Abstract
Background and aims
The impact of tobacco use and cessation on atherogenesis remains unclear. We aimed to study the association of tobacco use and prior cessation with the presence, extent and severity of atherosclerosis on coronary computed tomographic angiography (CTA).
Methods
We examined 1798 consecutive symptomatic patients without known coronary artery disease (CAD) referred for CTA, stratified by smoking status (never, current [within 30 days], or former [>30 days before CTA]). Plaque severity (none,<50%, ≥50% stenosis), composition (non-calcified [NCP], partially calcified [PCP], or calcified plaque [CP]), and segment involvement score (SIS) were visually graded. Multivariate analysis was performed, adjusting for CAD risk factors and cholesterol lowering medication use.
Results
The median age of patients was 50 years [IQR:42–58] (61% male), with 74% never smokers, 12% current smokers, and 14% former smokers (median quit duration=12 years [IQR:3–26]). Smoking exposure in former versus current smokers was 11 [IQR:5–25] and 10 [IQR:2–20] pack-years, respectively (p=0.01). Compared to never smokers, current smokers demonstrated an increased odds ratio of all plaque types (adjusted OR: any NCP=1.55 [95% CI 1.04–2.32], p=0.03; any PCP=1.61 [1.10–2.37], p=0.02; any CP=1.93 [1.32–2.81], p=0.001), non-obstructive CAD (aOR=1.47 [1.04, 2.07], p=0.03), obstructive CAD (aOR=1.81 [1.01–3.24], p=0.047), and SIS > 4 (aOR=1.60 [1.04–2.46], p=0.03). Compared to current smoking, prior smoking cessation (≥ 12 years) was associated with a decreased odds ratio of any NCP (aOR=0.42 [0.19–0.90], p=0.03), CP (aOR=0.43 [0.22–0.84], p=0.02), and obstructive CAD (aOR=0.40, [0.15–0.98], p=0.048).
Conclusions
Current smoking is independently associated with the presence and extent of coronary plaque, and a higher risk of non-obstructive and obstructive CAD compared to never smoking. Prior smoking cessation correlated with improvements in CTA-identified plaque measures.
Keywords: Atherosclerosis, coronary computed tomographic angiography, smoking, tobacco, coronary artery disease
Introduction
Cardiovascular disease (CVD) mortality has declined in recent decades owing to improvements in the management of CVD and its risk factors, including significant efforts to curb tobacco use (1). Still, the impact of smoking-related CVD on public health remains a global issue. In the United States, nearly 42 million (~1 in 5) adults are active smokers (2), contributing to the more than 16 million U.S. adults who live with a smoking-related disease (3). While smoking is well-known to increase the risk of major adverse cardiac events (MACE), the extent to which tobacco use and cessation impacts atherogenesis warrants further study.
Coronary computed tomographic angiography (CTA) is an established noninvasive technique used to visualize the presence, extent, severity and composition of atherosclerosis. Though prior studies have shown that the degree of coronary artery disease (CAD) can strongly predict future MACE (4), few studies have examined the relationship between smoking status and CTA-identified plaque (5, 6). Of these, the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes) multinational registry observed a higher rate of MACE in current but not former smokers undergoing coronary CTA by comparison to never smokers (5). Yet, few studies have examined the dose-dependent impact of smoking on CTA-identified atherosclerosis or controlled for cholesterol lowering medication use among potential covariates. We aimed to examine the relationship between tobacco use and prior cessation on the presence, extent, composition and severity of CTA-identified coronary atherosclerosis.
Patients and Methods
Patient population
We examined 1,798 consecutive symptomatic patients without known CAD clinically referred for coronary CTA between May 2006 – February 2013 at Walter Reed National Military Medical Center. Patient demographics, medical history, and CTA results were collected prospectively as described previously (7). After CTA, comprehensive prescription, laboratory data and smoking status were independently queried using all available electronic medical records of the Department of Defense Military Healthcare System - a large, closed international health care network providing comprehensive health care, labs and medications. Independent data extraction was blinded to CTA findings in order to verify: smoking status, smoking history (pack-years), prior smoking cessation (quit duration), most recent cholesterol panel and statin or nonstatin cholesterol lowering medication use within 12 months prior to CTA.
