Abstract
Progressive loss of tissue homeostasis is a hallmark of numerous age-related pathologies, including osteoarthritis (OA). Accumulation of senescent chondrocytes in joints contributes to the age-dependent cartilage loss of functions through the production of hypertrophy-associated catabolic matrix-remodeling enzymes and pro-inflammatory cytokines. Here, we evaluated the effects of the secreted variant of the anti-aging hormone α-Klotho on cartilage homeostasis during both cartilage formation and OA development. First, we found that α-Klotho expression was detected during mouse limb development, and transiently expressed during in vitro chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Genome-wide gene array analysis of chondrocytes from OA patients revealed that incubation with recombinant secreted α-Klotho repressed expression of the NOS2 and ZIP8/MMP13 catabolic remodeling axis. Accordingly, α-Klotho expression was reduced in chronically IL1β-treated chondrocytes and in cartilage of an OA mouse model. Finally, in vivo intra-articular secreted α-Kotho gene transfer delays cartilage degradation in the OA mouse model. Altogether, our results reveal a new tissue homeostatic function for this anti-aging hormone in protecting against OA onset and progression.
Keywords: α-Klotho, hormone, homeostasis, cartilage, aging
Introduction
Tissue homeostasis is ensured through self-repair of specialized cells and their replacements via differentiation of tissue-specific adult stem cells [1,2]. During aging, this equilibrium is gradually lost [2] through the expression of cellular senescence markers in tissues and the establishment of a senescence-associated secretory phenotype (SASP), that includes inflammatory and catabolic factors [2].
Osteoarthritis (OA) is the most common age-related osteoarticular disease characterized by the progressive loss of cartilage homeostasis, synovial activation and sub-chondral bone remodeling [3,4]. The molecular mechanisms responsible for OA onset have just started to be deciphered [4]. Repetitive mechanical stress and the resulting synovial inflammation trigger the acquisition of a p16INK4A–dependent senescence phenotype by articular chondrocytes [5] that resembles to their terminal differentiation program during endochondral ossification [5,6]. For instance, the production of many deleterious factors that alters the balance between anabolic and catabolic articular functions is a central event. Particularly, IL-1β plays a key role in OA onset by inducing in articular chondrocytes: (1) DNA damage accumulation through nitric oxide synthase 2 (NOS2)-dependent nitric oxide production [7], (2) the expression of senescence marker p16INK4A [6] (3) the onset of the hypoxia-inducible factor 2 (HIF2)-dependent terminal phase of endochondral ossification [8,9], and (4) the activation of the catabolic axis formed by the zinc importer ZIP8, metal-regulatory transcription factor-1 (MTF1) and the matrix metalloproteinase-13 (MMP13) [10].
Besides aging, genetic predisposition also favors OA development [11]. For instance, two single nucleotide polymorphisms in α-KL, the gene encoding the anti-geronic hormone α-Klotho, predispose to OA onset [12–14]. The human α-KL gene encodes four proteins: full length α-Klotho, two soluble variants, and one secreted form. Secreted α-Klotho results from alternative splicing of exon 3 and contains a N-terminal signal peptide and only one glycosyl hydrolase (KL) domain (KL1) with trophic factor-binding properties and low syalidase activity (for review [12],). The full-length transmembrane form contains two KL domains (KL1 and KL2) and acts as co-receptor of growth factors (such as FGF23 and VEGF) [12,15]. This membrane-bound form can also be cleaved by a disintegrin and metalloprotease (ADAM) cell surface proteases to form the two soluble KL1-KL2 and KL1 forms [16] that control the Ca2+/K+ reabsorption activity of transient receptor potential cation channel subfamily V member 5 (TRPV5) in kidney epithelial cells (for review [12],). Although the secreted and the short soluble form of α-Klotho are very similar (both harbor only the KL1 domain), the former has in addition a C-terminal extension of few amino acids [17].
The serum level of secreted α-Klotho decreases with aging in mice and humans [18], and secreted α-Klotho can inhibit TGFβ1-induced fibrosis and signaling pathways induced by Wnt/β-catenin and Insulin-like Growth factor-1 (IGF-1) (for review [12],). Finally, mice in which α-KL was knocked out show growth retardation, osteoporosis, ectopic calcification in soft tissue, premature tissue aging and death at 9 weeks of age [12,19].
In the present study, we evaluated the link between secreted α-Klotho expression, cartilage homeostasis and OA onset/progression. We determined the expression level of α-Klotho (all variants) during mouse limb development, and that of the secreted form during in vitro differentiation of bone marrow-derived mesenchymal stem cell. To determine the role of this alternative-spliced form for α-Klotho in cartilage homeostasis, we compared the expression profile of primary human chondrocytes from OA patients incubated with recombinant secreted α-Klotho or after α-KL silencing using a RNA interference approach. We identified a set of genes encoding catabolic factors that are repressed upon incubation with secreted α-Klotho and that are known to control OA-dependent tissue degeneration and hypertrophy. Using in vitro and in vivo OA models, we determined the expression level of secreted α-Klotho during OA and tested whether intra-articular α-KL gene transfer has chondroprotective effects.
