Abstract
Exogenous dendritic cells (bone marrow-derived dendritic cell (BMDC)) display restricted trafficking in vivo after injection into mice, but the route(s) by which they generate gut-homing effector cells is unclear. Mesenteric lymph nodes (LN) and spleen were differentially targeted by i.p. and i.v. administration of BMDC, respectively, whereas mediastinal LN were targeted by both routes. BMDC injected by either route activated CD8+ T cells to up-regulate both α4β1 and α4β7 integrins. However, the lymphoid compartment in which activation occurred determined their expression kinetics, magnitude, and population distribution. Only T cells activated in mesenteric LN after i.p. immunization expressed high levels of α4β7, which also correlated with localization to small intestine. These α4β7high cells also redistributed to mediastinal LN in a manner sensitive to treatment with α4β7 blocking Abs, but not to mucosal addressin cell adhesion molecule-1 blocking Abs. Our results demonstrate the importance of lymphoid compartment, as dictated by immunization route, in determining integrin expression on activated T cells and their distribution in lymphoid and nonlymphoid tissues.
Appropriately activated dendritic cells (DC)4 are potent APC, and exogenously administered Ag-pulsed DC induce specific CD8+ T cell responses against viral and tumor Ags (1–4). However, the cellular nature and migration properties of exogenous DC limit their distribution in lymphoid compartments in an injection route-dependent manner (3, 5–9), leading to regionally constrained immune responses (3, 5). Thus, in contrast to replicating viral or bacterial immunogens that distribute widely in the body, immunization with bone marrow-derived DC (BMDC) creates the possibility to study the time course and characteristics of the immune response initiated in individual lymph nodes (LN) in exquisite detail.
Taking full advantage of this capability requires greater understanding of the properties of BMDC as immunogens. One issue is how to target BMDC and the resulting T cell responses to different lymphoid compartments. Exogenous DC injected by i.v. or s.c. routes traffic into spleen alone or spleen and draining peripheral LN, respectively (3, 5–9), and induce primary responses localized to these same compartments (3). Importantly, neither s.c. nor i.v. immunization leads to localization of BMDC or T cell responses to mesenteric LN. Thus, it is currently unclear how to deliver BMDC to induce activation of CD8+ T cells in lymphoid compartments associated with robust immunity in the gut.
Even if appropriately targeted, a second issue is to what extent BMDC matured in vitro induce molecules that mediate tissue-specific homing of T cells. CD8+ T cells activated in vitro with endogenous DC enriched from mesenteric LN and Peyer’s patches up-regulate α4β7 integrin and CCR9, which are associated with gut homing (10–13). Conversely, peripheral LN-derived DC induce E- and P-selectin ligands that are associated with homing to the skin (13–15). A single study demonstrated that semimature BMDC have a limited ability to induce homing receptor expression in vitro (14), whereas more robust expression was observed after in vivo immunization. However, it was not established whether this difference was due to BMDC maturation in vivo, or alternatively, by different LN microenvironments.
In this study, we examined the CD8+ T cell response that develops in individual lymphoid compartments after immunization with fully mature, CD40L-activated BMDC by different routes. We used T cell adoptive transfer to detect early priming events and track the ensuing immune response with great precision over time and space. Up-regulation of α4 integrin expression on activated T cells was evaluated, and the relative roles of the BMDC and the lymphoid microenvironment in imprinting integrin expression were determined. Our results demonstrate route-dependent differences in activated T cell distribution in peripheral tissues. They also establish that α4β7high cells activated in mesenteric LN redistribute into mediastinal LN via an α4β7-dependent, but mucosal addressin cell adhesion molecule-1 (MAdCAM-1)-independent, mechanism.
Materials and Methods
Animals
C57BL/6 and OT-1 RAG1−/− mice were obtained from Charles River Laboratories and Taconic Farms, respectively. C57BL/6 Thy-1.1 and B6.C-H2bm1/ByJ (bm1) mice were obtained from The Jackson Laboratory. All animals were maintained in pathogen-free facilities at the University of Virginia. All protocols were approved by the Institutional Animal Care and Use Committee.
Adoptive transfer of OT-1 cells
Single-cell suspensions from spleen and pooled LN of OT-1 RAG1−/− mice were enriched for CD8+ T cells by negative selection (StemCell Technologies). Preparations were consistently 97–99% CD8+ by flow cytometry. In some cases, cells were labeled with 4 μM CFSE (Molecular Probes) in PBS for 15 min at 37°C before injection. Unless otherwise stated, 4 × 106 cells were injected i.v. into the dorsal tail vein.
DC culture and immunizations
BMDC were generated, as previously described, with slight modifications (16). Briefly, bone marrow cells removed from mouse femurs and tibias were cultured for 7 days in the presence of GM-CSF and IL-4 (BD Bio-sciences), enriched for DC by negative selection (StemCell Technologies), and activated by culturing overnight with NIH-3T3 cells expressing CD40L (a gift from R. Lapoint, University of Montreal, Quebec, Canada). These activated BMDC were uniformly CD70highCD80highCD86high and CD62Lnegative, and expressed IL-12 (our unpublished observations). Before injection, BMDC were pulsed with 10 μM OVA257–264 for 1 h at 37°C in the presence of 10 μg/ml human β2-microglobulin (Calbiochem). Mice were immunized with 100,000 BMDC in 200 μl either i.v. into the dorsal tail vein or i.p. into the peritoneal cavity 18–36 h after adoptive transfer of OT-1 cells.
Flow cytometric analysis of surface markers
Samples were incubated with purified anti-CD16/CD32 (eBioscience) to block FcRs, and adoptively transferred cells were identified by staining with anti-CD8α PerCP, anti-CD8α PerCP-Cy5.5 (BD Biosciences), anti-Thy1.2 APC, anti-CD3ε biotin (eBioscience), and/or H-2Kb-OVA257 tetramer-APC generated in house by S. Lewis (University of Virginia Cancer Center, Charlottesville, VA). T cell activation status was evaluated with anti-CD25 FITC and anti-CD44 PE (eBioscience). Integrin expression was evaluated using anti-α4β7 PE, anti-α4 PE, or anti-α4 biotin, followed by streptavidin-PerCP (BD Biosciences). Expression after in vivo injection of anti-α4β7 was detected using PE goat anti-rat IgG (Jackson ImmunoResearch Laboratories). Chemokine receptor expression was evaluated using anti-CCR9 PE (R&D Systems). Samples were collected on a FACSCalibur (BD Biosciences) and analyzed using Flow Jo software.
FTY720 treatment and in vivo Ab blockade
Mice were injected i.p. with 1 mg/kg FTY720 (a gift from V. Brinkmann, Novartis Pharma, Basel, Switzerland) in 200 μl beginning 24 h after BMDC immunization and then every 24 h until harvest. Purified anti-CD62L (MEL-14) (17) (American Type Culture Collection), anti-α4β7 (DATK32) (18), anti-MAdCAM-1 (MECA367) (19) (both gifts from J. Rivera-Nieves, University of Virginia, Charlottesville, VA), or rat IgG (20) were administered i.v. at 100 μg per mouse. Anti-CD62L treatment was initiated 6 h after BMDC injection, and anti-α4β7 and anti-MAdCAM-1 treatments were initiated 24 h after BMDC injection. Ab injections were repeated every 24 h until harvest.
Activation of OT-1 cells in vitro
CD8-enriched CFSE-labeled OT-1 cells were cultured in a 96-well U-bottom plate at a 1:1 or 1:2 ratio with either CD40L-activated, OVA257–264-pulsed, or unpulsed BMDC, or endogenous DC from mediastinal LN and spleen of mice that had been treated with 10 μg of human fms-like tyrosine kinase-3 ligand (Amgen) daily for 10 days and enriched by negative selection (StemCell Technologies). Wells were harvested after 4–6 days and stained for Thy1.2, CD8α, α4β7, and/or α4 expression.