Consistent with National Cholesterol Education Program criteria, smoking status was defined as: never, current (cigarette or cigar use ≤ 30 days prior to CTA), or former smoker (use >30 days prior to CTA) (8). Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, and/or treatment with an antihypertensive medication (9). Diabetes mellitus was defined by a prior fasting blood glucose ≥ 126 mg/dL, 2-hour blood glucose ≥ 200 mg/dL during oral glucose tolerance testing, a hemoglobin A1c ≥ 6.5%, a random blood glucose ≥ 200 mg/dL, and/or use of insulin or an oral hypoglycemic agent (10). Dyslipidemia was defined as total cholesterol > 240 mg/dL, serum triglycerides > 150 mg/dL, high density lipoprotein cholesterol (HDL) < 40 mg/dL (male) or < 50 mg/dL (women), or treatment with a cholesterol lowering medication (8,11). Family history of early CAD was defined as clinical CAD involving a first-degree male relative less than 55 years old or first-degree female relative less than 65 years old.
Patients were excluded for unknown smoking status (n=51), or a history of known CAD defined as a prior myocardial infarction or coronary revascularization (n=64). The study was approved by local institutional review and compliant with Health Insurance Portability and Accountability Act guidelines.
Coronary CTA
Coronary CTA was performed by 64-slice CT (Lightspeed VCT or Discovery CT750 HD; GE Healthcare, WI), with heart rate control and pre-scan sublingual nitroglycerin according to guidelines (12). An initial non-contrast CT was acquired for calcium scoring in a majority of patients (n=1574, 88%), and coronary artery calcium (CAC) scores were graded by the Agatston method (13). Contrast-enhanced coronary CTA was obtained by prospective ECG-triggering (n=1159, 64%) or retrospective ECG-gating with dose modulation (n=639, 36%) following a timing bolus. CTA acquisition variables were adjusted by the CTA provider in accordance with guidelines to minimize patient radiation exposure (12, 14). CTA scans were interpreted jointly (consensus) by a level 3-certified cardiologist and radiologist as part of routine clinical care (15). Using an 18-segment model, each coronary segment >1.5-mm diameter was assessed for CAD presence and severity by visual grading defined as: normal (no plaque), non-obstructive (1-49% stenosis), obstructive (≥ 50%), or uninterpretable (≥ 1 uninterpretable segment). Consistent with prior research, we used an intention-to-diagnose approach where patients with ≥ 1 uninterpretable segment were categorized as having obstructive CAD (16). The number of coronary segments with any plaque was graded by the segment involvement score, as the sum of the number of segments with any plaque irrespective of the degree of luminal stenosis (17). In each segment with plaque, we performed a qualitative assessment of plaque composition defined as predominant non-calcified plaque (NCP), partially calcified plaque (PCP), or calcified plaque (CP) (15).
Statistical analysis
Continuous variables with normal distributions are expressed as mean ± 1 standard deviation (SD) and compared with the Student’s t test for two independent groups and 1-way analysis of variance between multiple groups. Continuous variables with non-normal distributions are expressed as median ± interquartile range (IQR) and compared with the Wilcoxon rank-sum test. Categorical variables are expressed as frequencies (%) and compared by the Pearson Chi-square test. Binary logistic regression was performed to obtain all odds ratios, with multivariate adjustment for age, gender, body mass index, diabetes, family history of early CAD, hypertension, and hyperlipidemia as a binary variable incorporating the use of a statin or non-statin cholesterol medication. To examine the dose-dependent effect of smoking on atherosclerotic plaque burden and composition, we performed adjusted odds ratios among smokers above the median exposure period (≥ 12 pack-years), with never smokers as a comparison group. Additionally, a 12-year period for quit duration was used to compare patients across the median value for prior smoking cessation, and considering evidence that the risk of coronary heart disease normalizes approximately 10–15 years after quitting (18, 19). Statistical analysis was performed using Stata (Version 13.1, Statacorp, TX), and a 2-tailed p-value < 0.05 was considered significant.
Results
Baseline characteristics
The median age of all patients was 50 years [IQR: 42–58 years] (61% male), including 74% never smokers, 14% former smokers, and 12% current smokers (Table 1). Former smokers were more likely to be older, with greater smoking exposure (pack-years), and had a higher prevalence of hypertension and diabetes. Conversely, current smokers were younger and more predominantly male. By comparison to never smokers, current smokers reported a higher frequency of any chest pain. Additionally, our study found a step-wise increase in triglyceride levels from never smokers, to former and current smokers (p < 0.001 between groups). Conversely, we identified a trend towards lower HDL in current smokers compared to former smokers and never smokers (p=0.10 between groups). While the rate of medication use was not significantly different between groups, a trend towards lower statin or non-statin cholesterol medication use was noted among current smokers (p=0.10 between groups).
Table 1.
