Abstract
Introduction and hypothesis
To compare treatment success and adverse events between women undergoing open sacrocolpopexy (ASC) versus vaginal repair (VAR) using data from women enrolled in one of three multicenter trials. We hypothesized that ASC would result in better outcomes than VAR.
Methods
Participants underwent apical repair of stage 2–4 prolapse. Vaginal repair included uterosacral, sacrospinous, and illiococcygeal suspensions; sacrocolpopexies were via laparotomy. Success was defined as no bothersome bulge symptoms, no prolapse beyond the hymen and no retreatment up to 24 months. Adverse events were collected at multiple time-points. Outcomes were analyzed using longitudinal mixed effects models to obtain valid outcome estimates at specific visit times, accounting for data missing at random. Comparisons were controlled for center, age, BMI, initial POPQ stage, baseline scores, prior prolapse repair and concurrent repairs.
Results
Of women who met inclusion criteria (1022 women included /1159 eligbile); 701 underwent vaginal repair. The sacrocolpopexy group (n=321) was older, more likely White, had prior prolapse repairs, and Stage 4 prolapse (all p<0.05). While POPQ measurements and symptoms improved in both groups, treatment success was higher in the sacrocolpopexy group (OR:6.00, 95% CI:3.45–10.44). The groups did not differ significantly in most questionnaire responses at 12 months and overall bowel and bladder function improvement. By 24 months, fewer patients had undergone retreatment (2% sacrocolpopexy vs 5% vaginal repair); serious adverse events did not differ significantly through 6 weeks (13% vs 5%, OR:2.0, 95% CI:0.9–4.7) and 12 months (26% vs 13%, OR:1.6, 95%CI:0.9–2.9) respectively.
Conclusions
Open sacrocolpopexy resulted in more successful prolapse treatment at 2 years.
Keywords: Sacrocolpopexy, native tissue vaginal repair, apical repair, prolapse
Introduction
Surgeons who practice evidence-based reconstructive pelvic surgery seek the highest level of evidence for their surgical recommendations. Unfortunately, a significant knowledge gap exists for pelvic reconstructive surgeons who counsel the many women planning surgery for prolapse repair. Ideally, well-trained surgeons should be able to counsel and offer patients a variety of approaches and discuss the balance of benefits and risks of techniques based on high quality evidence including counseling regarding the impact of repair on bowel, bladder and sexual function. Although previous randomized trials have compared outcomes of open sacrocolpopexy (ASC) vs vaginal prolapse repair (VAR), these trials have drawbacks including small sample sizes, different types of procedures for both the abdominal and vaginal approaches and lack of standardized outcome measures, particularly for bowel, bladder and sexual function. [1–4] While data from these trials do show more durability in the short term for ASC, it is unclear whether or not functional outcomes are similar between the two approaches.
We aimed to compare success and adverse events between ASC and VAR utilizing data from three multi-center randomized trials that used standardized outcomes of pelvic reconstructive surgery for stage 2–4 pelvic organ pro-lapse. In our study, the pre-existing data from NICHD trials included validated measurement of both anatomic, symptom and quality of life data as well as detailed descriptions of adverse events. We hypothesized that ASC would result in better anatomic and quality of life outcomes than VAR. Our ultimate goal is to inform the design, conduct and analysis of future randomized trials and to improve evidence regarding the surgical approach for women with stage 2–4 pelvic organ prolapse.
Materials and Methods
We present a retrospective study of participants enrolled in one of three NICHD Pelvic Floor Disorders Network trials conducted in a total of 17 centers throughout the United States; all women underwent surgical correction of stage 2–4 pelvic organ prolapse with an apical suspension technique. The trials included in this study are the Colpopexy and Urinary Reduction Efforts (CARE) [5], Outcomes following Vaginal Prolapse Repair and Midurethral Sling (OPUS) [6], and the Operations and Pelvic Muscle Training in the Management of Apical Support Loss (OPTIMAL) trials [7]. The study designs and results of these trials have been previously published. Briefly, in the CARE trial, stress continent women underwent an abdominal sacrocolpopexy surgery for POP and were randomized to prophylactic Burch cystourethropexy continence surgery versus no Burch to evaluate the effectiveness of concomitant prophylactic continence surgery at the time of open abdominal surgery for POP.[5] Participants in the OPUS trial underwent vaginal prolapse surgery with a retropubic midurethral sling compared to a sham procedure to evaluate the efficacy of concomitant prophylactic continence surgery at the time of vaginal surgery for POP.[7] In both CARE and OPUS, women were not randomized to different types of prolapse repair. However, the OPTIMAL trial was designed with the primary aim to compare two native tissue vaginal approaches for the repair of apical POP and women were randomized to sacrospinous or uterosacral colpopexy.[6] The CARE and OPTIMAL trials were designed to follow patients 2 years after surgery while the OPUS trial was designed to follow patients to one year after surgery.
