Abstract
The purpose of this longitudinal blood sampling study was to examine relationships between sex hormones and fibromyalgia pain. Eight women meeting case definition criteria for fibromyalgia provided venous blood samples and reported their fibromyalgia pain severity over 25 consecutive days. All women exhibited normal menstrual cycles and were not taking oral contraceptives. Cortisol, and the sex hormones estradiol, progesterone, and testosterone, were assayed from serum. A linear mixed model was used to determine if fluctuations of sex hormones were associated with changes in pain severity. In the entire sample, day-to-day changes in both progesterone (p = 0.002) and testosterone (p = 0.015) were significantly and inversely correlated with pain severity. There was no relationship between estradiol and pain (p = 0.551) or cortisol and pain (p = 0.633). These results suggest that progesterone and testosterone play a protective role in fibromyalgia pain severity. Sex and other hormones may serve to both increase and decrease fibromyalgia pain severity.
Keywords: fibromyalgia, pain, progesterone, testosterone, longitudinal
INTRODUCTION
Fibromyalgia, a chronic pain disorder of unknown etiology, disproportionately affects women over men.49 The condition is characterized by diffuse musculoskeletal pain, increased sensitivity to pressure at soft tissues, profound fatigue, and self-reported cognitive disruption.45 One notable feature of fibromyalgia is that the symptom severity can vary markedly over short periods of time, with severity on any particular day being largely unpredictable.43 The lack of predictability adds to the debilitating nature of the disorder, as planning future activities can be difficult for sufferers.
The higher prevalence of fibromyalgia in women shows biological sex to be an important consideration in the disorder.30 There is, however, only limited evidence supporting a link between sex hormones and fibromyalgia incidence or severity. The incidence of pediatric fibromyalgia has been shown to be similar in both genders until the onset of puberty, when the incidence rate increases in girls.25 In addition, it has been posited that the sex hormone testosterone may provide a protective effect for men against these types of chronic pain disorders7 with transdermal testosterone gel significantly reducing fibromyalgia pain in women.44
There are several mechanisms by which sex hormones could impact the experience of pain, which have been covered extensively in recent review articles.34,41 These mechanisms include effects on: peripheral nociceptors,14 central nociceptive processing,27 spinal inflammation,39 central microglia,35 affective brain systems that modulate pain,47,2 and opioid systems.23,40
Testosterone5 and progesterone9 are generally shown to be associated with lower experience of pain. Progesterone in particular appears to have central anti-inflammatory, neuroprotective, and analgesic effects.15,28,21 Progesterone may also have local effects, as it has been shown to be superior to corticosteroids in treating carpal tunnel syndrome.32
Estradiol effects on nociception and pain are more complex. Estradiol has been shown in many cases to be both anti-nociceptive,46,10 and pro-nociceptive,18,31 the latter effect being mediated partially by upregulation of vanilloid 1 (TRPV1) and anoctamin 1 (ANO1) in primary sensory neurons.48 It is likely that estradiol metabolites (estradiol-3-glucuronide and estradiol-17-glucuronide) have differential effects on pain.20 Also, specific estrogen receptor subtypes may play different roles in the relative pro- and anti-nociceptive properties of estrogen.8
In order to test if sex hormones affect fibromyalgia pain, we conducted this daily sampling study in eight normally-cycling women with fibromyalgia. Sex hormones (estradiol, progesterone, and testosterone) and self-reported pain were assessed daily during the 25-day protocol. We tested three specific hypotheses in this study: First, progesterone would be negatively associated with pain. Second, testosterone would be negatively associated with pain. Third, estradiol would be positively associated with pain. Convincing cases for either a positive estradiol-pain relationship or negative relationship could be made and supported by the literature. While, we hypothesized a positive relationship between estradiol and pain, all tests were conducted two-tailed. In addition, because prior literature has shown a positive relationship between cortisol levels and fibromyalgia pain13, we also assayed cortisol at all time-points. To our knowledge, this is the first study to use daily hormone measurements to test the relationship between sex hormones and fibromyalgia severity.
METHODS
Participant Selection
All procedures were approved by the University of Alabama at Birmingham (UAB) Institutional Review Board. This study was completed between June 2015 and February 2017. Potential participants were identified via the laboratory’s database of 1200 local individuals who had expressed an interest in research participation. Individuals from the online database were selected for an additional phone screening if they were female, were within 1 hour driving distance to the laboratory, were between the ages of 18 and 65, met American College of Rheumatology 2010 self-reported fibromyalgia case definition criteria,45 and indicated an average daily pain of at least 3 out of 10. Pain was assessed with a single item, “How would you rate your average daily pain on a scale from 0 (no pain at all) to 10 (worst possible pain).”. Individuals did not pass the online screening stage if they indicated a diagnosis or medications for any significant medical conditions (e.g., cancer, heart disease, diabetes, liver disease, neurological disorders, and neurodegenerative diseases). Individuals were also not considered if they indicated a diagnosis or medications for any autoimmune or rheumatologic disorder.