Baseline characteristics.
| All patients (n=1,798) |
Never smoked (n=1339, 74%) |
Former smoker (n=243, 14%) |
Current smoker (n=216, 12%) |
p-value | |
|---|---|---|---|---|---|
| Age, years | 50 [IQR: 42–58] | 50 [IQR: 42–58] | 53 [IQR: 45–61] | 44 [IQR: 35–55] | < 0.001 |
| Male, n (%) | 1103 (61%) | 784 (59%) | 150 (62%) | 169 (78%) | < 0.001 |
| Body mass index, kg/m2 | 29 ± 5 | 28 ± 5 | 30 ± 5 | 28 ± 5 | 0.57 |
| Smoking duration, pack-years | – | – | 11 [IQR: 5–25] | 10 [IQR: 2–20] | 0.01 |
| Time since last cigarette, years | – | – | 12 [IQR: 3–26] | (Less than 30 days) | — |
| Hypertension, n (%) | 851 (47%) | 627 (47%) | 139 (57%) | 85 (39%) | 0.003 |
| Hyperlipidemia, n (%) | 818 (45%) | 599 (45%) | 127 (52%) | 92 (43%) | 0.21 |
| Diabetes mellitus, n (%) | 178 (10%) | 123 (9%) | 38 (16%) | 17 (8%) | 0.008 |
| Family history of CAD, n (%) | 416 (23%) | 300 (22%) | 59 (24%) | 57 (26%) | 0.86 |
| Any chest pain, n (%) | 1274 (71%) | 922 (69%) | 177 (73%) | 175 (81%) | 0.001 |
| - Non-anginal chest pain | 181 (10%) | 147 (11%) | 19 (8%) | 15 (7%) | |
| - Atypical chest pain | 944 (53%) | 664 (50%) | 146 (60%) | 134 (62%) | 0.001 |
| - Typical chest pain | 149 (8%) | 111 (8%) | 12 (5%) | 26 (12%) | |
| Dyspnea, n (%) | 487 (27%) | 346 (26%) | 80 (33%) | 61 (28%) | 0.15 |
| Statin use, n (%) | 604 (34%) | 458 (34%) | 87 (36%) | 59 (27%) | 0.10 |
| Non-statin cholesterol med, n (%) | 159 (9%) | 113 (8%) | 30 (12%) | 16 (7%) | 0.10 |
| Aspirin use, n (%) | 613 (34%) | 445 (33%) | 94 (39%) | 74 (34%) | 0.27 |
| Total cholesterol, mg/dL a | 189 ± 38 [n=1721] | 190 ± 38 [n=1287] | 189 ± 39 [n=233] | 189 ± 37 [n=201] | 0.70 |
| LDL-C, mg/dLa | 114 ± 34 [n=1718] | 114 ± 34 [n=1286] | 113 ± 34 [n=233] | 115 ± 33 [n=199] | 0.86 |
| HDL-C, mg/dLa | 53 ± 17 [n=1720] | 54 ± 17 [n=1287] | 50 ± 15 [n=233] | 48 ± 15 [n=200] | 0.10 |
| Triglycerides, mg/dLa | 122 ± 72 [n=808] | 117 ± 67 [n=641] | 136 ± 75 [n=93] | 143 ± 100 [n=74] | < 0.001 |
| Systolic BP, mmHga | 122 ± 16 [n=1697] | 122 ± 16 [n=1266] | 125 ± 16 [n=228] | 124 ± 16 [n=203] | 0.84 |
| Diastolic BP, mmHga | 74 ± 11 [n=1696] | 73 ± 11 [n=1265] | 75 ± 11 [n=228] | 76 ± 10 [n=203] | 0.24 |
Among patients with cholesterol and blood pressure values recorded within 12 months prior to coronary CTA [n = # of patients].
All values are expressed as mean ± SD, median [interquartile range], or frequency (%).
BP, blood pressure; CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Current smoking associated with CAD presence, extent, and severity
Coronary CTA findings are demonstrated in Table 2 stratified by smoking status, with no significant difference in CTA settings between groups. By comparison to never smokers, former and current smokers demonstrated a significant difference in CAC scores and CAD severity. Unadjusted, this finding appears driven by a higher frequency of both non-obstructive and obstructive CAD in former smokers. Notably, the severity of CAD and CAC was similar among current and never smokers despite a significant age difference between these groups (median age: 44 years vs. 50 years, respectively, p<0.001). Though observational, this finding supports the hypothesis that smoking may accelerate vascular aging (20). When stratified by age tertile and smoking status, current smoking was associated with the highest prevalence of obstructive CAD (Fig. 1).
