Abstract
Black Band Disease (BBD) is a widely distributed and destructive coral disease that has been studied on a global scale, but baseline data on coral diseases is missing from many areas of the Arabian Seas. Here we report on the broad distribution and prevalence of BBD in the Red Sea in addition to documenting a bleaching-associated outbreak of BBD with subsequent microbial community characterization of BBD microbial mats at this reef site in the southern central Red Sea. Coral colonies with BBD were found at roughly a third of our 22 survey sites with an overall prevalence of 0.04%. Nine coral genera were infected including Astreopora, Coelastrea, Dipsastraea, Gardineroseris, Goniopora, Montipora, Pavona, Platygyra, and Psammocora. For a southern central Red Sea outbreak site, overall prevalence was 40 times higher than baseline (1.7%). Differential susceptibility to BBD was apparent among coral genera with Dipsastraea (prevalence 6.1%), having more diseased colonies than was expected based on its abundance within transects. Analysis of the microbial community associated with the BBD mat showed that it is dominated by a consortium of cyanobacteria and heterotrophic bacteria. We detected the three main indicators for BBD (filamentous cyanobacteria, sulfate-reducing bacteria (SRB), and sulfide-oxidizing bacteria (SOB)), with high similarity to BBD-associated microbes found worldwide. More specifically, the microbial consortium of BBD-diseased coral colonies in the Red Sea consisted of Oscillatoria sp. (cyanobacteria), Desulfovibrio sp. (SRB), and Arcobacter sp. (SOB). Given the similarity of associated bacteria worldwide, our data suggest that BBD represents a global coral disease with predictable etiology. Furthermore, we provide a baseline assessment of BBD disease prevalence in the Red Sea, a still understudied region.
Keywords: Coral reef, Coral disease, Red Sea, Coral bleaching, Microbiology, Metabarcoding
Introduction
The rise of coral disease outbreaks contributes to the decline of coral reefs globally (Cróquer & Weil, 2009; Harvell et al., 2009; Hoegh-Guldberg, 2012; McLeod et al., 2010; Randall & Van Woesik, 2015) and coral disease appears to be the most destructive factor on many reefs. For instances, the Caribbean has been named a “disease hot spot” due to the fast emergence, high prevalence, and virulence of coral diseases in this region (Rosenberg & Loya, 2013). Coral disease outbreaks in the last decades in the Caribbean have resulted in significant losses in coral cover, diversity, and habitat (Aronson & Precht, 2001; Bruckner, 2002; Hughes, 1994; Precht et al., 2016; Weil, 2002). Following the mass-bleaching event in 2005 in the US Virgin islands, coral disease outbreaks reduced coral cover by more than 50% (Cróquer & Weil, 2009; Miller et al., 2009).
Coral diseases were first reported in the Caribbean in the 1970s, including black band disease (BBD), which is considered the most studied coral disease due to its widespread occurrence on reefs around the world (Bourne, Muirhead & Sato, 2011; Richardson, 2004). Black band disease has been reported from reefs throughout the Caribbean, the Indo-Pacific regions, the Red Sea, and the Great Barrier Reef (Al-Moghrabi, 2001; Dinsdale, 2002; Green & Bruckner, 2000; Kaczmarsky, 2006; Lewis et al., 2017; Montano et al., 2012; Page & Willis, 2006; Sutherland, Porter & Torres, 2004; Weil et al., 2012). BBD is the first described coral disease (Antonius, 1973), affecting scleractinian and gorgonian corals (Green & Bruckner, 2000; Sutherland, Porter & Torres, 2004; Weil, 2004). BBD prevalence generally is considered low (Dinsdale, 2002; Edmunds, 1991; Weil, 2002); however, this disease is a serious threat to coral reef ecosystems worldwide due to its persistence, leading to coral mortality in the long-term (Bruckner & Bruckner, 1997; Green & Bruckner, 2000; Kaczmarsky, 2006; Kuta & Richardson, 1996; Page & Willis, 2006; Sutherland, Porter & Torres, 2004; Zvuloni et al., 2009). Susceptibility to BBD differs between coral taxa and may result in long-term changes to coral community structure (Bruckner & Bruckner, 1997; Page & Willis, 2006). The abundance of BBD is affected by several environmental factors, including seawater temperature, water depth, solar irradiance, host population diversity, and anthropogenic nutrients (Al-Moghrabi, 2001; Kaczmarsky, 2006; Kuta & Richardson, 2002; Montano et al., 2013). Interestingly, seasonal temperatures influence BBD prevalence, with increased virulence during warmer summer months (Richardson & Kuta, 2003; Rützler & Santavy, 1983; Willis, Page & Dinsdale, 2004), as for example in the Maldives where sea surface temperatures above 28 °C promoted BBD infections (Montano et al., 2013).
BBD manifests as a dark band that migrates across the coral colony at a rate of >1 cm/day (Richardson, 1998) leaving behind bare skeleton. The base of the BBD mat is anoxic and high in sulfide levels, causing damage and necrosis to coral tissue (Ainsworth et al., 2007; Carlton & Richardson, 1995; Richardson et al., 1997). The BBD mat is composed of a polymicrobial consortium, dominated by filamentous cyanobacteria, sulfate-reducing bacteria (SRB), including members of Desulfovibrio spp., sulfide-oxidizing bacteria (SOB) (Beggiatoa spp.), and other heterotrophic bacteria (Cooney et al., 2002; Miller & Richardson, 2011; Sato, Willis & Bourne, 2010). As a result of diel light changes, the microbial members of the BBD mat undergo vertical migrations, which causes the harmful microenvironment on top of the coral tissue (Carlton & Richardson, 1995; Miller & Richardson, 2011; Richardson, 1996). Oxygen depletion and high sulfide concentrations are produced by SRB, which is lethal to the coral tissues and considered the most important factor in BBD pathogenicity (Glas et al., 2012; Richardson, 1996; Richardson et al., 1997; Richardson et al., 2009). Although the functional composition of the BBD mat is conserved, the diversity of the microbial consortium in BBD differs according to geographic location and coral species (Cooney et al., 2002; Frias-Lopez et al., 2004; Sekar et al., 2006).
The occurrence of BBD in the Red Sea was first recorded by Antonius (1988) where the severity of BBD was measured from rare to moderate and mostly correllated with elevated temperatures and seawater pollution. However, baseline data on BBD prevalence in the Red Sea is still lacking. To fill this gap, we conducted surveys to determine the distribution and prevalence of BBD across central Red Sea reefs spanning 4 degrees of latitude. We also detected a bleaching-associated outbreak of BBD on a coral reef in the southern central Red Sea and characterized the microbial community of BBD microbial mats from Coelastrea sp., Dipsastraea sp., Goniastrea sp., and Platygra sp. using high-throughput sequencing. We compared the microbial consortium to that reported from other regions of the world in order to identify biogeographic patterns in the main BBD consortium members.
Material and Methods
Black band disease surveys
Coral community structure and BBD prevalence was recorded at 22 sites spanning approx. 535 km along the coast of Saudi Arabia in the Red Sea (Fig. 1, Table 1). At least six reefs per region (Yanbu, Thuwal, Al-Lith) were surveyed with three additional reefs in Thuwal and one reef in Jeddah (80 km from Thuwal) that were surveyed as time permitted. The reefs sampled/assessed in this study do not fall under any legislative protection or special designation as a marine/environmental protected area. Under the auspices of KAUST (King Abdullah University of Science and Technology), the Saudi Coastguard Authority issued sailing permits to the sites that include coral collection. At each site, divers counted coral colonies by genera along two replicate belt transects (25 m × 1 m). At the same time, point-intercept method was used to characterize the substrate at 25 cm intervals. All corals with BBD lesions were identified along wider 25 × 6 m transects and photographed. Depending on depth and time limits, the length of transects were adjusted as necessary. Survey sites ranged in depth between 3 and 7.6 m and all surveys were conducted from 19 October to 3 November 2015. The diver surveys were used to determine average percent coral cover, coral community composition, and colony densities. Underwater time constraints prevented counting all colonies within the larger 25 × 6 m belts surveyed for disease. Therefore, BBD prevalence was estimated by calculating the average colony density (by genus) within the 25 × 1 m transect and then extrapolating the colony counts to the wider 25 × 6 m disease survey area and using this as the denominator of prevalence calculations, i.e., (number of colonies with BBD lesions/total number of estimated colonies) * 100) (Aeby et al., 2015a). At the outbreak site, diseased coral colonies were so numerous that only 49 m2 of the transect could be surveyed. The frequency of disease occurrence (FOC) was calculated by dividing the number of sites having corals with BBD lesions by the total number of sites surveyed. At the localized BBD outbreak site a chi-square goodness-of-fit test was used to examine differential distribution of the number of BBD versus healthy colonies among the coral genera affected by the disease. The chi-square test compares the observed vs. expected number of infected colonies based on the abundance of each coral genus in the field. Statistical analysis was performed using JMP statistical software (v. 10.0.2, SAS Institute Inc., Buckinghamshire, UK).