For this study, we defined two separate groups; an abdominal approach repair group (ASC) and a vaginal approach repair group (VAR). We excluded women who underwent colpocleisis or vaginal mesh procedures for prolapse and women whose only apical procedure was ligation of the peritoneal sac or trachelectomy. For the abdominal ASC group we included women in the CARE trial. For the VAR group, we included women in the OPUS and OPTIMAL trials who underwent either illiococcygeal, McCall’s culdoplasty, sacrospinous or uterosacral colpopexy. We combined anterior colporrhaphy with abdominal paravaginal repairs to an “anterior repair” variable; all colporrhaphies were performed vaginally, and all paravaginal repairs were performed abdominally. We also combined posterior repair and perineorrhaphy into a “posterior repair” variable, as surgeons vary widely in their definition of how they perform these two repairs and there is likely crossover between the two techniques.[8]
Our primary outcome was success after POP surgery which was defined as the absence of bothersome bulge symptoms, no prolapse beyond the hymen, and no subsequent retreatment of prolapse up to 24 months. Bothersome bulge was measured by the Pelvic Organ Prolapse Distress Inventory (POPDI)(9) Question 5 (i.e. response of “No” to “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?” or “Not at all” to “If yes, how much does it bother you”). No prolapse beyond the hymen was measured on POPQ examination (i.e. measure of less than or equal to 0 on points Ba, C, and Bp).[10,11] Participants failing any one of three criteria above were considered to be surgical failures; women whose status could not be determined due to missing data for the three criteria were excluded from this analysis. Our secondary outcomes were symptom and quality of life measures. POPQ examinations, the Pelvic Floor Distress Inventory-20 (PFDI-20), the Pelvic Floor Impact Questionnaires-7 (PFIQ-7), and Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12 (PISQ-12) measures were administered at baseline and each follow-up visit.[9,12,13] The PFDI-20 comprised the Urinary Distress inventory (UDI), Colorectal Anal Distress Inventory (CRADI) and POPDI subscales. The PFIQ-7 comprised the Urinary Impact Questionnaire (UIQ), the Colorectal Anal Impact Questionnaire (CRAIQ) and the Pelvic Organ Prolapse Impact Questionnaire (POPIQ) subscales. Higher scores on the PFDI-20 and PFIQ-7 indicate greater bother and negative impact on quality of life, respectively, and higher PISQ-12 scores indicate better sexual function.
Complications and serious adverse events (SAE) were recorded on standardized forms for all trials. Perioperative complications were recorded up to 6 weeks postoperatively and included blood transfusions, deep vein thrombosis, pulmonary embolus and injuries to the ureters, bladder, urethra, bowel, vascular system and nerves. Only serious adverse events were recorded consistently across all studies after the perioperative period, from six weeks to 1–2 years postoperatively. These events were categorized using the Dindo complication scale.[14]
Demographics and baseline characteristics were compared between treatment groups using analysis of variance techniques for continuous normally distributed measures, Kruskal-Wallis tests for non-normally distributed scale measures, mean score tests using standardized rank scores for ordinal categorical measures, and chi-square tests for general association for categorical measures. By design, the timing of follow-up visits varied by study. Success and QOL outcomes measured at multiple visits were analyzed using longitudinal mixed effects models (for continuous measures) or generalized linear models with empirical variance estimates (for binary measures) to obtain valid outcome estimates at specific visit times, accounting for data missing at random or missing completely at random due to the varied planned visits. Comparisons between groups controlled for center where possible as well as age, BMI, initial POPQ stage, prior prolapse repair and concurrent anterior or posterior repair except for events that occurred rarely. Additionally, models for PFDI-20, PFIQ-7 and PISQ-12 scores also controlled for the corresponding baseline score. Hypotheses for these outcomes were tested using contrast statements for assessing whether differences existed for the two groups across study follow-up as well as at individual visit times. Serious adverse events were graded by the Dindo score and compared between groups using analysis of covariance techniques for continuous measures, covariate adjusted logistic models for categorical measures, and covariate adjusted mean score tests using modified ridit scores for ordinal categorical measures. Due to low rates of occurrence, the percent of subjects experiencing specific complications during the perioperative and six week postoperative period was compared between treatment groups using a Fisher’s exact test without adjustments.
Results
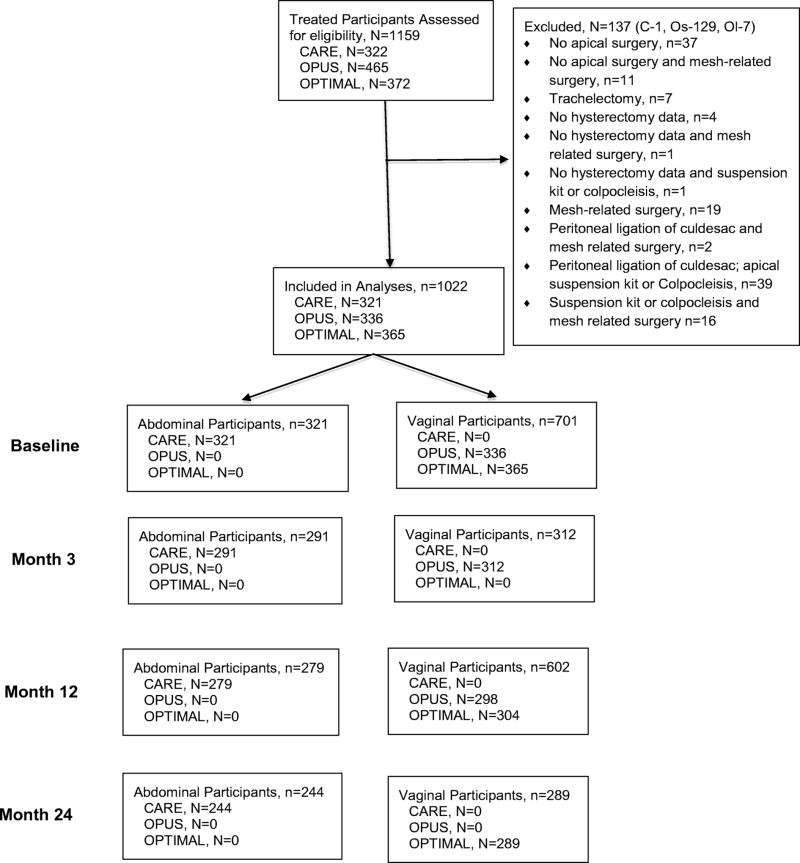
For these analyses 1022 women met inclusion criteria out of 1159 eligible from the parent studies. Figure 1 outlines the contribution to the study population from each of the parent studies. On average, participants in the ASC group were older, had lower BMI, and were more likely to be White, post-menopausal, and privately insured. Of the 701 patients who had vaginal surgery, 296 (42%) had outpatient surgery, 402 (57%) had inpatient surgery, and 3 (1%) had missing information. Of the 402 inpatients, the mean (SD) length of stay was 1.4 (0.7) days with a median length of stay of 1 day. All 321 patients who had ASC surgery were inpatients; they had a mean (SD) length of stay 0f 2.8 (1.1) days with a median length of stay of 3 days. In addition, they were more likely to have undergone prior incontinence or prolapse procedures. (Table1) Importantly, they did not differ in rates of sexual activity, mean number of vaginal births, smoking history or SF-36 physical component scores. Women in the ASC group had more severe prolapse as measured by the POPQ, and reported less bother preoperatively from urinary and colorectal symptoms on the PFDI-20, and less quality of life impact on the PFIQ-7. Interestingly, despite differences in severity of prolapse as measured by the POPQ, baseline POPDI subscale scores were not different between groups. (Table 2) As is common with pelvic reconstructive surgery, women underwent a variety of concomitant procedures. (Table 3)
Figure 1.
