Abstract
Introduction
Gait speed is recognized as an important predictor of adverse outcomes in older people. However, it is unknown if other more complex mobility tasks are better predictors of such outcomes.
Objective
To examine a range of clinic-based mobility tests and determine which were most strongly associated with measures of community performance and risk (CP&R).
Design
Cross-sectional study
Setting
Central Control Mobility and Aging Study, Westchester County, New York
Participants
Aged ≥ 65 years (n=424)
Methods
Clinic-based mobility measures included gait speed measured during normal and dual-task conditions, the Floor Maze Immediate and Delay tasks, and stair ascending and descending. CP & R measures were self-reported using standardized questionnaires and classified into measures of performance (distance walked; travel outside one’s home [life space]; activities of daily living, and participation in cognitive leisure activities) or risk (balance confidence, fear of falling and past falls). Linear and logistic regression were used to examine associations between the clinic-based mobility measures and CP&R measures adjusting for covariates.
Results
The mean age of the sample was 77.8 (SD 6.4) years and 55.2% (n=234) were female. In final models faster normal walking speed was most strongly associated with five out of the seven community measures (greater distance walked, greater life space, better activities of daily living function, higher balance confidence and less fear of falling; all p<.05). More complex tasks (walking while talking and maze immediate) were associated with cognitive leisure activity (p<.05), and ascending stairs was the only measure associated with a history of falls (p<.05).
Conclusion
Normal walking speed is a simple and inexpensive clinic-based mobility test that is associated with a wide range of CP&R measures. In addition, poorer performance ascending stairs may assist in identifying those at risk of falls. Poorer performance in more complex mobility tasks (walking while talking and maze immediate) may suggest inability to participate in cognitive leisure activities.
INTRODUCTION
An important role of geriatric care is in identifying those who have or are at risk of developing adverse outcomes in order to instigate effective therapeutic interventions. Gait speed has emerged as an inexpensive and time efficient clinic-based measure that is an important predictor of a number of ageing related adverse health outcomes such as falls 1, dementia 2, loss of independence 3,4 and even mortality 5. This is likely due to the impact of a wide range of impairments on gait, including chronic disease 6, reduced strength 7, balance 7, pain 8 and cognitive function 9. Consequently gait speed has been termed the sixth vital sign, reflecting a person’s overall health and ability to compensate for multiple impairments 10.
Other more complex mobility tests are available to health professionals in the clinic. Such tests include dual task or walking while talking (WWT) tests 11, time to ascend and descend stairs 12, and the Floor Maze Test 13. Due to these test placing greater stress on cognitive and motor systems, they may be more sensitive, or add value to normal gait speed in assessing an older person’s functional ability and risk of adverse health events outside of the clinic. Alternatively, normal walking speed may be sufficient to capture an individual’s performance in the community, and the extra time and space required by these more complex tests may not be required. A direct comparison of simple and complex mobility tasks has been lacking.
Therefore, using a large cohort study, we aimed to examine the associations between a number of objective clinic-based mobility tests and their associations with self-reported measure of community performance and risk (CP&R). In addition we aimed to explore which one or combination of mobility tests explained the greatest proportion of variance in each community measure. We hypothesized that 1) slow gait speed would be associated with all the community performance and risk measures but that 2) more complex mobility tests (WWT and the maze tasks) would be more strongly associated with community performance tasks requiring greater cognitive input (cognitive leisure activities, travelling outside one’s home environment and less ability to carry out instrumental activities of daily living).
METHODS
Subjects
Participants were people aged ≥ 65 years participating in the longitudinal Central Control of Mobility in Aging (CCMA) study. The aim of CCMA is to examine the role of the aging brain and cognition on mobility in older people. Participants were identified from a population list of lower Westchester County, New York. They were firstly sent a letter and then a follow-up telephone call inviting them to participant 14. As described previously 15, at study entry participants were excluded if they had dementia, were unable to walk, had severe neurological or psychiatric conditions, major visual or auditory loss, were receiving hemodialysis, or had recent or planned surgery that would interfere with assessments or restrict walking. The first assessment for each individual where the Floor Maze test was collected (phase 2 for most participants) was used in this analysis. Informed consent was obtained from all participants and the study was approved by the Albert Einstein College of Medicine Institutional Review Board.