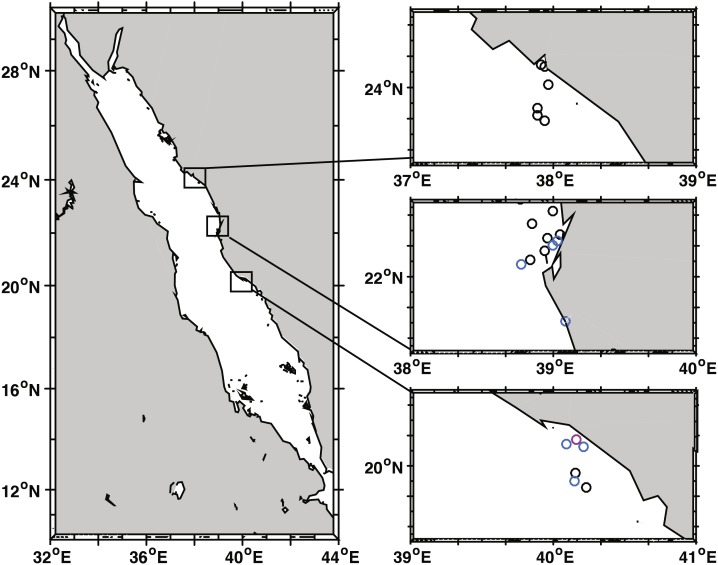
Figure 1. Black band disease survey locations of 22 reef sites along the central Red Sea coast of Saudi Arabia.
Survey points marked for Yanbu region (north), Thuwal (central), and Al-Lith (south). Sites without black band diseased coral colonies marked in black, sites with one or two diseased colonies in blue, and the site where a localized outbreak of BBD was observed is marked in pink.
Table 1. Survey of black band disease (BBD)-affected coral colonies at 22 reef sites in the central Red Sea. Coral genus counts denote number of BBD-affected colonies.
| Region | Reef site | GPS (latitude, longitude) | Depth (m) | Area colony count survey (m2) | Area BBD survey (m2) | Montipora | Dipsastraea | Psammocora | Gardineroseris | Astreopora | Pavona | Platygyra | Coelastreaa | Gonioporaa | Total no. of BBD | Total no. surveyed | BBD prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yanbu | Marker 32 | 23.8664, 37.8913 | 4 | 25 | 75.6 | 0 | 726 | 0 | |||||||||
| Marker 35 | 23.8207, 37.9350 | 4.6 | 25 | 78 | 0 | 930 | 0 | ||||||||||
| Abu Galaba | 23.7891, 37.9393 | 3 | 21 | 61.8 | 0 | 848 | 0 | ||||||||||
| Fringing reef 1 | 24.1362, 37.9396 | 5.2 | 25 | 150 | 0 | 1,308 | 0 | ||||||||||
| Marker 10 | 24.0189, 37.9666 | 4.6 | 25 | 126 | 0 | 1,749 | 0 | ||||||||||
| Fringing reef 2 | 24.1452, 37.9149 | 4.6 | 25 | 150 | 0 | 1,830 | 0 | ||||||||||
| Thuwal | Abu Madafi | 22.0766, 38.7751 | 4 | 25 | 300 | 1 | 1 | 2,184 | 0.05 | ||||||||
| Al Fahal | 22.1119, 38.8411 | 4.6 | 23.5 | 300 | 0 | 6,000 | 0 | ||||||||||
| Al-Mashpah | 22.0772, 38.7744 | 6.7 | 25 | 300 | 0 | 4,200 | 0 | ||||||||||
| Inner Fsar | 22.2358, 39.0304 | 4.6 | 25 | 300 | 1 | 1 | 6,852 | 0.01 | |||||||||
| Shaab | 22.2012, 38.9992 | 4.6 | 25 | 300 | 1 | 1 | 5,778 | 0.02 | |||||||||
| Shi’b Nazar | 22.3409, 38.8521 | 4.9 | 23.5 | 300 | 0 | 3,294 | 0 | ||||||||||
| Tahlah | 22.2750, 39.0497 | 5.2 | 25 | 300 | 0 | 3,780 | 0 | ||||||||||
| Qita al Kirsh | 22.4257, 38.9957 | 4.6 | 25 | 300 | 0 | 5,748 | 0 | ||||||||||
| Um Alkthal | 22.1653, 38.9391 | 7.6 | 25 | 300 | 0 | 5,208 | 0 | ||||||||||
| Jeddah | La Plage | 21.7092, 39.0832 | 4.6 | 25 | 300 | 1 | 1 | 474 | 0.21 | ||||||||
| Al-Lith | Abu Lath | 19.9554, 40.1543 | 5.5 | 20 | 240 | 0 | 5,556 | 0 | |||||||||
| South Reef | 19.8985, 40.1514 | 3.7 | 20 | 240 | 1 | 1 | 3,720 | 0.03 | |||||||||
| Al-Lith 3 | 19.8608, 40.2282 | 5.5 | 20 | 240 | 0 | 4,320 | 0 | ||||||||||
| Qita Al Kirsh | 20.1407, 40.0931 | 3 | 20 | 240 | 1 | 1 | 6,588 | 0.02 | |||||||||
| Fringing reef 1 | 20.1732, 40.1613 | 4.5 | 20 | 49 | 1 | 15 | 2 | 1 | 1 | 1 | 1 | 1 | 23 | 1,281 | 1.72 | ||
| Whaleshark reef | 20.1230, 40.2118 | 1.8 | 25 | 150 | 1 | 1 | 2 | 1,716 | 0.12 |
Notes.
Colonies of Coelastrea and Goniopora were only found outside the survey area.
Sample collection of black band disease microbial mats and 16S rRNA gene sequencing
Microbial mats were collected from BBD infected coral genera (one colony of Coelastrea sp., two colonies of Dipsastraea sp., three colonies of Goniastrea sp., and one colony of Platygra sp.) at the site of the observed BBD outbreak (Al-Lith fringing reef 1) in November 2015. Microbial mats were siphoned off the coral surface with Pasteur pipettes and transferred into ziplock bags under water. Sample replication was limited by obtainable coral species on this reef site due to environmental conditions.
Samples were homogenized using bead-beating via TissuLyser II (Qiagen, Hilden, Germany) twice for 30 sec at 30 Hz, 20 µl of the homogenate were boiled in sterile Milli-Q water at 99 °C for 5 min and subsequently 1 µl was directly used as PCR template. To amplify the variable region 4 of the 16S rRNA gene, the following primers were used: 515F [5′TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCC GCGGTAA′3] and 806RB [5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG GGACTACNVGGGTWTCTAAT′3] (Apprill et al., 2015; Caporaso et al., 2012; Kozich et al., 2013). Primer sequences contained sequencing adaptor overhangs (underlined above; Illumina, San Diego, CA, USA). Triplicate PCRs were performed for all samples with 0.2 µM of each primer in a total reaction volume of 25 µL using the Qiagen Multiplex PCR Kit. The following cycling conditions were used: 95 °C for 15 min, followed by 27 cycles of 94 °C for 45 s, 50 °C for 60 s, 72 °C for 90 s, and a final extension step of 72 °C for 10 min. Amplification was checked visually via 1% agarose gel electrophoresis. Triplicate samples were pooled and cleaned with ExoProStar 1-Step (GE Healthcare, Little Chalfont, UK). An indexing PCR was performed on the cleaned samples to add Nextera XT indexing and sequencing adaptors (Illumina, San Diego, CA, USA) following the manufacturer’s protocol and followed by sample normalization and library pooling. 16S rRNA gene amplicon libraries were sequenced on the Illumina MiSeq platform using 2*300 bp overlapping paired-end reads with a 10% phiX control at the KAUST Bioscience Core Laboratory. Sequence data determined in this study are available under NCBI Bioproject ID: PRJNA436216.
Sequence data processing and bacterial community analysis
Processing of raw sequence data was conducted in mothur (version 1.36.1; Schloss et al., 2009). Using the ‘make.contigs’ command, sequence reads were joined into contigs. Contigs longer than 310 bp and ambiguously called bases were excluded from the analysis. Subsequently, sequences that occurred only once across the entire dataset (singletons) were removed. The number of distinct sequences were identified and counted, and the total number of sequences per sample was determined using the ‘count.seqs’ command.
The remaining sequences were aligned against SILVA database (release 119; (Pruesse et al., 2007). Sequences were pre-clustered allowing for up to a 2 nt difference between the sequences (Huse et al., 2010). Chimeras were removed using UCHIME as implemented in mothur (Edgar et al., 2011). Next, sequences were classified with Greengenes database (release gg_13_8_99; bootstrap = 60; McDonald et al., 2012), followed by the removal of chloroplast, mitochondria, Archaea, and eukaryote sequences. Further, we found three abundant bacterial families (Dermabacteraceae, Dietziaceae, Brevibacteriaceae) that were present in all disease samples and at high abundance in our negative control. The negative control was a sample containing water as a template for the PCR reaction. As these bacterial families are also known as kit/reagent/lab contaminants, they were excluded from the dataset (Salter et al., 2014). Some additional bacterial taxa that were found in high numbers in the negative control with low abundance in coral samples were excluded (Comamonadaceae, Halomonadaceae, Staphylococcaceae). For further analyses, sequences were subsampled to 7,328 sequences per sample, which is the lowest number of sequences in a sample, and then clustered into OTUs (Operational Taxonomic Units) at a 97% similarity cutoff. Reference sequences for each OTU were determined by the most abundant sequence (Data S1). Alpha diversity indices (i.e., Chao1 Chao, 1984, Simpson evenness, and Inverse Simpson Index Simpson, 1949) were calculated as implemented in mothur.
For detecting similarity of the three main microbial consortium members in BBD microbial mats to previously reported taxa from other studies, the representative sequences of the most abundant OTUs were BLASTed against the NCBI database (https://blast.ncbi.nlm.nih.gov) using a 98% similarity cutoff. Subsequently, our sequences were compared to matches of highly similar bacterial taxa by obtaining the respective coral species, their location, and colony health status. Furthermore, low abundant OTUs not previously reported from BBD, but with related properties to SRB, SOB, or Cyanobacteria were also BLASTed against the NCBI database.
16S rRNA sequences of SOB and SRB were aligned and neighbor-joining trees were constructed based on Jukes-Cantor model with MAFFT (Katoh, Rozewicki & Yamada, 2017; Kuraku et al., 2013). All positions containing gaps and missing data were excluded and phylogenetic trees were visualized using Archaeopteryx.js.