Staining of LN high endothelial venules (HEV) by immunofluorescence
Mice were injected i.v. with 100 μg of anti-MAdCAM-1 (MECA367) or rat IgG. Thirty minutes after Ab injection, LN were resected and snap frozen. Acetone-fixed frozen sections of LN were incubated with Alexa594 goat anti-rat IgG (Molecular Probes) and FITC anti-smooth muscle α-actin (20). Images were captured on a Nikon TE-300 inverted microscope and Bio-Rad μ-Radiance confocal system.
Generation of bone marrow chimeras
Mice were irradiated (650 rad × 2) using a Gammacell 40 (Nordion International). Bone marrow cells from donor mice were depleted of T cells by incubation with biotinylated anti-CD4 and anti-CD8α (eBioscience), followed by negative selection (StemCell), and injected (2.5 × 106 in 200 μl) into the dorsal tail vein. Mice were used in experiments 10–14 wk after injection of donor bone marrow cells.
Lymphocyte recovery from peripheral tissues
Lymphocytes were recovered from lung by purging lungs of blood using a heparin solution, followed by digestion with 125 U/ml collagenase type XI, 60 U/ml DNase I, and 60 U/ml hyaluronidase type I-s (20), and lymphocytes were purified by centrifugation on a Histodenz gradient (20). Intraepithelial lymphocytes from the small intestine were isolated by excising from the duodenum to the cecum, removing Peyer’s patches, digesting with 1 mM DTT (Roche Applied Science), and isolating lymphocytes on a Percoll gradient (20, 21). Intrahepatic lymphocytes were isolated by flushing the liver with 0.05% collagenase IV (20) before removal, digestion with 0.05% collagenase IV, and isolation of lymphocytes on a Histodenz-based gradient (22).
Statistical analyses
Statistical analyses were performed using two-way ANOVA (Sigma-Aldrich Stat software).
Results
BMDC administered i.p. and i.v. prime naive CD8+ T cells in distinct lymphoid compartments
Previous studies had not established a route by which injected exogenous BMDC could infiltrate mesenteric LN and activate resident T cells. Consequently, we compared the localization of T cell activation after i.p. and i.v. injection of OVA257 peptide-pulsed CD40L-activated BMDC. To enhance detection of early activation events, CD8+ OT-1 TCR transgenic T cells were adoptively transferred into mice 18–36 h before immunization. Twenty-two hours after immunization, OT-1 cells in different lymphoid organs were identified by staining with H2-Kb-OVA257 tetramer, and their activation status was determined based on up-regulation of CD25 (Fig. 1a). Activated OT-1 cells were present in mesenteric LN following i.p., but not i.v. injection of BMDC. Conversely, T cell activation was observed in the spleen after i.v., but not i.p. injection. Whereas neither route of immunization led to activation of T cells in axillary/brachial LN, both led to T cell activation in mediastinal LN. These results were consistent in six independent experiments representing a total of 16 mice (Fig. 1b), and also using CD69 up-regulation as another early marker of activation (our unpublished observations). We also examined whether functional DC redistributed to additional lymphoid compartments with a delayed time course by injection of CFSE-labeled OT-1 cells into mice that had been immunized with BMDC 48 h previously. However, T cell activation at these later times remained localized to the same compartments, although the magnitude of activation waned somewhat by 48 h (Fig. 1c). These data demonstrate that activation of CD8+ OT-1 cells by exogenous BMDC was localized to distinct lymphoid compartments after i.p. and i.v. injection, and that mesenteric LN were targeted only by the i.p. route.
FIGURE 1.
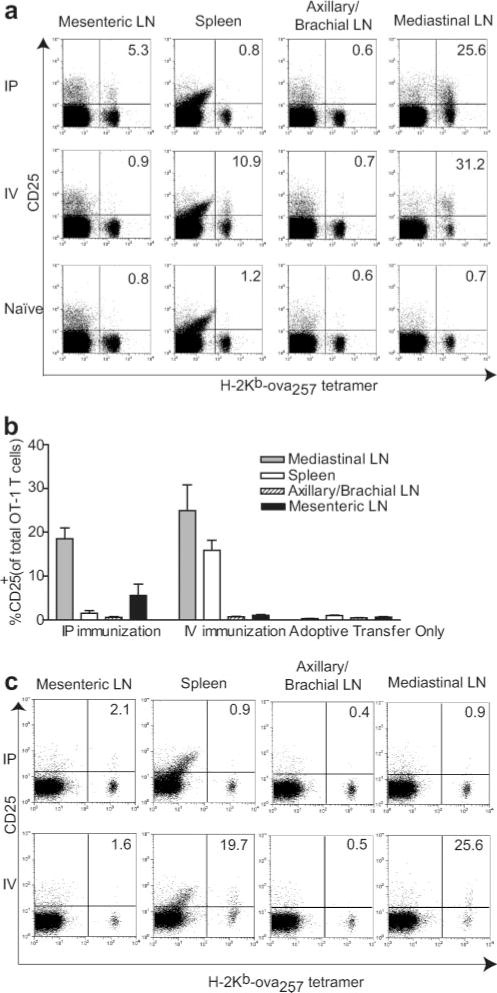
OT-1 cell activation is differentially localized following i.v. or i.p. injection of CD40L-activated OVA257-pulsed BMDC. a, Mice that had been adoptively transferred with CD8-enriched OT-1 cells were immunized, and lymphoid organs were harvested 22 h later for flow cytometry. OT-1 cells were identified by staining for CD8 and H-2Kb-OVA257-tetramer, and activation was determined by CD25 expression. Representative data from an individual experiment. Dot plots are gated on CD8+ lymphocytes, and numbers represent the percentage of H-2Kb-OVA257-tetramer+ cells that are CD25+. b, Aggregate data from six to nine mice in each group (±SEM) analyzed in six independent experiments. c, Mice were immunized with CD40L-activated OVA257-pulsed BMDC i.v. or i.p. CD8-enriched OT-1 cells were then adoptively transferred 48 h after BMDC injection. Lymphoid organs were harvested 24 h after T cell transfer and stained for flow cytometry, as described above. Data are individual animals from one experiment representative of four mice in immunized groups and two mice in adoptive transfer only group in two independent experiments.
Intraperitoneal but not i.v. immunization effectively generates gut-localized activated OT-1 cells
To determine whether the early activation of T cells in distinct lymphoid compartments led to a productive response, we examined the dilution of CFSE in labeled OT-1 cells 72 h after immunization. Activated OT-1 cells underwent substantial proliferation and expansion in all compartments in which up-regulation of CD25 on T cells had been observed (priming compartments) (Fig. 2a). Greater than 95% of OT-1 cells remained undivided in the same compartments of mice that had received either no BMDC or unpulsed BMDC (our unpublished observations). To evaluate whether activated OT-1 cells could localize to nonlymphoid tissues, peripheral tissues were harvested 6 days after immunization. At this time point, the percentages of OT-1 cells in spleen were similar after immunization by either route (Fig. 2b). In addition, substantial increases in OT-1 cells in lung and liver were evident after both i.p. and i.v. immunization. These cells were CD44high and CD62L−, consistent with an effector T cell phenotype (Fig. 2c). Activated OT-1 cells were equally prevalent in each of these tissues regardless of the immunization route. In contrast, the percentage of activated OT-1 cells in the small intestine was >6-fold greater after i.p. than i.v. immunization. Thus, only i.p. immunization effectively generated gut-resident OT-1 cells.
FIGURE 2.