Clinical mobility measures
The following tests were chosen as they are feasible in clinic-based settings and stress both cognitive (dual-task and the Floor Maze tests) and physical resources (stairs) to a greater extent than walking on the flat under single task conditions.
Normal gait speed under single-task conditions (cm/sec)
Participants were asked to complete one trial on a computerized mat (457.2 × 90.2 × 0.6 cm) with embedded pressure sensors (GAITRite; CIR Systems, PA, USA) at their ‘normal walking speed’. The GaitRite system has excellent test-retest reliability and validity in older people 16,17. Walking while talking (WWT) (cm/sec): Participants were asked to walk as above, but with the addition of a cognitive interference task (reciting alternate letters of the alphabet starting with the letter ‘A’) 18. They were instructed to pay equal attention to both the walking and talking tasks to avoid task prioritization effects. The Floor Maze Test (immediate and delay): The maze is based on the Porteus Maze test (extension VIII), and is a test of spatial navigation (ref) and has been shown to predict pre-dementia syndromes (ref). The maze is designed on the floor with yellow tape on a blue background measuring 7′×10′. For the immediate test, after a 15 second planning period (not included in the overall time), participants are timed with a stopwatch from the start to the exit of the maze (sec). For the delayed test, participants are asked to repeated the test after 10 minutes with no planning period 13. Stairs test (ascending and descending): Participants were timed with a stopwatch (sec) both ascending and descending 3 stairs (18cm in height, 26cm deep and 110cm wide). Participants were permitted to use a handrail and/or walking aid during the test. This test has been found to be a valid and reliable test in older adults, and predicts functional decline 19.
Community performance measures
The following measures were chosen as they represent a range of activities that an individual performs in the home or community including both everyday activities of daily living, socialization and using transportation.
Mobility distance was assessed using a standardized question “How far can you walk in one hour on level ground?”. Responses were coded as follows: 0=less than a quarter mile; 1=quarter to half mile; 2=half to one mile; 3=more than a mile. Travel outside of one’s home was measured using the Life Space Questionnaire 20. The Life Space questionnaire assesses mobility (not necessarily walking) with respect to reaching different locations or life-spaces. It has five levels, as described below, and includes the frequency of visits in the past month and any assistance required: “In the past 4 weeks, have you been to: 1) other rooms in your home besides the room where you sleep? 2) an area outside of your home such as your porch, patio, hallway, garage or yard? 3) different places in your neighborhood or your own backyard building? 4) Locations outside of your neighborhood but within your city? 5) Places outside your town?’.” A total score is calculated using the level, independence and the frequency (range 0 to 120 points). Activities of Daily Living (ADL) was measured with the Activities of Daily Living – Prevention Instrument 21, which includes questions on ability to manage money, drive, do the laundry, use appliances, shop, prepare meals, memory, writing, managing medications and complex activities. The questionnaire has been found to have adequate reliability, and discriminate between healthy and early signs of dementia 21.
Cognitive Leisure Activities: were recorded similar to our previous study 22. Activities included reading newspaper and books, knitting and art, crosswords, playing cards and bingo or board games, using a computer or email, writing for pleasure, music and singing, learning a program, and other mentally stimulating activities. For each activity, subjects received seven points for daily participation; four points for participating several days per week; one point for participating once a week; and zero points for participating monthly, occasionally, or never. Days for each activity were summed to generate a cognitive-activity score, ranging from 0 to 49.
Community risk measures
The risk measures were chosen to represent actual and perceived falls-risk.
Fear of falling and prior falls was assessed using a standardized question regarding whether an individual was fearful of falling (yes/no) and if the participant had fallen in the past year (yes/no). Confidence performing ambulatory activities was measured with the Activity Balance Confidence (ABC) scale. The scale has excellent reliability and validity in older people 23.