Results
Distribution and prevalence of black band disease on Red Sea reefs
We identified 30 coral genera within transects across 22 reef sites (Fig. 1) with an average density of 16 coral colonies / m2 (SE ± 1.2) and an average coral cover of 43.8% (SE ± 4.3). Colonies with BBD were found at 8 of 22 sites (Table 1, Fig. 1). Over all study sites, nine coral genera were infected and include Astreopora, Coelastrea, Dipsastraea, Gardineroseris, Goniopora, Montipora, Pavona, Platygyra, Psammocora. Approximately 74,090 colonies were examined for disease and overall BBD prevalence over all sites was low (0.04%) because most sites had no signs of BBD. At the sites where BBD occurred, seven of the eight sites had one to two colonies infected within survey areas (up to 300 m2) (avg. prevalence = 0.064%) and one site had a localized BBD outbreak (Al-Lith fringing reef 1) where 21 infected colonies were found within 49 m2 of the transect (prevalence = 1.7%) and an additional two colonies outside the transect (Table 1). At this site, 18 coral genera were found within transects, but only nine coral genera exhibited signs of disease suggesting differential BBD susceptibility among coral genera (x2 = 45.67 df = 6, P < 0.001). Dipsastraea appeared to be the most susceptible (prevalence = 6.1%) with more diseased colonies than expected based on its abundance within transects (Table 2). Dipsastraea represented 18.6% of the coral colonies within transects but 68.2% (15 of 22) of the BBD colonies.
Table 2. Survey of black band disease-affected coral genera at an outbreak site in the southern central Red Sea (Al-Lith fringing reef 1, Saudi Arabia).
| Coral species | No. of coral colonies/survey area (20 m2) | % of coral community | No. of BBD cases/survey area (49 m2) | Prevalence % |
|---|---|---|---|---|
| Astreopora | 23 | 4.24 | 1 | 1.8 |
| Coelastreaa | 0 | – | 1 | – |
| Dipsastraea | 101 | 18.63 | 15 | 6.1 |
| Gonioporaa | 0 | – | 1 | – |
| Montipora | 11 | 2.03 | 1 | 3.7 |
| Pavona | 5 | 0.92 | 1 | 8.2 |
| Platygyra | 28 | 5.17 | 1 | 1.5 |
| Psammocora | 28 | 5.17 | 2 | 2.9 |
| Other coral genera | 346 | 63.80 | 0 | 0 |
| Totala | 542 | 100 | 23 | 1.7 |
Notes.
Colonies of Coelastrea and Goniopora were only found outside the survey area and were not counted towards totals.
Bacterial community composition of black band disease microbial mats
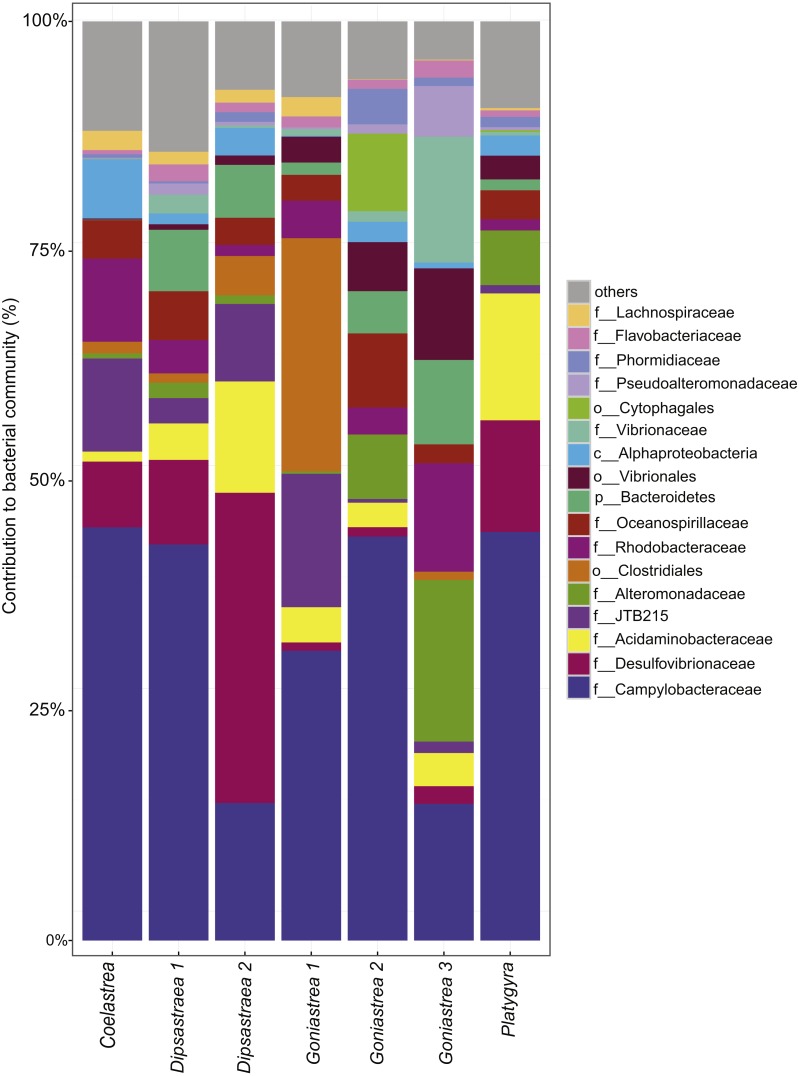
Besides the ecological survey of BBD prevalence, we investigated the microbial consortium of the BBD mat of corals from the outbreak site in the southern central Red Sea that was also subject to a bleaching event (Al-Lith fringing reef 1). We assessed whether the same bacterial taxa are associated with BBD in the Red Sea in comparison to other sites globally. Seven coral BBD microbial mat samples from the outbreak site included one colony of Coelastrea sp., two colonies of Dipsastraea sp., three colonies of Goniastrea sp., and one colony of Platygra sp., which together yielded 555,093 raw 16S rRNA gene sequences with a mean length of 298 bp (Table 3). After quality filtering and exclusion of chimeras and contaminant sequences, we retained 107,613 sequences for analysis of the BBD microbial mat microbiome. To assess bacterial community composition, sequences were classified to family level considering bacterial families that comprised >1% of the total sequence reads (Fig. 2). The presence of cyanobacteria, SRB, and SOB was confirmed but at varying abundance. For instance, Cyanobacteria such as Phormidiaceae ranged in proportion between 0 and 3.9%, SRB such as Desulfovibrionaceae between 0.9 and 33.8%, and SOB such as Campylobacteraceae between 15 to 45%. After subsampling to 7,328 sequences per sample, we found 351 distinct OTUs across the entire dataset (Data S1). Species richness (Chao1) and bacterial diversity (Inverse Simpson) were relatively similar between samples, ranging from 98 to 149 OTUs per sample (Table 3).
Table 3. Summary of sequencing information and alpha diversity measures of bacterial communities associated with black band disease microbial mats from coral colonies at an outbreak site in the southern central Red Sea (Al-Lith fringing reef 1, Saudi Arabia).
| Sample | No. of sequences | No. of OTUsa | Chao1a | Inv. Simpsona | Simpson evennessa |
|---|---|---|---|---|---|
| Coelastrea | 17,869 | 149 | 203 | 14.10 | 0.095 |
| Dipsastraea 1 | 7,328 | 146 | 240 | 22.33 | 0.153 |
| Dipsastraea 2 | 15,919 | 122 | 160 | 7.71 | 0.063 |
| Goniastrea 1 | 16,201 | 120 | 152 | 8.18 | 0.068 |
| Goniastrea 2 | 15,743 | 113 | 161 | 9.49 | 0.084 |
| Goniastrea 3 | 14,466 | 136 | 159 | 11.57 | 0.085 |
| Platygra | 20,037 | 98 | 125 | 8.50 | 0.087 |
Notes.
After subsampling to 7,328 sequences. Total number of OTUs: 315.
Figure 2. Bacterial community composition of black band disease microbial mats from four coral genera.
(one colony of Coelastrea, two colonies of Dipsastraea, three colonies of Goniastrea, and one colony of Platygyra) from an outbreak site in the southern central Red Sea (Al-Lith fringing reef 1, Saudi Arabia). Taxonomy stacked column plot on the phylogenetic level of family or to lowest resolved taxonomic level (f, family; o, order; p, phylum). Each color represents one of the 17 most abundant families. Remaining taxa are grouped under category ‘others’.
Black band disease representative bacterial consortia
We compared the sequences from representative bacterial BBD consortium members found in four coral genera in the southern central Red Sea to sequences obtained from other locations and coral taxa that were affected by BBD on a global scale. Coral disease microbial mat-associated OTUs that represent the three main bacterial consortium members in BBD were successfully identified in our samples:
Sulfide oxidizing bacteria (SOB)
Beggiatoa sp., a common BBD-SOB member was absent in our samples, despite microscopic white filaments in the disease lesions which suggested its presence. Another SOB-consortium member Arcobacter sp. was present in all samples, which has been associated previously with BBD and with white plague disease (WPD)(Sunagawa et al., 2009). The SOB-classified OTUs were the most abundant taxa in the dataset. Several OTUs were found to be associated with all coral genera (i.e., Coelastrea, Dipsastraea, Goniastrea, and Platygra) with proportions of up to 22.6% in all coral samples (OTU0001, 2, 4, 11, 16: all Arcobacter sp., OTU0010: Sulfurospirillum sp.). These SOB-associated OTUs were found to be similar to those found in different places around the world (e.g., Philippines (Garren et al., 2009) and in the Caribbean including the Netherlands Antilles (Klaus, Janse & Fouke, 2011), US Virgin Islands (Cooney et al., 2002), and Puerto Rico (Sunagawa et al., 2009) (Table 4), where they were associated with varying coral species (Fig. 3A).
