Abstract
The success of deep brain stimulation (DBS) surgeries for the treatment of movement disorders relies on the accurate placement of an electrode within the motor portion of subcortical brain targets. However, the high number of electrodes requiring relocation indicates that today’s methods do not ensure sufficient accuracy for all patients. Here, with the goal of aiding DBS targeting, we use 7 Tesla (T) MRI data to identify the functional territories and parcellate the globus pallidus interna (GPi) into motor, associative and limbic regions in individual subjects.
7T MRI scans were performed in seventeen patients (prior to DBS surgery) and one healthy control. Tractography-based parcellation of each patient’s GPi was performed. The cortex was divided into four masks representing motor, limbic, associative and “other” regions. Given that no direct connections between the GPi and the cortex have been shown to exist, the parcellation was carried out in two steps: 1) The thalamus was parcellated based on the cortical targets, 2) The GPi was parcellated using the thalamus parcels derived from step 1. Reproducibility, via repeated scans of a healthy subject, and validity of the findings, using different anatomical pathways for parcellation, were assessed. Lastly, post-operative imaging data was used to validate and determine the clinical relevance of the parcellation.
The organization of the functional territories of the GPi observed in our individual patient population agrees with that previously reported in the literature: the motor territory was located posterolaterally, followed anteriorly by the associative region, and further anteroventrally by the limbic territory. While this organizational pattern was observed across patients, there was considerable variability among patients. The organization of the functional territories of the GPi was remarkably reproducible in intra-subject scans. Furthermore, the organizational pattern was observed consistently by performing the parcellation of the GPi via the thalamus and via a different pathway, going through the striatum. Finally, the active therapeutic contact of the DBS electrode, identified with a combination of post-operative imaging and post-surgery DBS programming, overlapped with the high-probability “motor” region of the GPi as defined by imaging-based methods.
The consistency, validity, and clinical relevance of our findings have the potential for improving DBS targeting, by increasing patient-specific knowledge of subregions of the GPi to be targeted or avoided, at the stage of surgical planning, and later, at the stage when stimulation is adjusted.
Keywords: 7 Tesla MRI, Diffusion, Connectivity, Parcellation, Deep Brain Stimulation, Movement disorders
1. Introduction
The success of deep brain stimulation (DBS) surgery for the treatment of movement disorders, including Parkinson’s disease (PD), essential tremor (ET) and dystonia, relies on accurate placement of an electrode within the motor section of subcortical brain targets (Paek et al., 2013; Richardson et al., 2009; Starr et al., 2006; Welter et al., 2014). However, current neurosurgery stereotactic targeting methods do not ensure such accuracy. In fact, while some studies have reported procedure revision rates ranging from roughly 2% (Fenoy and Simpson, 2014) to 12.4% (Patel et al., 2015), a more recent study, reporting on over 28,000 cases from two large surgical databases, estimated that as many as 15–34% of DBS procedures require revisions (Rolston et al., 2016) due to no, or very limited, therapeutic benefit, poorly tolerated adverse effects, or complications from the surgery or the hardware. These revisions are commonly performed as a result of patients experiencing a lack of benefit by missing or skimming the motor territory or experiencing low threshold side effects that are likely due to activation of adjacent fiber pathways such as the internal capsule.
Currently, pre-operative targeting is commonly performed using 1.5 Tesla (T), or 3T magnetic resonance imaging (MRI). Typically, DBS planning involves indirect targeting methods such as fitting template data or a set of consensus coordinates adjusted onto the patient’s anatomy. Thus, a lot of efforts have focused on developing new image acquisitions at 3T (e.g. FGATIR – (Sudhyadhom et al., 2009; Tanner et al., 2012)) and pushing the resolution and contrast at 7T. However, these methods, while used by some, are not standard in most centers. There exists a high degree of variability in the size, shape, and location of the DBS targets for each subject (Duchin et al., 2014). Therefore, commonly used templates may not be suitable to accurately represent the reality of each patient’s anatomy. This may cause surgical teams to place an electrode in a non-motor territory of the target resulting in stimulation of a non-motor region, which has been associated with behavioral side effects (Mallet et al., 2007; Okun et al., 2009).
Ultra-high field MRI, such as 7T systems, enable researchers and clinicians to clearly visualize the DBS target nuclei (Abosch et al., 2010; Cho et al., 2010; Kerl et al., 2012; Lenglet et al., 2012). For movement disorders, these include the subthalamic nucleus (STN), the globus pallidus interna (GPi), and the ventralis intermedius (Vim) nucleus of the thalamus. These improvements are due to the enhanced image resolution and superior image contrast at 7T in comparison to a standard clinical scanner (1.5T or 3T). Additionally, these benefits are observed while keeping acquisition duration to clinically acceptable lengths (Abosch et al., 2010; Cho et al., 2010; Lenglet et al., 2012; Plantinga et al., 2014).
A recent study also demonstrated the ability to successfully integrate 7T data analysis in the pre-planning and post-operative stages of DBS surgeries in order to increase the likelihood of successfully intersecting the target nucleus and provide information on final electrode and contact location with a patient-specific model of the STN (Duchin et al., 2014). While such developments are essential to identifying the target, in order to reduce the number of non-optimal DBS lead placements, we must be able to define the relative location of motor, associative and limbic territories within the target. This is particularly true given the marked variability across patients of target shape and location.
Uncovering the patient-specific functional organization of DBS targets has the potential to improve targeting as well as patient care by increasing the therapeutic window (offering a wider range of useful settings available for programming), minimizing the patient’s side effects and potentially permitting DBS procedures under general anesthesia without the need for intra-operative micro-electrode recording. Additionally, if the location of each DBS contact is known relative to these regions (e.g. motor), algorithms can be developed to optimize stimulation settings and define optimal volumes and locations of tissue activation with DBS. This, may in turn, lead to drastically reduced programming time, speeding a process that can be burdensome to both clinicians and patients who may be off medication during programming sessions. This is particularly true when lead placements are suboptimal.