Other measures were collected via questionnaire and included age, gender, number of years of education, cognitive status with the Repeatable Battery for the Assessment of Neuropsychological Status 24 and self-reported information on medical history (Diabetes mellitus, high blood pressure, heart condition, arthritis, depression, COPD). Dementia was determined using the DSM-IV TR 25 at consensus diagnostic case conferences.
Statistical analysis
Participant characteristics were summarized using mean and standard deviation for continuous measures and number and percentage for dichotomous measures. Correlations between mobility measures were examined using Pearsons correlation coefficients, and between mobility measures and CP&R measures with Spearman correlation coefficients. Multivariable linear regression, adjusting for age and gender, was performed between each clinic-based mobility measure and each CP&R measure in separate models. The partial and pseudo R2 value were used to summarize the contribution and strength of associations of each mobility measure to the model. Final models were constructed by including each significant mobility measure from the previous analyses in the same model. Mobility variables were only maintained if they remained significant. For continuous outcomes Shapley values were computed to partition the contribution of each mobility measure and determine the strongest predictors to the final models. For dichotomous outcomes the pseudo r2 was calculated without, and then with, the mobility measure in the model. STATA 12 was used for all analyses.
RESULTS
Four hundred and twenty four people were included in the study. Characteristics of the sample are presented in Table 1. The mean age was 77.8 (SD 6.4) years, 55.2 percent (n=234) were female and the mean normal gait speed was 98.3 (SD 23.0) cm/sec. Correlations between mobility measures are shown in Table 2. Strong correlations were found between ascending and descending stairs (r=.97), normal walking and WWT (r=.68), normal walking and maze delay (−.34), and between maze immediate and maze delay (r=.60). Correlations between mobility and community performance measures are shown in Table 3. In regression analyses the ABC score was transformed using the boxcox command in STATA and then back transformed to present the results in their original units. The ADL score was positively skewed, but no adequate transformation could be found. Therefore the ADL score was divided into four categories based on its distribution (0; 1–3; 4–6; ≥7). Table 4 (and described in detail below) shows the results of multivariable regression for each clinic-based mobility measure in separate models with each CP&R measure adjusted for age and gender. The addition of education did not change the associations by more than 10% and was therefore not included in models. Medical history was also not included, as mobility was used to provide an overall marker of the accumulation of disease, so that their inclusion in the model would have been an over adjustment. Supplementary Table 1 provides a summary of the final models and Figure 1 shows the explained proportion of variance of each mobility measure in final models.
Table 1.
Participant characteristics (n=424)
| Characteristic | Mean or number | SD or % |
|---|---|---|
| Age, years | 77.8 | 6.4 |
| Female | 234 | 55.2 |
| Education, years | 14.8 | 3.1 |
| Diabetes Mellitus | 59 | 13.9 |
| High Blood pressure | 175 | 41.3 |
| Heart Attack or Congested Cardiac Failure | 13 | 3.1 |
| Arthritis (Osteo or Rheumatoid) | 240 | 56.6 |
| Depression | 28 | 6.6 |
| Chronic Obstructive Airways Disease | 25 | 5.9 |
| Dementia | 12 | 2.8 |
| RBANS index score | 93.5 | 12.6 |
| Normal gait speed, cm/sec | 98.3 | 23.0 |
| Walking while talking, cm/sec | 73.0 | 25.0 |
| Maze Immediate, sec | 30.6 | 32.0 |
| Maze Delay, sec | 22.43 | 19.6 |
| Ascending stairs, sec | 3.1 | 3.1 |
| Descending stairs, sec | 3.0 | 6.0 |
RBANS = Repeatable Battery for the Assessment of Neuropsychological Status
Table 2.