Table 4. Summary of bacterial taxa (OTUs) associated with black band disease (BBD) in corals from the southern central Red Sea and comparison with similar taxa from around the world, based on BLAST results (accession number, identity) of the BBD consortium of sulfide-oxidizing bacteria (SOB), sulfate-reducing bacteria (SRB), cyanobacteria, Firmicutes, and Vibrio sp.
| OTU | Count | Taxonomy | Identity | GenBank Acc No. | Reference | Health state | Host & location |
|---|---|---|---|---|---|---|---|
| SOB | |||||||
| Otu0001 | 913 | Arcobacter sp. | 99% | EF089456 | Barneah et al. (2007) | BBD | Favites and Dipsastraea, Red Sea |
| KC527436 | Roder et al. (2014) | WPD | Pavona duerdeni and Porites lutea, West Pacific | ||||
| HM768631 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae, Caribbean | ||||
| Otu0002 | 625 | Arcobacter sp. | 99% | GU319311 | Meron et al. (2010) | Healthy | Acropora eurystoma, Red Sea |
| FJ203140 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean | ||||
| AB235414 | Yasumoto-Hirose et al. (2006) | – | Non-coral species | ||||
| Otu0004 | 336 | Arcobacter sp. | 99% | KT973145 | Couradeau et al. (2017) | – | Non-coral species |
| JF344171 | Acosta-González, Rosselló-Móra & Marqués (2012) | – | Non-coral species | ||||
| FJ949362 | Suárez-Suárez et al. (2011) | – | Non-coral species | ||||
| Otu0010 | 246 | Sulfurospirillum sp. | 98% | LC026456 | K Yamaki, F Mori, R Ueda, R Kondo, U Umezawa, H Nakata & M Wada (2015, unpublished data) | – | Non-coral species |
| AF473976 | Cooney et al. (2002) | BBD | Faviidae, Caribbean | ||||
| GU472074 | L Arotsker, D Rasoulouniriana, N Siboni, E Ben-Dov, E Kramarsky-Winter, Y Loya & A Kushmaro (2010, unpublished data) | BBD | – | ||||
| Otu0011 | 208 | Arcobacter sp. | 99% | HM768558 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, and Gorgoniidae, Caribbean |
| GQ413587 | Garren et al. (2009) | – | Porites cylindrica, West Pacific | ||||
| Otu0016 | 117 | Arcobacter sp. | 98% | LC133150 | S Iehata, Y Mizutani & R Tanaka (2016, unpublished data) | – | Non-coral species |
| HE804002 | C Chiellini, R Iannelli, F Verni & G Petroni (2012, unpublished data) | – | Non-coral species | ||||
| KF185679 | J Vojvoda, D Lamy, E Sintes, JA Garcia, V Turk & GJ Herndl (2013, unpublished data) | – | Non-coral species | ||||
| SRB | |||||||
| Otu0005 | 322 | Desulfovibrio dechloracetivorans | 98% | AB470955 | K Yoshinaga, BE Casareto & Y Suzuki (2008, unpublished data) | Healthy | Montipora sp., West Pacific |
| FJ202627 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean | ||||
| EF123510 | Sekar, Kaczmarsky & Richardson (2008) | BBD | Siderastrea siderea, Caribbean | ||||
| Otu0006 | 294 | Desulfovibrio marinisediminis | 99% | MF039931 | R Keren, A Lavy, I Polishchuk, B Pokroy & M Ilan (2017, unpublished data) | – | Non-coral species |
| KY771114 | H Zouch, F Karray, A Fabrice, S Chifflet, A Hirschler, H Kharrat, W Ben Hania, B Ollivier, S Sayadi & M Quemeneur (2017, unpublished data) | – | Non-coral species | ||||
| KT373805 | K Alasvand Zarasvand & VR Rai (2015, unpublished data) | – | Non-coral species | ||||
| Cyanobacteria | |||||||
| Otu0023 | 81 | Oscillatoria sp. | 99% | KU579394 | Buerger et al. (2016) | BBD | Pavona, Great Barrier Reef |
| HM768593 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae,Caribbean | ||||
| GU472422 | L Arotsker, D Rasoulouniriana, N Siboni, E Ben-Dov, E Kramarsky-Winter, Y Loya & A Kushmaro (2010, unpublished data) | BBD | – | ||||
| Firmicutes | |||||||
| Otu0003 | 344 | family JTB215 | 99% | DQ647593 | R Guppy & JC Bythell (2006, unpublished data) | – | – |
| KC527313 | Roder et al. (2014) | WPD | Pavona duerdeni and Porites lutea, Caribbean | ||||
| HM768569 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae, Caribbean | ||||
| Otu0013 | 199 | Fusibacter sp. | 99% | GQ413281 | Garren et al. (2009) | – | Porites cylindrica, West Pacific |
| FJ202930 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean | ||||
| Otu0018 | 112 | Fusibacter sp. | 99% | KF179748 | Séré et al. (2013) | PWPS | Porites lutea, Western Indian Ocean |
| GU472060 | L Arotsker, D Rasoulouniriana, N Siboni, E Ben-Dov, E Kramarsky-Winter, Y Loya & A Kushmaro (2010, unpublished data) | BBD | – | ||||
| EU780347 | SE Godwin, J Borneman, E Bent & L Pereg-Gerk (2008, unpublished data) | SWS | Turbinaria mesenterina, | ||||
| EF089469 | Barneah et al. (2007) | BBD | Favites, Dipsastraea, Red Sea | ||||
| Otu0022 | 82 | family Lachnospiraceae | 99% | HM768582 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae, Caribbean |
| 98% | AF473930 | Cooney et al. (2002) | BBD | Faviidae, Caribbean | |||
| DQ647585 | R Guppy & JC Bythell (2006, unpublished data) | – | – | ||||
| Otu0027 | 62 | Fusibacter sp. | 99% | JX391361 | YYK Chan, AL Li, S Gopalakrishnan, RSS Wu, SB Pointing & JMY Chiu (2012, unpublished data) | – | Non-coral species |
| HM768587 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae, Caribbean | ||||
| FJ202981 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean | ||||
| Otu0031 | 48 | WH1-8 sp. | 99% | KF179804 | Séré et al. (2013) | PWPS | Porites lutea, Western Indian Ocean |
| KC527300 | Roder et al. (2014) | WPD | Pavona duerdeni and Porites lutea, West Pacific | ||||
| FJ203165 | Sunagawa et al. (2009) | WPD | Faviidae, Caribbean | ||||
| Otu0039 | 30 | Defluviitalea saccharophila | 99% | DQ647556 | R Guppy & JC Bythell (2006, unpublished data) | – | – |
| FJ202907 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean | ||||
| AF473925 | Cooney et al. (2002) | BBD | Faviidae, Caribbean | ||||
| Vibriosp. | |||||||
| Otu0015 | 170 | Vibrio sp. | 99% | KT974549 | Couradeau et al. (2017) | – | Non-coral species |
| 98% | GU471972 | L Arotsker, D Rasoulouniriana, N Siboni, E Ben-Dov, E Kramarsky-Winter, Y Loya & A Kushmaro (2010, unpublished data) | BBD | – | |||
| 98% | MF461384 | Keller-Costa et al. (2017) | – | Eunicella labiata | |||
| Otu0029 | 56 | order Vibrionales | 99% | JQ727003 | Witt, Wild & Uthicke (2012) | – | Non-coral species, GBR |
| HM768601 | Klaus, Janse & Fouke (2011) | BBD | Faviidae, Meandrinidae, Gorgoniidae, Caribbean | ||||
| FJ202558 | Sunagawa et al. (2009) | WPD | Orbicella faveolata, Caribbean |
Figure 3. Overview and phylogenetic relationship of coral black band disease bacterial consortium members from the southern central Red Sea (Al-Lith, Saudi Arabia) and other regions.
(A) Sulfide-oxidizing bacteria (SOB); (B) Sulfate-reducing bacteria (SRB). Phylogenetic trees were calculated using the neighbor-joining method, bootstrap values are indicated at the branches. The phylogenetic trees show NCBI accession numbers and sample name, health state of the coral species, host name, and region. Sequences from this study are in bold. The ‘*’ indicates that the bacterial species were not found in coral species.
Sulfate-reducing bacteria (SRB)
Two abundant OTUs were found to be associated with BBD samples. These OTUs were annotated to Desulfovibrio sp. (OTU0005, OTU0006) with abundance ranges of 0.01–30.7% in all coral samples. Similar SRB-OTUs were found in the Caribbean (Sekar, Kaczmarsky & Richardson, 2008; Sunagawa et al., 2009) and Japan in different coral species (e.g., Montipora sp., Orbicella faveolata, and Siderastrea sidereal, Table 4). OTU0005 (Desulfovibrio dechloracetivorans) clustered together with SRB previously found in corals diseased with WPD (Sunagawa et al., 2009) and BBD (Sekar et al., 2006), while OTU0006 (Desulfovibrio marinisediminis) clustered away (Fig. 3B), indicating that this is not a typical BBD consortium member.
Cyanobacteria
One cyanobacterium (OTU0023, Oscillatoria sp.) was found at proportions of up to 4% in our coral samples. Cyanobacteria of the same genus (99% sequence similarity) have previously been found in BBD infected Pavona sp. in the GBR (Buerger et al., 2016) and from other regions, e.g., the Caribbean (Casamatta et al., 2012), Hawaii (Aeby et al., 2015b), and Palau (Sussman, Bourne & Willis, 2006) (Table 4).