In PD patients, we have previously demonstrated that 7T diffusion weighted imaging is capable of generating reproducible, individualized parcellation of the STN into functional territories including motor, associative and limbic regions (Plantinga et al., 2016). In this study, we seek to uncover patient-specific functional organization of the GPi, another DBS target nucleus for the treatment of PD (Xu et al., 2016) and Dystonia (Fox and Alterman, 2015) in order to aid DBS targeting. A recent study, demonstrated the feasibility of parcellating the GPi in individual subjects based on its connection to other structures and found GPi clusters mostly connected to other subcortical regions as well as the prefrontal cortex (da Silva et al., 2017). While the above study is relevant to DBS, it was not conducted in patients, and relied heavily on templates for defining all the anatomical structures. Here, we aim at uncovering functional territories, especially the motor territory, in PD and dystonia patients using the patient’s own GPi. Furthermore, we demonstrate the clinical relevance of this study’s findings based on real-life data and patient’s outcomes. Performing such analyses at 7T is also of significant importance now that the 7T platform received an FDA approval in the USA for clinical use and that more centers will have access to such data. This is, to date, the first such study at 7T. Correctly targeting the motor GPi is all the more important since, contrary to STN stimulation, for which benefits are usually quickly identifiable, benefits during GPi stimulation may be delayed making programming difficult (Vitek, 2002), this is particularly true for dystonia.
2. Methods
Some elements of this “Methods” section were previously described (Plantinga et al., 2016).
2.1. Participants
Eighteen participants (5 female, age: 59.1 +/− 12.5 years) were recruited for this study. The cohort includes 17 DBS patients (15 PD, 2 Dystonia), and 1 healthy control. Patients were referred for DBS surgery at the University of Minnesota (Minneapolis, MN, USA). Surgical candidates were evaluated and identified through a DBS surgical candidacy consensus meeting, which includes experts in stereotactic and functional neurosurgery, movement disorder neurology, neuropsychology and nursing. Specific inclusion criteria included for DBS: clinical findings consistent with idiopathic PD or primary dystonia, inadequate control of symptoms with optimal nonsurgical treatment in the Parkinson’s patients severe ON/OFF fluctuations and/or dyskinesias and good initial L-dopa response. Exclusion criteria for MRI included: subject claustrophobia or other contra-indications for 7T MRI (such as pacemakers and metallic implants). This study was approved by the Institutional Review Board at the University of Minnesota. More information about the participants, including clinically relevant details, can be found in Table 1. The clinical team choose the DBS target, in a given patient, based on individual patient characteristics. For example, PD patients with neuropsychological abnormalities, prominent dyskinesias, or prominent dystonia were more likely to receive GPi DBS, while patients with prominent tremor, or in whom medication dose reduction was a high priority were more likely to receive STN DBS. Our dystonia patients (including cervical dystonia, i.e. spasmodic torticolis) all received GPi DBS.
Table 1.
Participants demographics. DYS = Dystonia, PD = Parkinson’s disease, C = Control, M = Male, F = Female, STDEV = Standard Deviation, LED = Levodopa equivalent dose (daily). At the time of analysis, only one patient was implanted bilaterally. Except for P006, the lead analyzed was the only one implanted. For P006, the outcome metrics reported are after the implantation of the second lead.
| Subject | Age at 7T | Sex | Diagnosis | Disease duration at surgery (years) | Surgery Target | Clinical Score (off/off) | Clinical Score (off/on DBS) | Comment | Medication Dose Pre-op (mg) | Medication Dose Post-op (mg) | Optimal Contact | Amplitude | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| DYS001 | 52 | M | D | 3 | GPi | 28.5 (disability = 15) | 18.5 (disability = 12) | The Fahn-Marsden | 6mg (Valium) + 0 unit (botulinum toxin) | 4mg (Valium) + 0 unit (botulinum toxin) | C+, 1− & 2− | 3.5V | at 7 months |
| DYS002 | 45 | M | D | 33 | GPi | 49 | 11.25 | TWSTRS | 16mg (Valium) + 95 unit (botulinum toxin) | 16mg (Valium) + 0 unit (botulinum toxin) | C+, 1− & 2− | 3.6V | at 6months |
|
| |||||||||||||
| PD001 | 69 | F | PD | 12 | GPi | 41 | 31 | UPDRS | 700 | 850 | C+, 2− | 3.0V | LED at 9months |
| PD002 | 76 | M | PD | 8 | GPi | 30 | 9 | UPDRS | 1000 | 700 | C+, 1− | 5.0V | LED at 7months |
| PD003 | 56 | M | PD | 8 | GPi | 33 | N/A | UPDRS | 1875 | 1875 | C+, 2− | 2.8V | LED at 5months |
| PD004 | 53 | M | PD | 10 | GPi | 21 | 10 | UPDRS | 1260 | 1110 | C+, 2− | 3.5V | LED at 10months |
| PD005 | 67 | M | PD | 6 | GPi | 24 | N/A | UPDRS | 1200 | 1596 | C+, 1− | 4.0V | LED at 6months |
| PD006 | 74 | M | PD | 13 | GPi | 26 | 21 | UPDRS | 800 | 1150 | C+, 2− (Left), C+, 3− (Right) | 3.3V (Left), 4.0V (Right) | LED at 6months after the second surgery |
| PD007 | 60 | M | PD | 12 | GPi | 29 | N/A | UPDRS | 1700 | 1600 | C+, 2− | 2.5V | Only initial programming data available |
| PD008 | 48 | F | PD | 8 | STN | 53 | N/A | UPDRS | 946 | 1068.75 | C+, 2− | 1.2V | LED Pre-op dose found 5–6m prior to surgery |
| PD009 | 53 | M | PD | 8 | GPi | 45 | N/A | UPDRS | 852 | 750 | C+, 9− | 2.5V | Pre-op dose found 5–6m prior to surgery |
| PD010 | 68 | M | PD | 3 | STN | 49 | N/A | UPDRS | 850 | 805 | C+, 2− | 2.3V | LED Pre-op dose found 5-m prior to surgery |
| PD011 | 72 | M | PD | 4 | STN | 34 | N/A | UPDRS | 670 | 210 | C+, 2− (Left), C+, 10− (Right) | 2.0V (Left), 2.0V (Right) | LED at 6months after the second surgery |
| PD012 | 61 | F | PD | 10 | GPi | 26 pre-op 57 at time of programming | N/A | UPDRS | 1896 | 1800 | C+.9− | 2.0V | LED at 10months (first programming) |
| PD013 | 55 | F | PD | 6 | GPi | 16 | N/A | UPDRS | 400 | 400 | 10+,11− | 4.6V | LED at 6months |
| PD014 | 69 | M | PD | 5 | GPi | 31 | N/A | UPDRS | 950 | 750 | C+, 2− | 3.6V | LED at 9months |
| PD015 | 60 | F | PD | 13 | GPi | 38 | 20 | UPDRS | 820 | 480 | C+, 3AB | 3.2mA | LED at 1m monopolar review |
|
| |||||||||||||
| C001 | 25 | M | C | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Subject | Mean Age (STDEV) | Sample size (F) |
|---|---|---|
| DYS | 48.5 (4.9) | 2 (0) |
| PD | 62.7 (8.6) | 15(5) |
| Control | 25 (0) | 1(0) - scanned 3 times |
|
| ||
| Overall | 59.1 (12.5) | 18 (5) |
2.2. Scanning Protocol
Patients were scanned on a 7T MRI scanner (Magnetom 7T Siemens, Erlangen, Germany). The scanner was equipped with SC72 gradients capable of 70 mT/m and a 200 T/m/s slew rate using a 32-element head array coil (Nova Medical, Inc., Burlington, MA, USA). On the day of scanning, the patients were instructed to take their usual medication in order to optimize patient comfort and minimize motion. Whenever patient head size enabled enough space in the coil, dielectric pads were utilized in order to enhance signal in the temporal regions (Teeuwisse et al., 2012).