Correlations between clinical mobility measures
| Normal walking n=424 |
WWT n=424 |
Maze Immediate n=409 |
Maze Delay n=404 |
Ascending stairs n=410 |
|
|---|---|---|---|---|---|
| Normal walking | – | ||||
| WWT | 0.68 | – | |||
| Maze Immediate | −0.29 | −0.21 | – | ||
| Maze Delay | −0.34 | −0.27 | 0.60 | – | |
| Ascending stairs | −0.19 | −0.15 | 0.07 | 0.09 | – |
| Descending stairs | −0.13 | −0.09 | 0.04 | 0.06 | 0.97 |
WWT = walking while talking
Table 3.
Correlations between clinic-based mobility measures and community performance and risk measures
| Distance walked | Life Space | ADL | Leisure | ABC | FOF | Falls | |
|---|---|---|---|---|---|---|---|
| Normal walking | 0.50* | 0.32* | −0.19* | 0.15** | 0.49* | −0.24* | −0.19* |
| WWT | 0.35* | 0.22* | −0.13** | 0.16* | 0.35* | −0.20* | −0.13** |
| Maze immediate | −0.29* | −0.26* | 0.18* | −0.23* | −0.34* | 0.12*** | 0.08 |
| Maze delay | −0.34* | −0.28* | 0.20* | −0.22* | −0.31* | 0.17* | 0.12*** |
| Ascending stairs | −0.39* | −0.27* | 0.10*** | −0.05 | −0.42* | 0.16* | 0.21* |
| Descending stairs | −0.40* | −0.31* | 0.12*** | −0.05 | −0.47* | 0.21* | 0.16* |
ADL=Activities of daily living; ABC=Activity Balance Confidence Scale; FOF= fear of falling; WWT=walking while talking
p<.001
p<.01;
p<.05
Table 4.
Multivariable linear regression between each clinical mobility measure and the community performance and risk measures
| Community Performance measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Distance walked | Life space | Activities of Daily Living | Leisure Activity | |||||||||
|
| ||||||||||||
| β | 95% CI | R2 | β | 95% CI | R2 | β | 95% CI | R2 | β | 95% CI | R2 | |
| Normal walking | 0.02 | 0.02,0.03* | 0.31 | 0.26 | 0.17,0.35* | 0.13 | −0.004 | −0.008,−0.002*** | 0.10 | 0.05 | 0.01,0.08** | 0.01 |
| WWT | 0.01 | 0.01,0.02* | 0.22 | 0.14 | 0.06,0.22* | 0.08 | −0.002 | −0.005,0.001 | 0.09 | 0.05 | 0.03,0.08* | 0.03 |
| Maze immediate | −0.01 | −0.01,−0.00** | 0.20 | −0.10 | −0.15,−0.04** | 0.08 | 0.000 | −0.000,0.000 | 0.09 | −0.04 | −0.06,−0.02* | 0.03 |
| Maze delay | −0.01 | −0.02,−0.01** | 0.21 | −0.20 | −0.30,−0.11** | 0.10 | 0.005 | 0.000,0.009* | 0.10 | −0.06 | −0.10,−0.03** | 0.02 |
| Ascending stairs | −0.06 | −0.09,−0.02* | 0.17 | −0.13 | −0.73,0.48 | 0.05 | −0.011 | −0.037,0.015 | 0.09 | 0.00 | −0.23,0.23 | 0.00 |
| Descending stairs | −0.02 | −0.04,−0.00** | 0.16 | 0.09 | −0.22,0.40 | 0.05 | −0.007 | −0.020,0.007 | 0.09 | 0.03 | −0.09,0.14 | 0.00 |
| Community Risk Measures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fear of falling | ABC score | Falls is in the past year | |||||||
|
| |||||||||
| OR | 95% CI | R2 | β | 95% CI | R2 | OR | 95% CI | R2 | |
| Normal walking | 0.98 | 0.97,0.99 | 0.08 | 0.20 | 0.16,0.24 | 0.30 | 0.98 | 0.97,0.99 | 0.04 |
| WWT | 0.99 | 0.98,0.99** | 0.07 | 0.11 | 0.07,0.14* | 0.18 | 0.99 | 0.98,0.99*** | 0.02 |
| Maze immediate | 1.00 | 0.99,1.00 | 0.05 | −0.05 | −0.08,−0.02** | 0.14 | 1.01 | 0.99,1.01 | 0.01 |
| Maze delay | 1.00 | 0.98,1.00 | 0.05 | −0.06 | −0.11,−0.01*** | 0.13 | 1.01 | 1.00,1.02 | 0.02 |
| Ascending stairs | 1.01 | 0.95,1.08 | 0.05 | −0.48 | −0.77,−0.19** | 0.12 | 1.49 | 1.21,1.84*** | 0.05 |
| Descending stairs | 1.00 | 0.97,1.04 | 0.05 | −0.17 | −0.32,−0.02*** | 0.11 | 1.25 | 1.07,1.45** | 0.03 |
WWT = Walking while talking; ABC = Activity Balance Confidence Score;
p<.001
p<.01;
p<.05
Figure 1.
