) were also detected.
3.6. Genomic Comparison
Circular visualization and comparison of the genomic sequences were generated using CGViewer server. E. coli EC-IMP153 was compared with 50579417 and E. coli K-12 MG1665 (Figure 4) and with LAU-EC4 and LAU-EC5 (Figure 5).
Figure 4.
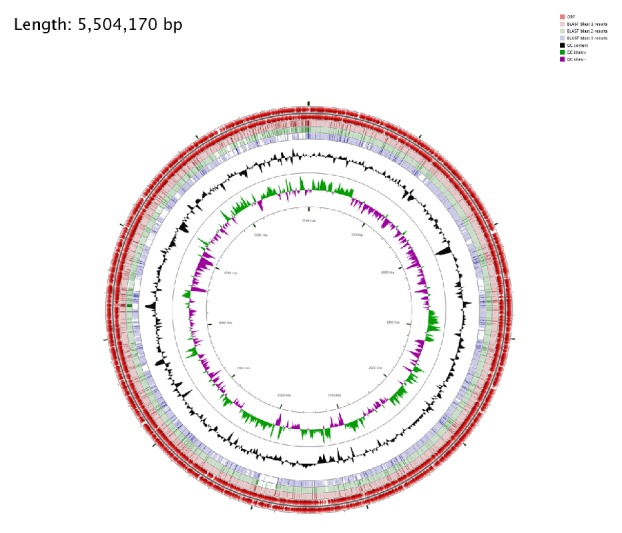
Circular genome representation of EC-IMP153 compared with E. coli 50579417 and E. coli K-12 MG1665. The outermost ring: EC-IMP153 open-reading frames (ORF) on both forward and reverse strands (red), E. coli blast 1 results for EC-IMP153 (light pink), E. coli blast 2 results 50579417 (green), E. coli blast 3 results K-12 MG1665 (purple) representing the positions covered by the BLASTN alignment, G+C content (black), G+C positive skew (green), and G+C negative skew (purple). Image created using CGview Server.
Figure 5.
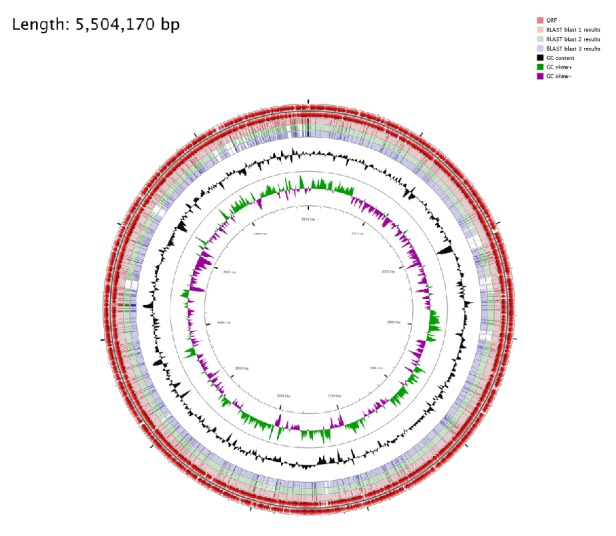
Circular genome representation of EC-IMP153 compared with E. coli LAU-EC4 and E. coli LAU-EC5. The outermost ring: EC-IMP153 open-reading frames (ORF) on both forward and reverse strands (red), E. coli blast 1 results for EC-IMP153 (light pink), E. coli blast 2 results LAU-EC4 (green), E. coli blast 3 results LAU-EC5 (purple) representing the positions covered by the BLASTN alignment, G+C content (black), G+C positive skew (green), and G+C negative skew (purple). Image created using CGview Server.