The scan protocol consisted of: T1-weighted whole brain scan (0.6mm3 isotropic), T2-weighted axial slab covering the whole pallidum (0.4 × 0.4 × 1.0 mm3), and diffusion-weighted images, covering the whole brain (50 directions, b-value = 1500 s/mm2, 4 additional b0-volumes, 1.5mm3 isotropic). The diffusion images were acquired twice, each with different phase encoding directions: anterior-posterior and posterior-anterior More information about the scanning protocol can be found in Table 2. An example of a complete dataset is given in Figure 1.
Table 2.
Scanning protocol. GR/IR = gradient-recalled echo/inversion recovery. TE = echo time. TR = repetition time. SE = spin echo. EP = echo planar
| Weighting | Sequence | TE (ms) | TR (ms) | Flip angle (°) | Matrix (x × y × z) | size Resolution (mm3) (x × y × z) | Acquisition time (min) |
|---|---|---|---|---|---|---|---|
| T1 | 3D GR/IR | 3.5 | 3100 | 5 | 312 × 384 × 256 | 0.6 × 0.6 × 0.6 | 6.5 |
| T2 | 2D SE | 58 | ± 8000 | 150 | 512 × 26 × 512 | 0.39× 1.0 × 0.39 | 6.5 |
| Diffusion | 2D EP | 55.6 | ± 5000 | 90 | 136 × 136 × 66 | 1.5 × 1.5 × 1.5 | 2 × 4.5 |
Figure 1.
Top: Example of 7T images (T1, T2, and B0) and zoomed in of 7T T2 with and without manual segmentation of the GPi/GPe. Bottom: 3D rendering of the segmentation in one patients. A: manual segmentation of the GPi/GPe. Pink = GPi, Peach = GPe. B: Cortical targets. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region. C: Thalamus segmentation.
2.3. Image Processing and Analysis
2.3.1 Analysis Approach
Probabilistic tractography is chosen as a primary tool to parcellate the GPi into motor, associative, limbic, and “other” regions (see (Calabrese, 2016) for review of tractography in DBS). This method has been proven reasonably reproducible and reliable in other studies (Behrens et al., 2003; Plantinga et al., 2016). The GPi lacks direct connections to the cortex (Da Cunha et al., 2015; Gunaydin and Kreitzer, 2016; Martinu and Monchi, 2013); therefore, the analysis was based on GPi’s efferent projection to thalamus in two stages, as follows:
Identification of functional territories within the thalamus.
Parcellation of the GPi using the thalamic parcellations obtained in stage 1 as tractography targets
Several steps are required in order to complete this two-staged approach. The steps were:
Diffusion MR imaging preprocessing
GPi/GPe segmentation
Cortical masks generation
Thalamus segmentation
Parcellation of the thalamus
Parcellation of the GPi via the thalamus
Parcellation of the GPi via the striatum
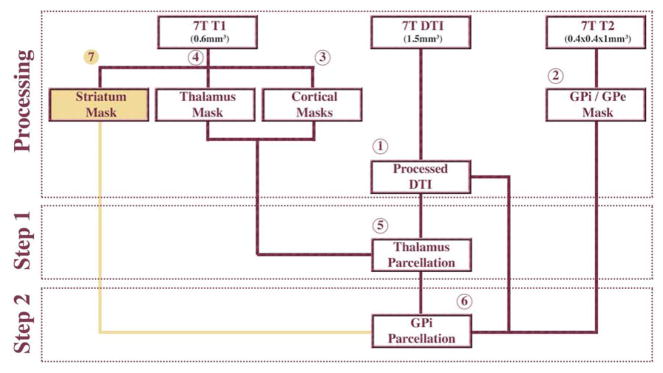
The following sections explain the different steps in greater detail. See Figure 2 for the approach in diagram form.
Figure 2.
Analysis approach in diagram form. Steps 1 through 6 are explained in greater details in the “Methods” section. 7T = 7Tesla MRI, DTI = Diffusion Tensor Imaging, GPi = Globus Pallidus interna, GPe = Globus Pallidus externa. Gold represents a validation step.
2.3.2 Diffusion MR Imaging Preprocessing
Preprocessing steps included: motion, susceptibility, and eddy current distortions correction using FSL’s eddy and topup algorithms (Andersson et al., 2003). Then, diffusion parameters were estimated using a three-fiber model with FSL’s bedpostX (Behrens et al., 2007).