RESULTS
α-Klotho is expressed during cartilage formation in mouse embryos
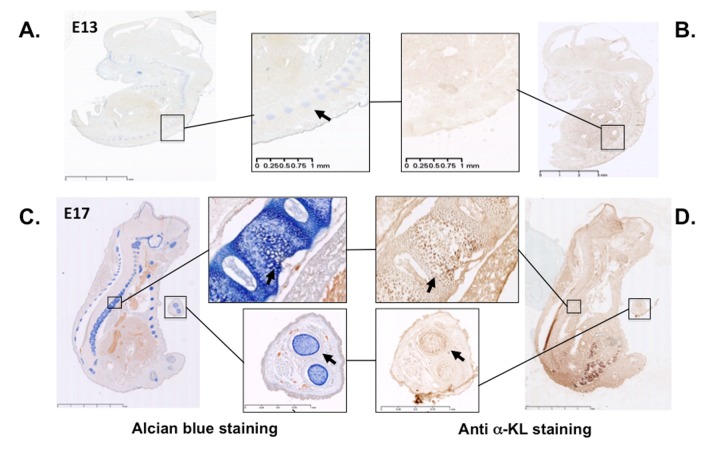
In mice, α-Klotho expression is detected in proliferative chondrocytes within the growth plate during endochondral ossification [20], as expected on the basis of the growth retardation phenotype described in α-KL knock-out mice [19]. These findings prompted us to assess α-Klotho expression during limb development using immunostaining approach with an antibody against all α-Klotho variants. Incubation of cryo-sections of mouse embryos at day 13 and 17 of embryonic development (E13 and E17) with this antibody showed that α-Klotho expression was barely detectable at E13, during formation of the cartilage primordia (Figure 1A-B). In contrast, it was clearly detected at E17 within cartilage rich-tissues, such as the spine and digits, as revealed by co-staining with Alcian blue (Figure 1C-D).
Figure 1.
α-Klotho expression in mouse embryos during cartilage formation. Serial sections of E13 and E17 mouse embryos were stained with Alcian blue to detect cartilage (A and C) or with an anti- pan α-Klotho antibody (B and D). Arrows are showing cartilaginous tissues expressing also α-Klotho.
Secreted α-KL is expressed during in vitro chondrogenesis
To develop potential innovative articular therapeutic approaches, we next wanted to specifically evaluate the expression level of secreted α-Klotho during cartilage formation. To do so, we ordered primers that allowed the specific detection of only this form by RT-qPCR [17,21]. We used bone marrow-derived mesenchymal stem cells (BM-MSC) in micropellet culture with TGFβ3 as in vitro chondrogenic progenitors to recapitulate in vitro the different stages of chondrogenic differentiation that occur during cartilage formation and in vivo endochondral ossification [22]. We exploited our previously published chondrogenic kinetic experiments, where Col2B and MMP13 mRNA expression levels, which encode for markers of mature chondrocytes and terminally hypertrophic differentiated chondrocytes respectively, reached their peak of expression at day 21 [6]. We monitored on the same samples, the expression of secreted α-KL by Rt-qPCR and western-blot during 21 days (Figure 2). At day 0, secreted α-KL was barely detectable in proliferating BM-MSCs (Figure 2 A-B). Upon TGFβ3 addition, the expression of secreted α-KL increased and peaked at day 7 and then progressively decreased until day 21 (Figure 2B), when Col2B and MMP13 reached their maximum [6]. The temporal changes in the expression of secreted α-Klotho were confirmed also by western blot analysis of conditioned medium from BM-MSCs in micropellet culture (Figure 2B). In summary, expression of α-Klotho is up regulated during early in vitro chondrocyte differentiation and in vivo during cartilage formation.
Figure 2.
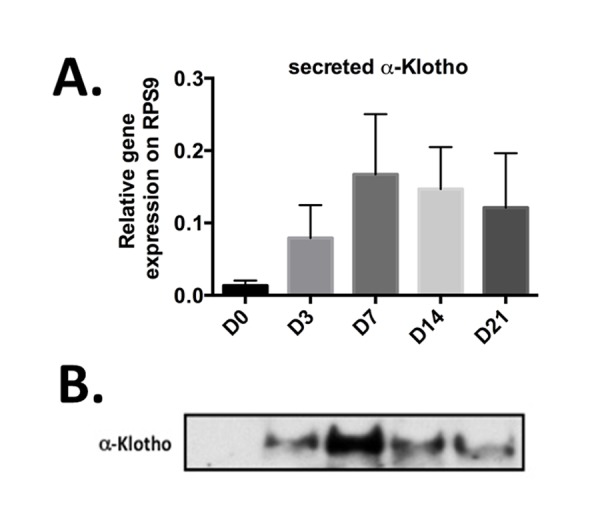
Secreted α-Klotho expression during in vitro chondrogenesis. TGFβ3-driven chondrogenic differentiation of BM-MSCs (osteochondral progenitors) in micropellet culture (n=3). Expression analysis of secreted α-KL (A) by RT-qPCR (gene expression level relative to that of RPS9) at different time points during BM-MSC differentiation into chondrocytes. (B) α-Klotho protein expression detectable below 65kDa marker by western blotting in conditioned medium from BM-MSCs in micromass culture at the different time points during chondrogenesis. Data are represented as mean -/+ SEM.
Secreted α-Klotho regulates the expression of major homeostatic pathways in human articular chondrocytes
To determine the specific role played by secreted α-Klotho in articular chondrocytes, we used primary human chondrocytes isolated from cartilage of patients after arthroplasty surgery. We incubated these isolated cells or not with recombinant secreted α-Klotho, purified as previously described [21], or we transfected them with an irrelevant siRNA or with a siRNA targeting all α-KL variants. After 7 days of culture, we isolated total RNA from cells in these different culture conditions and performed a genome-wide microarray analysis to identify genes the expression of which was modulated by recombinant secreted α-Klotho and by α-KL silencing compared with the respective controls. To strictly select gene transcripts modulated by secreted α-Klotho, we choose only genes for which the expression was affected in the opposite direction by α-Klotho addition and α-KL silencing (Figure 3A). We then used the Ingenuity® software to identify among these 748 differentially expressed genes, known factors with osteoarticular homeostatic functions. We could restrict the list to 38 genes that were positively or negatively regulated upon secreted α-Klotho addition or α-KL silencing (Figure 3B). Among the genes upregulated upon α-KL silencing and repressed by incubation with secreted α-Klotho, we found NOS2 and MMP13, two major cartilage homeostatic players, and also SLC39A8 that encodes the Zin2+ transporter ZIP8 (Figure 3B, arrows). NOS2 and the ZIP8-MMP13 axis are central actors in OA-dependent cartilage alteration. Moreover, NOS2 and MMP13 are involved also in endochondral ossification [8,10,23]. Interestingly, our data also revealed that secreted a-Klotho modulate the expression of senescence regulatory genes such as HMGB1, recently associated with OA onset [5], ATM and DOT1L, an epigenetic regulator found in GWAS analysis from OA patients [24]. Altogether, these suggest that α-Klotho might have a role in cartilage homeostasis by preventing OA onset/progression.