Others
Although not belonging to the three main BBD bacterial consortium members, Firmicutes have previously been reported in coral BBD (Barneah et al., 2007; Cooney et al., 2002; Klaus, Janse & Fouke, 2011). Members were also found in our dataset at proportions of up to 24.8% (OTU0003, OTU0009, OTU0013, OTU0018). Furthermore, the Firmicutes-associated OTUs in our data were similar to those found in Porites white patch syndrome (PWPS) (Séré et al., 2013) and WPD (Roder et al., 2014; Sunagawa et al., 2009) (Table 4).
We also retrieved sequences of Vibrio sp. (OTU0015, OTU0029) from our dataset, at proportions of up to 12.8%. These OTU sequences also had a high similarity (98–99%) to sequences from BBD and WPD (Klaus, Janse & Fouke, 2011; Sunagawa et al., 2009) (Table 4).
Discussion
In this study, we report on the distribution and prevalence of coral black band disease in the Red Sea. Our surveys ranged from 19.9 to 24.1 degrees of latitude and confirm the presence of BBD across the central Red Sea. Molecular characterization of the bacterial community identified the three main bacterial members of the disease consortium across coral species at a BBD outbreak site in the southern central Red Sea.
Black band disease distribution and prevalence in the Red Sea in comparison to other global sites
BBD is a global disease found in numerous regions, but its prevalence on coral reefs is generally low compared to other diseases such as white syndrome (WS) (Dinsdale, 2002; Edmunds, 1991; Page & Willis, 2006; Willis, Page & Dinsdale, 2004). The low prevalence recorded in this study is similar to levels reported elsewhere across the globe (Sutherland, Porter & Torres, 2004) with localized outbreaks of BBD also reported in the GBR (Sato, Bourne & Willis, 2009), Hawaii (Aeby et al., 2015b), Jamaica (Bruckner & Bruckner, 1997), Venezuela (Rodríguez & Cróquer, 2008), and the Red Sea (Al-Moghrabi, 2001). In the Red Sea, BBD was first discovered in the 1980s (Antonius, 1981) and our study confirms that BBD is a chronic threat to coral reefs in the Red Sea with localized outbreaks continuing to occur.
BBD is not a selective disease; multiple species and various levels of severity can affect colonies within and between coral species and across reefs (Bruckner, Bruckner & Williams, 1997; Dinsdale, 2002; Green & Bruckner, 2000; Peters, 1993). This was also observed in our study, where multiple species were infected, but with differences in prevalence among coral taxa. At the outbreak site, we found BBD prevalence to be highest in the genus Dipsastraea, which suggests that this genus may be an important host for BBD in the Red Sea. Our observations match previous reports and shows that this pattern is consistent through time (Antonius, 1985). Interestingly, although differential susceptibility to BBD among coral taxa has been found globally, the most vulnerable taxa differ by region. For example, in the Caribbean Montastraea/Orbicella are commonly infected (Bruckner & Bruckner, 1997; Porter et al., 2001), Montipora in Hawaii (Aeby et al., 2015b), and Acropora on the GBR (Page & Willis, 2006). It would be fruitful to examine the underlying defense mechanisms in the different coral taxa that lead to these differences in BBD occurrence.
BBD, climate change, and coral bleaching
The occurrence of BBD has been linked to elevated seawater temperatures (Boyett, Bourne & Willis, 2007; Kuta & Richardson, 2002; Muller & Van Woesik, 2011). The occurrence of a BBD outbreak during a bleaching event in the present study reflects previous reports from the Caribbean, where the positive correlation between bleaching events and BBD incidence was proposed first (Brandt & McManus, 2009; Cróquer & Weil, 2009). For instance, in the Florida Reef Tract, the prevalence of BBD increased from 0 to 6.7% following bleaching events in 2014 and 2015 (Lewis et al., 2017). Also, Cróquer & Weil (2009) found a significant linear correlation between coral bleaching and the prevalence of two other virulent diseases (yellow band disease and white plague) affecting Montastraea/Orbicella species. This further supports a strong relationship between bleaching events and the emergence of some coral diseases on a global scale. Understanding how climate change-related thermal anomalies and coral bleaching drive the emergence and virulence of coral diseases is essential for future research.
It has further been suggested that other anthropogenic activities, such as coastal pollution or ocean acidification, contribute to the increase of coral disease incidents (Jackson et al., 2001; Muller et al., 2017; Rosenberg & Ben-Haim, 2002). The surveyed outbreak area was adjacent to the outflow of a large aquaculture facility, which might have further aggravated the effects of the bleaching event due to increased nutrient availability (Roder et al., 2015; Ziegler et al., 2016). In comparison, other reefs in the Al-Lith area that were farther away from the coast displayed similar levels of bleaching, but BBD prevalence stayed at baseline levels in these locations. This suggests that bleaching alone was not the only factor that could have contributed to the BBD outbreak. The synergistic effects of high temperatures and nutrient pollution find further support in the Caribbean where BBD prevalence increased in reef sites with direct sewage input compared to control sites (Sekar, Kaczmarsky & Richardson, 2008) and in the Bahamas where BBD migration was faster in nutrient-enriched areas (Voss & Richardson, 2006). Further work is needed to directly examine the relationship between bleaching, nutrient stress, and BBD susceptibility.
Bacterial community composition of BBD microbial mats from the southern central Red Sea reflects global microbial patterns with local characteristics
Our results verify the presence of the three main consortium members in BBD microbial mats (Cyanobacteria, SOB, SRB) of corals from the southern central Red Sea. We identified Oscillatoria sp. as a BBD-associated cyanobacterium, which is similar to the BBD-associated cyanobacteria in other regions of the world (Aeby et al., 2015b; Arotsker et al., 2015; Buerger et al., 2016; Casamatta et al., 2012; Cooney et al., 2002; Frias-Lopez et al., 2003; Gantar, Sekar & Richardson, 2009; Glas et al., 2010; Meyer et al., 2016; Miller & Richardson, 2011; Rasoulouniriana et al., 2009; Sato, Willis & Bourne, 2010; Sussman, Bourne & Willis, 2006). However, we retrieved only a low number of cyanobacterial sequences, although cyanobacterial filaments were visually abundant in the sampled microbial mats, which could possibly be related to primer amplification bias. In addition, members of the SOB and SRB functional groups (Arcobacter sp. and Desulfovibrio sp., respectively) from BBD microbial mats in the southern central Red Sea were similar to those found in other BBD-affected corals worldwide (Barneah et al., 2007; Cooney et al., 2002; Klaus, Janse & Fouke, 2011; Sekar, Kaczmarsky & Richardson, 2008). This confirms that BBD-associated bacteria are not restricted to a specific coral species or region (Barneah et al., 2007; Cooney et al., 2002; Dinsdale, 2002; Frias-Lopez et al., 2003). Interestingly, we did observe white filaments within lesions that were morphologically similar to Beggiatoa, a sulfide-oxidizing bacterium associated with BBD in other regions (Cooney et al., 2002; Miller & Richardson, 2011; Sato, Willis & Bourne, 2010). However, we found no sequences aligning with Beggiatoa in our study. This suggests that either the white filaments were not Beggiatoa or that the methods used were not adequate to extract and identify Beggiatoa. Aeby et al. (2015b) sequenced Beggiatoa from BBD lesions in Hawaii by first culturing the white filaments from lesions and then using universal bacterial primers 8F and 1513R for sequencing. However, they found that no DNA sequences were available for Beggiatoa found in BBD from other regions even though numerous studies using molecular techniques have been published. Further work is needed to clarify these discrepancies.
Besides the three main bacterial consortium members that dominate BBD microbial mats, we detected other bacterial families as part of the BBD consortium. Members of the Firmicutes were abundant in BBD microbial mats, which is consistent with other studies (Arotsker et al., 2016; Arotsker et al., 2009; Barneah et al., 2007; Cooney et al., 2002; Frias-Lopez et al., 2002; Miller & Richardson, 2011; Richardson, 2004; Sekar, Kaczmarsky & Richardson, 2008). In addition, we detected the presence of Vibrio species. The pathogenicity of this genus has been documented previously in corals and other marine organisms (Ben-Haim, Zicherman-Keren & Rosenberg, 2003; Harvell et al., 1999; Kushmaro et al., 1996), and more broadly Vibrios have been characterized as opportunistic taxa (Cervino et al., 2004; Rosenberg & Falkovitz, 2004; Thompson et al., 2004; Ziegler et al., 2016). To date it is unknown whether this group plays a role in the etiology of BBD (Arotsker et al., 2009; Barneah et al., 2007) (Meyer et al., 2016), or whether the high number of Vibrios could be related to seasonal increases in the coral microbiome and coral bleaching (reviewed in Rosenberg & Koren, 2006; Tout et al., 2015).
Conclusions
Our study represents the first comprehensive assessment of Black Band Disease in the central Red Sea. Elucidation of the bacteria associated with BBD microbial mats of corals at a southern reef site confirms that BBD represents a disease with predictable etiology where the three main bacterial players are globally distributed with regional differences. Notably, our reef survey data, in line with data from other regions, identify BBD as a widespread disease, but as one with low prevalence in comparison to other coral diseases. Additional surveys including other coral diseases as well as pathogen infection experiments with Red Sea corals could further increase our understanding of coral stress tolerance in this understudied coral reef region. Importantly, the prevalence of BBD might increase with ongoing ocean warming and thermal anomalies, as supported by the here-documented disease outbreak coinciding with a thermal anomaly and widespread coral bleaching. The collection of long-term monitoring disease data in the Arabian Seas is important in order to establish baselines, which can then assist in more accurate prediction of disease prevalence and potential impact of climate change on coral communities in this region.