Strobe outline of participants.
Table 2.
Baseline prolapse measurements, Pelvic Floor Distress Inventory-20, Pelvic Floor Impact Questionnaire-7 and Pelvic Organ Prolapse Incontinence Sexual Questionnaire-12
| Characteristic | Abdominal (N=321) |
Vaginal (N=701) |
P-value [1] |
|
|---|---|---|---|---|
| POPQ* stage, n (%) | <0.001 | |||
| 2 | 44 (14%) | 227 (32%) | ||
| 3 | 217 (68%) | 434 (62%) | ||
| 4 | 60 (19%) | 39 (6%) | ||
| Unknown | 0 (0%) | 1 (0%) | ||
| Ba, mean (SD) | 3.3 (2.7) | 2.2 (2.3) | <0.001 | |
| C, mean (SD) | 1.4 (4.6) | −0.6 (3.7) | <0.001 | |
| Bp, mean (SD) | 1.1 (3.6) | −0.5 (2.4) | <0.001 | |
| UDI**, mean (SD) | 61.0 (40.7) | 92.1 (61.4) | <0.001 | |
| POPDI***, mean (SD) | 107.5 (67.4) | 109.1 (67.4) | 0.730 | |
| CRADI+, mean (SD) | 70.4 (69.4) | 84.8 (78.1) | 0.005 | |
| UIQ#, mean (SD) | 47.0 (50.4) | 101.8 (97.1) | <0.001 | |
| POPIQ$, mean (SD) | 43.7 (56.4) | 81.4 (96.7) | <0.001 | |
| CRAIQ^, mean (SD) | 29.3 (50.0) | 53.2 (82.2) | <0.001 | |
| PISQ-12^^, mean (SD) | 33.7 (7.1) | 32.5 (7.2) | 0.091 | |
P-values based on comparing characteristics between groups while excluding responses of unknown
Pelvic Organ Prolapse Quantification Scale;
Urinary Distress Inventory;
Pelvic Organ Prolapse Distress Inventory;
Colorectal Anal Distress Inventory;
Urinary Impact Questionnaire;
Pelvic Organ Prolapse Impact Questionnaire;
Colorectal Anal Impact Questionnaire;
Pelvic Organ Prolapse Urinary Incontinence Sexual Questionnaire
Table 3.
Surgical procedures
| Surgery | Abdominal (N=321) |
Vaginal (N=701) |
P-value [1] | |
|---|---|---|---|---|
| Anterior repair, n (%) | <0.001 | |||
| Anterior Colporrhaphy with/without Paravaginal Repair from vaginal approach | 0 (0%) | 442 (63%) | ||
| Abdominal Paravaginal Repair | 59 (18%) | 0 (0%) | ||
| Posterior Colporrhaphy/Perineorrhaphy, n (%) | 89 (28%) | 341 (49%) | <0.001 | |
| Hysterectomy and/or Oophorectomy, n (%) | 176 (55%) | 494 (70%) | <0.001 | |
| Stress incontinence procedures, n (%) | <0.001 | |||
| Burch | 154 (48%) | 0 (0%) | ||
| Retropubic Midurethral Sling | 0 (0%) | 526 (75%) | ||
| General Anesthesia, n (%) | 318 (99%) | 684 (98%) | 0.062 | |
| Operating Room Time, mean (SD) | 180.2 (57.9) | 148.7 (49.0) | <0.001 | |
P-values based on comparing characteristics between groups while excluding responses of unknown
For our composite definition of success, outcomes were better in the ASC group at every time point. Overall treatment success was higher in the sacrocolpopexy group (OR:6.00, 95% CI:3.45–10.44). (Table 4) Likewise POPQ measurement of points Ba and C were better in the ASC group at all time points. Outcomes in the posterior compartment were similar between groups at all time points. Retreatment of prolapse was low in both groups with only 2% of the ASC and 5% of the VAR group reporting retreatment prior to 24 months.
Table 4.