Figure 3.
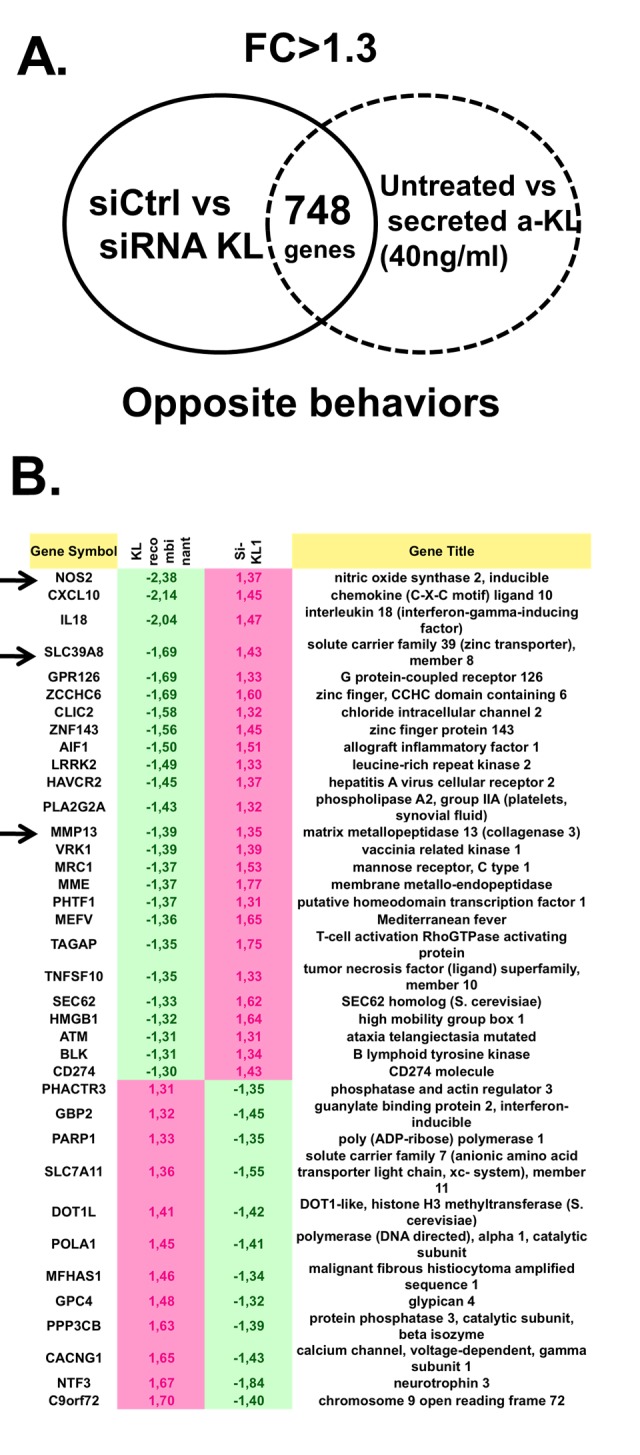
Identification of genes regulated by secreted α-Klotho in human chondrocytes. (A) Genome-wide microarray analysis of human primary chondrocytes incubated or not with recombinant secreted α-KL or after siRNA-mediated α-KL silencing identified 748 genes with opposite behavior in these two experimental conditions. FC, fold change. (B) List of the 38 genes (among the common 748 differentially expressed genes) that are involved in osteoarticular diseases according to the Ingenuity® software.