2.3.3 GPi/GPe Segmentation
Given the large variability in size, shape, and orientation of small sub-cortical structures like the STN (Duchin et al., 2014) and the GPi, using a template may not be suitable for DBS targeting. Therefore, using a high contrast, high-resolution, 7T images we acquired, manual segmentation was preferred over using a template. For each subject, the GPi and the globus pallidus externa (GPe) were manually segmented on the 7T T2 axial image. The border between the two structures was visible in the images and determined to be the internal medullary lamina (Figure 1 bottom, Figure 1 bottom A). The segmentation was carried out using the Avizo software (FEI, Hillsboro, OR, USA). Along with the T2-weighted images, these binary segmentations were co-registered to each subject’s T1-space using the Advanced Normalization Tools (Avants et al., 2011). These segmentations were then binarized using a threshold of 0.45, which was empirically determined to least affect the volumes of the segmentations and correct for interpolation error. The good intra-observer agreement index for segmentations of the basal ganglia based on similar images was demonstrated previously (Lenglet et al., 2012). In this study, GP segmentations were reviewed by a cohort of the authors of this work.
2.3.4 Cortical Masks Generation
Given the large volume of these cortical masks the use of a template was deemed appropriate. A 1mm3 isotropic T1-weighted reference brain, developed by the Montreal Neurological Institute (MNI) (Grabner et al., 2006), was manually divided into four cortical areas: the motor, associative, and limbic cortical areas, and the remaining cortex was grouped into region defined as “other” (Figure 1B). More details about this segmentation are available elsewhere (Plantinga et al., 2016). The T1-image from each patient was non-linearly registered to MNI space using the Human Connectome Project’s minimal processing pipelines (Glasser et al., 2013). The inverse registration was used to bring the cortical masks from MNI space to the patient’s native T1-space. The cortical masks were, in turn, masked with gray matter segmentations of the patient’s T1-image produced with AFNI’s 3dSeg that were dilated by one voxel. This resulted in four gray matter cortical masks in each subject’s native space.
2.3.5 Thalamus Segmentation
For a similar reason outlined in 2.3.4, the thalamus was extracted from the Destrieux Atlas 2009 (Destrieux et al., 2010) in T1 native space as obtained using the Human Connectome minimal preprocessing pipeline (Glasser et al., 2013) (Figure 1C).
2.3.6 Parcellation of the Thalamus
Tractography analysis was carried out ipsilaterally with FSL’s probtrackX2 and was performed in T1-space with the voxels of the thalamus as seed regions and the cortical masks as targets. Other fiber tracking settings included FSL’s default curvature threshold of 0.2, maximum number of steps per sample of 2000, step length of 0.5 mm, subsidiary fiber volume threshold of 0.01, and termination of pathways that looped back on themselves. Using the ipsilateral cortex as inclusion region and the contralateral cortex as exclusion region, the number of tracts reaching any of the four ipsilateral cortical regions was computed for each voxel in the thalamus. Since the goal of this step is to create targets for the parcellation of the GPi, parcellation masks were created by assigning each voxel to one cortical region by following a winner-take-all approach.
2.3.7 Parcellation of the GPi
For the parcellation of the GPi, the parameters for probtrackX2 were kept the same as for the parcellation of the thalamus. The only difference being that the voxels of the GPi were used as the seed regions and the thalamus parceled regions were used as targets. Using the ipsilateral thalamus parceled regions as inclusion region and the contralateral cortex as exclusion region, the percentage of tracts reaching any of the four ipsilateral cortical regions was computed for each voxel in the GPi. A voxel was considered to be connected to that thalamus parcelled area if at least 25% of its probabilistic tracts connected the two, allowing for one voxel to be connected to more than one cortical area. This threshold was preferred over a winner-takes-all approach, because with a diffusion resolution of 1.5 mm isotropic, one voxel might contain several white matter tracts.
2.4. Validation & Reproducibility
As a first step to validate our parcellation method, each thalamus parcellation was compared to the thalamic connectivity-based segmentation as demonstrated by Behrens et al. (Behrens et al., 2003). Second, in order to assess the intra-subject reproducibility of our method, a 25-year-old healthy male subject was scanned three times on two different days with the same scanning protocol. The three datasets were then analyzed as described above and the reproducibility of the outcome results was assessed by comparing the overlap of each functional region. Third, to validate the organization of the GPi territory as based on the pathway we chose (i.e. the GPi’s efferent projection to thalamus), we repeated the tractography-based parcellation of the GPi using the GPi’s afferent pathway from striatum (step 7 in Figure 2). It has been shown that connectivity pathways between the basal ganglia and the cortex are organized in “parallel loops;” therefore, we expect to observe a similar organizational pattern within the GPi with both the afferent and efferent pathways (Kelly and Strick, 2004). The striatum was used as a waypoint and the cortical regions as targets. The striatum masks were extracted from the Destrieux Atlas 2009 (Destrieux et al., 2010) in T1 native space as obtained using the Human Connectome minimal preprocessing pipeline (Glasser et al., 2013).
2.5 Clinical Relevance
We used postoperative imaging (CT) as well as information contained in each patient’s DBS monopolar review of clinical outcomes to build a postoperative 3D model for each patient including final electrode location as well as the location of the therapeutic (also called “active”) contact(s). The active contact(s) was chosen clinically based on a systematic assessment of benefits and side effects for each contact over a range of stimulation amplitudes (i.e. monopolar review) and verified by assessing therapeutic results over subsequent weeks to months of stimulation with the selected contact.
3. Results
3.1. Parcellation of the Thalamus
Figure 1 bottom C shows one example of thalamus segmentation in one patient. The average volume of the thalamus, in native space, for all patients was 7291 ± 2285 mm3. Figure 3 shows the parcellation of the thalamus for all patients and how it compares to the Behrens Atlas (Behrens et al., 2003). The parcellation of the thalamus served two purposes: validation of the methods and generation of the target for GPi parcellation. Thalamus parcellation patterns were found to be comparable to the ones described in previous studies (Behrens et al., 2003; Plantinga et al., 2016). The limbic region encompassed the anterior, posterior, and medial areas of the thalamus. The “other” region was immediately anterior to the posterior part of the limbic region, followed anteriorly by the motor and associative regions. The parcellations were then used as targets for the GPi parcellations.
Figure 3.
The organization of individualized parcellation of the thalamus in patients matches that of the template. Top: Cortical masks in the same space as the Behrens atlas (left) and snapshot of the Behrens template using the same colors as the Behrens et al manuscript (Behrens et al., 2003) on the right using the colors from the present study. Bottom: parcellation of the thalamus for each one of the patients. The screenshots were taken at a similar slice for each patient in their native T1 space. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region.