Table 2.
CTA characteristics.
| All patients (n=1,798) |
Never smoked (n=1339, 74%) |
Former smoker (n=243, 14%) |
Current smoker (n=216, 12%) |
p-value | |
|---|---|---|---|---|---|
| Pre-scan heart rate, bpm | 57 ± 7 | 57 ± 7 | 57 ± 7 | 57 ± 6 | 0.23 |
| Tube current, mA | 539 ± 73 | 534 ± 73 | 562 ± 72 | 550 ± 74 | 0.97 |
| Tube potential, kV | 113 ± 12 | 112 ± 12 | 115 ± 12 | 113 ± 13 | 0.69 |
| Scan type | |||||
| - Prospective triggered | 1159 (64%) | 860 (64%) | 161 (66%) | 138 (64%) | 0.82 |
| - Retrospective gating | 639 (36%) | 479 (36%) | 82 (34%) | 78 (36%) | |
| CTA radiation dose, mSva | 3.9 ± 3.7 | 3.8 ± 3.6 | 4.6 ± 4.1 | 3.7 ± 3.3 | 0.15 |
| CAD severity | |||||
| - No CAD | 995 (55%) | 758 (57%) | 117 (48%) | 120 (56%) | |
| - CAD 1–49% stenosis | 604 (34%) | 438 (33%) | 95 (39%) | 72 (33%) | 0.04 |
| - CAC ≥ 50% stenosis | 139 (8%) | 97 (7%) | 26 (11%) | 20 (9%) | |
| - ≥ 1 uninterpretable segment | 60 (3%) | 46 (3%) | 5 (2%) | 4 (2%) | |
| Segment involvement score > 4 | 239 (13%) | 163 (12%) | 49 (20%) | 27 (13%) | 0.003 |
| CAC score obtained (# of patients) | 1574 (88%) | 1165 (87%) | 219 (90%) | 190 (88%) | 0.93 |
| - CAC = 0 Agatston units | 915/1574 (58%) | 699/1165 (60%) | 106/219 (48%) | 110/190 (58%) | |
| - CAC = 1–99 | 414/1574 (26%) | 296/1165 (25%) | 66/219 (30%) | 52/190 (27%) | 0.03 |
| - CAC = 100–399 | 157/1574 (10%) | 106/1165 (9%) | 34/219 (16%) | 17/190 (9%) | |
| - CAC ≥ 400 | 88/1574 (6%) | 64/1165 (5%) | 13/219 (6%) | 11/190 (6%) | |
Includes effective radiation for CTA only (excluding scout topogram, timing bolus and CAC scan).
CAC, coronary artery calcium; CAD, coronary artery disease; CTA, computed tomographic angiography.
Fig. 1. Rate of obstructive CAD stratified by smoking status and age tertile.
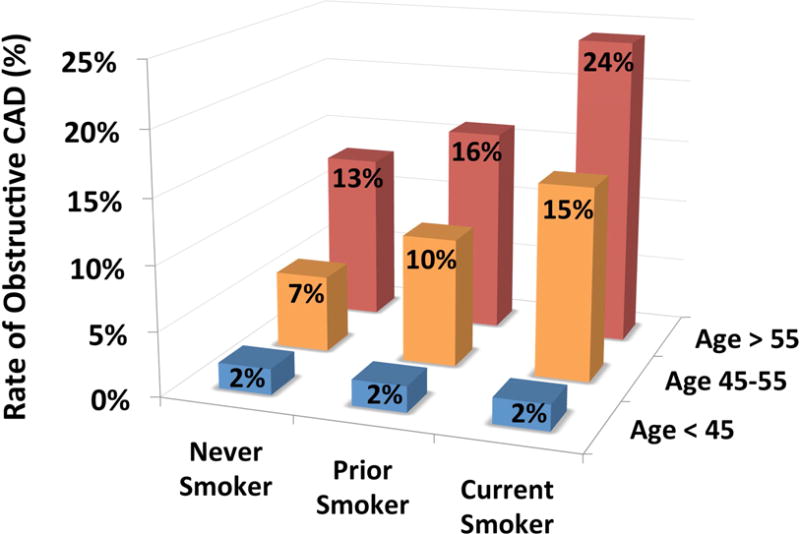
Demonstrates association of current smoking with the highest rate of obstructive CAD as a percentage of patients across age tertiles.
Considering baseline differences between groups noted in Table 1, we performed multivariate analyses to control for age, gender, CAD risk factors and cholesterol medication use. By comparison to never smokers, current but not former smoking was independently associated with an increased adjusted odds ratio of all plaque types, plaque extent (SIS > 4), and the presence of non-obstructive or obstructive CAD (Table 3).