Supplemental Information
Acknowledgments
We would like to thank the KAUST Coastal and Marine Resources Core Lab (CMOR) for their assistance and support in field operations and the KAUST Bioscience Core Lab (BCL) for sequencing. We wish to thank Craig Michell (KAUST) for sequence library preparation and Nikolaos Zarokanellos (KAUST) for help with Fig. 1.
Funding Statement
Research reported in this publication was supported by baseline research funds to Christian R. Voolstra and Red Sea Research Center funded project FCC/1/1973-21-01 by KAUST. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Greta Aeby, Email: greta@hawaii.edu.
Christian R. Voolstra, Email: christian.voolstra@kaust.edu.sa.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ghaida Hadaidi performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Maren Ziegler conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Amanda Shore-Maggio performed the experiments, analyzed the data, approved the final draft.
Thor Jensen performed the experiments, approved the final draft.
Greta Aeby and Christian R. Voolstra conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
Sequence data determined in this study is available at NCBI under BioProject ID PRJNA436216. Abundant coral bacterial microbiome OTU reference sequences are available under GenBank Accession numbers MH341637–MH341689.
Data Availability
The following information was supplied regarding data availability:
The OTU abundance table is provided as a Data S1.
References
- Acosta-González, Rosselló-Móra & Marqués (2012).Acosta-González A, Rosselló-Móra R, Marqués S. Characterization of the anaerobic microbial community in oil-polluted subtidal sediments: aromatic biodegradation potential after the Prestige oil spill. Environmental Microbiology. 2012;15:77–92. doi: 10.1111/j.1462-2920.2012.02782.x. [DOI] [PubMed] [Google Scholar]
- Aeby et al. (2015a).Aeby GS, Tribollet A, Lasne G, Work TM. Assessing threats from coral and crustose coralline algae disease on the reefs of New Caledonia. Marine and Freshwater Research. 2015a;34:393–406. doi: 10.1071/MF14151. [DOI] [Google Scholar]
- Aeby et al. (2015b).Aeby GS, Work TM, Runyon CM, Shore-Maggio A, Ushijima B, Videau P, Beurmann S, Callahan SM. First record of black band disease in the Hawaiian archipelago: response, outbreak status, virulence, and a method of treatment. PLOS ONE. 2015b;10:e0120853. doi: 10.1371/journal.pone.0120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth et al. (2007).Ainsworth T, Kramasky-Winter E, Loya Y, Hoegh-Guldberg O, Fine M. Coral disease diagnostics: what’s between a plague and a band? Applied and Environmental Microbiology. 2007;73:981–992. doi: 10.1128/AEM.02172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Moghrabi (2001).Al-Moghrabi S. Unusual black band disease (BBD) outbreak in the northern tip of the Gulf of Aqaba (Jordan) Coral Reefs. 2001;19:330–331. doi: 10.1007/s003380000127. [DOI] [Google Scholar]
- Antonius (1973).Antonius A. New observations on coral destruction in reefs. Tenth meeting of the association of Island Marine Laboratories of the Caribbean; Mayaguez. 1973. [Google Scholar]
- Antonius (1981).Antonius A. Coral reef pathology: a review. In: Gomez ED, Birkeland CE, Buddemeier RW, Johannes RE, Marsh Jr JA, Tsuda RT, editors. Proceedings of the 4th international coral reef symposium, Vol. 2. Marine Science Center, University of the Philippines, Manila, Philippines; Waco. 1981. [Google Scholar]
- Antonius (1985).Antonius A. Coral diseases in the Indo-Pacific: a first record. Marine Ecology. 1985;6:197–218. doi: 10.1111/j.1439-0485.1985.tb00322.x. [DOI] [Google Scholar]
- Antonius (1988).Antonius A. Distribution and dynamics of coral diseases in the Eastern Red Sea. In: Choat JH, Barnes D, Borowitzka MA, Coll JC, Davies PJ, Flood P, Hatcher BG, Hopley D, Hutchings PA, Kinsey D, Orme GR, Pichon M, Sale PF, Sammarco P, Wallace CC, Wilkinson C, Wolanski E, Bellwood O, editors. Proceedings of the 6th international coral reef symposium: vol. 2: contributed papers. Townsville, Australia; Waco. 1988. pp. 293–298. [Google Scholar]
- Apprill et al. (2015).Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology. 2015;75:129–137. doi: 10.3354/ame01753. [DOI] [Google Scholar]
- Aronson & Precht (2001).Aronson RB, Precht WF. White-band disease and the changing face of Caribbean coral reefs. In: Porter JW, editor. The ecology and etiology of newly emerging marine diseases. Springer; Dordrecht: 2001. pp. 25–38. [Google Scholar]
- Arotsker et al. (2016).Arotsker L, Kramarsky-Winter E, Ben-Dov E, Kushmaro A. Microbial transcriptome profiling of black band disease in a Faviid coral during a seasonal disease peak. Diseases of Aquatic Organisms. 2016;118:77–89. doi: 10.3354/dao02952. [DOI] [PubMed] [Google Scholar]
- Arotsker et al. (2015).Arotsker L, Kramarsky-Winter E, Ben-Dov E, Siboni N, Kushmaro A. Changes in the bacterial community associated with black band disease in a Red Sea coral, Favia sp. in relation to disease phases. Diseases of Aquatic Organisms. 2015;116:47–58. doi: 10.3354/dao02911. [DOI] [PubMed] [Google Scholar]
- Arotsker et al. (2009).Arotsker L, Siboni N, Ben-Dov E, Kramarsky-Winter E, Loya Y, Kushmaro A. Vibrio sp. as a potentially important member of the Black Band Disease (BBD) consortium in Favia sp. corals. FEMS Microbiology Ecology. 2009;70:515–524. doi: 10.1111/j.1574-6941.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- Barneah et al. (2007).Barneah O, Ben-Dov E, Kramarsky-Winter E, Kushmaro A. Characterization of black band disease in Red Sea stony corals. Environmental Microbiology. 2007;9:1995–2006. doi: 10.1111/j.1462-2920.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- Ben-Haim, Zicherman-Keren & Rosenberg (2003).Ben-Haim Y, Zicherman-Keren M, Rosenberg E. Temperature-regulated bleaching and lysis of the coral pocillopora damicornis by the novel pathogen vibrio coralliilyticus. Applied and Environmental Microbiology. 2003;69:4236–4242. doi: 10.1128/aem.69.7.4236-4242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, Muirhead & Sato (2011).Bourne DG, Muirhead A, Sato Y. Changes in sulfate-reducing bacterial populations during the onset of black band disease. ISME Journal. 2011;5:559–564. doi: 10.1038/ismej.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett, Bourne & Willis (2007).Boyett HV, Bourne DG, Willis BL. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Marine Biology. 2007;151:1711–1720. doi: 10.1007/s00227-006-0603-y. [DOI] [Google Scholar]
- Brandt & McManus (2009).Brandt ME, McManus JW. Disease incidence is related to bleaching extent in reef-building corals. Ecology. 2009;90:2859–2867. doi: 10.1890/08-0445.1. [DOI] [PubMed] [Google Scholar]
- Bruckner & Bruckner (1997).Bruckner A, Bruckner R. The persistence of black band disease in Jamaica: impact on community structure. In: Lessios HA, Macintyre IG, editors. Proceedings of the 8th international coral reef symposium vol. 1. Smithsonian Tropical Research Institute, Panama; Waco. 1997. pp. 601–606. [Google Scholar]
- Bruckner, Bruckner & Williams (1997).Bruckner AW, Bruckner RJ, Williams JEH. Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bulletin of Marine Science. 1997;61:919–928. [Google Scholar]
- Bruckner (2002).Bruckner US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Servicehttps://www.fisheries.noaa.gov/resource/document/priorities-effective-management-coral-diseases Priorities for effective management of coral diseases. 2002
- Buerger et al. (2016).Buerger P, Alvarez-Roa C, Weynberg KD, Baekelandt S, Van Oppen MJ. Genetic, morphological and growth characterisation of a new Roseofilum strain (Oscillatoriales, Cyanobacteria) associated with coral black band disease. PeerJ. 2016;4:e2110. doi: 10.7717/peerj.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso et al. (2012).Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME journal. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton & Richardson (1995).Carlton RG, Richardson LL. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: black band disease of corals. FEMS Microbiology Ecology. 1995;18:155–162. doi: 10.1016/0168-6496(95)00052-C. [DOI] [Google Scholar]
- Casamatta et al. (2012).Casamatta D, Stanić D, Gantar M, Richardson LL. Characterization of Roseofilum reptotaenium (Oscillatoriales, Cyanobacteria) gen. sp. nov. isolated from Caribbean black band disease. Phycologia. 2012;51:489–499. doi: 10.2216/11-10.1. [DOI] [Google Scholar]
- Cervino et al. (2004).Cervino JM, Hayes RL, Polson SW, Polson SC, Goreau TJ, Martinez RJ, Smith GW. Relationship of Vibrio species infection and elevated temperatures to yellow blotch/band disease in Caribbean corals. Applied and Environmental Microbiology. 2004;70:6855–6864. doi: 10.1128/AEM.70.11.6855-6864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao (1984).Chao A. Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics. 1984;11:265–270. [Google Scholar]
- Cooney et al. (2002).Cooney RP, Pantos O, Tissier MDAL, Barer MR, O’Donnell AG, Bythell JC. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environmental Microbiology. 2002;4:401–413. doi: 10.1046/j.1462-2920.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- Couradeau et al. (2017).Couradeau E, Roush D, Guida BS, Garcia-Pichel F. Diversity and mineral substrate preference in endolithic microbial communities from marine intertidal outcrops (Isla de Mona, Puerto Rico) Biogeosciences. 2017;14:311. [Google Scholar]