Prolapse Treatment Outcomes
| Outcomes at 3,12 and 24 month follow-up |
Abdominal (N=321) |
Vaginal (N=701) |
Adjusted difference or odds ratio [1] |
Raw P- value [2] |
Adjusted P-value [2] |
|---|---|---|---|---|---|
| Change in Ba from baseline, mean cm (SD) | |||||
| Overall | −1.86 | −1.01 | −0.84 | <0.001 | <0.001 |
| Month 3 | −2.02 | −1.46 | −0.56 | <0.001 | <0.001 |
| Month 12 | −1.81 | −0.84 | −0.97 | <0.001 | <0.001 |
| Month 24 | −1.74 | −0.74 | −0.99 | <0.001 | <0.001 |
| Change in C from baseline, mean cm (SD) | |||||
| Overall | −8.13 | −5.63 | −2.50 | <0.001 | <0.001 |
| Month 3 | −8.30 | −5.88 | −2.42 | <0.001 | <0.001 |
| Month 12 | −8.19 | −5.48 | −2.71 | <0.001 | <0.001 |
| Month 24 | −7.90 | −5.53 | −2.37 | <0.001 | <0.001 |
| Change in Bp from baseline, mean cm (SD) | |||||
| Overall | −2.18 | −2.09 | −0.08 | 0.381 | 0.416 |
| Month 3 | −2.36 | −2.21 | −0.16 | 0.184 | 0.166 |
| Month 12 | −2.09 | −2.09 | −0.01 | 0.842 | 0.939 |
| Month 24 | −2.07 | −1.99 | −0.08 | 0.458 | 0.525 |
| Absence of bothersome bulge, n (%) | |||||
| Overall | 7.82 (2.75,22.23) | <0.001 | <0.001 | ||
| Month 3 | 301 (100%) | 318 (98%) | 11.37 (1.23,105.11) | 0.022 | 0.019 |
| Month 12 | 298 (98%) | 576 (90%) | 7.99 (2.75,23.26) | <0.001 | <0.001 |
| Month 24 | 279 (96%) | 261 (87%) | 5.25 (2.25,12.28) | <0.001 | <0.001 |
| Support above hymen, n (%) | |||||
| Overall | 6.69 (3.74,11.98) | <0.001 | <0.001 | ||
| Month 3 | 301 (98%) | 278 (88%) | 9.62 (3.87,23.96) | <0.001 | <0.001 |
| Month 12 | 269 (95%) | 503(81%) | 6.03 (3.17,11.49) | <0.001 | <0.001 |
| Month 24 | 235 (94%) | 258(85%) | 5.17 (2.60,10.28) | <0.001 | <0.001 |
| No report of retreatment, n (%) | |||||
| Overall | # | # | # | ||
| Month 3 | 319 (99%) | 331 (100%) | # | # | # |
| Month 12 | 316 (98%) | 659 (98%) | # | # | # |
| Month 24 | 313 (98%) | 331 (95%) | # | # | # |
| Success*, n (%) | |||||
| Overall | 6.00 (3.45,10.44) | <0.001 | <0.001 | ||
| Month 3 | 282 (97%) | 271 (87%) | 6.36 (2.90,13.92) | <0.001 | <0.001 |
| Month 12 | 259 (93%) | 446 (74%) | 7.04 (3.79,13.11) | <0.001 | <0.001 |
| Month 24 | 216 (99%) | 212 (73%) | 4.83 (2.75,8.49) | <0.001 | <0.001 |
For continuous outcomes, adjusted means and differences are presented based on repeated measure mixed models adjusting for center where possible as well as apical surgical type, occurrence of anterior or posterior surgeries, prior POP surgery, age, BMI and POP-Q stage. For binary outcomes, proportions and adjusted odds ratios of vault vs. uterovaginal prolapse are presented based on subjects with available data at the visit.
Raw p-values based on analyses with no adjustments. Adjusted p-values based on repeated measure mixed models adjusting for center where possible as well as apical surgical type, occurrence of anterior or posterior surgeries, prior POP surgery, age, BMI and POP-Q stage
Success defined as absence of bothersome bulge symptoms as measured by the PFDI Question #5 (i.e. response of “No” to “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?” or “Not at all” to “If yes, how much does it bother you”), no prolapse beyond the hymen on POPQ examination (i.e. measure of less than or equal to 0 on Ba, C, and Bp) and no subsequent treatment for prolapse.
Not estimated
Bowel and bladder function as measured by the PFDI-20 and PFIQ-7 improved in both groups. (Table 5) Overall, adjusted results showed no difference in improvement between groups for PFDI-20 or PFIQ-7, including no difference in the UDI, POPDI, CRADI, UIQ, POPIQ and CRAIQ subscale scores. Improvement of POPDI scores were slightly greater at 24 months in the ASC group, but no difference was seen before this time point. PISQ-12 scores showed no difference in improvement between groups.
Table 5.