Table 3.
Smoking association with plaque present, extent and severity. Adjusted for risk factors and medications.
| Never smoked (n=1339) |
Former smoker (n=243) | Current smoker (n=216) | |||||
|---|---|---|---|---|---|---|---|
| Adjusted ORa | 95% CI | p-value | Adjusted ORa | 95% CI | p-value | ||
| Any non-calcified plaque | Reference | 1.35 | [0.94, 1.94] | 0.11 | 1.55 | [1.04, 2.32] | 0.03 |
| Any partially calcified plaque | 1.19 | [0.84, 1.68] | 0.33 | 1.61 | [1.10, 2.37] | 0.02 | |
| Any calcified plaque | 1.24 | [0.87, 1.76] | 0.23 | 1.93 | [1.32, 2.81] | 0.001 | |
| Segment involvement score > 4 | 1.35 | [0.94, 1.95] | 0.11 | 1.57 | [1.03, 2.42] | 0.04 | |
| Normal CTA | 1.00 | [0.72, 1.40] | 0.98 | 0.64 | [0.45, 0.90] | 0.01 | |
| Non-obstructive CAD (< 50%) | 1.02 | [0.74, 1.39] | 0.93 | 1.47 | [1.04, 2.07] | 0.03 | |
| Obstructive CAD (≥ 50%) | 1.18 | [0.70, 1.98] | 0.53 | 1.81 | [1.01, 3.24] | 0.047 | |
All odds ratios obtained by binary logistic regression adjusted for age, gender, body mass index, diabetes, family history of early CAD, hypertension, hyperlipidemia, and use of a statin or nonstatin cholesterol medication.
CAD, coronary artery disease; CI, confidence interval; CP, calcified plaque; CTA, coronary computed tomographic angiography; NCP, non-calcified plaque; OR, odds ratio; PCP, partially calcified plaque.
Dose-dependent effect of smoking on CAD
To examine the dose-dependent effect of smoking on atherosclerotic plaque burden and composition, we performed multivariate analysis in nonsmokers versus smokers stratified above the median exposure period. As shown in Fig. 2, patients with ≥ 12 pack-year exposure were more likely to have partially calcified or calcified plaque, and more extensive plaque, by comparison to never smokers. When stratified by pack-decades, smoking exposure had a dose-dependent association with the presence of all plaque types by adjusted comparison to never smokers (Supplemental Fig. 1).
Fig. 2. Association of smoking history with CAD measures.
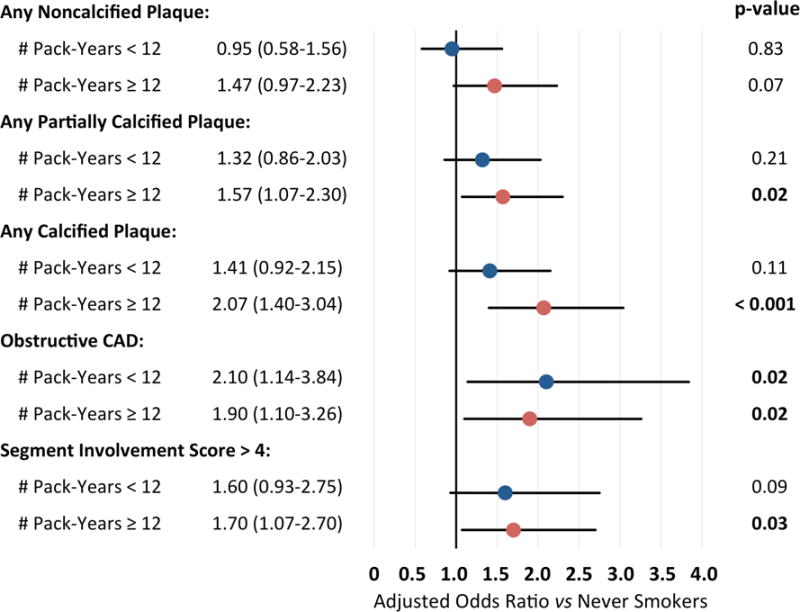
Adjusted odds ratio of CAD measures by comparison to never smokers (n=1339), among patients with complete pack-year information and < 12 pack-year (n=179) or ≥ 12 pack-year smoking history (n=149). Demonstrates that patients with ≥ 12 pack-year exposure had a higher likelihood of all CAD measures versus never smoking. Adjusted for age, gender, BMI, diabetes, family history of early CAD, hypertension, hyperlipidemia, and use of a statin or non-statin cholesterol medication.