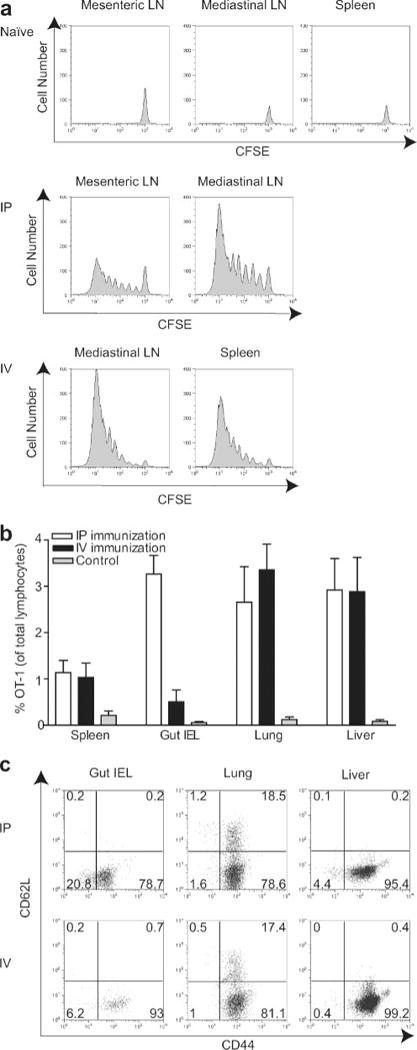
Activated OT-1 cells proliferate in all priming compartments, but distribution in peripheral tissues is dependent on route of BMDC immunization. a, Mice that had been adoptively transferred with CD8-enriched OT-1 cells were immunized, and lymphoid organs representing priming compartments defined in Fig. 1 were harvested 3 days later for flow cytometry. Histograms are gated on Thy1.2+ lymphocytes. b and c, Mice that had been adoptively transferred with 1 × 106 CD8-enriched OT-1 cells were immunized with BMDC, and lymphocytes were isolated from the indicated peripheral tissues 6 days later. OT-1 cells were identified by Thy1.2 or CD8 and H-2Kb-OVA257-tetramer staining. b, Data are mean values pooled from six mice per condition (±SEM) analyzed in five independent experiments. c, Representative dot plots from individual mice.
Both routes of immunization generate α4β7+ -activated OT-1 cells, but the level of α4β7 expressed is route dependent
Because of the known role of α4β7 integrin expression in homing of T cells to the gut, the expression of α4 integrins on activated OT-1 cells in priming compartments was evaluated. After i.p. immunization, divided OT-1 cells in both mesenteric and mediastinal LN gradually up-regulated α4 integrin expression, becoming nearly 100% α4+ by the sixth division (Fig. 3a). The majority of cells in mesenteric LN also up-regulated α4β7 expression by the sixth division. However, α4β7 expression was delayed relative to that of α4 by ~2 cell divisions (Fig. 3b). Because α4 only heterodimerizes with β1 and β7, this observation indicates that these cells up-regulated α4β1 first. Interestingly, α4 expression on cells in mediastinal LN was delayed relative to that on cells in mesenteric LN by ~2 cell divisions (Fig. 3b). Also, whereas most activated cells in mediastinal LN after i.p. immunization expressed α4β1, a significant fraction was α4β7+ (Fig. 3a). Following i.v. immunization, divided OT-1 cells in the mediastinal LN and spleen also became nearly 100% α4+ by the sixth division (Fig. 3, c and d). The majority of these cells failed to express α4β7; thus, the dominant population that developed in both lymphoid compartments was α4β1+. Unexpectedly, however, a small, but reproducible proportion of cells activated in both spleen and mediastinal LN became α4β7+. Activated cells with a similar distribution of α4 integrins were also evident in mesenteric LN (Fig. 3, c and e). This is consistent with the earlier demonstration that the mesenteric LN was not a priming site after i.v. immunization, and that it is not the source of the α4β7+ cells observed. Collectively, these results establish that the kinetics and relative expression of α4β1 and α4β7 integrins after BMDC immunization were lymphoid compartment dependent.
FIGURE 3.
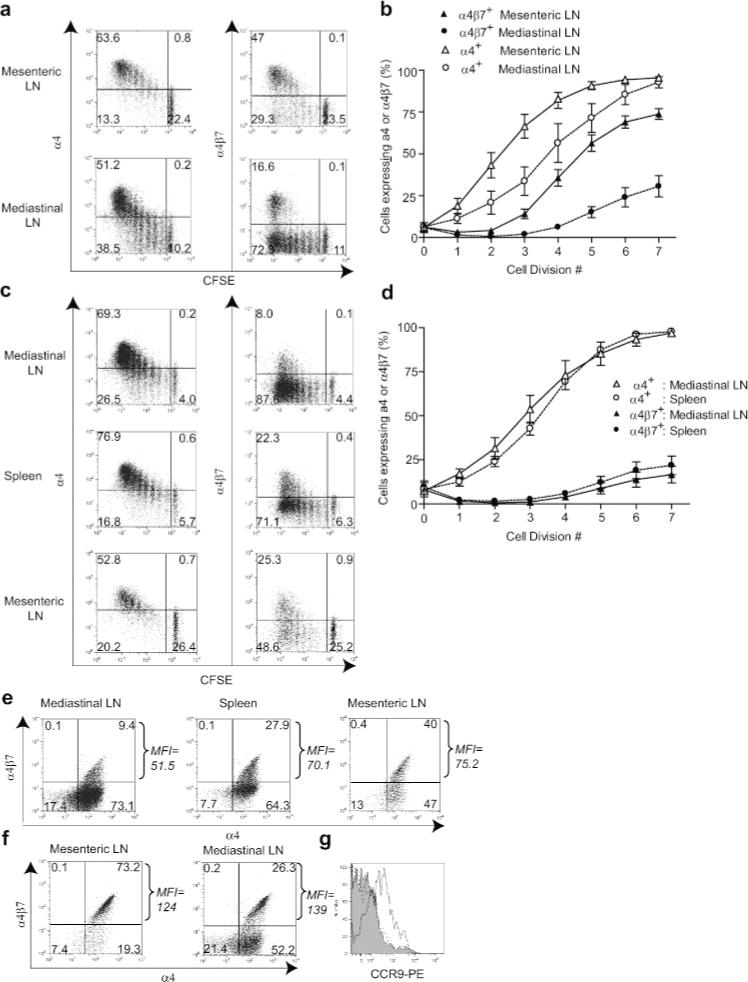
Kinetics, level, and type of α4 integrin expression differs in priming compartments after BMDC immunization. Mice that had been adoptively transferred with CD8-enriched OT-1 cells were immunized with BMDC by i.p. (a, b, f, and g) or i.v. (c, d, e, and g) routes, and lymphoid organs representing priming compartments defined in Fig. 1 were harvested 3 days later for flow cytometry. a and c, Representative data from individual mice. b and d, Percentage of OT-1 cells that were α4+ or α4β7+ at each cell division based on CFSE dilution. Data are mean values from five to six mice (±SEM) pooled from four to five independent experiments. e and f, Integrin expression on extensively divided OT-1 cells. Dot plots are on Thy1.2+ lymphocytes that had undergone five or more cell divisions as determined by CFSE dilution. MFI of α4β7 staining in upper right quadrant is indicated for each dot plot. g, CCR9 expression on α4β7+ divided OT-1 cells from the mesenteric LN after i.p. immunization (open), mediastinal LN after i.v. immunization (shaded), and mesenteric LN with no immunization (dotted).