α-Klotho expression is repressed in in vitro human and in vivo mouse models of OA
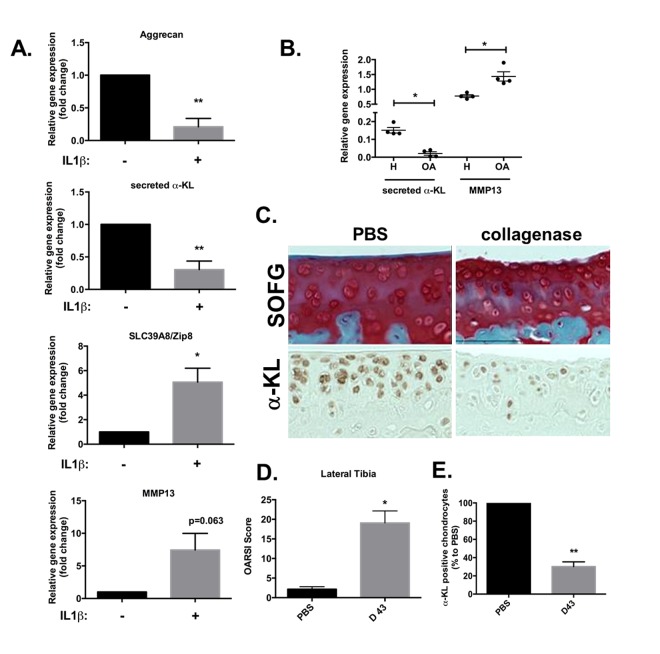
Therefore, we determined whether the expression level of secreted α-Klotho was affected during OA. To this aim, we first used an in vitro mimetic model of OA based on human primary chondrocytes chronically exposed to IL-1β. After 10 days of incubation with IL-1β, the expression levels of secreted α-KL and of ACAN, a cartilage matrix encoding protein, were significantly reduced, while the expression of SLC39A8 and MMP13 catabolic axis was increased as expected (Figure 4A) [10]. This showed an inverse correlation between the expression of secreted α-KL and this major OA-promoting catabolic axis and suggested that α-KL repression is a driving force in OA onset. We confirmed this finding on human cartilage, by analyzing through RT-qPCR, the expression level of secreted α-Klotho and MMP13 (Figure 4B) in cartilage samples from OA patients and healthy controls that we used in a previous study [6]. To finally in vivo validate these results on an OA experimental model, we relied on intra-articular injections of collagenase in mice (Figure 4C-E) [25]. In this model, both inflammation and mechanical stress contribute to cartilage loss of function [26]. At day 43 after collagenase injection in the knee joint, mice were sacrificed and limbs isolated to determine the level of cartilage degradation, compared with the contralateral joint that received an injection of phosphate buffer saline (PBS), using the international scoring (OARSI) method [26,27] after staining with Safranin-O Fast-Green (Figure 4C). The OARSI score for the lateral tibio-femoral component of the knee joint was significantly increased in the treated compared with the untreated (PBS) contralateral joint, thus confirming OA induction in our model (Figure 4D). Moreover, analysis of α-Klotho expression in these joints (as described in Figure 1) showed that the number of α-Klotho-positive chondrocytes was significantly decreased in collagenase-treated knees compared with the contralateral PBS-treated joints (Figure 4C and E). Altogether, these in vivo findings validated the results obtained in human chondrocytes and cartilages strongly suggesting that reduced level of secreted α-Klotho is associated with chondrocyte loss of function in OA.
Figure 4.
α-Klotho expression is reduced in osteoarthrosis models. (A) ACAN, secreted α-KL, SLC39A8 and MMP13 mRNA expression analysis by RT-qPCR of human chondrocytes incubated or not with IL-1β for 10 days. (B) Secreted α-KL and MMP13 expression levels in human OA (n=4) versus healthy cartilage (n=4). (C) α-Klotho expression by immunostaining (bottom panel) in joint knee cartilage at day 43 after intra-articular injection of PBS (n=5 mice) or collagenase (contralateral knee). Top panels show Safranin-O Fast-Green (SOFG) staining of the same joints. (C) OARSI scoring of cartilage degradation and (D) quantification of α-Klotho-positive chondrocytes in joints at day 43 after intra-articular injection of PBS or collagenase. Data are represented as mean -/+ SEM. *=p<0.05; **=p<0.01.
Intra-articular secreted α-KL gene transfer reduces disease severity in an OA mouse model
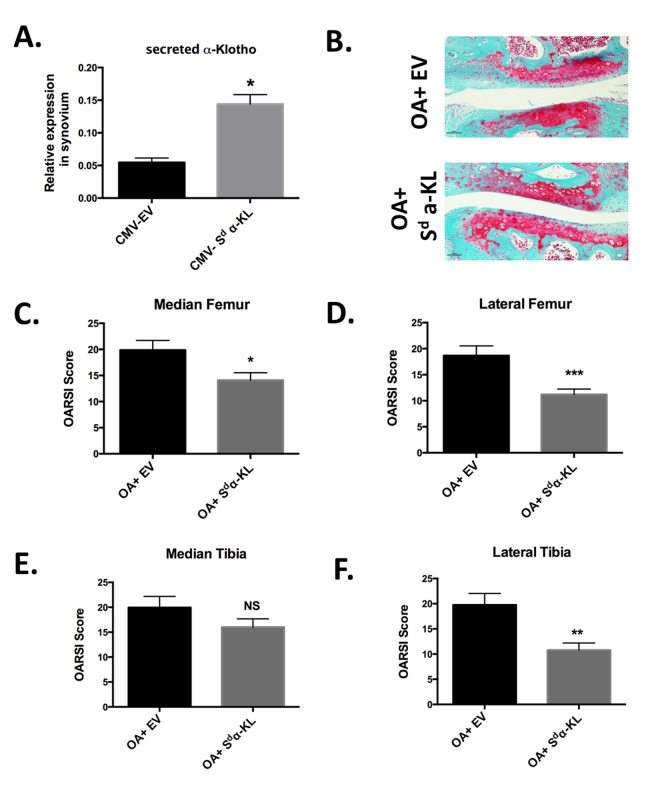
As secreted α-Klotho could downregulate three major catabolic actors of OA and α-Klotho-positive chondrocytes were significantly decreased in collagenase-treated knees, we next asked whether in vivo intra-articular secreted α-KL gene transfer in our mouse model of OA could have chondroprotective effects. First, we tested the efficiency of the gene transfer method by electrotransferring in the knee joint a DNA plasmid to express secreted mouse α-Klotho (CMV-Sd-α-KL) [17] or the corresponding empty vector (CMV-EV) [27]. Using this method, ectopic expression of the protein of interest is detected mainly in joint synoviocytes [28], which are articular specialized fibroblastic cells that provide nutrients and homeostatic factors to chondrocytes within the knee. Indeed, by RT-qPCR we detected a 2.5-fold increase in secreted α-KL expression level using total mRNA isolated from dissected synovial membrane of joints after electroporation of CMV-Sd-α-KL compared with controls (CMV-EV) (Figure 5A).
Figure 5.