Prior smoking cessation is associated with reduced CAD severity
Adjusted odds ratio of CAD measures stratified by duration of prior smoking cessation are shown in Fig. 3, using current smokers as the reference group. Among former smokers, we included patients with known quit date information, categorized as cessation within 12 years prior to CTA (n=179) and quit duration ≥ 12 years (n=149). By this analysis, patients who quit ≥ 12 years previously had a lower likelihood of non-calcified plaque, calcified plaque and obstructive CAD than current smokers.
Fig. 3. Association of prior smoking cessation with CAD measures.
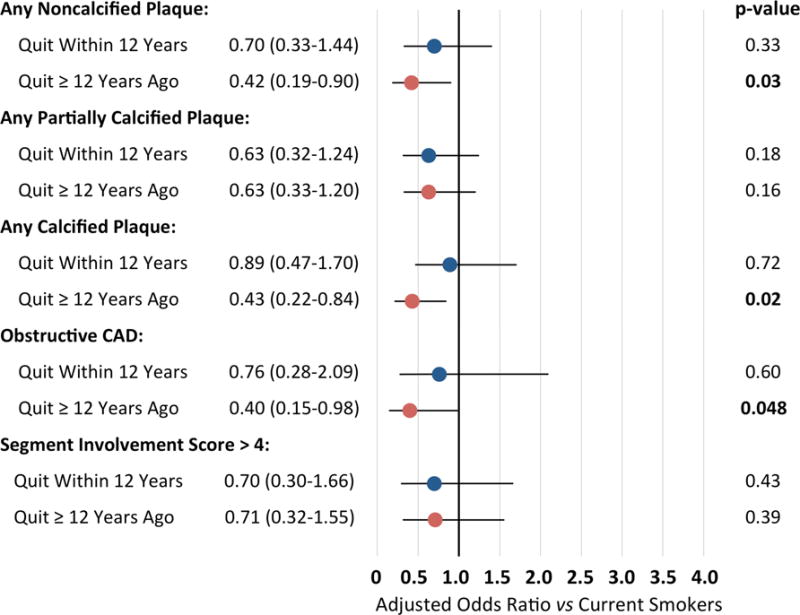
Adjusted odds ratio of plaque type, severity and extent by comparison to current smokers (n=216), among patients who quit smoking within 12 years prior to CTA (n=90) and ≥ 12 years prior to CTA (n=94). Note that patients who quit ≥ 12 years previously had a lower likelihood of non-calcified plaque, calcified plaque and a lower likelihood of obstructive CAD. Analyses performed for patients with complete smoking cessation information, adjusted for age, gender, BMI, diabetes, family history of early CAD, hypertension, hyperlipidemia, and statin or non-statin cholesterol medications.
Sensitivity analysis
Considering the potential impact of cholesterol medications on plaque analysis, we performed an additional sensitivity analysis excluding patients on a statin (n=604, 34%) or non-statin cholesterol lowering medication (n=159, 9%). The exclusion of patients on cholesterol lowering medications did not result in any significant differences in our primary analysis or associations between prior smoking cessation and plaque measures (Supplemental Tables 1 and 2).
Discussion
Among patients without known CAD referred for CTA, the main findings of this study are: (i) current but not former smoking was associated with the presence, extent and severity of CAD versus never smoking; (ii) smoking demonstrated a dose-dependent association with plaque presence and extent by comparison to never smokers; and (iii) patients who quit smoking ≥ 12 years prior to CTA had a lower likelihood of non-calcified plaque, calcified plaque and obstructive CAD by comparison to current smokers.
To date, several studies have shown that tobacco smoke contributes free radical-induced oxidative stress on the vascular endothelium, triggering an inflammatory response, hypercoagulable state and alterations in lipid profiles (21, 22). Conversely, smoking cessation has been shown to reverse the risk of MACE, with normalization of pro-inflammatory and hypercoagulability states to that of nonsmokers (23). Yet, the extent to which tobacco use and cessation impacts atherosclerosis remains unclear.