The above results suggested that the presence of gut-resident OT-1 cells after i.p. immunization was correlated with a dominant population of α4β7+ cells in mesenteric LN, which is in keeping with previous work from many laboratories. Indeed, the entry of these cells into the gut was blocked by in vivo administration of Abs against either α4β7 or its ligand, MAdCAM-1 (see Fig. 4i). However, significant numbers of divided α4β7+ OT-1 cells were also found in other priming compartments after both i.p. and i.v. immunization. A possible explanation for the route-dependent difference in localization of OT-1 cells to gut is that i.p. immunization generated larger numbers of α4β7+ cells. However, despite the greater proportion of α4β7+ OT-1 cells generated in mesenteric LN after i.p. immunization, the absolute number of α4β7+ cells generated after i.v. immunization was 2- to 3-fold greater because the spleen contained 10–15 times as many OT-1 cells as the mesenteric LN (our unpublished observations). This indicated that the paucity of activated OT-1 cells in gut after i.v. immunization was not due to lower absolute numbers of α4β7+ cells generated. However, we also noted that the level of α4β7 expression on extensively divided OT-1 cells was route dependent. Whereas T cells activated after i.v. immunization expressed intermediate levels of α4β7 (α4β7int) (Fig. 3e), those in i.p. immunized animals expressed ~2.3 times more (α4β7high) (Fig. 3f) based on mean fluorescence intensity (MFI) (23). The MFI of α4β7+ cells in the mediastinal LN varied in different experiments based on staining conditions, and was in some cases lower than that of cells in mesenteric LN (see Figs. 5 and 6). However, it was always higher than that of cells isolated from the mediastinal LN of mice immunized by the i.v. route in the same experiment. Additionally, cells expressing intermediate levels of α4β7 were found in the mesenteric LN after i.v. immunization. This suggests that the level of α4β7 integrin either directly determines the ability to home to the gut, or serves as a phenotypic marker for expression of other molecules that do so. To examine the latter possibility, the expression of CCR9 was examined due to the involvement of this receptor in migration of cells to the gut. Cells that had divided and expressed α4β7 in the mesenteric LN after i.p. BMDC immunization expressed CCR9. Conversely, divided cells expressing α4β7 in the mediastinal LN after i.v. BMDC immunization did not express CCR9 over levels found on undivided cells in a mouse receiving no BMDCs (Fig. 3g). This reveals two different α4β7+ populations that arise in different lymphoid organs.
FIGURE 4.
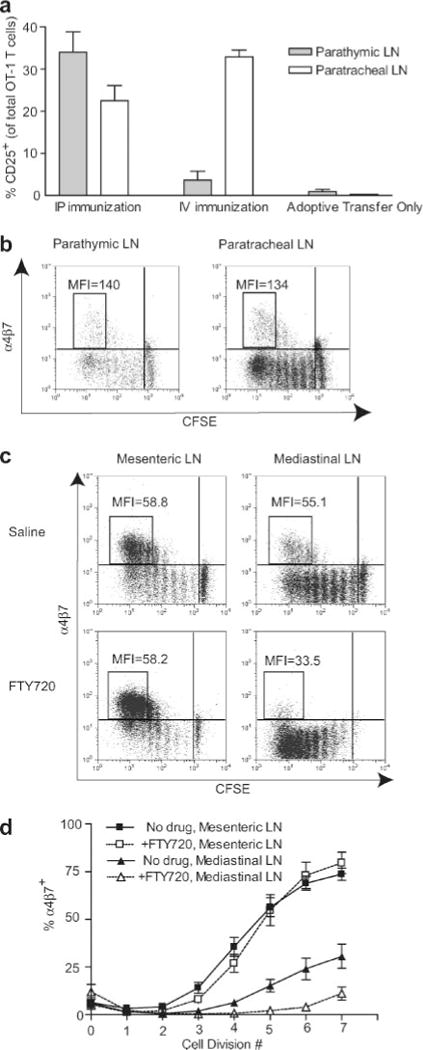
The α4β7high subpopulation of activated OT-1 cells in mediastinal LN after i.p. immunization originates in mesenteric LN. a, Mice that had been adoptively transferred with CD8-enriched OT-1 cells were immunized i.p. with BMDC, and the indicated LN were harvested 22 h or 3 days (40) later. OT-1 cells were identified by gating on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. a, OT-1 cell activation in separated components of the mediastinal LN. Data are mean values from five mice in immunized groups and three mice in adoptive transfer only group (±SEM). One of three independent experiments is shown. b, α4β7 expression on activated OT-1 cells in parathymic and paratracheal LN. MFI values refer to α4β7 staining of cell populations in boxes. Data are representative of eight mice in two independent experiments. c, Effect of in vivo administration of FTY720 on α4β7 expression on activated OT-1 cells in priming compartments. MFI values refer to α4β7 staining of cell populations in boxes. d, Percentage of OT-1 cells that were α4β7+ at each cell division based on CFSE dilution. Data are mean values from six mice (±SEM) analyzed in three independent experiments. There was no significant difference between mesenteric LN between FTY720-treated and control mice (p = 0.828), but differences in mediastinal LN were significantly different (p < 0.001) as determined by two-way ANOVA.
FIGURE 5.
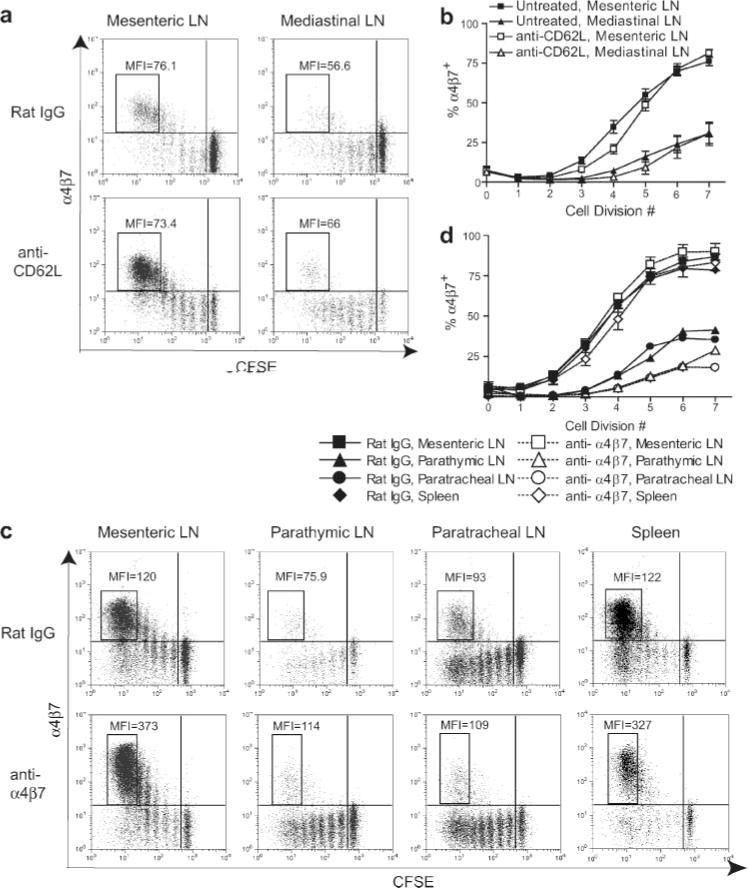
α4β7high OT-1 cells are activated in mesenteric LN and redistribute into mediastinal LN. Mice were adoptively transferred with CFSE-labeled OT-1 cells before i.p. immunization. Ab treatment was initiated 6 h (anti-CD62L) or 24 h (anti-α4β7) after BMDC injection and continued until harvest at 72 h. Dot plots are gated on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. MFI values refer to α4β7 staining of cell populations in boxes. a and b, Effects of blockade with anti-CD62L. a, Data from individual mice representative of eight mice treated with each reagent in three independent experiments. b, Data are mean values (±SEM) from eight mice per group analyzed in three independent experiments. c and d, Effects of blockade with anti-α4β7. c, Data from mesenteric LN and spleen of individual mice and parathymic and paratracheal LN pooled from two mice. Representative of eight mice treated with each reagent in four independent experiments. Because of the presence of in vivo bound anti-α4β7 Ab on OT-1 cells in anti-α4β7-treated mice, these cells were stained with PE anti-rat IgG, leading to an elevated MFI. Cells from mice treated with rat IgG were stained normally, as described in Materials and Methods. d, Data are mean values (±SEM) from two mice per group, except parathymic and paratracheal LN, which were values obtained from pooling samples from two mice. Data are representative of four independent experiments.