Twenty individuals from the online database were contacted via phone for a second screening, conducted by a member of the research team. In the phone screening, individuals were excluded from participation if they indicated any blood clotting disorders or other contraindications for phlebotomy, hysterectomy, current pregnancy or active plans to become pregnant. Participants must also have indicated having normal and predictable menstrual cycles. Medications were reviewed and individuals were excluded if taking blood thinning, anti-inflammatory, rheumatologic, antibiotic, hormone-based contraceptive, opioid analgesic medications, or any hormone therapies. During the phone screening, the study was also described in-depth and individuals were asked whether they were interested in participating.
Nine women who passed the screenings and were interested in participation were invited to a laboratory screening session at the Clinical Research Unit at UAB. At the screening visit, participants provided written, informed consent and provided a blood sample for screening tests. Participants were excluded from the study if they had abnormal renal function, triiodothyronine (T3), thyroxine (T4), thyroxin binding globulin (TBG), thyroid stimulating hormone (TSH), complete blood count with differential (CBC) or 25-hydroxy vitamin D. Participants were also excluded if they presented with detectable rheumatoid factor (Rf) or anti-nuclear antibody (ANA), or had an erythrocyte sedimentation rate (ESR) greater than 60mm/hr. All screening blood results were reviewed by Dr. Timothy Ness (UAB Department of Anesthesiology and Perioperative Medicine).
During the screening visit, participants also completed several self-report questionnaires that were not used in analyses, but instead used to capture additional exclusionary criteria. Depressed mood and anxiety was measured with the Hospital Anxiety and Depression Scale.50 The depressed mood and anxiety subscales yield possible scores of 0 – 7 (normal), 8 – 10 (mild), 11 –14 (moderate) and 15 – 21 (severe), with scores over 15 being exclusionary. Pain severity was assessed with the Brief Pain Inventory (BPI).26 The BPI provides scores for pain intensity (range 0 – 10) and pain interference (range 0 – 10). A minimum of 3 for pain intensity was required for participation. Participants also completed the Fibromyalgia Assessment Form45 that yields a widespread pain index (range 0 – 19) and a symptom severity score (range 0 – 12). Finally, participants completed a clinician-guided Mini International Neuropsychiatric Interview (MINI),37 and were excluded if meeting clinician criteria for major depressive disorder. All selected screening tools are commonly used in the pain literature and have demonstrated acceptable reliability and validity.
Sample size
Sample size was based on a minimal correlation of interest of r = 0.3 and desired power of 95%. With a p-value threshold of 0.05, 25 repeated measures per individual, and assumed repeated measures correlation of 0.5, 8 individuals would be needed to achieve 95% power. While our main analyses (see Statistical Analyses) examined correlations in participant-nested data, the power analyses provided a rough approximation of required sample size. However, some features of the acquired data (for example, autocorrelation) were not accounted for in the sample size calculation, as power calculators for linear mixed models are not adequately developed at this time.
Study Procedures
The study was a six-week observational project, including a two-week baseline period followed by a 25-day daily sample collection period. Immediately following the on-site screening, participants started an observational baseline period of two weeks. Daily symptoms were obtained through a questionnaire delivered on Android-based tablets running Qualtrics Survey Software (Qualtrics; Provo, UT). Fibromyalgia pain severity was rated on a 0 – 100 visual and numerical scale with the question, “Overall, how severe has your pain been today?” The far left of the scale was anchored at “no pain” and the far right anchored at “severe pain”. The daily questionnaire, completed at the end of the day, also contained items to measure fatigue, mood, stress, sleep quality, physical activity, and gastrointestinal complaints. Those items were not analyzed in this study. In order to reduce bias, participants were not informed of the exact blood tests to be conducted, and were not informed of the study hypotheses until their completion of the protocol. During the study period, participants were asked to avoid taking over-the-counter analgesics, and to report any such use on the daily questionnaire.
At the end of the baseline period, participants began the daily blood draws. Laboratory visits were scheduled for 25 consecutive days, including weekends. Appointments were held at the same time each day for the individual in order to minimize diurnal effects. Trained phlebotomists or research nurses drew 8mL of blood with a 21- to 23-guage butterfly needle into serum separating tubes (BD Vacutainer). Using standard processing protocols, the blood samples were kept at room temperature for 30 minutes and then centrifuged at 1300G for 15 minutes. The serum layer was extracted and stored at −80°C. Throughout this phase of the study, participants continued to complete their daily pain reports. Participants were paid $50 for each laboratory visit.