In a study of 6,814 adults without known CVD undergoing CAC scoring in the Multi-Ethnic Study of Atherosclerosis (MESA), McEvoy et al compared smoking status with inflammatory biomarkers and subclinical CAD (24). By comparison to never smoking, current smoking was associated with an increased hsCRP > 2 mg/dL (adjusted odds ratio = 1.7 [95% CI 1.5–2.1]) and subclinical CAD (aOR CAC >0 = 1.8 [95% CI 1.5–2.1]). Quitting smoking was independently associated with lower inflammation and atherosclerosis (aOR hsCRP>2 mg/dL = 0.91 [0.88–0.95]; aOR CAC>0 = 0.94 [0.90–0.97], respectively) for every 5-year cessation interval. Similarly, Kim et al. examined 7,104 asymptomatic Korean adults self-referred for screening coronary CTA, observing a higher prevalence of any plaque, non-calcified plaque, obstructive CAD, and CAC score > 100 by comparison to never-smokers (6). Adjusting for CVD risk factors, atherosclerosis increased in a dose-dependent fashion with increasing tobacco exposure in their study.
Among mechanisms that may contribute to atherosclerosis development in smokers, our study found a step-wise increase in triglyceride levels from never smokers (mean=117 mg/dL), to former smokers (136 mg/dL) and current smokers (143 mg/dL) (p< 0.001 between groups). Conversely, we identified a trend towards lower HDL in current smokers (mean=48 mg/dL) compared to former smokers (50 mg/dL) and never smokers (54 mg/dL) (p=0.10 between groups). While further studies are needed to examine the mechanisms of smoking-related atherogenesis, recent evidence suggests that alterations in endothelial progenitor cells, plasma microparticles and micro-RNA may offer potential targets to examine the impact of smoking on vascular injury and atherosclerosis (25–28).
Finally, our study adds to prior literature by providing an association of dose-dependent tobacco use and prior cessation across various plaque measures in a cohort of symptomatic patients clinically referred for coronary CTA. Though our study does not assess the relationship of smoking status, CAD severity and plaque composition with clinical outcomes, the international CONFIRM registry has previously observed a higher rate of MACE in current but not former smokers undergoing coronary CTA by comparison to never smokers (5). Considering available evidence, future studies are warranted to prospectively examine the long-term impact of smoking and tobacco cessation on atherogenesis and major adverse cardiac events.
We recognize several inherent limitations to our study. This is a cross-sectional analysis performed at a single-center, limiting generalizability. Second, our accounting of smoking status relied on accurate patient self-reporting and provider documentation, and provided no confirmation of smoking status by serum or urine cotinine levels. Further, we did not have information on second hand smoke exposure, which has been shown to contribute to inflammation, endothelial dysfunction and the development of atherosclerosis (29, 30). Finally, while we adjusted for CVD risk factors and cholesterol medications, unobserved confounders including diet and exercise may contribute to variations in plaque burden. Consequently, our results must be interpreted as hypothesis generating and not proof of causality. Acknowledging these limitations, our study adds to prior data regarding the association of tobacco use and cessation across a broad range of CTA-identified atherosclerosis measures.
Current smoking is significantly and independently associated with the presence and extent of coronary atherosclerosis, as well as an increased risk of non-obstructive and obstructive CAD by comparison to never smoking. Additionally, our study supports a dose-dependent relationship between smoking and atherogenesis in symptomatic patients clinically referred for CTA. Furthermore, prior smoking cessation correlated with improved plaque measures and strengthens the importance of smoking cessation to minimize the risk of cardiovascular disease.
Supplementary Material
Supplemental Fig. 1. Dose Dependent Effect of Smoking on Plaque Presence and Composition. Demonstrates increased odds ratio of all plaque types with increased pack-year exposure. Adjusted odds ratio and p-values by comparison to never smokers (n=1339), among patients with <10 pack-year (n=148), 10–19 pack-year (n=87), 20–29 pack-year (n=43), and ≥ 30 pack-year smoking history (n=59). All values adjusted for age, gender, BMI, diabetes, family history of early CAD, hypertension, hyperlipidemia, and use of a statin or nonstatin cholesterol medication.
Highlights.
Current smokers demonstrated an increase in coronary artery disease (CAD) extent and severity vs. never smokers
Ever smokers had a dose-dependent association with the presence and extent of CAD
Smoking cessation correlated with improved plaque measures vs. current smoking
Acknowledgments
Financial support
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Abbreviations
- CAD
coronary artery disease
- CP
calcified plaque
- CTA
computed tomographic angiography
- CVD
cardiovascular disease
- MACE
major adverse cardiac events
- NCP
non-calcified plaque
- PCP
partially calcified plaque
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest and no relationship with industry related to this work. The opinions herein and assertions contained are the authors’ alone, and do not constitute endorsement by the Department of the Army, Department of Defense or the United States Government. The content is solely the responsibility of the authors and does not represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Author contributions
All authors contributed significantly to the drafting of the manuscript, approve the work in its entirety, and meet criteria for authorship.