FIGURE 6.
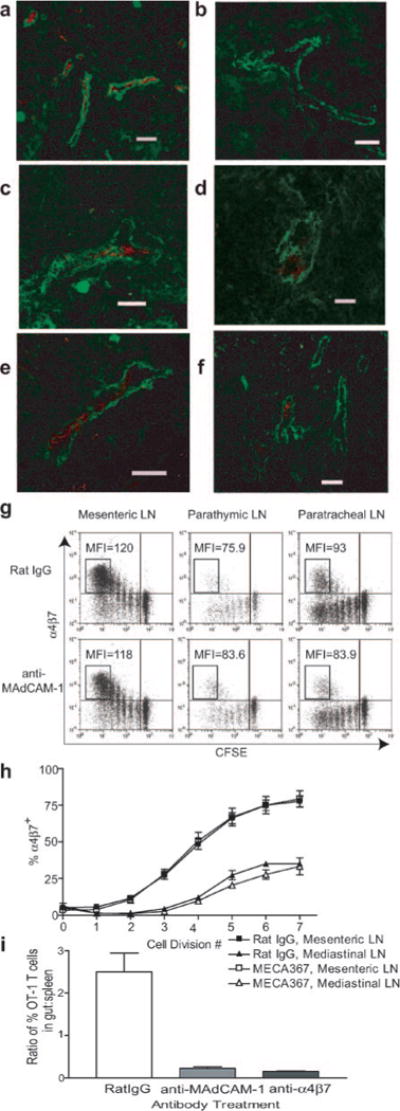
MAdCAM-1 expression and its role in the entry of α4β7high cells into mediastinal LN. a–f, Expression of MAdCAM-1 in mesenteric (a), brachial (b), parathymic (c), and paratracheal (d) LN in naive mice, as well as parathymic (e) and paratracheal (f) LN of i.p. immunized mice. MAdCAM-1 staining is red, and smooth muscle α-actin staining (to delineate HEV) is green. Scale bar equals 50 μm. g and h, Entry of α4β7high cells into mediastinal LN is MAdCAM-1 independent. Mice were adoptively transferred with CFSE-labeled OT-1 cells before i.p. immunization. Ab treatment was initiated 24 h after BMDC injection and continued until harvest at 72 h. g, Dot plots are gated on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. MFI values refer to α4β7 staining of cell populations in boxes. h, Percentage of OT-1 cells that were α4β7+ at each cell division based on CFSE dilution. Data are mean values from six to nine mice (±SEM) analyzed in three independent experiments. i, Mice were adoptively transferred with CFSE-labeled OT-1 cells before i.p. immunization. Ab treatment was initiated 24 h after BMDC injection and continued every 24 h. Tissues were harvested 3 days after BMDC injection. Intraepithelial lymphocytes were recovered, as described in Materials and Methods. Data are mean values (±SEM) from three to four mice analyzed in three independent experiments.
The α4β7high subpopulation present in mediastinal LN following i.p. immunization originates in mesenteric LN
To investigate the basis for immunization route-dependent differences in α4β7+ populations in mediastinal LN, we first tested the hypothesis that this difference was due to T cell activation in distinct LN within the mediastinum after i.v. and i.p. immunization. The mediastinal LN used in our experiments were collected from the anterior and posterior mediastinum (also known as parathymic and paratracheal LN, respectively). Therefore, T cell activation was evaluated separately in parathymic and paratracheal LN 22 h after BMDC injection. Following i.p. administration, OT-1 cells in both LN were activated, although the fraction activated in parathymic LN was consistently higher (Fig. 4a). However, after i.v. injection, OT-1 cell activation was almost completely confined to the paratracheal LN. This suggested that BMDC injected into the peritoneal cavity accessed afferent lymphatics of parathymic LN, whereas BMDC present in lung after i.v. injection could not. In contrast, afferent lymphatics of the paratracheal LN were accessed by BMDC draining from both the lungs and peritoneal cavity. Whereas these results were consistent with the hypothesis that differential mediastinal priming after i.p. and i.v. immunization might lead to different α4β7 expression levels, the levels of α4β7 on extensively divided cells in parathymic and paratracheal LN were similar 3 days after i.p. immunization (Fig. 4b). Thus, despite the activation of T cells in different mediastinal LN after i.v. and i.p. immunization, this did not account for differences in the expression of α4β7 because when activation occurred in both mediastinal compartments, α4β7 expression levels on cells in those compartments were equivalent to each other.
We next tested an alternate hypothesis that α4β7high OT-1 cells were generated in mesenteric LN and subsequently recirculated into mediastinal LN. To do this, we treated animals with FTY720, a drug that inhibits lymphocyte egress from LN (24), to sequester T cells in the compartments in which they were activated. In i.p. immunized mice treated with FTY720, the MFI of extensively divided α4β7+ cells in mediastinal LN was only about half that of the α4β7+ population in mesenteric LN of the same animal (Fig. 4c), and was similar to what was observed in mediastinal LN and spleen after i.v. immunization. Additionally, the percentage of α4β7+ cells in mediastinal LN of FTY720-treated mice was significantly reduced, whereas there was no effect on cells in the mesenteric LN (Fig. 4d). These data demonstrated that the subpopulation of α4β7high OT-1 cells in mediastinal LN after i.p. immunization resulted from the redistribution of cells activated in mesenteric LN.
Entry of α4β7high OT-1 cells in mediastinal LN is not mediated by CD62L, but is α4β7 dependent
The redistribution of activated α4β7high T cells from mesenteric to mediastinal LN was unexpected. Because CD62L expression on naive T cells mediates entry into LN, we used in vivo blockade with anti-CD62L Ab (25) to test the hypothesis that entry of α4β7high cells into mediastinal LN would also be mediated by this selectin. As expected, the cellularity of the peripheral LN in mice treated with anti-CD62L for 66 h was substantially reduced, whereas the cellularity of the spleen was slightly increased (our unpublished observations). However, the MFIs of extensively divided α4β7+ cells in mesenteric and mediastinal LN were not significantly diminished in i.p. immunized mice treated with anti-CD62L compared with those treated with rat IgG as a control (Fig. 5a). Additionally, there was no diminution in the percentage of α4β7+ cells as a function of cell division number (Fig. 5b). Together, these data indicated that entry of α4β7high cells into mediastinal LN was CD62L independent.
We next used in vivo blockade with anti-α4β7 (25) to evaluate whether entry of α4β7high cells into mediastinal LN was mediated by this integrin. Because α4β7 blockade was achieved with the same Ab used for staining ex vivo, PE-conjugated anti-rat IgG was used to detect α4β7+ cells that had bound Ab in vivo. This increased the MFI of all α4β7+ cells isolated from these animals, regardless of compartment, relative to the MFI of cells isolated from mice treated with rat IgG and stained directly (Fig. 5c). Despite this, the percentages of α4β7+ cells detected in mesenteric LN of i.p. immunized mice that had been treated with either anti-α4β7 or rat IgG were comparable (Fig. 5d). The percentages of these cells that had migrated through the circulation to spleen at this time were also comparable. However, anti-α4β7 blockade substantially reduced the percentage of divided α4β7high cells in both parathymic and paratracheal LN of i.p. immunized mice. This effect was limited to these LN, and there was no impact on the percentage of α4β7+ in the spleen. In the experiment shown, the MFI of extensively divided α4β7+ OT-1 cells in parathymic and paratracheal LN of rat IgG-treated mice was only ~65–80% of the MFI of cells from mesenteric LN and spleen. However, in anti-α4β7-treated mice, the MFI of cells from parathymic and paratracheal LN was reduced to only ~30% of that in mesenteric LN of the same animal, whereas the relative MFI of cells in the spleen remained at 90% (Fig. 5c). We attribute the somewhat lower MFI of cells from parathymic and paratracheal LN of rat IgG-treated mice to somewhat lower infiltration of α4β7high cells relative to α4β7int cells activated in the same compartment. The selective reduction in both the MFI and the percentage α4β7+ cells in parathymic and paratracheal LN was observed in four independent experiments. These results demonstrate that entry of α4β7high cells into both components of the mediastinal LN is mediated by α4β7 itself.