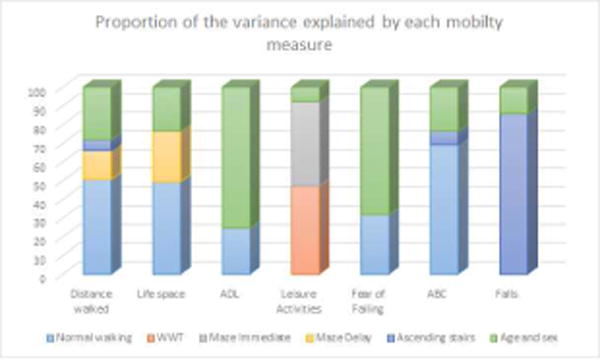
The proportion of the Community performance and risk outcome measures explained by each clinic-based mobility test
ADL=Activities of daily living; ABC=Activities Balance Confidence score; WWT=Walking While Talking
Performance measures
Distance walked in 1 hour: In separate models poorer performance on all clinic based mobility tests were associated with less distance walked in 1 hour (p<.05; Table 4). In final models slower normal walking speed (β .02 95%CI .01, .02; p<.001); longer maze delay time (β −.01 95%CI −.01, −.00; p=.004) and longer time to ascend stairs (β −.03 95%CI −.06, −.00; p=.045) remained statistically significant, explaining 34% of the variance of the final model. Of this 34%, normal walking speed explained the greatest proportion (50.8%; Figure 1).
Life space
In separate models slower normal walking (p<.001), WWT (p<.001), maze immediate (p=.002) and delay times (p<.001) were all associated with poorer scores on the life space questionnaire (Table 4). In final models slower normal walking speed (β .20 95%CI .11, .29; p<.001) and longer maze delay times (β −.15 95%CI −.24,−.05; p=.004) remained significant and explained 15% of the variance. Of this normal walking speed explained the greatest proportion (49.5%; Figure 1).
ADL function
Slower normal walking speed (p=.04) and maze delay times (p=.03) were associated with poorer ADL function (Table 4). When both mobility measures were included in the same model neither remained significant. In individual models the explained proportion of was variance was similar for both models (10%), but when this was partitioned, normal walking speed explained a higher proportion (24.7% vs 18.2%), and so was chosen for the final model.
Cognitive leisure activities
In separate models slower normal walking (p=.005), WWT (p<.001), maze immediate (p<.001) and delay times (p=.001) were associated with doing less cognitive leisure activities (Table 4). In final models slower WWT (β .04 95%CI .02, .07;p=.002) and longer maze immediate times (β−.03 95%CI −.06, −.01; p=.003) remained significant, with the model explaining 5% of the variance. Of this WWT contributed 47.2% and maze immediate 45.2% of the variance (Figure 1).
Community risk measures
Fear of Falling
Slower normal walking (p=.001) and WWT (p=.006) were associated with fear of falling (Table 4), but in final models only normal walking was significant (OR .98 95% CI .97,.99; p=.001). The pseudo R2 for the model was 8% with normal walking contributing 31.8% of 8%.