Effect of intra-articular secreted α-KL gene transfer in an experimental murine OA model. (A) Secreted α-KL gene expression in mice synovium after electrotransfer of empty vector (CMV-EV) or CMV-Sd-α-KL expressing vector. (B) Representative Safranin-O Fast-Green staining of knee joints from OA mice treated with empty vector (OA+EV) or CMV-Sd-α-KL expressing vector (OA+Sd-α-KL). (C-F) OARSI scores in the different joint localizations from OA mice after intra-articular electrotransfer of empty vector (OA+EV; n=15) or CMV-Sd-α-KL expressing vector (OA+Sd-α-KL; n=25). Data are represented as mean -/+ SEM. *=p<0.05; **=p<0.01.
We next determined whether ectopic intra-articular expression of secreted α-KL modulated disease severity in the mouse model of OA. To this aim, at day 13 after collagenase injection, we intra-articulary electroporated the CMV-Sd-α-KL construct (n=25 mice) or the empty vector (n=15 mice) in the knee of each mouse. At day 43, OARSI scoring of knee joints after staining with Safranin-O Fast-Green revealed that the OARSI score, and therefore OA severity, was significantly reduced in the lateral femur, median femur and lateral tibia, but not in the median tibia joint component, of treated mice (OA+ Sd-α-KL) compared with controls (OA+EV) (Figure 5B-F). Altogether, our results demonstrate the protective effects of secreted α-Klotho during OA.
DISCUSSION
In OA, loss of the balance between anabolic and catabolic factors that ensures joint tissue homeostasis promotes the establishment of a senescence-associated phenotype. Indeed, articular OA chondrocytes are characterized by the production of deleterious inflammatory, reactive oxygen species and p16INK4a-dependent expression of tissue remodeling enzymes [6] leading to cartilage degradation. The restoration of the catabolic/anabolic equilibrium is a target of choice for innovative therapeutic interventions in patients with OA.
Here, we found that the secreted form of the anti-aging factor α-Klotho is a new homeostatic regulator of cartilage integrity. This hormone is locally expressed by articular chondrocytes during early chondrogenesis (Figures 1 and 2), and its level is reduced at the terminal differentiation stage (Figure 2). Moreover, in human primary chondrocytes, secreted α-Klotho regulates negatively the expression of the NOS2 and MMP13 genes that encode two major articular catabolic factors (Figure 3). NOS2 and MMP13 are indeed central players in OA onset and during endochondral terminal differentiation within the growth plate [29]. Nos2-deficient mice show cartilage developmental defect [30], while NOS2-dependent nitric oxide production triggers in vitro and in vivo OA phenotypes in chondrocytes [29]. The MMP13 catabolic axis has also this dual role by being important during endochondral ossification and OA development [6,31]. We also found that the level of secreted α-Klotho is already decreased when MMP13 reach its expression peak at the end of in vitro chondrogenesis (Figure 2) [6], as well as in human chondrocytes following IL1β treatment (Figure 4A) and in human OA cartilage compared to healthy donors (Figure 4B). These findings argue for a reverse correlation between expression of α-KL and these catabolic factors in physiological and pathological conditions. Finally, α-Klotho level is also reduced in murine articular chondrocytes after collagenase-induced OA (Figure 4B). Conversely, ectopic expression of secreted α-KL in joints reduces collagenase-induced cartilage degeneration (Figure 5), thus revealing its chondroprotective function. Importantly, our findings cannot rule out a role or even a lack of role(s) for other forms of α-Klotho in cartilage homeostasis as recently published for FGF23/membrane-bound α-Klotho axis [32].
It is not known how secreted α-Klotho can trigger such effects on cartilage. This hormone has no known receptor on the cell surface and its mode of action seems to depend on its ability to bind to and modify glycoproteins (for review [12]). By this way, secreted α-Klotho can directly inhibit several signaling molecules involved in various processes, such as TGFβ1-dependent fibrosis [33], insulin-like growth factor (IGF-1)-induced oxidative stress [34] and Wnt/β-catenin-dependent gene expression [33]. Remarkably, all these three signaling factors have important functions in cartilage homeostasis. Variation in TGFβ1 level indeed controls endochondral ossification and its overexpression can induce OA in mice [35]. IGF-1 regulates articular chondrocyte proliferation and anabolic/catabolic functions [36], and the canonical Wnt signaling cascade promotes expression of matrix-remodeling enzymes by chondrocytes during OA progression [37].
TGFβ1, Wnt/β-catenin-dependent signaling and IGF-1 can also be senescence-promoting factors [38,39] whereas articular OA chondrocytes and OA synovium were recently proven to harbor cellular senescence features during OA onset [5]. Remarkably α-Klotho has been shown to prevent cellular senescence onset in fibroblasts [15], endothelial [40] and epithelial kidney cells [41] meanwhile α-Klotho improves stem cell proliferation and cell survival [42]. Therefore, we could hypothesize that, in healthy joints, the local production of secreted α-KL by chondrocytes modulates the damaging activity of the mechanical loading stress that can drive production of OA-/hypertrophic-associated and pro-senescent factors, exemplified by TGFβ1. With aging, such repeated stresses in articular joints might result in the progressive reduction of secreted α-Klotho level, thus increasing for instance TGFβ1 activity to drive cellular senescence and OA onset. Thus, restoring the articular level of secreted α-Klotho in OA joints could help to reduce cartilage degeneration as shown in Figure 5 through blockade of these deleterious factors.