MAdCAM-1 is expressed on HEV in parathymic and paratracheal LN, but entry of α4β7high OT-1 cells into mediastinal LN is not MAdCAM-1 dependent
It has been established that the major ligand for α4β7 enabling entry into small intestine and its associated lymphoid tissue is mucosal vascular addressin MAdCAM-1 (25). However, its expression in mediastinal LN has not been reported. When we examined parathymic and paratracheal LN in naive and i.p. immunized animals by immunofluorescence, MAdCAM-1 expression was observed on HEV (as identified by costaining with anti-smooth muscle α-actin) (Fig. 6, a–e). By comparison with mesenteric LN, this expression was limited to a subset of HEV. To test the hypothesis that this ligand was binding α4β7 on OT-1 cells to allow entry into mediastinal LN, i.p. immunized mice were treated with anti-MAdCAM-1 Ab (19). Surprisingly, MAdCAM-1 blockade had no effect on the presence of α4β7high cells in either component of the mediastinal LN, based either on a reduction in the MFI of extensively divided α4β7high cells in treated mice relative to their rat IgG-treated counterparts (Fig. 6f), or the percentage of α4β7+ cells (Fig. 6g). In contrast, MAdCAM-1 blockade almost completely inhibited entry of activated OT-1 cells into small intestine (Fig. 6i). In total, these data demonstrated that despite expression of MAdCAM-1 on HEV, entry of α4β7high cells into mediastinal LN was MAdCAM-1 independent.
Ag-presenting BMDC require the lymphoid microenvironment to induce α4β7, but not α4β1 up-regulation on activated T cells
The results of Figs. 3 and 4 established that the kinetics, level, and type of α4 integrin expression induced by immunization with exogenous BMDC varied with the lymphoid compartment in which activation occurred. Therefore, we examined the relative contributions of the BMDC themselves and the lymphoid microenvironment in imprinting α4 integrin expression. To assess the ability of BMDC to induce α4β1 and α4β7 expression on activated T cells in the absence of the lymphoid microenvironment, OT-1 cells were stimulated with OVA257-pulsed CD40L-activated BMDC in vitro. Divided OT-1 cells did not express α4β7 after 4 days (Fig. 7a) and remained α4β7negative after 6 days (our unpublished observations), indicating that the lymphoid microenvironment was necessary for imprinting both α4β7int and α4β7high expression. In contrast, virtually all of these cells became α4β1+ by the second cell division. Interestingly, peptide-pulsed endogenous DC enriched from mediastinal LN or spleen did not significantly increase α4β1 expression on OT-1 cells during activation in vitro, despite inducing robust proliferation (Fig. 7b). This indicates that the simple acquisition of α4β1 on activated CD8 T cells is a property of exogenous BMDC and not of the lymphoid microenvironment.
FIGURE 7.
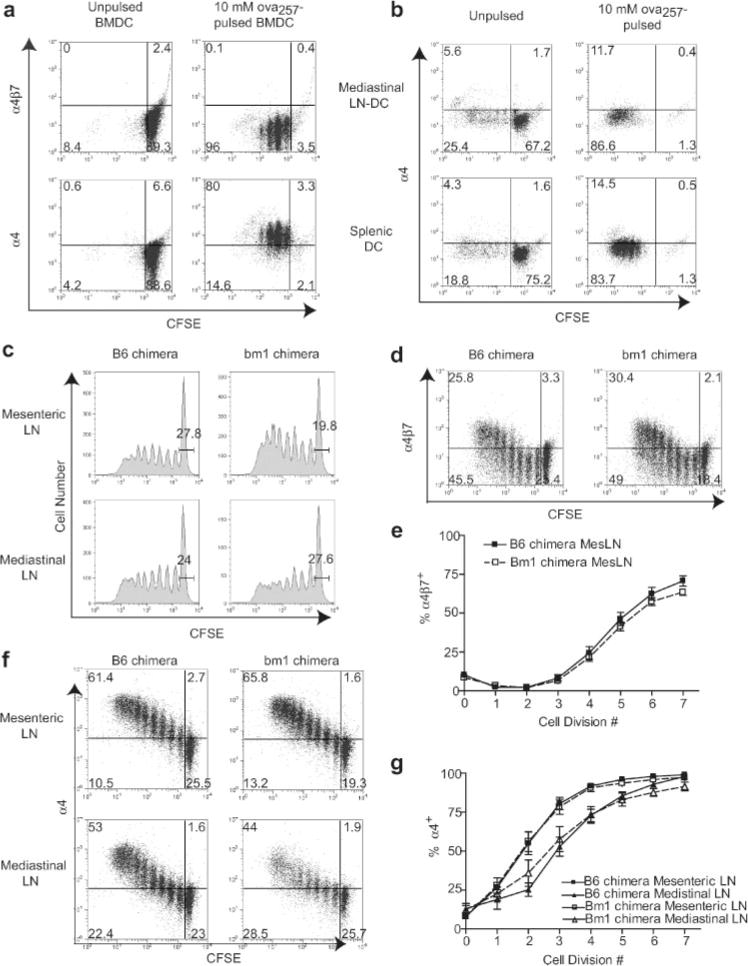
Roles of BMDC and endogenous DC in imprinting of α4 integrin expression on activated OT-1 cells. a and b, CFSE-labeled, CD8-enriched OT-1 cells were cultured in vitro for 4 days with unpulsed or 10 μM OVA257-pulsed CD40L-activated BMDC (a) or DC enriched from mediastinal LN or spleen (b) from fms-like tyrosine kinase-3 ligand-treated mice. Dot plots are gated on CD8+ (a) or Thy1.2+ (b) lymphocytes. Data are representative of three independent experiments. c–g, Lethally irradiated B6 mice were reconstituted with bone marrow from either bm1 or B6 mice. At least 10 wk after bone marrow transplant, these bone marrow were adoptively transferred with CFSE-labeled, CD8-enriched OT-1 cells, and then immunized i.p. with CD40L-activated OVA257-pulsed BMDC. Lymphoid organs were collected 3 days after immunization. c, OT-1 cell proliferative responses in priming compartments of bm1 and B6 chimeras. Histograms are gated on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. Values are percentage of CFSE undiluted of total OT-1 cells. d, Expression of α4β7 on OT-1 cells in the mesenteric LN of bm1 and B6 chimeras. Dot plots are gated on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. e, Percentage of OT-1 cells that were α4β7+ at each cell division in the mesenteric LN as determined by CFSE dilution. Data are mean values (±SEM) from five bm1 chimeras and four B6 chimeras analyzed in three independent experiments. f, Expression of α4 on OT-1 cells in priming compartments of bm1 and B6 chimeras. Dot plots are gated on CD8+H-2Kb-OVA257-tetramer+ lymphocytes. g, Percentage of OT-1 cells that were α4+ at each cell division in priming compartments as determined by CFSE dilution. Data are mean values (±SEM) from five bm1 chimeras and four B6 chimeras analyzed in three independent experiments.