Activities and Balance Confidence Scale
Poorer performance on all mobility measures was associated with lower ABC scores (p<.05; Table 4). In final models only slower normal walking speed (β .20 95% CI .15, .24; p<.001) and time to ascend stairs (β−.28 95%CI −.56,−.01; p=.04) remained significant, explaining 30% variance. Of this normal walking speed contributed the greatest proportion (69.3%; Figure 1).
Falls
In separate models slower normal walking (p<.001), ascending (p<.001) and descending stairs (p=.002) were associated with falls in the previous year. In final models only ascending stairs (OR 1.33 95%CI 1.05, 1.68; p=.02) remained significant. The pseudo R2 was 5% with ascending stairs contributing 81.7%.
Removing 12 people with dementia did not meaningfully change any of the associations.
Finally to increase the clinical relevance of our study we present (in Supplementary Table 2) the associations between normal walking speed and the above significant CP&R measures using gait thresholds of 80 and 100 cm/s previously described in the literature5,26,27.
DISCUSSION
Loss of independence, social isolation, and adverse events such as falls are major problems in older age 3. Identifying those in need of preventative interventions is an important component of geriatric care. We examined a range of clinic-based mobility tests and their associations with CP&R in order to determine which tests may best identify older people who may require further assessment and therapeutic interventions. We found that slower normal walking speed was the strongest predictor of all but two of the seven CP&R measures. In contrast, complex mobility tasks (WWT and Floor Maze test) were associated with greater participation in cognitive leisure activities, and slower time to ascend stairs was associated with having had a fall in the past 12 months. This study provides useful information for clinicians suggesting that, in most cases, a simple test of normal walking speed is the strongest predictor of CP&R in older people.
Slower walking speed at an individual’s normal pace was associated with all the CP&R measures in individual models. It explained the greatest proportion of the variance in all but the cognitive leisure score and reporting a fall in the past year. In the final models where other mobility measures were included, slow gait speed continued to be the mobility measure most strongly associated with shorter distances walked, smaller life space, greater impairment in ADLs, fear of falling and lower balance confidence. These results add to prior findings that slow walking speed is a good summary measure of an older persons functional ability as well as an indicator of risk of adverse outcomes 1,3-5,28. Slow gait speed can be thought of as the final expression of a person’s ability to compensate for a wide range of impairments including pain 8, cognitive (e.g. executive function and processing speed) 9, and sensory motor deficits (e.g. Strength, balance, vision, proprioception and simple reaction time)7. For clinicians, the implications of our results are useful, in that measuring normal walking speed is relatively quick and inexpensive to perform. Compared with other tests that require equipment (e.g.stairs) or more space (e.g a Maze drawn on the floor), gait speed under single task conditions can be simply measured in a corridor using a stopwatch, and has existing threshold values for which walking below these speeds predicts future adverse health outcomes 10,29. For example gait speed of lower than 1.0 m/s predictor of hospitalisation26,27, disability26,27 and survival5,26.
In agreement with our hypothesis, better performance on more complex mobility tests, such as the WWT and the Maze immediate tasks, were the only measures independently associated with participating in more cognitive leisure activities. This may reflect that cognition, more than physical function, is important for these activities. WWT requires more attentional resources than normal walking 30 and slower times on the Maze immediate task are associated with a decline in processing speed 13. In addition, both are associated with greater levels of cognitive impairment 13,31-33. Our results suggest that these mobility tests are required to expose subtle deficits in cognition important for participating in leisure activities. However, it cannot be ruled out that participating in these leisure activities might improve cognitive performance22 and subsequently complex mobility tasks.