3.7. SNPs Based Phylogenetic Analysis
wgSNPs-based phylogenetic analysis of EC-IMP153 with 36 reference genomes downloaded from NCBI separated the isolates into three major clades (I, II, and III). Clade II was further subdivided into four subclades. Four ST-405 isolates including EC-IMP153, two CTX-M-15, and OXA-1 producing isolates (LAU-EC4 and LAU-EC5) [26], E. coli 50579417 harboring CTX-M-1 and OXA-48 genes isolated from Norway [24], and E. coli Z1002 belonging to ST-11 all clustered together. The distribution of the isolates correlated mainly with the area of isolation and ST (Figure 6).
Figure 6.
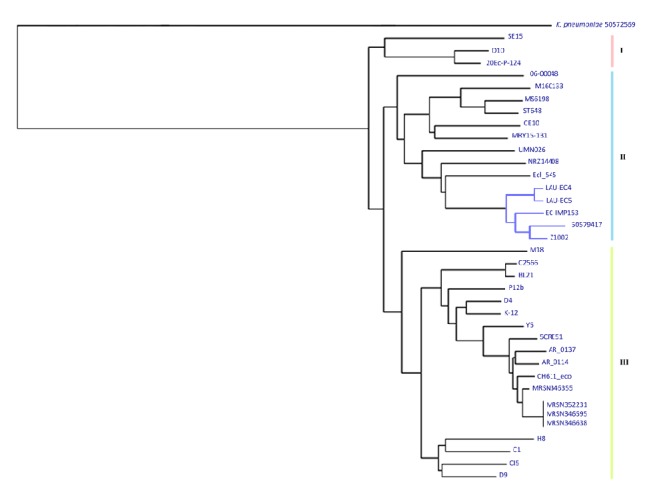
SNPs based phylogenetic tree. EC-IMP153 (LJOJ01000000), 50579417 (NZ_LNHL00000000), OXA-48 producing E. coli isolated from Norway, LAU-EC4 (AYOP0100000000), LAU-EC5 (AYOG0100000000) isolated from Lebanon, and Z1002 (AE005174) clustering in one clade.
4. Discussion
This study, to the best of our knowledge, is the first in-depth comparative genomic analysis of ST-405 OXA-48 producing MDR E. coli, linked to bacteremia isolated from a patient in Lebanon. Bloodstream infections (BSIs) caused by E. coli have been associated with prolonged hospital stay [25]. ST-405 is classified as one of the important ExPEC lineages [27] and has been involved in the spread of genes encoding ESBLs, cephamycinases, and carbapenemases [28, 29]. Previous surveillance studies conducted across European countries and North and South America have shown that around 20 to 45% of ExPEC were resistant to the first line of antibiotics including cephalosporins, fluoroquinolones, and trimethoprim-sulfamethoxazole [30]. EC-IMP153 was of serotype O102:H6, previously linked to E. coli ST-405 strains in France [31], and to ST964 (O102:H6) strains in Norway [32].
EC-IMP153 was positive for the blaOXA-48, an important carbapenem-resistant determinant. The OXA carbapenemases are presented by different class D OXA enzymes such as OXA-23-like, OXA-24-like, OXA-48, OXA-51-like, and OXA-58-like [33]. Poirel et al. (2011) [34] linked the distribution of OXA-48 producers, particularly in the Mediterranean region and in Western Europe, to the spread of the IncL/M-type plasmid, which carries the blaOXA-48 gene as the only resistant determinant. This was in harmony with our results, and EC-IMP153 was positive for blaOXA-48 and carried the IncL plasmid type as confirmed by WGS, PBRT testing, and PLACNETw. Earlier studies indicated that plasmids from different countries around the world carrying the OXA-48 gene shared related features; being self-conjugative, similar in size and not having other resistance determinants [22, 35]. Sequence analysis of IncL/M plasmid showed that the blaOXA-48 gene is bracketed by two copies of IS1999, giving rise to a composite transposon Tn1999 [4].
