To the Editor
Asthma is a chronic inflammatory disorder of the airways with airflow obstruction that may initially be reversible but progresses to an irreversible status due to airway remodeling. These structural changes, which occur in both the large and small airways of asthmatics, are characterized by subepithelial fibrosis, goblet cell hyperplasia, angiogenesis, and airway smooth muscle cell hypertrophy.1
Mast cells (MCs) play an important role in tissue fibrosis in asthma.2 MC-deficient mice showed reduced deposition of collagen in the airways in a murine model of asthma, which was restored by MC reconstitution.2 However, the mechanisms and mediators of MCs involved in asthmatic airway remodeling remain unclear. Plasminogen activator inhibitor type 1 (PAI-1) is the primary inhibitor of urokinase-and tissue-type plasminogen activators (uPA and tPA). We previously reported that the levels of PAI-1 were elevated in the airways of a murine model of asthma and that genetic deletion of PAI-1 was associated with diminished airway fibrosis.3 PAI-1 inhibitor treatment also reduced airway inflammation and tissue remodeling in these mice.4,5 We have also demonstrated that human MCs are a major source of PAI-1 in the airways of asthmatic patients. 6,7 In this study, we hypothesized that MC-derived PAI-1 plays a major role in airway remodeling.
In order to verify the role of MC-derived PAI-1 in asthma, experiments using MC-deficient C57BL/6J- Kitw-sh/w-sh mice were performed. MC-deficient C57BL/6J- Kitw-sh/w-sh mice were reconstituted with bone marrow cultured MCs (BMCMCs) from PAI-1−/− and wild type (WT) mice. The functionality of activated BMCMCs from PAI-1−/− mice was confirmed by the equal amount of histamine release compared with activated BMCMCs from WT mice (Supplementary Fig. 1) before reconstitution, and similar numbers of tissue MCs in the lungs from both BMCMC reconstituted groups were also observed after reconstitution (Supplementary Fig. 2). Detailed experimental methods and statistical analyses are described in the Supplementary methods section.
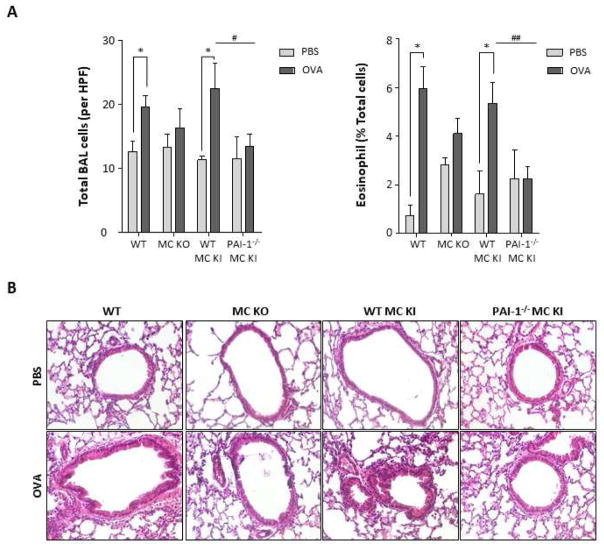
The numbers of total inflammatory cells and eosinophils in BALF from Kitw-sh/w-sh mice reconstituted with BMCMCs from PAI-1−/− mice (PAI1−/− BMCMCs→ Kitw-sh/w-sh mice) were significantly decreased compared with those from Kitw-sh/w-sh mice reconstituted with BMCMCs from WT mice (WT BMCMCs→ Kitw-sh/w-sh mice) following OVA challenge (Fig 1A, total inflammatory cells: 13.38±5.29 vs 22.46±7.92 cells/high power field (HPF), p<0.05; eosinophils: 2.24±1.28 vs 5.34±1.72 percent, p<0.01, respectively). H&E staining showed recruitment of inflammatory cells in the peribronchial and perivascular areas of OVA-challenged WT and WT BMCMCs→ Kitw-sh/w-sh mice. However, OVA-challenged PAI1−/− BMCMCs→ Kitw-sh/w-sh mice displayed reduced infiltration of inflammatory cells (Fig 1B). Collectively, these results show that MC-derived PAI-1 promotes inflammation in this murine model of asthma.
Figure 1. Inflammation in the lung tissues of PBS- or OVA- challenged mice.
(A) Number of total inflammatory cells and eosinophils per high power field (HPF) in the BALF from PBS or OVA-challenged mice. * p <0.05 versus PBS group. (B) Representative hematoxylin and eosin (H&E) staining for histopathologic changes in lung sections of PBS- or OVA-challenged mice. Data are expressed as the means ± SE. A, WT mice; B, Kitw-sh/w-sh mice; C, WT BMCMCs→ Kitw-sh/w-sh mice; D, PAI1−/− BMCMCs→ Kitw-sh/w-sh mice.