Sample Processing
Sex hormone concentrations were analyzed in serum samples by the Metabolism Core at UAB, under the direction of Dr. Barbara Gower. Total progesterone, estradiol and testosterone assays were conducted using standard assay manufacturer protocols on an Automated Immunoassay Analyzer (AIA)-900 (TOSOH Bioscience, South San Francisco, CA), using the Fluorescent Enzyme Immunoassay (FEIA) method. Cortisol was also assayed in sera samples using the same procedures. Laboratory technicians were blinded to the study protocol.
Statistical Analyses
The three primary hypotheses were tested in a single, multivariate linear mixed model (LMM) approach in SPSS v.24 (IBM), using the restricted maximum likelihood (REML) estimation procedure and an autoregressive covariance structure. Daily pain was the dependent variable. Daily values of all three sex hormones (estradiol, testosterone, progesterone) were entered as independent predictors. Data were nested by subject, with study day as the repeated-measures index variable. All time-series data were person-centered (z-scored) to remove the influence of between-subject differences. A Bonferroni-adjustment was used for the three main predictors to hold the overall chance of error to 0.05, yielding a p-value threshold of 0.017. The decision to employ a correction for multiple comparisons was made after data collection and was therefore not accounted for in the sample size calculation performed before data collection.
RESULTS
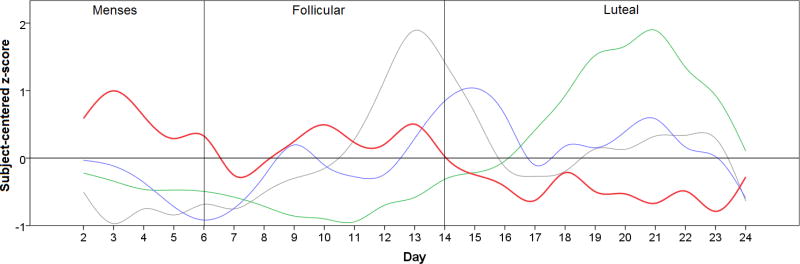
One individual was excluded at baseline due to abnormally elevated ESR values. Eight individuals, aged 33+/− 8.6, met all criteria for participation. Average depression symptom severity (6.9, SD = 3.9) was in the normal range, as was anxiety (5.6, SD = 2.4). The group showed moderately-high pain severity (6.8, SD = 3.8) and moderate pain interference (4.6, SD = 1.5). The Fibromyalgia Assessment Form widespread pain index was 10.3 (SD = 4.6) and the somatosensory scale was 9.6 (SD = 2.5). No participants met criteria for major depressive disorder in the MINI. One participant took pregabalin for the duration of the study, and one took gabapentin (see Table 1). No participants reported use of over-the-counter analgesics/anti-inflammatories during the study. Table 1 shows additional demographic information on the final study cohort. On visual review of individual-level sex hormone plots, all participants demonstrated the expected peaks of estrogen and progesterone, suggesting normal menstrual cycles. Across the 25-day study period, average progesterone was 4.11+/− 5.64 ng/ml), estradiol 92.56+/−65.62 pg/ml and testosterone 42.36+/−17.49 ng/ml. Pain level across all days was 56.49+/−26.54. Average cortisol was 12.92+/−6.4 µg/dl. Sex hormone and pain levels across time can be seen for the entire sample in Figure 1.
Table 1.
Demographics and screening blood test values.
| ID | Age | Race | Pain | Free T3 | Free T4 | TSH | Vit-D | hsCRP | ESR | RBC | WBC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANALGESICS | |||||||||||||
| 1 | 39 | Caucasian | 9 | 3.2 | 1.15 | 1.23 | 14.6 | 1.47 | 10 | 4.56 | 9.08 | ||
| 2 | 37 | Caucasian | 4 | 86 (total) | 1.3 | 0.99 | 27.8 | 0.86 | 6 | 4.24 | 8.38 | ||
| 3 | 21 | Caucasian | 6 | 2.8 | 1.23 | 0.60 | 55.9 | 0.9 | 0 | 4.42 | 2.67 | pregabalin | |
| 4 | 46 | Caucasian | 3 | 2.1 | 0.77 | 2.10 | 29.3 | 3.66 | 14 | 4.55 | 8.35 | ||
| 5 | 27 | Asian | 7 | 3.4 | 11.3 (total) | 3.00 | 6.3 | 0.32 | 4 | 5.11 | 6.09 | ||
| 6 | 24 | Caucasian | 5 | 90 (total) | 6.3 | 0.92 | 16.7 | 0.45 | 5 | 4.41 | 6.84 | ||
| 7 | 26 | African A | 8 | 3.0 | 1.03 | 0.73 | 15.3 | <0.2 | 10 | 4.69 | 4.41 | ||
| 8 | 39 | African A | 9 | 88 (total) | 0.86 | 1.59 | 15.2 | 34.2 | 36 | 4.22 | 8.22 | gabapentin | |
T3 = triiodothyronine (nanograms per deciliter; free unless indicated as total), T4 = thyroxine (nanograms per deciliter; free unless indicated as total), TSH = thyroid stimulating hormone (international units per milliter), Vit-D = vitamin D (nanograms per milliliter), hsCRP = high sensitivity C-reactive protein (milligrams per liter), ESR = erythrocyte sedimentation rate (millimeters per hour). RBC = red blood cell count (million per microliter), WBC = white blood cell count (thousand per microliter). No participants had detectable levels of anti-nuclear antibody or rheumatoid factor (not shown).