3.2. Segmentation of the GPi
Figure 1 bottom A shows one example of GPi/GPe segmentation in one patient. The average volume of the segmented GPi, in native space, was 573± 122 mm3. There was no statistical difference between the size of the left and right GPi volumes (P ≈ 0.9). The volumes of the segmented GPi are shown in Table 3. There were not enough dystonia patients available in this study to meaningfully compare whether there are differences in GPi volumes between PD and dystonia patients. Finally, for reproducibility testing, the healthy subject GPi segmentation across the three repeated measurements yielded GPi volumes within one standard deviation of the patient’s GPi volumes (629 ± 44 mm3).
Table 3.
GPi segmentation volumes. DYS = Dystonia, PD = Parkinson’s Disease, GPi = Globus Pallidus pars interna.
| Subject | GPi_L (stdev) mm3 | GPi_R (stdev) mm3 |
|---|---|---|
|
| ||
| DYS | 685 (77) | 608 (71) |
| PD | 560 (139) | 567 (114) |
| Control | 600 (27) | 659 (38) |
|
| ||
| Overall | 575 (138) | 572 (109) |
3.3. Parcellation of the GPi
Figure 4 shows the results of a 3D reconstruction for the parcellation of the GPi of 5 representative patients. Figure 5 shows the organizational pattern of the functional territories for 3 patients in axial cross-section views. The organization of the functional territories of the GPi observed here follows a reproducible and consistent pattern across all subjects as well as across left and right GPi. The description of the organization of the functional territories of the GPi is done here with respect to the long (A – P) axis of the GPi. The motor region, in general, is located posteriorly but shifts to a posterolateral position in the inferior slices. In 31 out of 34 GPi parcellations, the motor territory is located posterolaterally followed anteriorly by the associative territory. In 29 of those parcellations the limbic territory is immediately anterior and inferior to the associative territory. In 27 of the 34 GPi parcellations, the “other” territory is located at the posterior tip of the GPi.
Figure 4.
Parcellation of the GPi for five patients. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region. Light peach color = GPe.
Figure 5.
Axial snapshots of the parcellation of the left and right GPi for three patients (Threshold = 0.25). L = Left, R = Right, Post = Posterior.
3.4. Validation and Reproducibility of GPi Segmentation and Parcellation
The repeatability of the parcellation analysis was tested by repeating the acquisition and analysis on data acquired from a healthy control over two days (three total sessions). The segmentation of the GPi for this subject was very consistent across sessions yielding very low intra-subject variability in volume size when compared to inter-subject variability measured in the patient population (about one-third as much). Figure 6 shows the parcellation results for the three sessions. The pattern is consistent across the three sessions with only minor differences. Thresholding out the 25% lowest values, the Dice coefficients for the intra-subject comparison were 0.65, 0.63, and 0.59 for the associative, motor and limbic regions, respectively.
Figure 6.
GPi Parcellation of a healthy control from three different scans. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region. Peach color = GPe
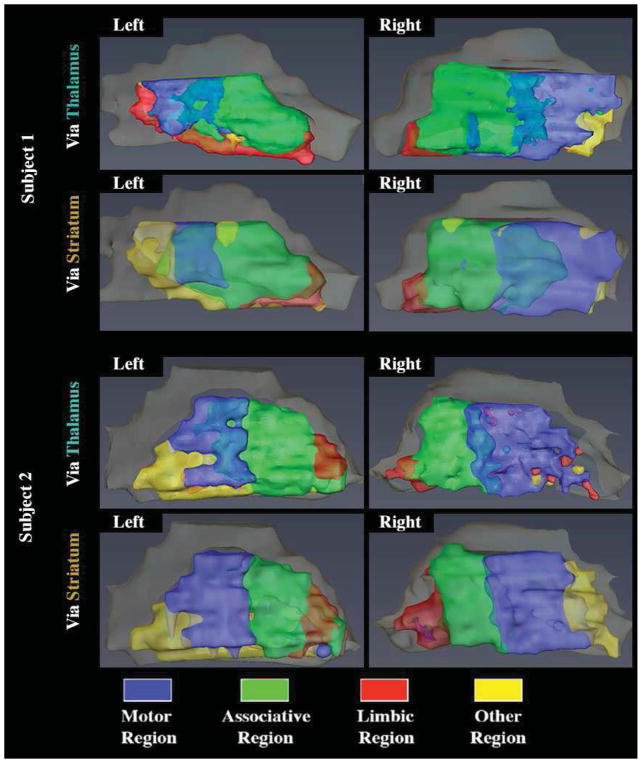
Another validation for the consistency of the functional organization of the GPi was obtained by parcellation of the GPi based on a different pathway. Figure 7 shows two examples of parcellation of the GPi using the striatum as a waypoint mask and the comparison to what was obtained with the thalamus. A similar and consistent pattern was observed when the parcellation was performed via the thalamus or via the striatum. Thresholding to discard the bottom 25% of values, the Dice coefficients, comparing the overlap between the parcels obtained via Thalamus and those obtained via the Striatum, were 0.65, 0.53, and 0.68 for the associative, motor and limbic regions, respectively.
Figure 7.
Parcellation of the GPi based on the striatum. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region. Peach color = GPe.
3.5 Clinical Relevance
Out of the seventeen patients, fourteen underwent GPi DBS surgery and the remaining three underwent DBS of the STN. For the fourteen patients who underwent DBS surgery of the GPi (including one staged bilateral procedures for a total of 15 surgeries), the post-surgical CT scan was merged with the patient-specific anatomical model. Following the initial DBS programming, it was found that 15 out 15 of the DBS electrodes (100%) were placed in the GPi. 14 out of the 15 (93%) DBS leads had an active contact which overlapped with the high probability “motor” region of the GPi as defined by imaging-based methods (see Figure 8 for four examples) or in its immediate vicinity (about 1mm). In one case, while the deepest contact was located ventrally in the GPi, the most therapeutically beneficial contact was determined to be more superior and lateral, located within the GPi-GPe border area.
Figure 8.
DBS electrode active contact overlaps with the “Motor” region. Blue = Motor region, Green = Associative region, Red = Limbic region, Yellow = “Other” region. Peach color = GPe. White cylinder = DBS electrode shaft, small grey cylinder = DBS electrode contact, small red cylinder = DBS electrode active contact.
