Abstract
Nanomedicines can be multifunctional drug delivery agents for cancer therapies. However, they have faced several challenges in clinical trials owing to poor targeting ability, insufficient tumor penetration, difficulty in synthesis and scale up, and limited understanding of interactions between a tumor and nanoparticles. In this regard, tumor muticomponent targeting drug delivery systems are a rational approach for developing tumor-site-specific therapeutics. One of the goals is to arrive at a ready-to-configure, combinatorial, reagent-free click-chemistry-based tumor multicomponent targeting nanoparticle library. The nanoparticles can be co-loaded with drugs, genes and imaging agents, surface decorated with varying targeting ligands that can home to varying tumors and/or tumor multicomponents. This library of nanocarriers could be utilized for early tumor diagnosis and therapy based on individual patient needs for personalized medicine, with a high degree of success in the clinic.
Keywords: Tumor multicomponent targeting, polypharmacy payload delivery, nanomedicine, nanoparticles, ligand library, click chemistry, personalized cancer therapy, immune modulation, immunotherapy
Introduction
Cancer is one of the major causes of death, annually claiming more than half a million lives and there are an estimated 1.5 million new cases diagnosed in the USA alone each year [1]. Thus, there is an urgent need to improve diagnostic tools for early detection and to develop more-selective drug delivery agents for therapy of cancer with the least-toxic side effects. Conventional nanomedicine approaches have faced challenges in clinical trials, especially in terms of targeting the cancer tissues and the ease of preparing the delivery system itself, making them suitable for personalized medicine. Thus, targeted drug delivery that utilizes tumor multicomponent homing ligands to recognize specific biomarkers of the tumor environment, such as tumor epithelial cells, angiogenic blood vessels, tumor stroma, hypoxia and infiltrating immune cells, has become an urgent requirement for selective delivery of drugs, thereby enhancing therapeutic efficacy. Tumor multicomponent-targeted cancer treatment differentiates between healthy and cancer tissues. The targeted nanoparticle has dual ability to accumulate in the tumor lesion by the enhanced permeability (EPR) effect [2–22], followed by receptor-mediated tumor-cell-specific endocytosis and delivery of the polypharmacy payload [14,19,22–33]. The dual property of passive and active targeting nanoparticles is effective for delivering payload to the primary as well as metastatic tumor lesion. Developing a library of ligands with a ‘clickable’ functional group has several advantages [34,35], such as (i) high stereoselectivity, (ii) high yield, (iii) free from toxic reagent and chemical condition, and (iv) instant yield of product upon mixing of reactant. This advanced method of chemical conjugation provides a rational way of potentially transforming the area of personalized cancer treatment with improved quality of life while providing the opportunity to integrate chemistry [11,36–38], drug delivery and cancer research elements.
General targeting strategies for cancer therapy
Fundamental studies have documented that tumors are infiltrated by immune cells, and their influx has been considered as evidence that the host is battling against developing a tumor. Once a tumor starts growing, the tumor-killing immune cells appear to be ineffective or reprogrammed as pro-tumorigenic entities, resulting in evasion of immune surveillance in association with tumor-helping factors, such as the tumor stroma, blood vessels and a variety of associated tissue cells [39]. The following research objectives could be pursued.
Synthesis, functional modification and characterization of high-affinity tumor-specific antigen-targeting small molecules, peptides, antibodies and antibody fragments [29,40–44].
Self-assembly of polymer, lipid, metallic-based nanodrug-carrier, and biomanufacturing of endogenous cells (red blood cells and exosomes) and protein (serum albumin and transferrin) based cargo carrier [9,10,23,25,27,45–60].
Functionalization of targeting ligands with the cargo carriers using reagent-free synthesis approaches, such as an alkyne–azide click reaction, thiol-ene Michael-type and alkyne–nitrone cycloadditions [9,10,46–53,61].
The new knowledge gained through this interdisciplinary effort will provide a unique repertoire of advancements in drug delivery, with rational design of the targeting ligand customized cargo carrier to exert distinct function in the tumor microenvironment. Thus, the prime objective of modern research in cancer therapy should be to develop a universal approach to target all the significant components of the tumor microenvironment for early detection and therapy. It is only noticeable that this is one of the most logical and economical approaches to develop an off-the-shelf library of targeting agents and functionally variable, various size-and-shaped drug–carrier combinations based on the phenotype of the tumor milieu and get a license to destroy them. This combination approach will serve as an off-the-shelf instant drug formulation platform for selective delivery of payload based on tumor biomarkers and the stage of the tumor. An outline strategy for the multicomponent extracellular biomarker targeting of the tumor (Figure 1) could include:
tumor epithelial cells overexpressing receptors such as folate receptors (FR) [52,62], HER-2 [63,64], CD44 [65] and several types of G-glycoprotein receptor (GPCR) [66];
tumor angiogenic blood vessel targeting, vascular endothelial growth factor receptor (VEGFR)-2 [67] and tumor necrosis factor (TNF)-α [68];
tumor hypoxia marker targeting using antibodies or small molecules;
cancer stem cell (CSC) biomarker targeting using CD44 [65];
tumor stroma, fibroblast and extracellular matrix marker targeting fibroblast-activated protein (FAP);
folate receptors (FRs) and PD-1/PD-L1 overexpressed in tumor-associated immune cells [69], such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and inflammatory leukocytes [70].