Pain values were collected from a single-item measure, “How would you rate your average daily pain on a scale from 0 (no pain at all) to 10 (worse possible pain)?”
Figure 1.
Relationship between progesterone (green), testosterone (blue), estradiol (gray) and pain (red) over 25 days in eight women with fibromyalgia. Individuals began their participation at different points of their cycle, therefore time courses have been shifted to allow representation of group averages. The “day” index variable was shifted so that the first estradiol peak occurred in the same time period for all participants. The fit was confirmed by observing that the progesterone peak also was temporally aligned for all participants. All y-axis values have been subject-centered (z-scored) to allow variables to be plotted on the same scale, and represent standard deviations from the subject mean.
Sex hormone data were not obtained for 6 out of 200 visits (3%) due to missed laboratory visits. These days were treated as missing values and were not imputed. There were no missing pain reports.
The inter-assay coefficients of variation (CV) were: progesterone (2.26%), estradiol (1.42%) testosterone (7.49%), and cortisol (2.46%). Intra-assay CV’s were 1.04%, 2.31%, 11.05%, and 5.19%; respectively. Minimum detection values were 0.10 ng/ml, 25.0pg/ml, 10 ng/ml, and 0.2µg/dl; respectively. All samples provided detectable levels of hormones.
There was a significant and inverse relationship between pain severity and both progesterone (F = −9.76, p = 0.002) and testosterone (F = −6.01, p = 0.015). Serum levels of estradiol were not associated with pain (F = 0.36, p = 0.551). Because previous research has demonstrated a positive relationship between cortisol and fibromyalgia pain,13 we also included main effects for cortisol, which was not significant (F = 0.23, p = 0.633). The strongest relationship, based on statistical indices, was found between progesterone and pain, and Figure 2 presents individual plots for each participant.
Figure 2.
Relationship between progesterone and pain in all eight participants over 25 days (x-axis). The green line represents progesterone and the blue line represents pain. Progesterone and pain have been subject-centered (z-scored) to allow plotting on the same scale. Time courses have been shifted to reflect the expected menstrual cycle, so that the progesterone peak occurs at the end of each participants’ time series.
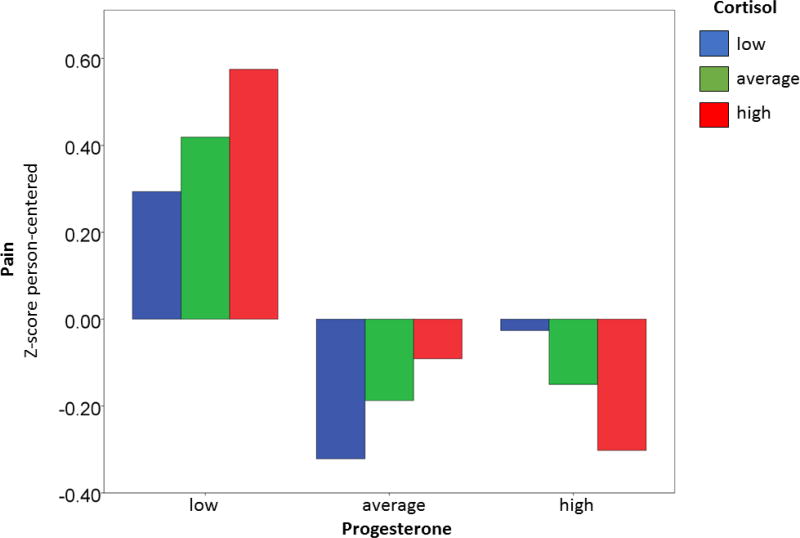
Post-hoc analyses were performed to examining possible interactions between cortisol and sex hormones. Interaction terms were entered into the same linear mixed model. Cortisol interacted with progesterone to influence pain (F = −11.20, p = 0.001). To explore the nature of the interaction, cortisol was split into tertiles (low, medium, and high). Progesterone was not correlated with pain during low cortisol (r = 0.10, p = 0.454) or during moderate cortisol (r = − 0.22, p = 0.096), but was during times of high cortisol (r = −.45, p = 0.0004). Figure 3 shows the interaction of cortisol and progesterone on pain. Pain was greatest on days when progesterone was low and cortisol was high. The correlation of progesterone and pain was not related to participants’ average cortisol over the 25 days (r = −.33, p = 0.420). Significant interactions were not identified between cortisol and other sex hormones.
Figure 3.