Furthermore, detailed analysis of resistance determinants revealed that EC-IMP153 was positive for blaCTX-M-3, blaTEM-1B, blaCMY-70, tet(A), tet(R), and aac(3)-lld. The CTX-M enzymes have been the most predominant among Enterobacteriaceae [36]. CTX-M group 1 is represented by blaCTX-M-1, blaCTX-M-3, and blaCTX-M-15, with blaCTX-M-1 and blaCTX-M-15 being associated with plasmids belonging to incompatibility groups IncI1, IncFII, and IncN [37], most of which were detected in this study. blaCTX-M-3 and blaOXA-1 were found coexisting on IncFII plasmid in EC-IMP153. IncFII originated from Kluyvera ascorbata through ISEcp1 that functions as a promoter for the expression of adjacent genes [38, 39]. Although ISEcp1 is bracketed by two 14-bp inverted repeats (IRL and IRR), it mobilizes adjacent regions through IRL and alternative similar sequences forming 5-bp direct repeats (DR) [38]. Studying the genetic content of IncF plasmid in EC-IM1P53 revealed that the transposition unit carrying blaCTX-M-3 is inserted in the Tn2/Tn3 hybrid, which also carries blaTEM-1B and was in harmony with previous reports [40, 41]. This model was also detected in blaCTX-M-15 positive E. coli isolates; blaCTX-M-3 is the progenitor of blaCTX-M-15 differing by a single amino acid substitution Asp-240 to Gly [38] and based on Ambler numbering [42]. Additionally, as previously reported, the downstream end of Tn2 was truncated by IS21 [43]. The association of Tn2 with ISs may also be important for the spread of blaCTX-M, especially as complete or partial copies of Tn2 are frequently found in MRRs and on plasmids [40].
The blaCMY-2 gene was first detected in 1990 [44]. Plasmid-mediated AmpC genes express resistance to broad-spectrum cephalosporins [45]. Latest reports highlighted new variants of CMY, CMY-70, which was detected in this study and was registered in Lahey database (http://www.lahey.org/Studies/other.asp#table1) [46]. On the other hand, sequence comparison at the amino acid level for gyrA and parC genes detected in EC-IMP153 revealed the presence of the single substitution S80I in ParC [47] and (S83L and D87N) in gyrA, conferring resistance to fluoroquinolones [48].
Replicon sequence typing of the detected IncF plasmid in EC-IMP153 revealed that it belonged to pMLST F31:A4:B1. This ST was found to be a common pMLST in E. coli ST617, ST131, and ST44. E. coli EC-IMP153 had other different virulence determinants, including genes linked to different STs (ST69, ST393, and ST405), associated with biofilm formation (fimH, papC and papG, fyuA or kpsMT II), which could favor persistence [49]. In addition, Col (BS512) plasmid detected in EC-IMP153 is known as the Shigella boydii plasmid pBS512 with replicon type FIIA and being categorized as invasive plasmid with relation to type three secretion system (T3SS) [50].
SNPs were considered to investigate the phylogeny and detect possible clonal links between EC-IMP153 and 36 other isolates. The wgSNPs-based phylogenetic analysis distributed the isolates depending on both the isolation site and STs. Similar to other reports, isolates clustering together on the same clade were of the same phylogenetic group [51] but with different resistance profiles [52]. Visual representation of circular genomes showed the presence of unique genes found in EC-IMP153 but not in 50579417, LAU-EC4, LAU-EC5, and K-12 MG1665.
5. Conclusion
In this study, we identified the resistome of IncF plasmids and the genetic environments surrounding blaOXA-48 producing E. coli isolated from Lebanon. The isolate was a MDR that coproduced ESBLs and other plasmid-mediated resistance determinants. Our findings suggest that many IncF as well other plasmids have incorporated into the OXA-48 positive isolate. Because of limited therapeutic options and higher mortality caused by these carbapenem-resistant Enterobacteriaceae, continuous surveillance and molecular characterization of OXA-48 producers are needed to shed light upon all of the transmission pathways.
Acknowledgments
This work was partly funded by the School of Arts and Sciences Research and Development Council.
Data Availability
Whole Genome Shotgun project of Escherichia coli isolate EC-IMP153 has been deposited at DDBJ/EMBL/GenBank under the accession number LJOJ00000000. The version described in this paper is version LJOJ00000000.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Reference genomes accession numbers retrieved from GenBank Nucleotide database.