- Cróquer & Weil (2009).Cróquer A, Weil E. Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Diseases of Aquatic Organisms. 2009;87:33–43. doi: 10.3354/dao02164. [DOI] [PubMed] [Google Scholar]
- Dinsdale (2002).Dinsdale E. Abundance of black-band disease on corals from one location on the Great Barrier Reef: a comparison with abundance in the Caribbean region. In: Moosa MK, Soemodihardjo S, Soegiarto A, Romimohtarto K, Nontji A, Soekarno, Suharsono, editors. Proceedings of the ninth international coral reef symposium, 23–27 October 2000; Waco. 2002. pp. 1239–1243. [Google Scholar]
- Edgar et al. (2011).Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds (1991).Edmunds PJ. Extent and effect of black band disease on a Caribbean reef. Coral Reefs. 1991;10:161–165. doi: 10.1007/bf00572175. [DOI] [Google Scholar]
- Frias-Lopez et al. (2003).Frias-Lopez J, Bonheyo GT, Jin Q, Fouke BW. Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Applied and Environmental Microbiology. 2003;69:2409–2413. doi: 10.1128/AEM.69.4.2409-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Lopez et al. (2004).Frias-Lopez J, Klaus JS, Bonheyo GT, Fouke BW. Bacterial community associated with black band disease in corals. Applied and Environmental Microbiology. 2004;70:5955–5962. doi: 10.1128/AEM.70.10.5955-5962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Lopez et al. (2002).Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Applied and Environmental Microbiology. 2002;68:2214–2228. doi: 10.1128/AEM.68.5.2214-2228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantar, Sekar & Richardson (2009).Gantar M, Sekar R, Richardson LL. Cyanotoxins from black band disease of corals and from other coral reef environments. Microbial Ecology. 2009;58:856–864. doi: 10.1007/s00248-009-9540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren et al. (2009).Garren M, Raymundo L, Guest J, Harvell CD, Azam F. Resilience of coral-associated bacterial communities exposed to fish farm effluent. PLOS ONE. 2009;4:e7319. doi: 10.1371/journal.pone.0007319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas et al. (2010).Glas MS, Motti CA, Negri AP, Sato Y, Froscio S, Humpage AR, Krock B, Cembella A, Bourne DG. Cyanotoxins are not implicated in the etiology of coral black band disease outbreaks on Pelorus Island, Great Barrier Reef. FEMS Microbiology Ecology. 2010;73:43–54. doi: 10.1111/j.1574-6941.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- Glas et al. (2012).Glas MS, Sato Y, Ulstrup KE, Bourne DG. Biogeochemical conditions determine virulence of black band disease in corals. The ISME journal. 2012;6:1526–1534. doi: 10.1038/ismej.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green & Bruckner (2000).Green EP, Bruckner AW. The significance of coral disease epizootiology for coral reef conservation. Biological Conservation. 2000;96:347–361. doi: 10.1016/S0006-3207(00)00073-2. [DOI] [Google Scholar]
- Harvell et al. (1999).Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus ADME, Overstreet RM, Porter JW, Smith GW, Vasta GR. Emerging marine diseases—climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Harvell et al. (2009).Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E. Climate change and wildlife diseases: when does the host matter the most? Ecology. 2009;90:912–920. doi: 10.1890/08-0616.1. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg (2012).Hoegh-Guldberg O. Coral reefs, climate change, and mass extinction. In: Hannah L, editor. Saving a million species: extinction risk from climate change. Island Press/Center for Resource Economics; Washington, D.C.: 2012. pp. 261–283. [Google Scholar]
- Hughes (1994).Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Huse et al. (2010).Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environmental Microbiology. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson et al. (2001).Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Kaczmarsky (2006).Kaczmarsky L. Coral disease dynamics in the central Philippines. Diseases of Aquatic Organisms. 2006;69:9–21. doi: 10.3354/dao069009. [DOI] [PubMed] [Google Scholar]
- Katoh, Rozewicki & Yamada (2017).Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2017 doi: 10.1093/bib/bbx108. Epub ahead of print Sep 6 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Costa et al. (2017).Keller-Costa T, Eriksson D, Gonçalves JMS, Gomes NCM, Lago-Lestón A, Costa R. The gorgonian coral Eunicella labiata hosts a distinct prokaryotic consortium amenable to cultivation. FEMS Microbiology Ecology. 2017;93:fix143–fix143. doi: 10.1093/femsec/fix143. [DOI] [PubMed] [Google Scholar]
- Klaus, Janse & Fouke (2011).Klaus JS, Janse I, Fouke BW. Coral black band disease microbial communities and genotypic variability of the dominant cyanobacteria (CD1C11) Bulletin of Marine Science. 2011;87:795–821. doi: 10.5343/bms.2010.1050. [DOI] [Google Scholar]
- Kozich et al. (2013).Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraku et al. (2013).Kuraku S, Zmasek CM, Nishimura O, Katoh K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Research. 2013;41:W22–W28. doi: 10.1093/nar/gkt389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmaro et al. (1996).Kushmaro A, Loya Y, Fine M, Rosenberg E. Bacterial infection and coral bleaching. Nature. 1996;380:396–396. doi: 10.1038/380396a0. [DOI] [Google Scholar]
- Kuta & Richardson (1996).Kuta KG, Richardson LL. Abundance and distribution of black band disease on coral reefs in the northern Florida keys. Coral Reefs. 1996;15:219–223. doi: 10.1007/bf01787455. [DOI] [Google Scholar]
- Kuta & Richardson (2002).Kuta K, Richardson L. Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs. 2002;21:393–398. doi: 10.1007/s00338-002-0261-6. [DOI] [Google Scholar]
- Lewis et al. (2017).Lewis CL, Neely KL, Richardson LL, Rodriguez-Lanetty M. Temporal dynamics of black band disease affecting pillar coral (Dendrogyra cylindrus) following two consecutive hyperthermal events on the Florida Reef Tract. Coral Reefs. 2017;36:427–431. doi: 10.1007/s00338-017-1545-1. [DOI] [Google Scholar]
- Meron et al. (2010).Meron D, Atias E, Iasur Kruh L, Elifantz H, Minz D, Fine M, Banin E. The impact of reduced pH on the microbial community of the coral Acropora eurystoma. The ISME Journal. 2010;5:51. doi: 10.1038/ismej.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald et al. (2012).McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod et al. (2010).McLeod E, Moffitt R, Timmermann A, Salm R, Menviel L, Palmer MJ, Selig ER, Casey KS, Bruno JF. Warming seas in the coral triangle: coral reef vulnerability and management implications. Coastal Management. 2010;38:518–539. doi: 10.1080/08920753.2010.509466. [DOI] [Google Scholar]
- Meyer et al. (2016).Meyer JL, Gunasekera SP, Scott RM, Paul VJ, Teplitski M. Microbiome shifts and the inhibition of quorum sensing by black band disease cyanobacteria. The ISME Journal. 2016;10:1204–1216. doi: 10.1038/ismej.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller & Richardson (2011).Miller AW, Richardson LL. A meta-analysis of 16S rRNA gene clone libraries from the polymicrobial black band disease of corals. FEMS Microbiology Ecology. 2011;75:231–241. doi: 10.1111/j.1574-6941.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2009).Miller J, Muller E, Rogers C, Waara R, Atkinson A, Whelan KRT, Patterson M, Witcher B. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs. 2009;28:925–937. doi: 10.1007/s00338-009-0531-7. [DOI] [Google Scholar]
- Montano et al. (2012).Montano S, Strona G, Seveso D, Galli P. First report of coral diseases in the Republic of Maldives. Diseases of Aquatic Organisms. 2012;101:159–165. doi: 10.3354/dao02515. [DOI] [PubMed] [Google Scholar]
- Montano et al. (2013).Montano S, Strona G, Seveso D, Galli P. Prevalence, host range, and spatial distribution of black band disease in the Maldivian Archipelago. Diseases of Aquatic Organisms. 2013;105:65–74. doi: 10.3354/dao02608. [DOI] [PubMed] [Google Scholar]
- Muller et al. (2017).Muller EM, Leporacci NM, Macartney KJ, Shea AG, Crane RE, Hall ER, Ritchie KB. Low pH reduces the virulence of black band disease on Orbicella faveolata. PLOS ONE. 2017;12:e0178869. doi: 10.1371/journal.pone.0178869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller & Van Woesik (2011).Muller EM, Van Woesik R. Black-band disease dynamics: prevalence, incidence, and acclimatization to light. Journal of Experimental Marine Biology and Ecology. 2011;397:52–57. doi: 10.1016/j.jembe.2010.11.002. [DOI] [Google Scholar]
- Page & Willis (2006).Page C, Willis B. Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Diseases of Aquatic Organisms. 2006;69:41–51. doi: 10.3354/dao069041. [DOI] [PubMed] [Google Scholar]
- Peters (1993).Peters EC. Diseases of other invertebrate phyla: porifera, cnidaria, ctenophora, annelida, echinodermata. In: Couch JA, Fourine JW, editors. Pathology of marine and estuarine organisms. Boca Raton: CRC Press. International Society for Reef Studies; Waco: 1993. pp. 393–449. [Google Scholar]
- Porter et al. (2001).Porter JW, Dustan P, Jaap WC, Patterson KL, Kosmynin V, Meier OW, Patterson ME, Parsons M. Patterns of spread of coral disease in the Florida keys. Hydrobiologia. 2001;460:1–24. doi: 10.1023/a:1013177617800. [DOI] [Google Scholar]
- Precht et al. (2016).Precht WF, Gintert BE, Robbart ML, Fura R, Van Woesik R. Unprecedented disease-related coral mortality in Southeastern Florida. Scientific Reports. 2016;6:31374. doi: 10.1038/srep31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse et al. (2007).Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall & Van Woesik (2015).Randall CJ, Van Woesik R. Contemporary white-band disease in Caribbean corals driven by climate change. Nature Climate Change. 2015;5:375–379. doi: 10.1038/nclimate2530. [DOI] [Google Scholar]