4. Discussion
In this study, we aim at identifying, on a subject-by-subject basis, the functional territories of the GPi with the intention of localizing the motor territory for the purpose of enhancing future DBS targeting on a finer scale. To achieve this goal, 7T MRI scans were done in 17 patients (prior to DBS surgery) and one control subject. Using 7T MRI, a similar and consistent organizational pattern was found across all subjects. The observed functional organization of the GPi was supported by multiple cross references including repeated scans from a given control and by using parcellation via both afferent and efferent pathways. Furthermore, using post-surgery CT imaging, final DBS lead and contact locations were found to overlap with the motor region making the organizational pattern of clinical relevance. The focus on actual movement disorder patients bridges the gap and demonstrates a clear translational application between basic research and potential clinical use. Contrary to previous reports focusing on the methodological aspect using healthy controls as input data, we are able to directly relate our parcellation results to each individual patient’s DBS device and its programming outcomes. Finally, we expand on the previous approaches and report here a novel parcellation technique that is a two-staged and may prove to be more specific as it is more constrained due to use of thalamic sub-territories.
The GPi volumes obtained from manual segmentation of the DBS patients were in agreement with values reported in the literature (Vasques et al., 2009). The motor parcel of the GPi was consistently located in the posterolateral portion immediately followed anteriorly by associative and limbic regions, respectively. The limbic region was anterior and inferior to the associative region. This was found to be true across 31 out of 34 GPis as well as across all three sessions acquired for a healthy control (6 GPis). This finding is consistent with a previous human study in which calbindin immunoreactivity was studied in the GPi, among other basal ganglia structures, and a gradient corresponding to the intensity of labeling was observed (Karachi et al., 2002). The authors identified three main regions within the gradient corresponding to weak, intermediate, and strong calbindin labeling moving from the posterior to the anterior portions of the GPi. Strong calbindin labeling was linked to regions receiving sensorimotor, associative and limbic projections from the striatum (Karachi et al., 2002). In monkeys, immunohistochemistry studies have shown that limbic projections within the GPi are located in the most anterior part of the structure (Côté et al., 1995), which agrees well with the observations made in this study. Furthermore, a labeling study focusing on macaques to investigate the topological organization of the pallidum and the substantia nigra with respect to associative and sensorimotor projections found that, akin to our findings, the sensorimotor region was located posterior to the associative region (François et al., 1994); this finding was also found in a study using striatal stimulation in the awake monkey (Tremblay and Filion, 1989) as well as a Golgi study in the macaque (Percheron et al., 1984), and a tracing study in the squirrel monkey (Smith and Parent, 1986). This pattern is also consistent with recent findings in which the motor territory found here corresponds to a thalamic cluster believed to represent the GPi’s main efferent sensorimotor tracts(da Silva et al., 2017). Finally, the relatively low Dice values reported here can be explained by the small volumes of the different parcels in respect to the input imaging voxel size (Shamir et al., 2016; Shamir et al., 2018). This is especially true since those small volumes are determined from 1.5mm3 isotropic diffusion data. Further, given that the pallidothalamic and pallidostriatal tracts do not necessarily take on the same roles, one cannot expect a parcellation from the two pathways to be an exact match. However, both methods yielded a very similar spatial organization of the different functional territories within the GPi
The consistent organizational pattern of the functional territories of the GPi seem to align with the organizational pattern found in the STN (Supplemental Figure 1; (Plantinga et al., 2016)) and the thalamus (Behrens et al., 2003). This would further suggest that fiber pathways, within the basal ganglia-thalamocortical pathways, connect similar functional territories (e.g. STN Motor -> GPi Motor -> Thalamus Motor -> Cortex Motor) from bundles that run parallel to one another. The presence of segregated circuits, organized in parallel, has been hypothesized previously (Alexander et al., 1986). Recent imaging studies using DTI in healthy individuals uncovered similar parallel arrangements in the sensorimotor tract (Archer et al., 2017) as well as the thalamocortical pathway (Behrens et al., 2003); here, we show that this organization is likely preserved between the thalamus, the GPi and the STN and can be visualized in vivo and in individual patients using high resolution imaging techniques.
Patient-specific models in which the visualization of the patient’s own anatomy is being used for targeting, combined with parcellation methods derived in this study, have the potential to significantly improve surgical accuracy. By delineating the motor region derived from the patient’s own imaging data, targeting can be made more accurate and precise requiring fewer trajectory passes during the micro-electrode recording stage to delineate the target, resulting in faster surgeries, and ultimately decreased the risk of infection or hemorrhage. Precise patient specific targeting of the sensorimotor territory has many advantages the least of which is providing patients with better, more consistent outcomes and less side effects with a procedure that can be performed in less time for both implantation and programming. Greater consistency in lead placement in the sensorimotor territory will decrease the variability in motor improvement between patients and centers due to more precise electrode placement. Finally, patient specific models can be used post-operatively to visualize the final location of the DBS electrode and contacts. Accurate delineation of contact locations within the target structure relative to the motor, associative and limbic territories can be used to develop probabilistic maps of improvement in individual motor signs within the STN and GPi as well as characterize the type and incidence of non-motor side effects when volume of tissue activation fields encroach on these non-motor territories. These data can be used to develop patient specific automated programming algorithms that restrict current fields to the sensorimotor territory while avoiding spread encroachment into non-motor associative and limbic territories thus reducing side effects while optimizing motor outcomes (Pena et al., 2017). This is all the more relevant now that industry and academic centers work on DBS electrodes with more contact (segmented) elements making the number of possible settings ever more complicated and lengthening clinic time required for optimizing these settings.
Limitations
Scanning patients with movement disorders, such as Parkinson’s disease, dystonia or essential tremor, bring about challenges in terms of image quality due to excessive motion that is detrimental for any MR images. To minimize these effects, we used shorter scanning protocols specially optimized for imaging of movement disorder patients; this protocol has been discussed elsewhere in greater detail (Abosch et al., 2010; Lenglet et al., 2012). Future development of MRI sequences that are faster and less susceptible to motion will significantly advance similar studies.