Pelvic Floor Distress Inventory -20 (PFDI-20), Pelvic Floor Impact Questionnaire-7 (PFIQ-7) Subscale Scores and Pelvic Organ Prolapse Urinary Incontinence Sexual Questionnaire 12 (PISQ-12) Results
| Outcome | Time of follow-up |
Abdominal, Change from Baseline (N=321) |
Vaginal, Change from Baseline (N=701) |
Adjusted difference [1] |
Raw P- value [2] |
Adjusted P- value [2] |
|---|---|---|---|---|---|---|
| UDI**, mean (SD) | Overall | −51.16 | −55.16 | 4.00 (−3.09,11.10) | <0.001 | 0.269 |
| Month 3 | −48.96 | −58.34 | 9.38 (1.73,17.03) | <0.001 | 0.016 | |
| Month 12 | −53.30 | −58.81 | 5.52 (−1.92,12.95) | <0.001 | 0.146 | |
| Month 24 | −51.21 | −48.32 | −2.88 (−10.76,4.99) | <0.001 | 0.473 | |
| POPDI***, mean (SD) | Overall | −77.50 | −77.10 | −0.40 (−8.62,7.83) | 0.243 | 0.925 |
| Month 3 | −77.30 | −83.65 | 6.35 (−2.74,15.45) | 0.966 | 0.171 | |
| Month 12 | −78.09 | −80.40 | 2.31 (−6.42,11.04) | 0.641 | 0.604 | |
| Month 24 | −77.11 | −67.26 | −9.85 (−19.11,−0.60) | 0.009 | 0.037 | |
| CRADI+, mean (SD) | Overall | −42.01 | −43.40 | 1.39 (−7.29,10.07) | 0.081 | 0.753 |
| Month 3 | −42.75 | −47.41 | 4.66 (−5.01,14.33) | 0.142 | 0.345 | |
| Month 12 | −41.79 | −48.46 | 6.67 (−2.59,15.93) | 0.004 | 0.158 | |
| Month 24 | −41.49 | −34.34 | −7.15 (−17.01,2.70) | 0.672 | 0.155 | |
| UIQ#, mean (SD) | Overall | −45.42 | −51.81 | 6.39 (−4.69,17.46) | <0.001 | 0.258 |
| Month 3 | −39.35 | −53.91 | 14.55 (2.62,26.49) | <0.001 | 0.017 | |
| Month 12 | −49.19 | −58.32 | 9.13 (−2.45,20.71) | <0.001 | 0.122 | |
| Month 24 | −47.72 | −43.20 | −4.53 (−16.72,7.66) | <0.001 | 0.466 | |
| POPIQ$, mean (SD) | Overall | −44.40 | −51.67 | 7.26 (−1.81,16.33) | <0.001 | 0.116 |
| Month 3 | −42.70 | −56.31 | 13.61 (3.73,23.49) | <0.001 | 0.007 | |
| Month 12 | −44.95 | −57.64 | 12.70 (3.17,22.23) | <0.001 | 0.009 | |
| Month 24 | −45.57 | −41.05 | −4.52 (−14.55,5.51) | <0.001 | 0.377 | |
| CRAIQ^, mean (SD) | Overall | −21.27 | −25.95 | 4.68 (−4.42,13.78) | <0.001 | 0.313 |
| Month 3 | −19.66 | −30.69 | 11.04 (0.90,21.17) | <0.001 | 0.033 | |
| Month 12 | −23.85 | −33.49 | 9.63 (−0.07,19.34) | <0.001 | 0.052 | |
| Month 24 | −20.31 | −13.67 | −6.64 (−16.95,3.68) | 0.029 | 0.207 | |
| PISQ-12^^, mean (SD) | Overall | 3.41 | 4.47 | −1.06 (−2.49,0.36) | 0.088 | 0.142 |
| Month 3 | 3.51 | 4.37 | −0.87 (−2.49,0.75) | 0.426 | 0.294 | |
| Month 12 | 3.44 | 5.02 | −1.58 (−3.10,−0.07) | 0.022 | 0.041 | |
| Month 24 | 3.28 | 4.02 | −0.74 (−2.38,0.89) | 0.179 | 0.372 |
Urinary Distress Inventory
Pelvic Organ Prolapse Distress Inventory
Colorectal Anal Distress Inventory
Urinary Impact Questionnaire
Pelvic Organ Prolapse Impact Questionnaire
Colorectal Anal Impact Questionnaire
Pelvic Organ Prolapse Urinary Incontinence Sexual Questionnaire
Table 6 displays the adverse events. Although serious adverse events were more common in the open sacrocolpopexy group at 6 weeks and one year, after statistically adjusting for center, apical procedure type and occurrence of posterior or anterior procedure, the groups were similar. Visceral injury, transfusion, pulmonary embolus and deep vein thrombosis were rare. Mesh erosion rates have previously been reported in the parent studies; for the OPUS trial, there were no mesh erosions, for the OPTIMAL study there were two erosions and in the CARE trial there were twelve.5,6,7 Neither serious adverse events rates nor Dindo ratings were different over the follow-up period. (Table 6)
Table 6.
Complications and Adverse Events
| Adverse Event | Abdominal, (N=321) |
Vaginal, (N=701) |
P-value [1] |
Adjusted P-value [2] |
|
|---|---|---|---|---|---|
| Serious Adverse Events, n (%) | |||||
| During First 6 Weeks Post-op | 42 (13%) | 38 (5%) | <0.001 | 0.104 | |
| During First Year Follow-up | 83 (26%) | 93 (13%) | <0.001 | 0.098 | |
| Serious Adverse Events per subject, mean (SD) (range) | |||||
| During First 6 Weeks Post-op | 0.1 (0.4) (0–2) | 0.1 (0.2) (0–2) | <0.001 | 0.276 | |
| During First Year Follow-up | 0.3 (0.6) (0–3) | 0.2 (0.4) (0–3) | <0.001 | 0.431 | |
| Maximum reported Dindo score during first 6 weeks post-op, n (%) | 0.109 | 0.596 | |||
| 1 | 11 (3%) | 3 (0%) | |||
| 2 | 17 (5%) | 18 (3%) | |||
| 3 | 14 (4%) | 16 (2%) | |||
| 4 | 0 (0%) | 0 (0%) | |||
| 5 | 0 (0%) | 0 (0%) | |||
| Maximum reported Dindo score during first year follow-up, n (%) | 0.890 | 0.983 | |||
| 1 | 11 (3%) | 6 (1%) | |||
| 2 | 19 (6%) | 27 (4%) | |||
| 3 | 50 (16%) | 58 (8%) | |||
| 4 | 3 (1%) | 1 (0%) | |||
| 5 | 0 (0%) | 0 (0%) | |||
| Specific Adverse Events Intraoperative and Through 6 weeks Postoperatively | |||||
| Ureteral injury during surgery, n (%) | 1 (0%) | 16 (2%) | 0.018 | n/a | |
| Blood transfusion during hospitalization, n (%) | 12 (4%) | 13 (2%) | 0.081 | n/a | |
| Cystotomy/urethral injury, n (%) | 4 (1%) | 54 (8%) | <0.001 | n/a | |
| Bowel injury, n (%) | 0 (0%) | 1 (0%) | 1.000 | n/a | |
| Vascular injury, n (%) | 1 (0%) | 0 (0%) | 0.314 | n/a | |
| Nerve injury, n (%) | 0 (0%) | 2 (0%) | 1.000 | n/a | |
| Deep vein thrombosis, n (%) | 1 (0%) | 1 (0%) | 0.531 | n/a | |
| Pulmonary embolism, n (%) | 2 (1%) | 1 (0%) | 0.235 | n/a | |
P-values based on comparing outcomes between groups while excluding missing outcomes. For variables with small cell counts, p-values are based on unadjusted, Fisher exact tests.