- Rasoulouniriana et al. (2009).Rasoulouniriana D, Siboni N, Ben-Dov E, Kramarsky-Winter E, Loya Y, Kushmaro A. Pseudoscillatoria coralii gen. nov. sp. nov., a cyanobacterium associated with coral black band disease (BBD) Diseases of Aquatic Organisms. 2009;87:91–96. doi: 10.3354/dao02089. [DOI] [PubMed] [Google Scholar]
- Richardson (1996).Richardson LL. Horizontal and vertical migration patterns of Phorrnidium corallyticum and Beggiatoa spp. associated with black-band disease of corals. Microbial Ecology. 1996;32:323–335. doi: 10.1007/bf00183066. [DOI] [PubMed] [Google Scholar]
- Richardson (1998).Richardson LL. Coral diseases: what is really known? Trends in Ecology & Evolution. 1998;13:438–443. doi: 10.1016/S0169-5347(98)01460-8. [DOI] [PubMed] [Google Scholar]
- Richardson (2004).Richardson LL. Black band disease. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer; Berlin: 2004. pp. 325–336. [Google Scholar]
- Richardson & Kuta (2003).Richardson LL, Kuta KG. Ecological physiology of the black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiology Ecology. 2003;43:287–298. doi: 10.1016/S0168-6496(03)00025-4. [DOI] [PubMed] [Google Scholar]
- Richardson et al. (1997).Richardson LL, Kuta K, Schnell S, Carlton RG. Ecology of the black band disease microbial consortium. In: Lessios HA, Macintyre IG, editors. Proceedings of the 8th international coral reef symposium vol. 1. Smithsonian Tropical Research Institute, Panama; Waco. 1997. pp. 597–600. [Google Scholar]
- Richardson et al. (2009).Richardson LL, Miller AW, Broderick E, Kaczmarsky L, Gantar M, Stanić D, Sekar R. Sulfide, microcystin, and the etiology of black band disease. Diseases of Aquatic Organisms. 2009;87:79–90. doi: 10.3354/dao02083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder et al. (2014).Roder C, Arif C, Bayer T, Aranda M, Daniels C, Shibl A, Chavanich S, Voolstra CR. Bacterial profiling of white plague disease in a comparative coral species framework. The ISME Journal. 2014;8:31–39. doi: 10.1038/ismej.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder et al. (2015).Roder C, Bayer T, Aranda M, Kruse M, Voolstra CR. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Molecular Ecology. 2015;24:3501–3511. doi: 10.1111/mec.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez & Cróquer (2008).Rodríguez S, Cróquer A. Dynamics of black band disease in a diploria strigosa population subjected to annual upwelling on the northeastern coast of Venezuela. Coral Reefs. 2008;27:381–388. doi: 10.1007/s00338-007-0341-8. [DOI] [Google Scholar]
- Rosenberg & Ben-Haim (2002).Rosenberg E, Ben-Haim Y. Microbial diseases of corals and global warming. Environmental Microbiology. 2002;4:318–326. doi: 10.1046/j.1462-2920.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg & Falkovitz (2004).Rosenberg E, Falkovitz L. The vibrio shiloi/oculina patagonica model system of coral bleaching. Annual Review of Microbiology. 2004;58:143–159. doi: 10.1146/annurev.micro.58.030603.123610. [DOI] [PubMed] [Google Scholar]
- Rosenberg & Koren (2006).Rosenberg E, Koren O. The biology of vibrios. Washington, D.C.: ASM Press; 2006. Vibrios in coral health and disease. [Google Scholar]
- Rosenberg & Loya (2013).Rosenberg E, Loya Y. Coral health and disease. Heidelberg: Springer-Verlag, Berlin; 2013. [Google Scholar]
- Rützler & Santavy (1983).Rützler K, Santavy DL. The black band disease of atlantic reef corals. Marine Ecology. 1983;4:301–319. doi: 10.1111/j.1439-0485.1983.tb00116.x. [DOI] [Google Scholar]
- Salter et al. (2014).Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biology. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Bourne & Willis (2009).Sato Y, Bourne DG, Willis BL. Dynamics of seasonal outbreaks of black band disease in an assemblage of Montipora species at Pelorus Island (Great Barrier Reef, Australia) Proceedings of the Royal Society B: Biological Sciences. 2009;276:2795–2803. doi: 10.1098/rspb.2009.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Willis & Bourne (2010).Sato Y, Willis BL, Bourne DG. Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. The ISME Journal. 2010;4:203–214. doi: 10.1038/ismej.2009.103. [DOI] [PubMed] [Google Scholar]
- Schloss et al. (2009).Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75:7537–7541. doi: 10.1128/aem.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar, Kaczmarsky & Richardson (2008).Sekar R, Kaczmarsky LT, Richardson LL. Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Marine Ecology Progress Series. 2008;362:85–98. doi: 10.3354/meps07496. [DOI] [Google Scholar]
- Sekar et al. (2006).Sekar R, Mills DK, Remily ER, Voss JD, Richardson LL. Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band-diseased Siderastrea siderea. Applied and Environmental Microbiology. 2006;72:5963–5973. doi: 10.1128/AEM.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séré et al. (2013).Séré MG, Tortosa P, Chabanet P, Turquet J, Quod J-P, Schleyer MH. Bacterial communities associated with Porites white patch syndrome (PWPS) on three Western Indian Ocean (WIO) coral reefs. PLOS ONE. 2013;8:e83746. doi: 10.1371/journal.pone.0083746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson (1949).Simpson EH. Measurement of diversity. Nature. 1949;163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- Suárez-Suárez et al. (2011).Suárez-Suárez A, López-López A, Tovar-Sánchez A, Yarza P, Orfila A, Terrados J, Arnds J, Marqués S, Niemann H, Schmitt-Kopplin P. Response of sulfate-reducing bacteria to an artificial oil-spill in a coastal marine sediment. Environmental Microbiology. 2011;13:1488–1499. doi: 10.1111/j.1462-2920.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- Sunagawa et al. (2009).Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, DeSalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M. Bacterial diversity and white plague disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME Journal. 2009;3:512–521. doi: 10.1038/ismej.2008.131. [DOI] [PubMed] [Google Scholar]
- Sussman, Bourne & Willis (2006).Sussman M, Bourne DG, Willis BL. A single cyanobacterial ribotype is associated with both red and black bands on diseased corals from Palau. Diseases of Aquatic Organisms. 2006;69:111–118. doi: 10.3354/dao069111. [DOI] [PubMed] [Google Scholar]
- Sutherland, Porter & Torres (2004).Sutherland KP, Porter JW, Torres C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Marine Ecology Progress Series. 2004;266:273–302. doi: 10.3354/meps266273. [DOI] [Google Scholar]
- Thompson et al. (2004).Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF. Diversity and dynamics of a North Atlantic coastal Vibrio community. Applied and Environmental Microbiology. 2004;70:4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tout et al. (2015).Tout J, Siboni N, Messer LF, Garren M, Stocker R, Webster NS, Ralph PJ, Seymour JR. Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Frontiers in Microbiology. 2015;6:540–552. doi: 10.3389/fmicb.2015.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss & Richardson (2006).Voss JD, Richardson LL. Nutrient enrichment enhances black band disease progression in corals. Coral Reefs. 2006;25:569–576. doi: 10.1007/s00338-006-0131-8. [DOI] [Google Scholar]
- Weil (2002).Weil E. Coral disease epizootiology: status and research needs. 2002 Coral health and disease: developing a national research plan. Coral Health and Disease Consortium, Charleston, South Carolina, 14.
- Weil (2004).Weil E. Coral reef diseases in the Wider Caribbean. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer; Berlin: 2004. pp. 35–68. [Google Scholar]
- Weil et al. (2012).Weil E, Irikawa A, Casareto B, Suzuki Y. Extended geographic distribution of several Indo-Pacific coral reef diseases. Diseases of Aquatic Organisms. 2012;98:163–170. doi: 10.3354/dao02433. [DOI] [PubMed] [Google Scholar]
- Willis, Page & Dinsdale (2004).Willis BL, Page CA, Dinsdale EA. Coral disease on the great barrier reef. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer; Berlin: 2004. pp. 69–104. [Google Scholar]
- Witt, Wild & Uthicke (2012).Witt V, Wild C, Uthicke S. Terrestrial runoff controls the bacterial community composition of biofilms along a water quality gradient in the great barrier reef. Applied and Environmental Microbiology. 2012;78:7786–7791. doi: 10.1128/AEM.01623-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto-Hirose et al. (2006).Yasumoto-Hirose M, Nishijima M, Ngirchechol MK, Kanoh K, Shizuri Y, Miki W. Isolation of marine bacteria by in situ culture on media-supplemented polyurethane foam. Marine Biotechnology. 2006;8:227–237. doi: 10.1007/s10126-005-5015-3. [DOI] [PubMed] [Google Scholar]
- Ziegler et al. (2016).Ziegler M, Roik A, Porter A, Zubier K, Mudarris MS, Ormond R, Voolstra CR. Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Marine Pollution Bulletin. 2016;105:629–640. doi: 10.1016/j.marpolbul.2015.12.045. [DOI] [PubMed] [Google Scholar]
- Zvuloni et al. (2009).Zvuloni A, Artzy-Randrup Y, Stone L, Kramarsky-Winter E, Barkan R, Loya Y. Spatio-temporal transmission patterns of black-band disease in a coral community. PLOS ONE. 2009;4:e4993. doi: 10.1371/journal.pone.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The OTU abundance table is provided as a Data S1.