We demonstrate here for the first time that anti-geronic secreted α-Klotho has a chondroprotective role on articular cartilage and links its repression to OA onset. Recently, heterochronic parabiosis approaches whereby young mice share their blood circulation with old mice allowed the identification of other longevity factors, such as growth differentiation factor-11 (GDF11) (review in [43]). The circulating blood level of both secreted α-Klotho and GDF11 is reduced in elderly and aged mice [43]. Remarkably, systemic delivery of GDF11 in old mice can improve cerebral tissue functions [43] like secreted α-KL does [44]. Thus, through their systemic hormonal action, these two factors broadly control tissue homeostasis by either reducing the levels of oxidative stress, preventing cellular senescence, controlling ectopic tissue calcification, but also by improving cognitive, renal, muscular, cardiac or articular functions ([43,44] and this work). Altogether these bring new therapeutic perspectives based on the systemic or local delivery of such longevity factors to treat age-dependent pathologies.
MATERIALS AND METHODS
Cell types and cell culture conditions
Human primary chondrocytes and human primary BM-MSCs were isolated from cartilage and tibial subchondral bone of patients with OA undergoing knee arthroplasty, after informed written consent by the patients and approval by the local and national Ethical Committee “Cellule de bioéthique de la direction générale pour la recherche et innovation, Ministère de l’Enseignement Supérieur et de la Recherche (registration number: DC-2009-1052)” as described in [22]. BM-MSCs were used at passage 3 to 6 and were positive for CD44, CD73, CD90 and CD105, and negative for CD14, CD34 and CD45, as previously described [11]. Primary chondrocytes were cultured at passage 0 and 1 in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal calf serum. 5x105 confluent chondrocytes were maintained in DMEM supplemented with 0.1μM dexamethasone (Sigma, USA), 1mM sodium pyruvate (Invitrogen, UK), 0.17mM ascorbic acid (Sigma, USA), 0.35mM proline (Sigma, USA), 1% insulin-transferrin-selenic acid (ITS) (Lonza, CH), 2mM L-glutamine (Lonza, CH), 100U/ml penicillin and 100μg/ml streptomycin (Lonza, CH), as described [23]. Human recombinant secreted α-Klotho was purified as previously described from transfected NIH3T3 cells [18]. Confluent human primary chondrocytes were incubated or not with 40ng/ml recombinant secreted α-Klotho for 7 days (refreshed every day) as described [45], or with 10ng/ml recombinant human IL-1β (Bio-Techne, GER) for 10 days.
In vitro differentiation of BM-MSCs into chondrocytes
Chondrogenic differentiation of BM-MSCs was induced by 21-day culture in micropellets [12]. Briefly, 2.5 x 105 BM-MSCs were pelleted by centrifugation in 15ml conical tubes and cultured in chondrogenic medium (DMEM supplemented with 0.1μM dexamethasone, 0.17mM ascorbic acid and 1% ITS) with TGFβ3 (Bio-Techne, GER) [12]. Chondrogenic differentiation was monitored by assessing the expression of chondrogenic markers by RT-qPCR using previously published primers [6]. The expression of secreted α-Klotho was evaluated by western blotting using 20μg of TCA-precipitated total proteins from 2 ml of conditioned medium from BM-MSC cultures at the indicated times during differentiation [46]. Proteins were separated on 10% SDS-PAGE, transferred on PVDF membranes, saturated with 5% fat-milk in PBS and incubated with a rabbit polyclonal anti-αKlotho antibody (1:200; Abcam 75023) overnight. Antibody binding was revealed using a goat anti-rabbit antibody coupled to HRP and ECL, as published ([6].
Gene expression analysis and vector purification
Total RNA from cultured cells or mouse synovial tissues was extracted with the RNeasy Kit (Qiagen, USA) according to the manufacturer’s instructions. 250 ng of total RNA was reverse transcribed using the M-MLV Reverse Transcriptase Kit according to the manufacturer’s instructions (Thermo Fisher, UK) and using 10µM random hexamers (Thermo Fisher, UK), 1mM dNTP mix, 10mM DTT, 5X RT buffer, 50 units M-MLV Reverse Transcriptase. Complementary DNA (cDNA) was then mixed with the Sybr Green Master Mix (Roche, CH) and specific primers. The published sequences of human and mouse secreted α-KL [17] were used. The primer sequences for NOS2, SLC39A8, and MMP13 were previously described [10]. Gene expression analysis was performed as previously described using the 2-dCT method and RPS9 expression level as internal control for human and mouse samples [6].
The PCNA-neo vector (CM-EV) and PCNA-neo-secreted α-KL expressing plasmid (CMV-Sd-α-KL) used in our in vivo experiments were previously described [17]. They were purified from ampicillin-resistant E. coli colonies using the Endotoxin-Free Plasmid DNA Isolation Kit (Qiagen, USA) according to the manufacturer’s instructions. The intra-articular expression level of ectopic secreted α-KL was verified using total RNA from dissected synovial tissue.
RNA interference and genome-wide microarray analysis
Total RNA was extracted with the RNeasy Isolation Kit (Qiagen, USA) from 5x105 primary chondrocytes from three different patients with OA incubated or not with recombinant human α-Klotho (40ng/ml) for 7 days. In parallel, total RNA was obtained also from the same three independent human primary chondrocyte cultures transfected twice (at day 1 and 2) with 20nM of control siRNA or the previously described siRNA targeting all α-KL variants [47] using Oligofectamine according to the manufacturer’s protocol (Thermo Fisher Scientific, UK). Labeling and hybridization to the HTA-2_0 Human Transcriptome Array 2.0 (Affymetrix, UK) were performed using 200 ng of total RNA according to the manufacturer’s protocol. Raw data were normalized and additional data analysis was performed as described previously [25]. Gene array data are available at the NIH library under the accession number GSE80285.
Collagenase-induced OA and articular electroporation
Two-month-old C57BL6 male mice were housed and cared for according to the European Directive 2010/63/EU. The experimental protocol was approved by the “Regional ethical committee on animal experimentation” (approval CEEA-LR-10042). At day 0 and day 2, 1U/ml collagenase (Sigma-Aldrich (USA) was injected intra-articularly as previously described [48]. At day 13 post-injection, 10μg of each plasmid was electro-transferred intra-articularly, as previously described [28]. At day 43 post-injection, animals were sacrificed and their limb joints isolated for cartilage analysis.