Adjusted p-value controls for center, apical procedure type and occurrence of posterior or anterior procedure.
Discussion
Two years following surgery, open ASC is associated with better composite outcome success rates and no significant differences in adverse events than vaginal repair of prolapse. Patient quality of life and symptom bother outcomes were similar and low reoperation rates were observed for both groups. Published symptom and anatomic outcomes, complications and reoperation rates for these procedures have significant limitations. A meta-analysis of comparative studies reported improved anatomic outcomes with colpopexy over native tissue repair with an increased risk of adverse events; however there was insufficient evidence to make any statements regarding the impact on bowel or bladder function.[15] Three published randomized trials that have compared open ASC to SSLF are summarized in a second meta-analysis.[1–4] That study supported superior anatomic outcomes for open ASC, but with longer operating times and longer recovery. Because of the transition to minimally invasive routes for ASC, the authors of the meta-analysis concluded that further evidence comparing minimally invasive approaches to ASC and vaginal repairs with and without mesh are needed.[1] Thus, existing randomized data directly comparing ASC and VAR prolapse repairs do not allow an evidence-based decision regarding the best route of apical prolapse surgery for each patient with prolapse.[1] In our retrospective study, combining data from three NICHD trials allowed us to evaluate a large number of women with validated anatomical, quality of life, bowel and bladder and sexual function outcomes as well as co evaluate adverse events throughout postoperative follow-up, permitting a comparison of the risks and benefits that often inform surgical decision making.
Serious adverse events did not differ between groups. Individual events commonly associated with surgery including thrombosis, pulmonary embolis and ureteral injury were very low and we likely did not have enough power to detect differences between groups. Although perioperative complications after sacrospinous ligament fixation are uncommon, the consequences for individual affected patients can be significant. Buttock pain occurs in 3% but is usually self-limited, whereas sacral/pudendal neurovascular injury can result in transfusion in 2% and life-threatening hemorrhage in 0.2%.[16] In this study, transfusion was rare and occurred in both cohorts. Ureteral kinking or injury is commonly associated with transvaginal uterosacral colpopexy; however, most of these are identified intraoperatively and only 0.6% of patients require interval ureteral reimplantation.[17] Immediate perioperative complications of open ASC include wound complications in 4.6%, hemorrhage/transfusion in 4.4%, and visceral injury in 1–3.1%. We did find more ureteral kinking in our vaginal repair group but long-term sequelae from these injuries was low. Other visceral injuries were very low in both groups. Erosion of colpopexy material is one of the main concerns with ASC, occurring in 3.4% of 2,178 cases 3 years after surgery regardless of the type of material used, and requiring reoperation in 3% of women having ASC.[18] In the CARE trial from which our ASC cases were gathered, mesh erosion was thought to occur only in the first few years after surgery. However, longer follow-up in this cohort has documented a 10.5% rate of mesh erosion by seven years post operatively.[19] The CARE trial did allow multiple forms of synthetic mesh, some of which are no longer commonly used. In addition, the anatomical failure rate in the long term CARE followup was much higher than anticipated and ranged from 24 to 48%, depending on the definition used.
Our retrospective comparison benefited from the combined data from three large multicenter randomized trials with well characterized subjects using the same validated pelvic floor symptom distress and impact measures and systematic collection of adverse events. It allowed a robust comparison of prospectively collected one- and two-year outcomes in women undergoing a vaginal apical reconstructive surgery to those women undergoing an open abdominal approach. These data should be considered in light of their limitations. A comparative retrospective study has inherent biases regarding patient allocation. Patient characteristics were significantly different in the groups, with the ASC group being older, having greater prolapse, more previous surgeries for prolapse repair, and less bothersome urinary and bowel symptoms at baseline. Although we detected better outcomes in the ASC group, the baseline differences between groups may have diminished the differences observed. Despite excellent patient follow-up and standardized data collection, one of the two vaginal trials only collected 12-month outcomes, and the outcomes for times beyond 12 months were inferred using longitudinal mixed effects models (for continuous measures) or generalized linear models with empirical variance estimates (for binary measures) to obtain valid outcome estimates at specific visit times, accounting for data missing at random or missing completely at random due to the varied planned visits. These assumptions may not be correct and may have favored the VAR group underestimating the observed better outcomes in the ASC group.
Surgical techniques have evolved with time. The three studies in this analysis did not include transvaginal mesh or minimally invasive colpopexy procedures, and we do not have data on why patients were offered a vaginal or abdominal approach to their repair. Therefore, we are limited in our ability to comment on the role of these procedures in pelvic organ prolapse repair. A higher number of patients had undergone prior prolapse repairs, and a history of prior repair may have important implications on success of the current attempt at repair. Surgical technique varies with individual surgeons and technique may account for some of the differences seen. It is possible that there may have been other differences that we did not measure or control for that influenced our findings.