The Maze task also incorporates spatial navigation skills that are import in navigating outside one’s home. In agreement with this, we found the better performance on the Maze delay task was independently associated with greater life space and walking greater distances in one hour. However, in contrast to our hypothesis that the more complex mobility tasks would be more strongly associated with greater life space and ADLs, slower normal walking speed was the strongest predictor of these measures. Although surprising, possible explanations could be that normal gait speed is able to adequately capture deficits associated with these activities or that they rely more heavily on physical (e.g. items on the ADL questionnaire such as shopping, laundry, housework and repairs), rather than cognitive ability. In addition the majority of participants were independent in ADLs (median=1; 25-75% quartiles 0-18), potentially reducing the ability to identify a relationship with the more complex mobility measures. Alternatively there may be greater variation in response to more complex tasks. For example, during WWT some individuals may have inappropriately chosen to maintain their gait speed, whilst others appropriately decreased their speed for safety 34.
Finally, slower time to ascend stairs was independently associated with lower balance confidence and falls in the past year. This is important, as the ABC score and a history of past falls are both associated with future falls 35,36. These associations may be explained by the additional degrees of lower limb muscle strength, visual spatial ability and range of movement required to ascend stairs 37 when compared to walking on a flat surface. Indeed prior studies have shown links between lower limb muscle strength, visuospatial ability and falls 38. Alternatively, many falls occur on stairs 39 and reduced time ascending stairs may be a strategy to reduce the risk of future falls.
Strengths of this study include a well-characterized population from the large CCMA cohort study. The average gait speed of the sample was 98.3 cm/s, slightly lower than that reported in healthy populations (~120 cm/s)40, reflecting the inclusion of those with a range of chronic diseases. We included a range of quantitative clinic-based mobility measures, and carefully assessed their independence and contribution to the CP&R measures to determine which may be most useful to therapists. As well as including commonly used measures such as normal walking and WWT, we also included more novel measures such as the Floor Maze test. This study also has limitations. We did not have objective measurements of community performance and the self-reported measures could be subject to recall or reporting bias. The study was cross-sectional in nature and so we are unable to make causal inferences. Other gait parameters such as gait variability 41 were not included in this study as they are not used in regular clinical practice and require more expensive equipment such as an electronic mat. Finally for some outcomes the explained variance was small indicating that other measures are required to adequately assess community performance and risk in the clinic.
CONCLUSION
We provide evidence of associations between poorer performance in clinic-based mobility tasks and community performance in older people. A simple inexpensive measure of normal walking speed appears to be the strongest marker of CP&R. In addition, poorer performance ascending stairs may assist in identifying those at risk of falls. Poorer performance in more complex mobility tasks (e.g WWT and the maze test) may suggest inability to participate in cognitive leisure activities.
Supplementary Material
Acknowledgments
National Institute on Aging grants (R01 AG039330, RO1AGO44007, AGO44829 and R01AG036921)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest
Evidence level III.
References
- 1.Callisaya ML, Blizzard L, Schmidt MD, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40(4):481–487. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- 2.Beauchet O, Annweiler C, Callisaya ML, et al. Poor Gait Performance and Prediction of Dementia: Results From a Meta-Analysis. J Am Med Dir Assoc. 2016;17(6):482–490. doi: 10.1016/j.jamda.2015.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 4.Perera S, Patel KV, Rosano C, et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. J Gerontol A Biol Sci Med Sci. 2016;71(1):63–71. doi: 10.1093/gerona/glv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI, Li TC, Lin WY, et al. Combined association of chronic disease and low skeletal muscle mass with physical performance in older adults in the Sarcopenia and Translational Aging Research in Taiwan (START) study. BMC geriatrics. 2015;15:11. doi: 10.1186/s12877-015-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Lord SR, Srikanth VK. A population-based study of sensorimotor factors affecting gait in older people. Age Ageing. 2009;38(3):290–295. doi: 10.1093/ageing/afp017. [DOI] [PubMed] [Google Scholar]