Histology, immunohistochemistry and antibodies
C57BL6 mouse embryo cryo-sections were obtained from the RHEM collection (samples were collected according to the European Directive 2010/63/EU). This collection was approved by the regional ethics committee on animal experimentation (approval CEEA-LR-1194). For analysis of the OA mouse model, joints from PBS-treated or collagenase-treated animals were fixed in 4% paraformaldehyde at 4°C for 48h, washed in PBS and then processed for routine histology. Knees were decalcified in 14% EDTA/PBS for three weeks and then paraffin-embedded. Tissue sections (5 µm) were rehydrated through a gradient of ethanol and xylene. Sections were then stained with Safranin-O Fast-Green solution to evaluate cartilage degradation using the OARSI scoring system, or processed for α-Klotho detection with specific antibodies. For this, embryo cryo-sections and paraffin-embedded tissue sections were first incubated at room temperature (RT) with 10μl of pepsin for 20min for epitope retrieval, and then with 1% H2O2 at RT for 20min to block endogenous peroxidases. Sections were then pre-incubated with PBS/10% goat serum/0.1% Triton X-100 at RT for 30min. Endogenous biotin was blocked with the Streptavidin/Biotin Blocking Kit (Vector Laboratories, SP-2002) for 30min. Then, sections were incubated with the primary anti-α-Klotho polyclonal rabbit antibody (1:200; Abcam 75023) at 4°C overnight, followed by the secondary biotinylated antibody (1:200; Vectastain Elite ABC-HRP Kit, Vector Laboratories, PK6100) at RT for 1 hour. Finally, the Avidin/Biotinylated enzyme complex (ABC Vectastain Elite ABC-HRP Kit, Vector Laboratories, PK6100) was added at RT for 30min. Antibody binding was revealed with the DAB D-4146 kit revelation (Sigma-Aldrich, USA) according to the manufacturer’s instructions.
Statistical analysis
The unpaired Mann–Whitney test was used to compare the OARSI scores between treated versus untreated mice and human cartilage samples from OA versus healthy donors. Human chondrocyte experiments and cell counting were performed using at least three independent samples. Comparisons of two conditions for the same samples were performed using the paired t-test. All statistical analyses were performed with GraphPad Prism (San Diego, USA). P values <0.05 were considered significant.
ACKNOWLEDGEMENTS
We would like to thank all members of the INSERM 1183 and 1051 Units, including D. Philipot, A Sansaloni, D. Guérit, E. Middendorp, G. Tejedor, R. M Borzi and M-L Vignais for helpful technical supports and discussions all along this work. Thanks also to Veronique Pantesco from the Transcriptomic platform, CHU IRMB, Montpellier France, the Montpellier Histology Network (RHEM) and the animal facility staff at the Institute for Neurosciences of Montpellier (RAM network) for their expert animal care. We also want to acknowledge Drs Y-M Pers, R Ferriera, F Canovas for organizing the patients’ consents and providing human tissue samples. Special thanks to Prof Y. Nabeshima for the gift of the plasmids that encode the human and murine secreted forms of α-Klotho.
Footnotes
AUTHOR CONTRIBUTIONS: PC, CJ and JMB developed the concept and designed all experiments. PC, ALB, MM, FE, MT, KT, DN, FD and JMB designed, performed or analyzed the in vitro and in vivo experiments. CR, MT performed in situ experiments. ALB, MM and JMB supervised manuscript writing and corrections.
CONFLICTS OF INTEREST: The authors declare no competing interests.
FUNDING: This work was supported INSERM, by Fondation de l’Avenir grant ETS3-698 that was awarded to JMB. FE was recipient of a fellowship from the Chilean-French exchange program.
REFERENCES
- 1.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015; 21:854–62. 10.1038/nm.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014; 20:870–80. 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017; 23:1072–79. 10.1038/nm.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011; 23:492–96. 10.1097/BOR.0b013e3283494005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017; 23:775–81. 10.1038/nm.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philipot D, Guérit D, Platano D, Chuchana P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM, Jorgensen C, Noel D, Brondello JM. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther. 2014; 16:R58. 10.1186/ar4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008. (Suppl 2); 16:S15–20. 10.1016/S1063-4584(08)60008-4 [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, Min BH, Chun JS. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010; 16:687–93. 10.1038/nm.2153 [DOI] [PubMed] [Google Scholar]
- 9.Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci USA. 2013; 110:1875–80. 10.1073/pnas.1207458110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014; 156:730–43. 10.1016/j.cell.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 11.Goldring MB, Marcu KB. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol Med. 2012; 18:109–18. 10.1016/j.molmed.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015; 36:174–93. 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Zhai G, Kato BS, Hart DJ, Hunter D, Spector TD, Ahmadi KR. Association between KLOTHO gene and hand osteoarthritis in a female Caucasian population. Osteoarthritis Cartilage. 2007; 15:624–29. 10.1016/j.joca.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Tsezou A, Furuichi T, Satra M, Makrythanasis P, Ikegawa S, Malizos KN. Association of KLOTHO gene polymorphisms with knee osteoarthritis in Greek population. J Orthop Res. 2008; 26:1466–70. 10.1002/jor.20634 [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011; 13:254–62. 10.1038/ncb2167 [DOI] [PubMed] [Google Scholar]