Progesterone and cortisol interaction predicts fibromyalgia pain. Person-centered cortisol (x-axis) and progesterone (x-axis bin) have been split into low, average, and high tertiles. Person-centered pain is on the y-axis. Pain is highest when progesterone is low (left bin) while cortisol is high (red column).
As a post-hoc analysis, we further tested the relationship between progesterone and pain. A linear mixed model was constructed to determine if pain differences between high and low progesterone phases were statistically and clinically significant. We contrasted average pain in the 3-day progesterone nadir to the 3-day progesterone peak. When progesterone was lowest, average pain was 66.5. When progesterone was highest, pain was rated as 50.4. This 25.6% drop in pain was statistically significant (F = 9.1, p = 0.005).
DISCUSSION
In this study, we tested if the sex hormones estradiol, progesterone, and testosterone are associated with daily pain severity in women with fibromyalgia. We found that progesterone and testosterone, but not estradiol, were associated with day-to-day changes in self-reported pain severity. Both progesterone and testosterone were inversely associated with pain, with peaks of those hormones occurring on days with lower reported pain. Self-reported fibromyalgia pain was lowest in the mid-luteal phase, corresponding to high progesterone and moderate estradiol and testosterone levels. Pain was highest during the menstrual phase, when all sex hormones are at low levels. These results are consistent with previous reports that women with chronic pain show higher pain sensitivity in the menstrual phase.16
Our results largely agree with previous research. Decades of research have shown that sex hormones affect pain processing in animal models.11,39,41, 46 Not only do rodent and other animal models show greater pain sensitivity in females, but that sensitivity can be reliably affected with experimental manipulation of sex hormones.22 Large studies of humans have similarly identified relationships between sex hormones and pain. De Kruijf and colleagues reported in a large, population study of 9717 participants that lower levels of sex hormones (estrogen, testosterone, androstenedione, and 17-hydroxyprogesterone) were associated with the prevalence and incidence of chronic musculoskeletal pain.19 Another cross-sectional study of 188 women using hormonal contraceptives found that progestin-only contraceptive users had a higher pain tolerance than participants receiving a combined hormonal contraceptive.24 Our results do diverge from a previous study29 examining sex hormones and fibromyalgia pain in 74 women completing 3 laboratory sessions across a menstrual cycle. In that study, no significant relationship between sex hormones and pain threshold or tolerance was found. It is possible that our use of 25 consecutive days of sampling gave us greater power to detect mild-to-moderate relationships between hormones and pain.
We also observed that sex hormones may interact with other hormones to influence pain. Low progesterone was associated with higher pain, but particularly so during times of high cortisol (Figure 3). There are likely many more interactions of interest that could be further explored with this research model, including adrenal and thyroid hormones, neuropeptides, neurotransmitters, cytokines, and other factors. Using the same daily sampling approach, we have previously reported inflammatory and hormonal drivers of fatigue in chronic fatigue syndrome.42 While that study was conducted in chronic fatigue syndrome participants, many such individuals also meet criteria for fibromyalgia.38 It is possible that an optimized set of analytes could predict the majority of pain fluctuations in fibromyalgia. Comprehensively measuring chemistry related to pain is outside the scope of this pilot study, but is of great interest for future research. Daily sampling, while intensive and potentially burdensome on participants, can provide unique data to explore psychophysiological relationships.
While we found significant effects for progesterone and testosterone, we did not observe an effect for estradiol. It is possible that the effects of estradiol, which is preceded by an increase of testosterone and co-occurs with an increase of progesterone, cannot easily be temporally separated from the effects of other hormones. As noted in the introduction, the relationship between estrogens and pain is complex and unresolved, showing both pro-algesic and analgesic properties in the literature. Polymorphisms in estrogen receptors likely need to be closely studied13 as well as the differential effects of the various estrogen metabolites.33 It is also possible that the impact of sex hormones on pain will differ between men and women.
The change of pain associated with sex hormones may be clinically significant, as the progesterone peak is associated with 25.6% lower pain severity. It is unknown if the results from this and other studies indicate that manipulation of sex hormones may be used to modulate fibromyalgia pain. There is little experimental evidence in chronic pain conditions on this topic, and exogenous modulators of sex hormones could act differently than normally cycling hormones. We noted cases where sex hormones were administered systemically44 or locally32 to reduce pain and/or inflammation, and the use of sex hormones in treating autoimmune pain disorders is still a topic of interest.17 Effects of administered sex hormones on pain has also been studied in the context of transsexual individuals receiving hormone treatment. In Aloisi and colleagues’ study3 assessing varied chronic pain conditions, 55% of female-to-male transsexual individuals with chronic pain reported a reduction of pain after testosterone treatment (none reported increased pain). In male-to-female individuals receiving estrogen, 23% reported initiation of chronic pain after estrogen and anti-androgen therapy and another 18% reported a greater sensitivity to pain. This evidence collectively suggests that testosterone can reduce pain severity.