In conclusion, in a careful examination of pre-existing datasets from three large trials of prolapse repair, open sacrocolpopexy was more likely to result in prolapse treatment success and was not observed to be significantly associated with greater rates of serious adverse events following surgery compared to vaginal repair. Bowel, bladder and prolapse functional results improved in both groups similarly. A randomized trial of vaginal and minimally invasive abdominal surgical approaches in women with apical prolapse with longer-term outcome time-points accompanied by cost effectiveness and robust adverse event analyses would more fully inform surgical practice and counseling.
Table 1.
Patient Characteristics
| Characteristic | Abdominal (N=321) |
Vaginal (N=701) |
P-value [1] | |
|---|---|---|---|---|
| Age at surgery, mean years (SD) | 61.5 (10.2) | 59.5 (10.4) | 0.005 | |
| BMI* (kg/m2), mean (SD) | 27.0 (4.5) | 28.4 (5.4) | <0.001 | |
| Primary race, n (%) | <0.001 | |||
| Black/African American | 17 (5%) | 41 (6%) | ||
| White | 298 (93%) | 604 (86%) | ||
| Other | 6 (2%) | 54 (8%) | ||
| Unknown | ||||
| Hispanic/Latina Ethnicity, n (%) | 9 (3%) | 127 (18%) | <0.001 | |
| Insurance, n (%) | <0.001 | |||
| Medicaid/Medicare | 29 (9%) | 194 (28%) | ||
| Private/HMO | 279 (87%) | 496 (71%) | ||
| Self-Pay | 8 (2%) | 10 (1%) | ||
| Other | 5 (2%) | 1 (0%) | ||
| Vaginal deliveries, mean (SD) | 3.0 (1.4) | 3.0 (1.9) | 0.274 | |
| History of caesarean delivery, n (%) | 16 (5%) | 59 (8%) | 0.050 | |
| Prior urinary incontinence surgery, n (%) | 22 (7%) | 23 (3%) | 0.010 | |
| Prior prolapse surgery, n (%) | 125 (39%) | 58 (8%) | <0.001 | |
| Post-Menopausal, n (%) | 279 (87%) | 528 (75%) | <0.001 | |
| Currently using estrogen replacementtherapy, n (%) | 129 (40%) | 231 (33%) | 0.025 | |
| Current smoker, n (%) | 23 (7%) | 51 (7%) | 0.950 | |
| Diabetes Mellitus, n (%) | 16 (5%) | 84 (12%) | <0.001 | |
| Cardiac impairment, n (%) | 48 (15%) | 110 (16%) | 0.823 | |
| SF36 Physical component score, mean (SD) | 45.4 (9.6) | 45.3 (9.9) | 0.869 | |
| Sexually active, n (%) | 161 (50%) | 326 (47%) | 0.672 | |
P-values based on comparing characteristics between groups while excluding responses of unknown
Body Mass Index
Brief Summary.
In a retrospective analysis, open sacrocolpopexy when compared to vaginal apical repairs results in greater composite treatment success without increases in adverse events.
Acknowledgments
University of Iowa: CS Bradley, K Kreder; University of Alabama, KL Burgio, RE Varner, A Ballard, J Burge, K Carter, P Goode, AD Markland, C Parker-Autry, TS Wilson; University of Michigan YW Casher, B Marchant, JT Wei, PA Wren, YH Chen, D DiFranco, C Spino, B Marchant; University North Carolina of Chapel Hill AM Connolly, W Whitehead; Johns Hopkins GW Cundiff, VL Handa; Baylor P Fine; Magee Women’s Hospital J Gruss, P Moalli, HM Zyczynski; Duke AG Visco, CL Amundsen, I Harm-Ernandes, M Raynor, J Wu, NY Siddiqui; UCSD ME Albo, C Grimes, ES Lukacz, CW Nager; UTSW S Atnip, EK Moore, D Rahn, C Wai; University of Utah J Baker, M Masters, A Orr; Kaiser San Diego G Diwadkar, KY Dyer, LM Hall, LM Mackinnon, JN Nguyen, G Zazueta-Damian, J Tan-Kim; Cleveland Clinic A Frick, B O’Dougherty, L Pung, B Ridegeway, C Williams; RTI M Gantz, LK Warren, D Matthews, A Shaffer, KA Wilson, RE Whitworth, J Thornberry, TT Terry; Steering Committee Chair K Hartmann; Kaiser Bellflower S Jakus-Waldman; Loyola E Mueller, M Tulke; University of New Mexico Y Komesu, G Dunivan, C Cichowski, P Jeppson.
Funding: Research Support: Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (2U01HD41249, 2U10 HD41250, 2U10 HD41261, 2U10 HD41267, 1U10 HD54136, 1U10 HD54214, 1U10 HD54215, 1U10 HD54241, U10 HD069013, U10 HD069025, U10 HD069010, and U01 HD069031) and the National Institutes of Health Office of Research on Women’s Health.
Footnotes
Disclosures: Rogers: UptoDate-Royalties; Travel and stipend from ABOG for work on the board, Travel and Stipend from IUGA for work related to the editor in chief for the IUJ, DSMB Chair for the TRANSFORM trial sponsored by American Medical Systems. Richter: Pelvalon – Research Grant and Consultant, Kimberly Clark- consultant, UptoDate –royalties; Jelovsek: no conflict of interest; Shepherd – Site PI for the Synergy trial sponsored by Astellas; Harvie: no conflict of interest; Brubaker: Editorial stipends, Female Pelvic Medicine and Reconstructive Surgery and UptoDate. Royalties UpToDate; Menefee: no conflict of interest; Myers: no conflict of interest; Hsu: no conflict of interest; Schaffer: McGraw Hill- Royalties, Boston Scientific – Research, Astellas - Speaker; Wallace: no conflict of interest; Meikle: no conflict of interest.