- 16.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007; 104:19796–801. 10.1073/pnas.0709805104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998; 242:626–30. 10.1006/bbrc.1997.8019 [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010; 398:513–18. 10.1016/j.bbrc.2010.06.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390:45–51. 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 20.Kawai M, Kinoshita S, Kimoto A, Hasegawa Y, Miyagawa K, Yamazaki M, Ohata Y, Ozono K, Michigami T. FGF23 suppresses chondrocyte proliferation in the presence of soluble α-Klotho both in vitro and in vivo. J Biol Chem. 2013; 288:2414–27. 10.1074/jbc.M112.410043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998; 424:6–10. 10.1016/S0014-5793(98)00127-6 [DOI] [PubMed] [Google Scholar]
- 22.Guérit D, Philipot D, Chuchana P, Toupet K, Brondello JM, Mathieu M, Jorgensen C, Noël D. Sox9-regulated miRNA-574-3p inhibits chondrogenic differentiation of mesenchymal stem cells. PLoS One. 2013; 8:e62582. 10.1371/journal.pone.0062582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies CM, Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008; 16:624–30. 10.1016/j.joca.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaño Betancourt MC, Cailotto F, Kerkhof HJ, Cornelis FM, Doherty SA, Hart DJ, Hofman A, Luyten FP, Maciewicz RA, Mangino M, Metrustry S, Muir K, Peters MJ, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA. 2012; 109:8218–23. 10.1073/pnas.1119899109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989; 135:1001–14. [PMC free article] [PubMed] [Google Scholar]
- 26.ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PL. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012; 64:3604–13. 10.1002/art.34626 [DOI] [PubMed] [Google Scholar]
- 27.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010. (Suppl 3); 18:S17–23. 10.1016/j.joca.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 28.Denys A, Thiolat A, Descamps D, Lemeiter D, Benihoud K, Bessis N, Boissier MC. Intra-articular electrotransfer of mouse soluble tumour necrosis factor receptor in a murine model of rheumatoid arthritis. J Gene Med. 2010; 12:659–68. 10.1002/jgm.1482 [DOI] [PubMed] [Google Scholar]
- 29.Rosa SC, Judas F, Lopes MC, Mendes AF. Nitric oxide synthase isoforms and NF-kappaB activity in normal and osteoarthritic human chondrocytes: regulation by inducible nitric oxide. Nitric Oxide. 2008; 19:276–83. 10.1016/j.niox.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 30.Teixeira CC, Agoston H, Beier F. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev Biol. 2008; 319:171–78. 10.1016/j.ydbio.2008.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung UI, Kawaguchi H. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010; 16:678–86. 10.1038/nm.2146 [DOI] [PubMed] [Google Scholar]
- 32.Bianchi A, Guibert M, Cailotto F, Gasser A, Presle N, Mainard D, Netter P, Kempf H, Jouzeau JY, Reboul P. Fibroblast Growth Factor 23 drives MMP13 expression in human osteoarthritic chondrocytes in a Klotho-independent manner. Osteoarthritis Cartilage. 2016; 24:1961–69. 10.1016/j.joca.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol. 2013; 24:771–85. 10.1681/ASN.2012080865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005; 280:38029–34. 10.1074/jbc.M509039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013; 19:704–12. 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Li L, Yang W, Cao Y, Shi Y, Li X, Zhang Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthritis Cartilage. 2017; 25:309–20. 10.1016/j.joca.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 37.Blom AB, Brockbank SM, van Lent PL, van Beuningen HM, Geurts J, Takahashi N, van der Kraan PM, van de Loo FA, Schreurs BW, Clements K, Newham P, van den Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009; 60:501–12. 10.1002/art.24247 [DOI] [PubMed] [Google Scholar]
- 38.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014; 15:1139–53. 10.15252/embr.201439245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varela-Eirin M, Loureiro J, Fonseca E, Corrochano S, Caeiro JR, Collado M, Mayan MD. Cartilage regeneration and ageing: targeting cellular plasticity in osteoarthritis. Ageing Res Rev. 2018; 42:56–71. 10.1016/j.arr.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 40.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006; 339:827–32. 10.1016/j.bbrc.2005.11.094 [DOI] [PubMed] [Google Scholar]
- 41.Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, Liu Y, Levine B, Moe OW, Hu MC. αKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J Am Soc Nephrol. 2016; 27:2331–45. 10.1681/ASN.2015060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan J, Sun Z. The Antiaging Gene Klotho Regulates Proliferation and Differentiation of Adipose-Derived Stem Cells. Stem Cells. 2016; 34:1615–25. 10.1002/stem.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conese M, Carbone A, Beccia E, Angiolillo A. The Fountain of Youth: A Tale of Parabiosis, Stem Cells, and Rejuvenation. Open Med (Wars). 2017; 12:376–83. 10.1515/med-2017-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leon J, Moreno AJ, Garay BI, Chalkley RJ, Burlingame AL, Wang D, Dubal DB. Peripheral Elevation of a Klotho Fragment Enhances Brain Function and Resilience in Young, Aging, and α-Synuclein Transgenic Mice. Cell Reports. 2017; 20:1360–71. 10.1016/j.celrep.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000; 276:767–72. 10.1006/bbrc.2000.3470 [DOI] [PubMed] [Google Scholar]
- 46.Mathieu M, Vigier S, Labour MN, Jorgensen C, Belamie E, Noël D. Induction of mesenchymal stem cell differentiation and cartilage formation by cross-linker-free collagen microspheres. Eur Cell Mater. 2014; 28:82–96. 10.22203/eCM.v028a07 [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010; 29:99. 10.1186/1756-9966-29-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toupet K, Maumus M, Peyrafitte JA, Bourin P, van Lent PL, Ferreira R, Orsetti B, Pirot N, Casteilla L, Jorgensen C, Noël D. Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 2013; 65:1786–94. 10.1002/art.37960 [DOI] [PubMed] [Google Scholar]