A few limitations should be discussed. One limitation of the study was its observational approach. While animal studies demonstrate that sex hormones have an effect on nociception and pain behavior, very little experimental research similar research has been conducted in humans, especially in the context of chronic pain.12 Because all women showed the canonical sex hormone cycle, it is more likely that sex hormones influenced pain rather than vice-versa, but this study cannot definitively determine causation. It is also important to note that we did not observe any dysregulated sex hormones in this small sample. Consistent with previous studies29,36,1,6 sex hormones appear normal in women with fibromyalgia. There is no evidence that sex hormones are part of fibromyalgia pathology. Rather, sex hormones may be a moderator of pain in fibromyalgia. These effects are likely not unique to fibromyalgia, and could be studied in other chronic pain conditions.
The subject size of eight women is limited. While the study involves 200 independent laboratory visits, and therefore sufficient statistical power for our tests, using only eight subjects means we cannot confidentially generalize results to the entire fibromyalgia population. Replication in an independent and larger sample will be required. Collecting samples over 28 days instead of 25 days would also have been preferable, allowing more complete coverage of a full menstrual cycle. Collection was stopped at 25 days to avoid requiring laboratory visits over a fourth weekend, which is particularly burdensome for participants and staff. It is also possible that more sensitive or comprehensive measures of the primary pain outcome could be used, such as the pain intensity scale of the Brief Pain Inventory. Our single-item pain severity marker does not have demonstrated reliability and validity. The study may have also been strengthened by including a healthy control group. However, healthy women who have completed self-report measures in our protocol show insufficient variability of pain (often reporting 0 pain throughout) to allow correlational analyses between sex hormones and pain to be computed. Another limitation is that participant #8 returned a C-reactive protein result of 34.2 mg/L at baseline, which could indicate an inflammatory disorder or infection. The individual did not meet exclusionary criteria for the study and was retained in the analyses. There are likely important individual differences in the relationship between sex hormones and pain, especially as several participants showed no observable relationship between their hormone levels and pain. With a small sample size, we cannot account for those individual differences, and will explore possible fibromyalgia subgroups in future studies.
There are several additional questions regarding sex hormones and pain that could be answered with future applications of this study design. We are interested to know if sex hormones predict pain levels in post-menopausal women, and women taking oral contraceptives. It would also be interesting to examine other pain syndromes that can exhibit day-to-day fluctuations in severity. And we should determine if sex hormones can drive pain severity in men with fibromyalgia. Future studies may also examine the relationship of sex hormones on other aspects of fibromyalgia, such as fatigue and cognitive complaints. We conclude that this research adds to the strong literature showing a relationship between sex hormones and pain4, and suggest that those relationships have real-world consequences for individuals with chronic pain disorders. Sex hormones may be an important target for successfully managing some chronic pain conditions.
Highlights.
Relationships between sex hormones and fibromyalgia were assessed daily.
Both progesterone and testosterone were associated with lower self-reported pain.
Estradiol fluctuations were not correlated with self-reported pain.
Pain was particularly elevated when progesterone was low and cortisol was high.
PERSPECTIVE.
Sex hormones fluctuate normally in women with fibromyalgia, but may still contribute to pain severity.
Acknowledgments
This study was carried out under a NIH R01 grant “R01 AI107655”. We thank Dr. Timothy Ness, M.D., Ph.D. (UAB Department of Anesthesiology and Perioperative Medicine) for reviewing all screening blood tests. We also thank Barbara Gower, Ph.D. and the Metabolism Core at UAB for conducting all sex hormone assays used in this study. Funds for sex hormone assays were provided by the Department of Psychology and College of Arts and Sciences at UAB. Finally, we thank research assistants Natasha Mehra, Cooper Bailey, and Skylar McMahan for their help collecting these data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Akkus S, Delibas N, Tamer MN. Do sex hormones play a role in fibromyalgia? Rheumatology. 2000;39:1161–1163. doi: 10.1093/rheumatology/39.10.1161. [DOI] [PubMed] [Google Scholar]