Presentation Information: This was presented at the Society of Gynecologic Surgeons’ 2014 annual meeting in Orlando, Florida.
Authors’ contributions to the manuscript: All authors participated in protocol/project development and manuscript writing and editing.
Author’s contribution to the manuscript:
ROGERS Project development, data collection, data analysis, manuscript writing editing; NOLEN Project development, data collection, data analysis, manuscript writing editing; WEIDNER Project development, data collection, data analysis, manuscript writing editing; RICHTER Project development, data collection, data analysis, manuscript writing editing; JELOVSEK Project development, data collection, data analysis, manuscript writing editing; SHEPHERD Project development, data collection, data analysis, manuscript writing editing; HARVIE Project development, data collection, data analysis, manuscript writing editing; BRUBAKER Project development, data collection, data analysis, manuscript writing editing; MENEFEE Project development, data collection, data analysis, manuscript writing editing; MYERS Project development, data collection, data analysis, manuscript writing editing; HSU Project development, data collection, data analysis, manuscript writing editing; SCHAFFER Project development, data collection, data analysis, manuscript writing editing; WALLACE, PhD, Study design, analysis and manuscript writing and approval; MEIKLE Project development, data collection, data analysis, manuscript writing editing
Contributor Information
Rebecca G Rogers, Dell Medical School, University of Texas, Austin, Texas and Albuquerque, New Mexico, Department of Obstetrics and Gynecology, University of New Mexico Health Sciences Center.
Tracy L. Nolen, Research Triangle Park, NC, RTI International.
Alison C. Weidner, Durham, North Carolina, Department of Obstetrics and Gynecology, Duke University.
Holly E Richter, Birmingham, Alabama, Department of Obstetrics and Gynecology, University of Alabama at Birmingham.
J Eric Jelovsek, MMEd Cleveland, Ohio, Obstetrics, Gynecology & Women’s Health Institute, Cleveland Clinic.
Jonathan P Shepherd, Pittsburgh, Pennsylvania, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh Medical Center.
Heidi S Harvie, Philadelphia, Pennsylvania, Department of Obstetrics and Gynecology, University of Pennsylvania.
Linda Brubaker, Maywood, Illinois, Department of Obstetrics and Gynecology, Stritch School of Medicine.
Shawn A. Menefee, San Diego, California, Department of Obstetrics and Gynecology, Kaiser Permanente.
Deborah Myers, Providence, Rhode Island, Department of Obstetrics and Gynecology, Brown University.
Yvonne Hsu, Salt Lake City, Utah, Department of Obstetrics and Gynecology, University of Utah.
Joseph I Schaffer, Dallas Texas, Department of Obstetrics and Gynecology, University of Texas, Southwestern.
Dennis Wallace, Research Triangle Park, NC, RTI International.
Susan F. Meikle, Northwest Texas Physician Group, Amarillo, TX.
References
- 1.Maher C, Feiner B, Baessler K, Adams EJ, Hagen S, Glazener CM. Surgical management of pelvic organ prolapse in women. Cochrane database of systematic reviews. 2010;14(4):CD004014. [Google Scholar]
- 2.Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175(6):1418–21. doi: 10.1016/s0002-9378(96)70084-4. [DOI] [PubMed] [Google Scholar]
- 3.Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190(1):20–6. doi: 10.1016/j.ajog.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Lo TS, Wang AC. Abdominal colpopsacropexy and sacrospinous ligament suspension for severe uterovaginal prolapse: a comparison. J Gyn Surg. 1998;14(2):59–64. [Google Scholar]
- 5.Brubaker L, Cundiff GW, Fine P, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557–1566. doi: 10.1056/NEJMoa054208. [DOI] [PubMed] [Google Scholar]
- 6.Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311:1023–34. doi: 10.1001/jama.2014.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei JT, Nygaard I, Richter H, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. NEJM. 2012;366(25):2358–2367. doi: 10.1056/NEJMoa1111967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haylen BT, Younis M, Naidoo S, Birrell W. Perineorrhaphy quantitative assessment (Pe-QA) Int Urogynecol. 2014 doi: 10.1007/s00192-014-2528-1. J epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–95. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 10.Barber MD, Brubaker L, Nygaard I, et al. Pelvic Floor Disorders Network. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114(3):600–609. doi: 10.1097/AOG.0b013e3181b2b1ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber MD. Symptoms and outcome measures of pelvic organ prolapse. Clinical Obstetrics and Gynecology. 2005;48(3):648–661. doi: 10.1097/01.grf.0000170424.11993.73. [DOI] [PubMed] [Google Scholar]
- 12.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 13.Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the pelvic organ prolapse/urinary incontinence sexual questionnaire (PISQ-12) Int Urogynecol J Pelvic Floor Dys. 2003;14(3):164–8. doi: 10.1007/s00192-003-1063-2. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui NY, Grimes CL, Casiano ER, Abed HT, Jeppson PC, Olivera CK, et al. Mesh scrocolpopexy compared with native tissue vaginal repair. Obstet Gynecol. 2015;125(1):44–55. doi: 10.1097/AOG.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466–75. doi: 10.1016/S0029-7844(96)00337-7. [DOI] [PubMed] [Google Scholar]
- 17.Margulies RU, Rogers MA, Morgan DM. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol. 2010;202:124–34. doi: 10.1016/j.ajog.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104(4):805–823. doi: 10.1097/01.AOG.0000139514.90897.07. [DOI] [PubMed] [Google Scholar]
- 19.Nygaard I, Brubaker L, Zyczynski HM, et al. Long term outcomes following abdominosacralcolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024. doi: 10.1001/jama.2013.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]