References
- 1.Russo T. A., Johnson J. R. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes and Infection. 2003;5(5):449–456. doi: 10.1016/S1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 2.Riley L. W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clinical Microbiology and Infection. 2014;20(5):380–390. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 3.Jacoby G. A., Munoz-Price L. S. The new ß-lactamases. The New England Journal of Medicine. 2005;352:380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 4.Carrër A., Poirel L., Eraksoy H., Cagatay A. A., Badur S., Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrobial Agents and Chemotherapy. 2008;52(8):2950–2954. doi: 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y. Y., Wang Y., Walsh T. R. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 6.Matar G. M., Cuzon G., Araj G. F., et al. Oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Lebanon. Clinical Microbiology and Infection. 2008;14(9):887–888. doi: 10.1111/j.1469-0691.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 7.Poirel L., Potron A., Nordmann P. OXA-48-like carbapenemases: The phantom menace. Journal of Antimicrobial Chemotherapy. 2012;67(7):1597–1606. doi: 10.1093/jac/dks121.dks121 [DOI] [PubMed] [Google Scholar]
- 8.Schmiedel J., Falgenhauer L., Domann E., et al. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiology. 2014;14(1, article no. 187) doi: 10.1186/1471-2180-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L., Bonnin R. A., Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrobial Agents and Chemotherapy. 2011;56(1):559–562. doi: 10.1128/aac.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyrouthy R., Robin F., Dabboussi F., Mallat H., Hamzé M., Bonnet R. Carbapenemase and virulence factors of Enterobacteriaceae in North Lebanon between 2008 and 2012: Evolution via endemic spread of OXA-48. Journal of Antimicrobial Chemotherapy. 2014;69(10):2699–2705. doi: 10.1093/jac/dku181. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2016. (Twenty-Sixth Information Supplement M100-S26). [Google Scholar]
- 12.Aktas Z., Satana D., Kayacan C., et al. Carbapenem resistance in Turkey: repeat report on OXA-48 in Klebsiella pneumoniae and first report on IMP-1 beta-lactamase in Escherichia coli. African Journal of Microbiology Research. 2012;6(17):3874–3878. doi: 10.5897/ajmr12.036. [DOI] [Google Scholar]
- 13.Lob S. H., Kazmierczak K. M., Badal R. E., et al. Trends in susceptibility of Escherichia coli from intra-abdominal infections to ertapenem and comparators in the United States according to data from the SMART program, 2009 to 2013. Antimicrobial Agents and Chemotherapy. 2015;59(6):3606–3610. doi: 10.1128/AAC.05186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tritt A., Eisen J. A., Facciotti M. T., Darling A. E. An integrated pipeline for de novo assembly of microbial genomes. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0042304.e42304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen M. V., Cosentino S., Rasmussen S., et al. Multilocus sequence typing of total-genome-sequenced bacteria. Journal of Clinical Microbiology. 2012;50(4):1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B., Pop M. ARDB - Antibiotic resistance genes database. Nucleic Acids Research. 2009;37(1):D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zankari E., Hasman H., Cosentino S., et al. Identification of acquired antimicrobial resistance genes. Journal of Antimicrobial Chemotherapy. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carattoli A., Zankari E., Garciá-Fernández A., et al. In Silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrobial Agents and Chemotherapy. 2014;58(7):3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Research. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanza V. F., de Toro M., Garcillán-Barcia M. P., et al. Plasmid Flux in Escherichia coli ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences. PLoS Genetics. 