Ultra-high field diffusion imaging, e.g. at 7T, has been shown to experience signal loss in the temporal lobes (Polders et al., 2011). In this study, we used dielectric pads, whenever they could be fitted comfortably, to increase the signal to these regions (Teeuwisse et al., 2012). Nevertheless, this issue may affect our results by potentially lowering the estimation of connections to the limbic regions in the temporal and orbitofrontal areas. While this study uses high-resolution data at ultra-high field, partial volume effects likely play a role in our results, especially at the border between the GPi and GPe and also at the ventral and medial borders. This may introduce some degree of variability in our results and may partially explain the variance in the sizes and shapes of the overlapping functional regions seen across subjects.
The foundation of this method rests on the quality of the registration between the parcelled MNI brain and each individualized subject’s brain. While this non-linear registration was verified for each subject, it is possible that small inaccuracies impact the GPi parcellation. This is likely to be more impactful at the borders between the different territories.
Also, the GPi is in close proximity to the thalamus; therefore, it is natural to worry about confounding factors such as distance and volume. Distance confound relates to the higher probability for a tract to be identified between structures close to one another. Volume confounds relates to the higher probability for a tract to be identified if the target is larger. We assume that these confounds did not bias our results given that we found similar parcellations outcomes preformed via the thalamus and via the striatum, which are pathways with different distances and different structures size.
Additionally, the threshold method that was used to visualize the functional territories within the GPi displays the different regions as having distinct boundaries. However, this obscures the underlying data in which there is gradual overlap between the territories. The distinct boundaries will vary according to the threshold chosen.
Finally, the number of parcels in which the GPi was divided into corresponds directly to the number of predefined cortical segmentations. While the functional territories of interest were chosen because of their importance in the context of DBS surgeries, they can only result in a maximum of four GPi parcels; Using a clustering algorithm to parcellate the cortex and GPi might help provide insight as to a more realistic number of functional regions. Future studies, using submillimeter spatial resolution, will be key to determining accurately the number of zones independently for each patient.
5. Conclusion
The field of DBS is rapidly advancing with major manufacturers and startup companies developing new and impressive electrode and battery technology. While some of these improvements may help improve benefits for less than ideal electrode placement (i.e. current steering), accurate targeting remains essential for DBS to be effective. In this study, we demonstrate the feasibility of direct targeting using patient-specific models of the GPi constructed from 7T MRI data as well as the possibility to uncover functional territories within the GPi. These functional territories were found to be organized in a reproducible manner and were shown to be clinically relevant. These findings have the potential to improve DBS targeting, in general, by increasing the community’s knowledge about which specific regions of the GPi to target and, perhaps which regions to avoid. Additionally, the patient-specific methodology utilized in this study could facilitate DBS targeting at the individual level by providing the neurosurgeon and neurologist with a target area, within the GPi, specific for each patient. Such planning methods could potentially result in improved motor benefits while decreasing the risk of unwanted side effects associated with unintended stimulation of other functional territories, while aiding in the development of automated programming strategies. We believe that the validity and reliability of our results constitutes a step forward towards individualized DBS surgical treatment.
Supplementary Material
Highlights.
Patient-specific parcellation of the GPi using 7T MRI data is feasible prior to DBS
GPi functional regions followed a Motor, Associative, and Limbic organization (from posterior to anterior)
Similar functional organizational patterns were found using two different parcellation methods
The optimal therapeutic contact was located in the motor region
Acknowledgments
This study was supported by the NIH R01-NS085188; P41 EB015894; P30 NS076408 and the University of Minnesota Udall center P50NS098573. The funding sources had no involvement in study design, data collection, data analysis, result interpretation, manuscript writing, or any decision regarding the submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67:1745–1756. doi: 10.1227/NEU.0b013e3181f74105. discussion 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization fo functionally segregated circuits linking basal ganlgia and cortex. Annu Rev Neurosci. 1986 doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Archer DB, Vaillancourt DE, Coombes SA. A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cereb Cortex. 2017:1–15. doi: 10.1093/cercor/bhx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, KS, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Diffusion Tractography in Deep Brain Stimulation Surgery: A Review. Front Neuroanat. 2016;10:45. doi: 10.3389/fnana.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ZH, Min HK, Oh SH, Han JY, Park CW, Chi JG, Kim YB, Paek SH, Lozano AM, Lee KH. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010;113:639–647. doi: 10.3171/2010.3.JNS091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté PY, Levitt P, Parent A. Distribution of limbic system-associated membrane protein immunoreactivity in primate basal ganglia. Neuroscience. 1995;69:71–81. doi: 10.1016/0306-4522(95)00185-l. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Boschen SL, Gomez AA, Ross EK, Gibson WS, Min HK, Lee KH, Blaha CD. Toward sophisticated basal ganglia neuromodulation: Review on basal ganglia deep brain stimulation. Neurosci Biobehav Rev. 2015;58:186–210. doi: 10.1016/j.neubiorev.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva NM, Ahmadi SA, Tafula SN, Cunha JP, Botzel K, Vollmar C, Rozanski VE. A diffusion-based connectivity map of the GPi for optimised stereotactic targeting in DBS. NeuroImage. 2017;144:83–91. doi: 10.1016/j.neuroimage.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin Y, Saprio G, Baker K, McIver J, Vitek J, Harel N. Utility of 7T MRI for Deep Brain Stimulation (DBS) Applications. Organization for Human Brain Mapping; Hamburg, Germany: 2014. [Google Scholar]
- Fenoy AJ, Simpson RK., Jr Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120:132–139. doi: 10.3171/2013.10.JNS131225. [DOI] [PubMed] [Google Scholar]