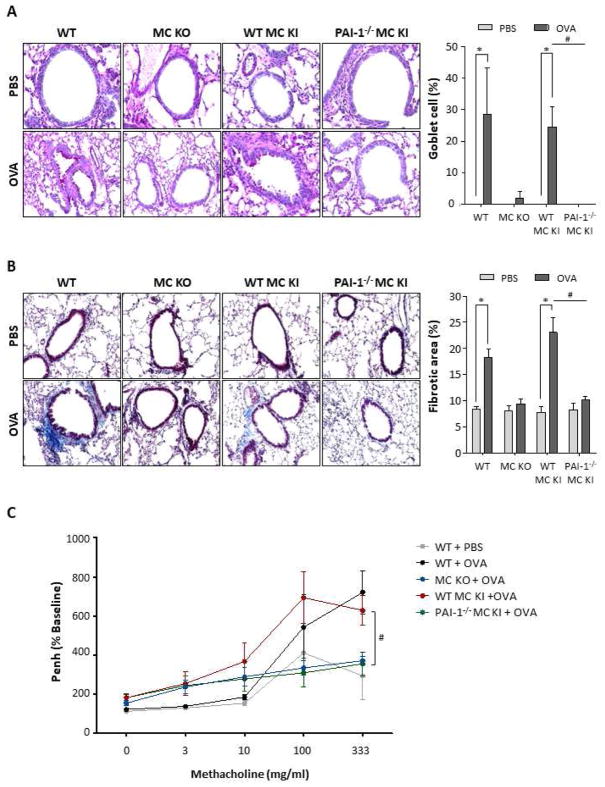
As shown in Fig 2A, PAI1−/− BMCMCs→ Kitw-sh/w-sh mice displayed markedly suppressed goblet cell hyperplasia compared to WT BMCMCs→ Kitw-sh/w-sh mice following OVA challenge (0.13±0.22 vs 24.52±12.75 percent, p<0.01). The accumulation of collagen in lung tissues was markedly increased in WT mice and WT BMCMCs→ Kitw-sh/w-sh mice after OVA challenge compared with PBS-challenged controls (WT: 18.26±5.94 vs 8.40±1.43 percent, p<0.01; WT BMCMCs→ Kitw-sh/w-sh: 23.06±10.37 vs 7.75±3.54 percent, p<0.01, respectively). In contrast, PAI1−/− BMCMCs→ Kitw-sh/w-sh mice showed remarkably decreased collagen deposition compared with WT BMCMCs→ Kitw-sh/w-sh mice after OVA challenge (10.21±2.17 and 23.06±10.37, p<0.01) (Fig 2B). Our results implicate MC-derived PAI-1 in OVA-induced goblet cell hyperplasia and collagen deposition. Furthermore, we measured the enhanced minute pause (Penh) to determine the physiologic effect on airway hyperresponsiveness (AHR). The Penh value of PAI1−/− BMCMCs→ Kitw-sh/w-sh mice was significantly decreased compared to WT BMCMCs→ Kitw-sh/w-sh mice after OVA challenge (355.6±60.4 vs 629.2±76.1 %Penh, p<0.05) (Fig 2C).
Figure 2. Airway remodeling in the lung tissues of PBS- or OVA- challenged mice.
(A) Lung sections were stained with periodic acid-Schiff (PAS) stain to assess goblet cell hyperplasia. Original magnification, 400x. The number of PAS-positive cells per 100 μm of basement membrane is visualized graphically using Image J (right panel). * p <0.05 versus PBS group. (B) Masson’s trichrome staining was conducted to evaluate collagen deposition (blue). Original magnification, 200x. * p <0.05 versus PBS group. (C) The Airway hyperresponsiveness (AHR) to increasing concentrations of methacholine (0–333 mg/ml) was measured by the Buxco apparatus, and was reported as percent change of enhanced minute pause (Penh) per baseline value. #p <0.05 versus OVA challenged WT BMCMCs→ Kitw-sh/w-sh mice. All data are expressed as the means ± SE. A, WT mice; B, Kitw-sh/w-sh mice; C, WT BMCMCs→ Kitw-sh/w-sh mice; D, PAI1−/− BMCMCs→ Kitw-sh/w-sh mice.
Lastly, we examined the expression of PAI-1 in each group using immunohistochemical (IHC) analysis. As expected, overall PAI-1 protein expression was significantly lower in the lungs of Kitw-sh/w-sh and PAI1−/− BMCMCs→ Kitw-sh/w-sh mice compared to WT and WT BMCMCs→ Kitw-sh/w-sh mice after OVA challenge (Supplementary Fig. 3A). MCs were strongly positive for PAI-1 staining and monocytes/macrophages were another sources of strong PAI-1 expression (Supplementary Fig. 3B).
Yu M et al. previously demonstrated that MCs play a critical role in the airway remodeling in asthma using a MC knock-out and reconstitution approach,2 which is similar to our current experimental approach. Emerging evidence from our laboratory, and that of others, shows that PAI-1 plays an important role in the pathogenesis of asthma and that MC are a major source of PAI-1. 5–7 Therefore, we further investigated the causative role of MC-derived PAI-1 in allergen-induced asthma using a MC knockout and reconstitution model. This study confirmed that MC-specific PAI-1 promotes airway inflammation, goblet cell hyperplasia, collagen deposition and airway hyperreactivity.