2014;10(12) doi: 10.1371/journal.pgen.1004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carattoli A. Plasmids in Gram negatives: Molecular typing of resistance plasmids. International Journal of Medical Microbiology. 2011;301(8):654–658. doi: 10.1016/j.ijmm.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K. L., Threlfall E. J. Identification of plasmids by PCR-based replicon typing. Journal of Microbiological Methods. 2005;63(3):219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Samuelsen Ø., Naseer U., Karah N., et al. Identification of Enterobacteriaceae isolates with OXA-48 and coproduction of OXA-181 and NDM-1 in Norway. Journal of Antimicrobial Chemotherapy. 2013;68(7):1682–1685. doi: 10.1093/jac/dkt058. [DOI] [PubMed] [Google Scholar]
- 24.Tokajian S., Salloum T., Eisen J. A., et al. Genomic attributes of extended-spectrum β-lactamase-producing Escherichia coli isolated from patients in Lebanon. Future Microbiology. 2017;12(3):213–226. doi: 10.2217/fmb-2017-0171. [DOI] [PubMed] [Google Scholar]
- 25.Diekema D. J., Beekmann S. E., Chapin K. C., Morel K. A., Munson E., Doern G. V. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. Journal of Clinical Microbiology. 2003;41(8):3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant J. R., Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Research. 2008;36:W181–184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naseer U., Sundsfjord A. The CTX-M conundrum: dissemination of plasmids and Escherichia coli clones. Microbial Drug Resistance. 2011;17(1):83–97. doi: 10.1089/mdr.2010.0132. [DOI] [PubMed] [Google Scholar]
- 28.Kim J., Bae I. K., Jeong S. H., Chang C. L., Lee C. H., Lee K. Characterization of IncF plasmids carrying the blaCTX-M-14 gene in clinical isolates of Escherichia coli from Korea. Journal of Antimicrobial Chemotherapy. 2011;66(6):1263–1268. doi: 10.1093/jac/dkr106.dkr106 [DOI] [PubMed] [Google Scholar]
- 29.Tian G.-B., Wang H.-N., Zhang A.-Y., et al. Detection of clinically important β-lactamases in commensal Escherichia coli of human and swine origin in western China. Journal of Medical Microbiology. 2012;61(2):233–238. doi: 10.1099/jmm.0.036806-0. [DOI] [PubMed] [Google Scholar]
- 30.Foxman B. The epidemiology of urinary tract infection. Nature Reviews Urology. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 31.Mihaila L., Wyplosz B., Clermont O., et al. Probable intrafamily transmission of a highly virulent CTX-M-3-producing Escherichia coli belonging to the emerging phylogenetic subgroup D2 O102-ST405 clone. Journal of Antimicrobial Chemotherapy. 2010;65(7):1537–1539. doi: 10.1093/jac/dkq155.dkq155 [DOI] [PubMed] [Google Scholar]
- 32.Naseer U., Haldorsen B., Tofteland S., et al. Molecular characterization of CTX-M-15-producing clinical isolates of Escherichia coli reveals the spread of multidrug-resistant ST131 (O25:H4) and ST964 (O102:H6) strains in Norway. APMIS-Acta Pathologica, Microbiologica et Immunologica Scandinavica. 2009;117(7):526–536. doi: 10.1111/j.1600-0463.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 33.Walther-Rasmussen J., Høiby N. OXA-type carbapenemases. Journal of Antimicrobial Chemotherapy. 2006;57(3):373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 34.Poirel L., Ros A., Carrër A., et al. Cross-border transmission of OXA-48-producing Enterobacter cloacae from Morocco to France. Journal of Antimicrobial Chemotherapy. 2011;66(5):1181–1182. doi: 10.1093/jac/dkr023. [DOI] [PubMed] [Google Scholar]
- 35.Carrër A., Poirel L., Yilmaz M., et al. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrobial Agents and Chemotherapy. 2010;54(3):1369–1373. doi: 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bush K. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Current Opinion in Microbiology. 2010;13(5):558–564. doi: 10.1016/j.mib.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Cullik A., Pfeifer Y., Prager R., Von Baum H., Witte W. A novel IS26 structure surrounds blaCTX-M genes in different plasmids from German clinical Escherichia coli isolates. Journal of Medical Microbiology. 2010;59(5):580–587. doi: 10.1099/jmm.0.016188-0. [DOI] [PubMed] [Google Scholar]