- 2.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloisi AM, Bachiocco V, Costantino A, Stefani R, Ceccarelli I, Bertaccini A, Meriggiola MC. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132:S60–67. doi: 10.1016/j.pain.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartley EJ, Palit S, Kuhn BL, Kerr KL, Terry EL, DelVentura JL, Rhudy JL. Natural variation on testosterone is associated with hypoalgesia in healthy women. Clin J Pain. 2015;31:730–739. doi: 10.1097/AJP.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 6.Bramwell BL. The role of sex hormones on fibromyalgia pain mediators. Int J Pharm Compd. 2010;14:193–199. [PubMed] [Google Scholar]
- 7.Cairns B, Gazerani P. Sex-related differences in pain. Maturitas. 2009;63:292–6. doi: 10.1016/j.maturitas.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Coulombe MA, Spooner MF, Gaumond I, Carrier JC, Marchand S. Estrogen receptors beta and alpha have specific pro- and anti-nociceptive actions. Neuroscience. 2011;184:172–182. doi: 10.1016/j.neuroscience.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 9.Coronel MF, Villar MJ, Brumovsky PR, Gonzalez SL. Spinal neuropeptide expression and neuropathic behavior in the acute and chronic phases after spinal cord injury: effects of progesterone administration. Peptides. 2017;88:189–195. doi: 10.1016/j.peptides.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Deng C, Gu Y-J, Zhang H, Zhang J. Estrogen affects neuropathic pain through upregulating N-methyl-D-asparate acid receptor 1 expression in the dorsal root ganglion of rats. Neural Regen Res. 2017;12:464–469. doi: 10.4103/1673-5374.202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fávaro-Moreira N, Okoti L, Furini R, Tambeli C. Gonadal hormones modulate the responsiveness to local β-blocker-induced antinociception in the temporomandibular joint of male and female rats. European Journal of Pain. 2014;19:772–780. doi: 10.1002/ejp.601. [DOI] [PubMed] [Google Scholar]
- 12.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. The Journal of Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K, Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome – the role of cortisol and alphaamylase. Psychoneuroendocrinology. 2016;63:68–77. doi: 10.1016/j.psyneuen.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Greaves E, Grieve K, Horne AW. Saunders PTK: Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J Clin Endocrinol Metab. 2014;99:E1738–E1743. doi: 10.1210/jc.2014-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez SL, Coronel MF. Beyond reproduction: the role of progesterone in neuropathic pain after spinal cord injury. Neural Regen Res. 2016;11:1238–1240. doi: 10.4103/1673-5374.189177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellström B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Perceptual and Motor Skills. 2003;96:201–211. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nature Reviews Rheumatology. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152:1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kruijf M, Stolk L, Zillikens MC, de Rijke YB, Bierma-Zeinstra SM, Hofman A, Huygen FJ, Uitterlinden AG, van Meurs JB. Lower sex hormone levels are associated with more chronic musculoskeletal pain in community-dwelling elderly women. Pain. 2016;157:1425–1431. doi: 10.1097/j.pain.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SS, Hutchinson MR, Frick MM, Zhang Y, Maier SF, Sammakia T, Rice KC, Watkins LR. Select steroid hormone glucuronide metabolites can cause Toll-like receptor 4 activation and enhanced pain. Brain Beh Immun. 2015;44:128–136. doi: 10.1016/j.bbi.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Li W, Dai L, Zhang T, Xia W, Liu H, Ma K, Xu J, Jin Y. Early repeated administration of progesterone improved the recovery of neuropathic pain and modulates spinal 18 kDA-translocator protein (TSPO) expression. Journal of Steroid Biochemistry and Molecular Biology. 2014;143:130–140. doi: 10.1016/j.jsbmb.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Ching C, Wang S, Wu F. 17 -Estradiol Mediates the Sex Difference in Capsaicin-Induced Nociception in Rats. Journal of Pharmacology and Experimental Therapeutics. 2009;331:1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- 23.Macedo CG, Fanton LE, Fishcer L, Tambeli CH. Coactivation of mu- and kappa-opioid receptors may mediate the protective effect of testosterone on the develop of temporomandibular joint nociception in male rats. J Oral Facial Pain Headache. 2016;30:61–7. doi: 10.11607/ofph.1298. [DOI] [PubMed] [Google Scholar]
- 24.Máximo MM, Silva PS, Vieira CS, Gonçalvez TM, Rosa-E-Silva JC, Candido-Dos-Reis FJ, Nogueira AA, Poli-Neto OB. Low-dose progestin-releasing contraceptives are associated with a higher pain threshold in healthy women. Fertility and Sterility. 2015;104:1182–1189. doi: 10.1016/j.fertnstert.2015.07.1165. [DOI] [PubMed] [Google Scholar]
- 25.Mcleod JD. Juvenile Fibromyalgia Syndrome and Improved Recognition by Pediatric Primary Care Providers. Journal of Pediatric Health Care. 2014;28:9–18. doi: 10.1016/j.pedhc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Naderi A, Asgari AR, Zahed R, Ghanbari A, Samandari R, Jorjani M. Estradiol attenuates spinal cord injury-related central pain by decreasing glutamate levels in thalamic VPL nucleus in male rats. Metab Brain Dis. 2014;29:763–770. doi: 10.1007/s11011-014-9570-z. [DOI] [PubMed] [Google Scholar]