- 8.Manty M, Thinggaard M, Christensen K, Avlund K. Musculoskeletal pain and physical functioning in the oldest old. Eur J Pain. 2014;18(4):522–529. doi: 10.1002/j.1532-2149.2013.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin KL, Blizzard L, Wood AG, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68(6):726–732. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- 10.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32(2):46–49. [PubMed] [Google Scholar]
- 11.Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60(10):1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nightingale EJ, Pourkazemi F, Hiller CE. Systematic review of timed stair tests. J Rehabil Res Dev. 2014;51(3):335–350. doi: 10.1682/JRRD.2013.06.0148. [DOI] [PubMed] [Google Scholar]
- 13.Verghese J, Lipton R, Ayers E. Spatial navigation and risk of cognitive impairment: A prospective cohort study. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr) 2014;36(1):373–381. doi: 10.1007/s11357-013-9570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allali G, Ayers EI, Verghese J. Multiple modes of assessment of gait are better than one to predict incident falls. Arch Gerontol Geriatr. 2015;60(3):389–393. doi: 10.1016/j.archger.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20(1):20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 17.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 18.Brandler TC, Oh-Park M, Wang C, Holtzer R, Verghese J. Walking while talking: investigation of alternate forms. Gait Posture. 2012;35(1):164–166. doi: 10.1016/j.gaitpost.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh-Park M, Wang C, Verghese J. Stair negotiation time in community-dwelling older adults: normative values and association with functional decline. Arch Phys Med Rehabil. 2011;92(12):2006–2011. doi: 10.1016/j.apmr.2011.07.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51(11):1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D, Bennett DA, Sano M, et al. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):S152–169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 22.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 23.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 24.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders - text revision ed. Washington DC: 2000. [Google Scholar]
- 26.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 27.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 28.Verghese J, Wang C, Holtzer R. Relationship of clinic-based gait speed measurement to limitations in community-based activities in older adults. Arch Phys Med Rehabil. 2011;92(5):844–846. doi: 10.1016/j.apmr.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornyak V, JM VS, Brach J. Measurement of gait speed. Topics in Geriatric Rehabilitation. 2013;28(1):27–32. [Google Scholar]
- 30.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 31.Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, et al. Association of Dual-Task Gait With Incident Dementia in Mild Cognitive Impairment: Results From the Gait and Brain Study. JAMA Neurol. 2017;74(7):857–865. doi: 10.1001/jamaneurol.2017.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35(1):96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Tangen GG, Engedal K, Bergland A, Moger TA, Hansson O, Mengshoel AM. Spatial navigation measured by the Floor Maze Test in patients with subjective cognitive impairment, mild cognitive impairment, and mild Alzheimer’s disease. Int Psychogeriatr. 2015;27(8):1401–1409. doi: 10.1017/S1041610215000022. [DOI] [PubMed] [Google Scholar]
- 34.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1-2):196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Cleary K, Skornyakov E. Predicting falls in community dwelling older adults using the Activities-specific Balance Confidence Scale. Arch Gerontol Geriatr. 2017;72:142–145. doi: 10.1016/j.archger.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? Jama. 2007;297(1):77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 37.Startzell JK, Owens DA, Mulfinger LM, Cavanagh PR. Stair negotiation in older people: a review. J Am Geriatr Soc. 2000;48(5):567–580. doi: 10.1111/j.1532-5415.2000.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin KL, Blizzard L, Srikanth VK, et al. Cognitive Function Modifies the Effect of Physiological Function on the Risk of Multiple Falls–A Population-Based Study. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt010. [DOI] [PubMed] [Google Scholar]
- 39.Afram B, Stephan A, Verbeek H, et al. Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc. 2014;15(2):108–116. doi: 10.1016/j.jamda.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Beauchet O, Allali G, Sekhon H, et al. Guidelines for Assessment of Gait and Reference Values for Spatiotemporal Gait Parameters in Older Adults: The Biomathics and Canadian Gait Consortiums Initiative. Front Hum Neurosci. 2017;11:353. doi: 10.3389/fnhum.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Ageing and gait variability–a population-based study of older people. Age Ageing. 2010;39(2):191–197. doi: 10.1093/ageing/afp250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