- Fox MD, Alterman RL. Brain Stimulation for Torsion Dystonia. JAMA Neurol. 2015;72:713–719. doi: 10.1001/jamaneurol.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François C, Yelnik J, Percheron G, Fénelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp Brain Res. 1994:102. doi: 10.1007/BF00227517. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, Consortium WUMH. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Kreitzer AC. Cortico-Basal Ganglia Circuit Function in Psychiatric Disease. Annu Rev Physiol. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Karachi C, Francois C, Parain K, Bardinet E, Tande D, Hirsch E, Yelnik J. Three-dimensional cartography of functional territories in the human striatopallidal complex by using calbindin immunoreactivity. J Comp Neurol. 2002;450:122–134. doi: 10.1002/cne.10312. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Progress in Brain Research. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Kerl HU, Gerigk L, Pechlivanis I, Al-Zghloul M, Groden C, Nolte IS. The subthalamic nucleus at 7.0 Tesla: evaluation of sequence and orientation for deep-brain stimulation. Acta Neurochir (Wien) 2012;154:2051–2062. doi: 10.1007/s00701-012-1476-0. [DOI] [PubMed] [Google Scholar]
- Lenglet C, Abosch A, Yacoub E, De Martino F, Sapiro G, Harel N. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PLoS One. 2012;7:e29153. doi: 10.1371/journal.pone.0029153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet L, Schupbach M, N’Diaye K, Remy P, Bardinet E, Czernecki V, Welter ML, Pelissolo A, Ruberg M, Agid Y, Yelnik J. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci U S A. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinu K, Monchi O. Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson’s disease: pathophysiology or compensation? Behav Neurosci. 2013;127:222–236. doi: 10.1037/a0031226. [DOI] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson CEt, Wang X, Gordon CW, Jr, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez RL, Foote KD. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE trial. Ann Neurol. 2009;65:586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek SH, Yun JY, Song SW, Kim IK, Hwang JH, Kim JW, Kim HJ, Kim HJ, Kim YE, Lim YH, Kim MR, Huh JH, Lee KM, Park SK, Kim C, Kim DG, Jeon BS. The clinical impact of precise electrode positioning in STN DBS on three-year outcomes. J Neurol Sci. 2013;327:25–31. doi: 10.1016/j.jns.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse events associated with deep brain stimulation for movement disorders: analysis of 510 consecutive cases. Neurosurgery. 2015;11(Suppl 2):190–199. doi: 10.1227/NEU.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena E, Zhang S, Deyo S, Xiao Y, Johnson MD. Particle swarm optimization for programming deep brain stimulation arrays. J Neural Eng. 2017;14:016014. doi: 10.1088/1741-2552/aa52d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheron G, Yelnik J, Francois C. A golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J Comp Neurol. 1984;227:214–227. doi: 10.1002/cne.902270207. [DOI] [PubMed] [Google Scholar]
- Plantinga BR, Temel Y, Duchin Y, Uludag K, Patriat R, Roebroeck A, Kuijf M, Jahanshahi A, Ter Haar Romenij B, Vitek J, Harel N. Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga BR, Temel Y, Roebroeck A, Uludag K, Ivanov D, Kuijf ML, Ter Haar Romenij BM. Ultra-high field magnetic resonance imaging of the basal ganglia and related structures. Front Hum Neurosci. 2014;8:876. doi: 10.3389/fnhum.2014.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polders DL, Leemans A, Hendrikse J, Donahue MJ, Luijten PR, Hoogduin JM. Signal to noise ratio and uncertainty in diffusion tensor imaging at 1.5, 3.0, and 7.0 tesla. J Magn Reson Imaging. 2011;33:1456–1463. doi: 10.1002/jmri.22554. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Ostrem JL, Starr PA. Surgical repositioning of misplaced subthalamic electrodes in Parkinson’s disease: location of effective and ineffective leads. Stereotact Funct Neurosurg. 2009;87:297–303. doi: 10.1159/000230692. [DOI] [PubMed] [Google Scholar]
- Rolston JD, Englot DJ, Starr PA, Larson PS. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: Analysis of multiple databases. Parkinsonism Relat Disord. 2016;33:72–77. doi: 10.1016/j.parkreldis.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir RR, Duchin Y, Kim J, Sapiro G, Harel N. Segmentation Overlap Measures are Biased to Structure’s Size but Correctable. Int J Comput Assist Radiol Surg. 2016;11:S44–S45. [Google Scholar]
- Shamir RR, Duchin Y, Kim J, Sapiro G, Harel N. Continuous Dice Coefficient: a Method for Evaluating Probabilistic Segmentations. 2018 bioRxiv. [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Starr PA, Turner RS, Rau G, Lindsey N, Heath S, Volz M, Ostrem JL, Marks WJ. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg. 2006;104:488–501. doi: 10.3171/jns.2006.104.4.488. [DOI] [PubMed] [Google Scholar]
- Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) NeuroImage. 2009;47(Suppl 2):T44–52. doi: 10.1016/j.neuroimage.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Tanner M, Gambarota G, Kober T, Krueger G, Erritzoe D, Marques JP, Newbould R. Fluid and white matter suppression with the MP2RAGE sequence. J Magn Reson Imaging. 2012;35:1063–1070. doi: 10.1002/jmri.23532. [DOI] [PubMed] [Google Scholar]
- Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magn Reson Med. 2012;67:1285–1293. doi: 10.1002/mrm.23108. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Filion M. Responses of pallidal neurons to striatal stimulation in intact waking monkeys. Brain Res. 1989;498:1–16. doi: 10.1016/0006-8993(89)90394-6. [DOI] [PubMed] [Google Scholar]
- Vasques X, Cif L, Hess O, Gavarini S, Mennessier G, Coubes P. Prognostic value of globus pallidus internus volume in primary dystonia treated by deep brain stimulation. J Neurosurg. 2009;110:220–228. doi: 10.3171/2008.3.17433. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Deep Brain Stimulation for Parkinson’s Disease. Stereotactic and Functional Neurosurgery. 2002;78:119–131. doi: 10.1159/000068959. [DOI] [PubMed] [Google Scholar]
- Welter ML, Schupbach M, Czernecki V, Karachi C, Fernandez-Vidal S, Golmard JL, Serra G, Navarro S, Welaratne A, Hartmann A, Mesnage V, Pineau F, Cornu P, Pidoux B, Worbe Y, Zikos P, Grabli D, Galanaud D, Bonnet AM, Belaid H, Dormont D, Vidailhet M, Mallet L, Houeto JL, Bardinet E, Yelnik J, Agid Y. Optimal target localization for subthalamic stimulation in patients with Parkinson disease. Neurology. 2014;82:1352–1361. doi: 10.1212/WNL.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Ma W, Huang Y, Qiu Z, Sun L. Deep brain stimulation of pallidal versus subthalamic for patients with Parkinson’s disease: a meta-analysis of controlled clinical trials. Neuropsychiatr Dis Treat. 2016;12:1435–1444. doi: 10.2147/NDT.S105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.