Several studies have demonstrated that DC enriched from mesenteric LN can imprint α4β7 expression on T cells during activation in vitro (10–12). Because BMDC were unable to up-regulate α4β7, this raised the question of whether the endogenous cells in the LN that imprinted expression of this integrin in vivo also were responsible for presenting Ag. To address this, we used irradiated C57BL/6 mice reconstituted with bone marrow from bm1 mice, which have a mutated H-2Kb molecule that is unable to present OVA257 to OT-1 cells (26). After i.p. immunization with OVA257-pulsed BMDC derived from B6 mice, OT-1 cells in both bm1 and B6 bone marrow chimeras showed comparable overall activation based on CFSE dilution profiles (Fig. 7c). Importantly, α4β7 expression occurred with similar kinetics and expression levels in both chimeras (Fig. 7, d and e). This same result was also observed by ablation of endogenous Ag presentation using β2-microglobulin-deficient mice (our unpublished observations). These results demonstrated that α4β7 integrin expression after BMDC immunization did not require Ag presentation by endogenous DC. Interestingly, we found that the delayed kinetics of α4β1 up-regulation in mediastinal LN as compared with mesenteric LN (previously shown in Fig. 3b) was also observed in i.p. immunized bm1 chimeras (Fig. 7, f and g). These results establish that the induction of α4β7 expression and modulation of α4β1 expression kinetics on T cells activated by BMDC are properties of the lymphoid microenvironment, and are separable from the cells in LN that present Ag.
Discussion
In this study, we have used BMDC to gain insight into the activation of CD8+ T cells occurring in individual lymphoid compartments. BMDC introduced by different routes entered distinct lymphoid compartments, enabling us to compare T cell responses in these specific environments. By using preactivated, peptide-pulsed BMDC that traffic rapidly, we avoid the more prolonged accumulation of cells presenting Ag, as well as their uncertainty about their distribution, which would occur using virus or peptide in adjuvant. This enabled us to evaluate the kinetics of T cell activation and differentiation with a high degree of precision. Activated OT-1 cells in all priming compartments up-regulated α4β1 integrin. This was mediated by BMDC, although the lymphoid microenvironment impacted the kinetics of up-regulation. In contrast, α4β7 expression was imprinted by the lymphoid microenvironment, but endogenous Ag presentation was not required. The microenvironment controlled not only the proportion of cells that ultimately expressed this integrin, but also its level on individual cells. Only those cells expressing high levels of α4β7 after activation in mesenteric LN were able to home to the gut. These same cells redistributed into both parathymic and paratracheal LN early after activation by an α4β7-dependent, but MAdCAM-1- and CD62L-independent mechanism. These results identify distinct roles for BMDC and the lymphoid microenvironment in determining integrin expression on activated T cells and the redistribution of these cells into both lymphoid and nonlymphoid tissues.
Our work offers new insights into the precise sites of BMDC trafficking after introduction by different routes. Whereas prior work established localization of BMDC to spleen following i.v. introduction (3, 5–9), their entry into paratracheal LN has not been reported. This observation, however, is consistent with the involvement of the paratracheal LN as the major receptacle of lung lymphatic drainage (27), and the entry of BMDC into lung shortly after i.v. introduction (5, 6, 9, 27, 28). In contrast, there have been significant discrepancies among studies evaluating cell migration out of the peritoneal cavity. Lymphocytes introduced i.p. migrated to mediastinal, but not mesenteric LN (29). Conversely, another study that used indium-111-labeled BMDC found migration only to the spleen (6). However, the association of radioactivity with viable and functional BMDC was not established. In this study, we have demonstrated that Ag-presenting BMDC introduced i.p. trafficked to mesenteric LN and both parathymic and paratracheal LN. Whereas BMDC may have specific migratory capacities to access mesenteric LN that lymphocytes lack, we found no evidence for delayed entry of functional BMDC into any other lymphoid compartment after either i.p. or i.v. introduction, even after waiting up to 48 h. Because only targeting of mesenteric LN led to gut-localized effectors, these data suggest that i.p., but not i.v. immunization would be likely to provide protection against infection or tumor in the gut.
The use of BMDC as immunogens allowed us to follow the process of T cell activation in individual LN with a new level of precision, and to establish that the details are different in distinct lymphoid compartments. Whereas the expression of α4β7 integrin by a majority of T cells activated within mesenteric LN is consistent with earlier studies (11, 30), our work demonstrates that it is also expressed at lower levels on small, but significant populations of cells activated in other compartments. These α4β7int cells do not express CCR9 and do not home to the gut, and their functional significance remains to be investigated. In addition, the expression of α4β7 on cells activated in mesenteric LN was significantly delayed relative to that of α4β1. Because we have established that BMDC suffice to induce α4β1, whereas α4β7 is controlled by the microenvironment, these results suggest that the microenvironmental influence is delayed, and that it operates to control expression of β7. Although it is not clear whether these cells continue to express α4β1, they do remain β1 positive (our unpublished observations). Thus, the use of BMDC has illuminated details of the T cell differentiation process in mesenteric LN that were not previously appreciated. Finally, we have demonstrated that the kinetics of α4β1 expression as a function of cell division were delayed in mediastinal LN as compared with mesenteric LN. This might reflect the influence of microenvironmental factors that promote or delay expression that is induced by BMDC. Alternatively, it may result from differences in BMDC number or kinetics of infiltration in different lymphoid compartments. Regardless of the mechanism, the results establish both qualitative and quantitative differences in the differentiation of activated T cells in individual LN.
One hypothesis to explain these compartmental differences is that they reflected the entry of distinct functional subsets of BMDC that developed under the influence of the nonlymphoid environment at the site of injection. Indeed, Calzascia et al. (31) concluded that α4 integrin and E- and P-selectin ligand expression on activated T cells in different LN was determined by the site from which DC trafficked, and not the LN microenvironment. However, we found that α4 integrin expression of T cells activated in mediastinal LN after i.v. and i.p. immunization was the same, despite BMDC entry via different lymphatics. In addition, BMDC injected into the peritoneal cavity generated predominantly α4β7high cells in mesenteric LN, but predominantly α4β1+ cells in mediastinal LN. Thus, different T cell differentiation programs were induced in distinct lymphoid compartments infiltrated by BMDC from the same source. One important difference is that Calzascia et al. (31) used locally injected tumor to introduce Ag into resident DC in different peripheral compartments, rather than the exogenous BMDC used in this study. These BMDC are uniformly highly activated, with no evidence of functional heterogeneity, and are not likely to be susceptible to further maturation under the influence of the injection environment. Whereas we cannot rule out peripheral tissue-dependent differences in DC maturation, our data clearly point to an important role of the lymphoid environment in determining the kinetics and extent to which T cells acquire integrin expression that is independent of this possibility.
This conclusion is in keeping with the demonstration that resident DC from different LN compartments imprint homing receptor expression on T cells (13, 32), and the identification of retinoic acid as a soluble mediator of α4β7 imprinting (33). It is also in keeping with the demonstration that α4β7 expression on circulating effectors was much higher after BMDC immunization in vivo than after in vitro culture (14). Because the BMDC used were immature, this was interpreted to reflect their maturation in vivo. Conversely, we have demonstrated in this study that whereas CD40L-activated, peptide-pulsed BMDC were unable to induce α4β7 expression in vitro, its induction in vivo was not dependent on Ag presentation by endogenous DC. Thus, our data demonstrate that Ag presentation and induction of α4β7 expression need not be mediated by the same cell in the LN.