Activated MC in the airways of asthmatics are important not only in initiating allergic inflammation after allergen challenge, but also in maintaining inflammation via interaction with resident cells and migratory inflammatory cells in the airways. Endothelial cells, macrophages and other inflammatory cells tend to produce both PAI-1 and tPA simultaneously.7,8 MC, however, release tPA constitutively in their resting state and PAI-1 only upon activation.6 Consequently, activated MC-derived PAI-1 converts a fibrinolytic environment to a fibrogenic environment by overcoming tPA activity, which is dominant during the resting state.6 We previously reported that MC-derived PAI-1 is highly expressed in human lung samples from fatal asthmatic patients.6 Taken together, allergen-induced MC activation results in elevated levels of PAI-1, which in turn converts fibrinolytic environment to fibrogenic environment.
It is interesting that the PAI1−/− BMCMCs→ Kitw-sh/w-sh mice had reduced eosinophilic inflammation. Our previous study showed that PAI-1 inhibition by tiplaxtinin decreased the degree of eosinophilic airway inflammation and AHR in an OVA-challenge model of allergic asthma.4 Miyamoto et al.9 also reported that intra-airway administration of small interfering RNA (siRNA) against PAI-1 reduced eosinophilic airway inflammation and AHR in a murine model of acute asthma. They suggest that elevation of hepatocyte growth factor (HGF) by PAI-1 inhibition may be a mechanism of attenuation in airway inflammation and AHR in their model of acute asthma. We also measured HGF and other growth factors such as TGF-β1 and VEGF, but could not find any significant difference between the groups. Interestingly, however, our in vitro study showed that PAI-1 enhanced IL-4/dsRNA-induced thymic stromal lymphopoietin (TSLP) production from airway epithelial cells (Supplementary Fig. 4), suggesting a possible feedback loop of IgE-PAI-1-TSLP-IL-4/5-IgE/eosinophil inflammation. Based on this result, we speculate that deletion of MC-derived PAI-1 may reduce eosinophilic airway inflammation, at least in part, via attenuation of the PAI-1-TSLP feedback pathway.
In conclusion, our experiments demonstrated that MCs are an important source of PAI-1 and that MC-derived PAI-1 contributes to airway inflammation, goblet cell hyperplasia, collagen deposition, and AHR. These data suggest that MC-derived PAI-1 can be a novel therapeutic target for airway remodeling.
Supplementary Material
Acknowledgments
Declaration of all sources of funding: This study was supported by the American Heart Association (AHA) grant 11SDG7590063 and NIH grant 1K23AI110731.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orihara K, Dil N, Anaparti V, Moqbel R. What’s new in asthma pathophysiology and immunopathology? Expert Rev Respir Med. 2010;4:605–29. doi: 10.1586/ers.10.57. [DOI] [PubMed] [Google Scholar]
- 2.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–41. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002;294:1155–60. doi: 10.1016/S0006-291X(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Eren M, Vaughan DE, Schleimer RP, Cho SH. A plasminogen activator inhibitor-1 inhibitor reduces airway remodeling in a murine model of chronic asthma. Am J Respir Cell Mol Biol. 2012;46:842–6. doi: 10.1165/rcmb.2011-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto S, Hattori N, Senoo T, Onari Y, Iwamoto H, Kanehara M, et al. Intra-airway administration of small interfering RNA targeting plasminogen activator inhibitor-1 attenuates allergic asthma in mice. Am J Physiol Lung Cell Mol Physiol. 2011;301:L908–16. doi: 10.1152/ajplung.00115.2011. [DOI] [PubMed] [Google Scholar]
- 6.Cho SH, Tam SW, Demissie-Sanders S, Filler SA, Oh CK. Production of plasminogen activator inhibitor-1 by human mast cells and its possible role in asthma. J Immunol. 2000;165:3154–61. doi: 10.4049/jimmunol.165.6.3154. [DOI] [PubMed] [Google Scholar]
- 7.Cho SH, Lee SH, Kato A, Takabayashi T, Kulka M, Shin SC, et al. Cross-talk between human mast cells and bronchial epithelial cells in plasminogen activator inhibitor-1 production via transforming growth factor-beta1. Am J Respir Cell Mol Biol. 2015;52:88–95. doi: 10.1165/rcmb.2013-0399OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van De Craen B, Declerck PJ, Gils A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012;130:576–85. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto S, Hattori N, Senoo T, Onari Y, Iwamoto H, Kanehara M, et al. Intra-airway administration of small interfering RNA targeting plasminogen activator inhibitor-1 attenuates allergic asthma in mice. Am J Physiol Lung Cell Mol Physiol. 2011;301:L908–16. doi: 10.1152/ajplung.00115.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.