- 38.Poirel L., Decousser J.-W., Nordmann P. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrobial Agents and Chemotherapy. 2003;47(9):2938–2945. doi: 10.1128/AAC.47.9.2938-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez M. M., Power P., Radice M., et al. Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: A possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrobial Agents and Chemotherapy. 2004;48(12):4895–4897. doi: 10.1128/AAC.48.12.4895-4897.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey J. K., Pinyon J. L., Anantham S., Hall R. M. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. Journal of Antimicrobial Chemotherapy. 2011;66(4):745–751. doi: 10.1093/jac/dkq529. [DOI] [PubMed] [Google Scholar]
- 41.Partridge S. R., Hall R. M. Evolution of Transposons Containing blaTEM Genes. Antimicrobial Agents and Chemotherapy. 2005;49(3):1267–1268. doi: 10.1128/AAC.49.3.1267-1268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karim A., Poirel L., Nagarajan S., Nordmann P. Plasmid-mediated extended-spectrum β-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiology Letters. 2001;201(2):237–241. doi: 10.1016/s0378-1097(01)00276-2. [DOI] [PubMed] [Google Scholar]
- 43.Partridge S. R. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiology Reviews. 2011;35(5):820–855. doi: 10.1111/j.1574-6976.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 44.Bauernfeind A., Schweighart S., Grimm H. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18(5):294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 45.Philippon A., Arlet G., Jacoby G. A. Plasmid-determined AmpC-type β-lactamases. Antimicrobial Agents and Chemotherapy. 2002;46(1):1–11. doi: 10.1128/aac.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porres-Osante N., Sáenz Y., Somalo S., Torres C. Characterization of Beta-lactamases in Faecal Enterobacteriaceae Recovered from Healthy Humans in Spain: Focusing on AmpC Polymorphisms. Microbial Ecology. 2015;70(1):132–140. doi: 10.1007/s00248-014-0544-9. [DOI] [PubMed] [Google Scholar]
- 47.Alouache S., Estepa V., Messai Y., Ruiz E., Torres C., Bakour R. Characterization of ESBLs and associated quinolone resistance in Escherichia coli and Klebsiella pneumoniae isolates from an urban wastewater treatment plant in Algeria. Microbial Drug Resistance. 2014;20(1):30–38. doi: 10.1089/mdr.2012.0264. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrobial Agents and Chemotherapy. 1990;34(6):1271–1272. doi: 10.1128/AAC.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco J., Mora A., Mamani R., et al. National survey of Escherichia coli causing extraintestinal infections reveals the spread of drug-resistant clonal groups O25b:H4-B2-ST131, O15:H1-D-ST393 and CGA-D-ST69 with high virulence gene content in Spain. Journal of Antimicrobial Chemotherapy. 2011;66(9):2011–2021. doi: 10.1093/jac/dkr235.dkr235 [DOI] [PubMed] [Google Scholar]
- 50.Johnson T. J., Nolan L. K. Pathogenomics of the Virulence Plasmids of Escherichia coli. Microbiology and Molecular Biology Reviews. 2010;74(3):477–478. doi: 10.1128/MMBR.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hommais F., Pereira S., Acquaviva C., Escobar-Páramo P., Denamur E. Single-nucleotide polymorphism phylotyping of Escherichia coli. Applied and Environmental Microbiology. 2005;71(8):4784–4792. doi: 10.1128/AEM.71.8.4784-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forde B. M., Ben Zakour N. L., Stanton-Cook M., et al. The complete genome sequence of escherichia coli EC958: A high quality reference sequence for the globally disseminated multidrug resistant E. coli O25b:H4-ST131 clone. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0104400.e104400 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reference genomes accession numbers retrieved from GenBank Nucleotide database.
Data Availability Statement
Whole Genome Shotgun project of Escherichia coli isolate EC-IMP153 has been deposited at DDBJ/EMBL/GenBank under the accession number LJOJ00000000. The version described in this paper is version LJOJ00000000.