References
- 1.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. The New England journal of medicine. 2007;356(23):2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults—United States, 2005–2013. Morbidity and Mortality Weekly Report. 2014;63(47):1108–12. [accessed 2015 Jun 30] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [accessed 2015 Jun 30] [Google Scholar]
- 4.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2011;57(10):1237–47. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi R, Berman DS, Budoff MJ, Gransar H, Achenbach S, Al-Mallah M, et al. Current but not past smoking increases the risk of cardiac events: insights from coronary computed tomographic angiography. European heart journal. 2015;36(17):1031–40. doi: 10.1093/eurheartj/ehv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JA, Chun EJ, Lee MS, Kim KJ, Choi SI. Relationship between amount of cigarette smoking and coronary atherosclerosis on coronary CTA in asymptomatic individuals. The international journal of cardiovascular imaging. 2013;29(Suppl 1):21–8. doi: 10.1007/s10554-013-0224-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheezum MK, Hulten EA, Smith RM, Taylor AJ, Kircher J, Surry L, et al. Changes in preventive medical therapies and CV risk factors after CT angiography. JACC Cardiovasc Imaging. 2013;6(5):574–81. doi: 10.1016/j.jcmg.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA: the journal of the American Medical Association. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine. 2016 doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 11.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, et al. Prognostic value of non-obstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 12.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3(3):190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5(4):198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–58. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Cheezum MK, Subramaniyam PS, Bittencourt MS, Hulten EA, Ghoshhajra BB, Shah NR, et al. Prognostic value of coronary CTA vs. exercise treadmill testing: results from the Partners registry. Eur Heart J Cardiovasc Imaging. 2015;16(12):1338–46. doi: 10.1093/ehjci/jev087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. Journal of the American College of Cardiology. 2007;50(12):1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 18.Tobacco Control: Reversal of Risk After Quitting Smoking. IARC Handbooks of Cancer Prevention. 2007;11:11. [Google Scholar]
- 19.van Domburg RT, Meeter K, van Berkel DF, Veldkamp RF, van Herwerden LA, Bogers AJ. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. Journal of the American College of Cardiology. 2000;36(3):878–83. doi: 10.1016/s0735-1097(00)00810-x. [DOI] [PubMed] [Google Scholar]
- 20.Le J, Zhang D, Menees S, Chen J, Raghuveer G. “Vascular age” is advanced in children with atherosclerosis-promoting risk factors. Circulation Cardiovascular imaging. 2010;3(1):8–14. doi: 10.1161/CIRCIMAGING.109.880070. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T. Cigarette smoking as a risk factor of coronary artery disease and its effects on platelet function. Tob Induc Dis. 2004;2(1):27–33. doi: 10.1186/1617-9625-2-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8(7):917–32. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(4):1002–10. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanta S, Balasubramanian S, Rajasingh S, et al. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc Med. 2016;26:407–19. doi: 10.1016/j.tcm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobarrez F, Antoniewicz L, Bosson JA, Kuhl J, Pisetsky DS, Lundback M. The effects of smoking on levels of endothelial progenitor cells and microparticles in the blood of healthy volunteers. PloS one. 2014;9:e90314. doi: 10.1371/journal.pone.0090314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015;213:60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 28.Novak J, Olejnickova V, Tkacova N, Santulli G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. Adv Exp Med Biol. 2015;887:79–100. doi: 10.1007/978-3-319-22380-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferis BJ, Lowe GD, Welsh P, Rumley A, Lawlor DA, Ebrahim S, et al. Secondhand smoke (SHS) exposure is associated with circulating markers of inflammation and endothelial function in adult men and women. Atherosclerosis. 2010;208(2):550–6. doi: 10.1016/j.atherosclerosis.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallio K, Jokinen E, Saarinen M, Hamalainen M, Volanen I, Kaitosaari T, et al. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circulation Cardiovascular quality and outcomes. 2010;3(2):196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Dose Dependent Effect of Smoking on Plaque Presence and Composition. Demonstrates increased odds ratio of all plaque types with increased pack-year exposure. Adjusted odds ratio and p-values by comparison to never smokers (n=1339), among patients with <10 pack-year (n=148), 10–19 pack-year (n=87), 20–29 pack-year (n=43), and ≥ 30 pack-year smoking history (n=59). All values adjusted for age, gender, BMI, diabetes, family history of early CAD, hypertension, hyperlipidemia, and use of a statin or nonstatin cholesterol medication.