A final important observation in this work was that α4β7high T cells rapidly redistributed into mediastinal LN from their site of activation in mesenteric LN. The redistribution of activated T cells is not readily apparent when examining immune responses to systemically distributing virus or peptide because of the inability to distinguish cells that were primed in a compartment from cells recirculating into that compartment. In a small number of studies using localized Ag challenge (34, 35), redistribution to noncontiguous LN was inferred from the extent of cell division of Ag-specific cells, but more extensive Ag presentation in these additional compartments due to Ag spreading was not excluded. The use of localized BMDC immunization and FTY720 to block T cell egress from LN definitively demonstrates the existence of this LN to LN redistribution process. We have characterized this process in greater detail elsewhere (S. Sheasley-O’Neill, C. C. Brinkman, and V. H. Engelhard, manuscript submitted). This work has demonstrated that redistribution is widespread in different LN compartments and usually mediated by CD62L. However, in the present work, we have made the novel observation that the entry of these cells into mediastinal LN is mediated by α4β7, not CD62L, but apparently via recognition of a ligand other than MAdCAM-1. This is surprising because MAdCAM-1 is the major ligand for α4β7-mediated entry into gut and GALT (25), and we found it on a subset of mediastinal LN HEV. However, MAdCAM-1 is also expressed in the sinus-lining cells of the spleen, but is irrelevant for homing to that organ (36, 37). We considered the possibility that α4β7high cells enter mediastinal LN via afferent lymphatics from lung or peritoneal cavity rather than from the bloodstream. However, this is unlikely because we have shown that entry into mediastinal LN is blocked using anti-α4β7 Ab in vivo, whereas this maneuver has no effect on lymphocyte entry into lung (25). Additionally, lymphocyte entry into the peritoneal cavity has been shown to be dependent on P- or E-selectin ligands (38, 39). Together, this suggests that entry of activated α4β7high cells is mediated by α4β7 integrin binding to an as yet unidentified ligand expressed on mediastinal LN HEV.
In summary, this study demonstrates that BMDC immunization route determines the sites of T cell priming, which then impacts integrin expression on activated T cells and the distribution of activated cells in lymphoid and nonlymphoid tissues. This work has important clinical implications because BMDC-based immunotherapeutic strategies to date have not specifically addressed the route of immunization as a variable, nor considered the possibility that routing might alter the ability to generate gut-associated immunity. These results underscore the importance of site of BMDC targeting in inducing differentially targeted immune responses, and provide insight into mechanisms of homing receptor imprinting resulting from BMDC immunization.
Acknowledgments
We thank Janet V. Gorman and Sarah T. Lewis for excellent technical assistance. We also thank Dr. Victoria Camerini, Dr. Young Hahn, and Dr. Thomas Braciale for providing the lymphocyte recovery protocols for the small intestine, liver, and lung, respectively. We thank T. J. O’Neill for assistance with the analysis of LN HEV by immunofluorescence. Finally, we thank Dr. Klaus Ley, Dr. Timothy Bullock, and Lisa Nichols for their insightful discussions.
Footnotes
This work was supported by U.S. Public Health Service Grants CA78400, AI20963, and AI059996 (to V.H.E.). S.L.S.-O. was supported by Training Grant GM07627.
Abbreviations used in this paper: DC, dendritic cell; BMDC, bone marrow-derived DC; HEV, high endothelial venule; intintermediate; LN, lymph node; MAdCAM-1, mucosal addressin cell adhesion molecule-1; MFI, mean fluorescence intensity.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. [Published erratum appears in 1990 J Exp Med 172: 1275] J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins DW, Bullock TN, Colella TA, Robila VV, Engelhard VH. Immune responses to the HLA-A*0201-restricted epitopes of tyrosinase and glycoprotein 100 enable control of melanoma outgrowth in HLA-A*0201-transgenic mice. J Immunol. 2001;167:4853–4860. doi: 10.4049/jimmunol.167.9.4853. [DOI] [PubMed] [Google Scholar]
- 3.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarei S, Abraham S, Arrighi JF, Haller O, Calzascia T, Walker PR, Kundig TM, Hauser C, Piguet V. Lentiviral transduction of dendritic cells confers protective antiviral immunity in vivo. J Virol. 2004;78:7843–7845. doi: 10.1128/JVI.78.14.7843-7845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lappin MB, Weiss JM, Delattre V, Mai B, Dittmar H, Maier C, Manke K, Grabbe S, Martin S, Simon JC. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181–188. doi: 10.1046/j.1365-2567.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 7.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 8.Okada N, Tsujino M, Hagiwara Y, Tada A, Tamura Y, Mori K, Saito T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br J Cancer. 2001;84:1564–1570. doi: 10.1054/bjoc.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olasz EB, Lang L, Seidel J, Green MV, Eckelman WC, Katz SI. Fluorine-18 labeled mouse bone marrow-derived dendritic cells can be detected in vivo by high resolution projection imaging. J Immunol Methods. 2002;260:137–148. doi: 10.1016/s0022-1759(01)00528-2. [DOI] [PubMed] [Google Scholar]
- 10.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of α4 β7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 13.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 14.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 15.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullock TNJ, Colella TA, Engelhard VH. The density of peptides displayed by dendritic cells affects immune responses to human tyrosinase and gp100 in HLA-A2 transgenic mice. J Immunol. 2000;164:2354–2361. doi: 10.4049/jimmunol.164.5.2354. [DOI] [PubMed] [Google Scholar]
- 17.Gallatin WM, I, Weissman L, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 18.Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, Kilshaw PJ, Butcher EC. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]
- 19.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 20.Levelt CN, Mombaerts P, Wang B, Kohler H, Tonegawa S, Eichmann K, Terhorst C. Regulation of thymocyte development through CD3: functional dissociation between p56lck and CD3σ in early thymic selection. Immunity. 1995;3:215–222. doi: 10.1016/1074-7613(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 21.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system: intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–1776. [PubMed] [Google Scholar]
- 22.Cruise MW, Melief HM, Lukens J, Soguero C, Hahn YS. Increased Fas ligand expression of CD4+ T cells by HCV core induces T cell-dependent hepatic inflammation. J Leukocyte Biol. 2005;78:412–425. doi: 10.1189/jlb.0105005. [DOI] [PubMed] [Google Scholar]
- 23.Shurin MR, Pandharipande PP, Zorina TD, Haluszczak C, Subbotin VM, Hunter O, Brumfield A, Storkus WJ, Maraskovsky E, Lotze MT. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–184. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–575. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 25.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 26.Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110–117. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 27.Tilney NL. Patterns of lymphatic drainage in the adult laboratory rat. J Anat. 1971;109:369–383. [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat. 1995;186:453–467. [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade W, Johnston MG, Hay JB. The exit of lymphocytes and RBCs from the peritoneal cavity of sheep. Immunobiology. 1996;195:77–90. doi: 10.1016/S0171-2985(96)80007-6. [DOI] [PubMed] [Google Scholar]
- 30.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Dudda JC, Martin SF. Tissue targeting of T cells by DCs and microenvironments. Trends Immunol. 2004;25:417–421. doi: 10.1016/j.it.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Lambrecht BN, Pauwels RA, de St Groth BF. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000;164:2937–2946. doi: 10.4049/jimmunol.164.6.2937. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 36.Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147:763–771. [PMC free article] [PubMed] [Google Scholar]
- 37.Nolte MA, Hamann A, Kraal G, Mebius RE. The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunology. 2002;106:299–307. doi: 10.1046/j.1365-2567.2002.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4+ and CD8+ T cells. J Exp Med. 1999;189:1765–1776. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thoma S, Bonhagen K, Vestweber D, Hamann A, Reimann J. Expression of selectin-binding epitopes and cytokines by CD4+ T cells repopulating scid mice with colitis. Eur J Immunol. 1998;28:1785–1797. doi: 10.1002/(SICI)1521-4141(199806)28:06<1785::AID-IMMU1785>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Schneck J, Munitz T, Coligan JE, Maloy WL, Margulies DH, Singer A. Inhibition of allorecognition by an H-2Kb-derived peptide is evidence for a T-cell binding region on a major histocompatibility complex molecule. Proc Natl Acad Sci USA. 1989;86:8516–8520. doi: 10.1073/pnas.86.21.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]