- 28.de Nicola AF, Garay LI, Meyer M, Guennoun R, Sitruk-Ware R, Schumacher M. Deniselle MCG: Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J Neuroendocrinol. doi: 10.1111/jne.12502. [2017, Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Okifuji A, Turk DC. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. J Pain. 2006;7:851–859. doi: 10.1016/j.jpain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Pieretti S, Giannuario AD, Givannandrea RD, Marzoli F, Piccaro G, Minosi P, Aloisi AM. Gender differences in pain and its relief. Annali dell'Istituto Superiore di Sanità. 2016;52:184–189. doi: 10.4415/ANN_16_02_09. [DOI] [PubMed] [Google Scholar]
- 31.Qu Z-W, Liu T-T, Ren C, Gan X, Qiu C-Y, Ren P, Rao Z, Hu W-P. 17beta-estradiol enhances ASIC activity in primary sensory neurons to produce sex difference in acidosis-induced nociception. Endocrinology. 2015;156:4660–4671. doi: 10.1210/en.2015-1557. [DOI] [PubMed] [Google Scholar]
- 32.Raeissadart SA, Shahraeeni S, Sedighipour L, Vahdatpour B. Randomized controlled trial of local progesterone vs corticoid injection for carpal tunnel syndrome. Acta Neurol Scand. 2017;2017:1–7. doi: 10.1111/ane.12739. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro-Dasilva MC, Peres Line SR, Leme Godoy dos Santos MC, Arthuri MT, Hou W, Fillingim RB, Rizzatti Barbosa CM. Estrogen Receptor-α Polymorphisms and Predisposition to TMJ Disorder. J Pain. 2009;10:527–533. doi: 10.1016/j.jpain.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. Journal of Neuroscience Research. 2017;95:500–508. doi: 10.1002/jnr.23831. [DOI] [PubMed] [Google Scholar]
- 35.Saghaei E, Abbaszadeh F, Naseri K, Ghorbanpoor S, Afhami M, Haeri A, Rahimi F, Jorjani M. Estradiol attenuates spinal cord injury-induced pain by suppressing microglial activation in thalamic VPL nuclei of rats. Neuroscience Research. 2013;75:316–323. doi: 10.1016/j.neures.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Samborski W, Sobieska M, Peita P, Drews K, Brzosko M. Normal profile of sex hormones in women with primary fibromyalgia. Ann Acad Med Stetin. 2005;51:23–26. [PubMed] [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller W, Hergueta T, Baker R, Dunbar GC. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–23. [PubMed] [Google Scholar]
- 38.Shipley M. Chronic widespread pain and fibromyalgia syndrome. Medicine. 2014;42:271–273. [Google Scholar]
- 39.Shivers K, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary– adrenal axis activity. Cytokine. 2015;72:121–129. doi: 10.1016/j.cyto.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta J-K. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorge RE, Totsch SK. Sex Differences in Pain. Journal of Neuroscience Research. 2017;95:1271–1281. doi: 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 42.Stringer EA, Baker KS, Carroll IR, Montoya JG, Chu L, Maecker HT, Younger JW. Daily cytokine fluctuations, driven by leptin, are associated with fatigue severity in chronic fatigue syndrome: evidence of inflammatory pathology. Journal of Translational Medicine. 2013;11:93. doi: 10.1186/1479-5876-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussaint L, Vincent A, Mcallister SJ, Whipple M. Intra- and Inter-Patient Symptom Variability in Fibromyalgia: Results of a 90-Day Assessment. Musculoskeletal Care. 2014;13:93–100. doi: 10.1002/msc.1090. [DOI] [PubMed] [Google Scholar]
- 44.White HD, Brown LA, Gyurik RJ, Manganiello PD, Robinson TD, Hallock LS, Lewis LD, Yeo KT. Treatment of pain in fibromyalgia patients with testosterone gel: Pharmacokinetics and clinical response. International Immunopharmacology. 2015;27:249–256. doi: 10.1016/j.intimp.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe F, Clauw DJ, Fitzcharles M, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care & Research. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 46.Vacca V, Marinelli S, Pieroni L, Urbani A, Luvisetto S, Pavone F. 17beta-estradiol counteracts neuropathic pain: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Scientific Reports. 2016;6:18980. doi: 10.1038/srep18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X, Yang Y, Zhang Y, Zhang X-M, Zhao Z-Q, Zhang Y-Q. Estrogen in the anterior cingulate cortex contributes to pain-related aversion. Cerebral Cortex. 2013;23:2190–2203. doi: 10.1093/cercor/bhs201. [DOI] [PubMed] [Google Scholar]
- 48.Yamagata K, Sugimura M, Yoshida M, Sekine S, Kawano A, Oyamaguchi A, Maegawa H, Niwa H. Estrogens exacerbate nociceptive pain via up-regulation of TRPV1 and ANO1 in trigeminal primary neurons of female rats. Endocrinology. 2016;157:4309–4317. doi: 10.1210/en.2016-1218. [DOI] [PubMed] [Google Scholar]
- 49.Yunus MB, Inanici F, Aldag JC, Mangold RF. Fibromyalgia in men: comparison of clinical features with women. The Journal of Rheumatology. 2000;27:485–490. [PubMed] [Google Scholar]
- 50.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]