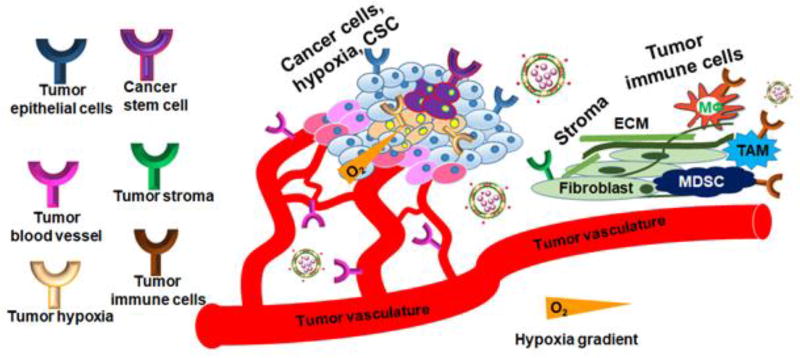
Figure 1.
Strategy for universal nanothernaostic delivery systems to target all major components of cancer cells and cancer-associated cells. The tumor microenvironment has been divided into six major components: tumor epithelial cells, tumor blood vessel, tumor hypoxia, cancer stem cells, tumor stroma and tumor immune cells. The epithelial cells function as a generator of tumor mass and proliferation, the tumor blood vessel is responsible for angiogenesis and supplying nutrients to the tumor, tumor hypoxia represents a drug-resistant population and works for tumor acidosis, cancer stem cells maintain stemness of the tumor environment and is responsible for metastasis and invasion. Tumor stroma is a dense layer of extracellular matrix and fibroblasts that prevents drug penetration, and tumor immune cells help to fight against antitumor immune surveillance.
Another way of tumor targeting is to develop a tumor-stimuli-responsive linker containing targeting ligands to target various receptors of the tumor microenvironment. For example, functionally variable linkers, such as an alkyl chain (CnH2n+2, n = 6, 12, 18), valine–citrulline (Val–Cit), dithiol (S–S) and thiol–maleimide (S–Mal), could be employed for designing all types of targeted nanosystems. For the payload carrier, various types of nanocarriers, such as liposomes [8], polymeric micelles [7,9,10,53,71,72], lipid–polymer hybrid nanoparticles, gold nanoparticles, ultrafine superparamagnetic iron oxide nanoparticles (USPIONs) [52] or human serum albumin (HSA) nanoparticles can be developed. These targeting ligands and nanocarriers could be coupled through reagent-free click reactions to arrive at the final targeted drug delivery vehicles. Specific complementary functional groups could be conjugated with targeting ligand and nanoparticles, so that they could be mixed and treat the relevant tumor. Because tumors have characteristic features such as acidic pH, reducing and a protease-rich microenvironment, the use of these stimuli-responsive linkers will assist the nanoparticles to deliver the payload inside the tumor selectively. For instance, the use of an alkyl chain will not only improve the self-assembly of nanoparticles (NPs) but the C–C bond will also provide rotational flexibility to the targeting ligand to efficiently bind with the cognate receptors. Likewise, the Val–Cit (dipeptide) or S–S linkers will be cleaved owing to the abundance of cathepsin B protease or glutathione reductase, respectively, thereby leading to selective release of cargo in the tumor [73,74]. Similarly, the purpose of using S-Mal linker chemistry is to ensure NP stability in plasma during circulation (FDA-approved Kadcyla® has high stability owing to the presence of the S–Mal linker). For improving the efficacy of the nanoformulations, consideration should be given to tumor heterogeneity and purpose of use while designing a variety of nanoparticles that could also be of variable sizes and shapes. For example, pancreatic ductal adenocarcinoma (PDAC) is a therapy-resistant and deadly disease in urgent need of effective drugs [75]. PDAC tumors are heterogeneous, harboring resistant CSCs, a characteristically dense desmoplastic stroma that prevents optimal drug penetration and an immune-compromised microenvironment that hinders a therapy-induced antitumor immunogenic response [76,77]. Therefore, strategies guided toward tumor CSCs that can also penetrate the stroma and reactivate the immune response are required for meaningful therapeutic outcomes in PDAC patients. Researchers have already shown the possibility of designing USPIONs of 5–10 nm [78] and ultra-small lipid–polymer hybrid nanoparticles with 20–25 nm particle size to enhance tumor penetration, and target stemness of the tumor [79].
Target-specific precision medicine for cancer treatment
The specific targets that show promise for target-specific cancer therapy are described below. A summary of the strategies is summarized in Figure 2.
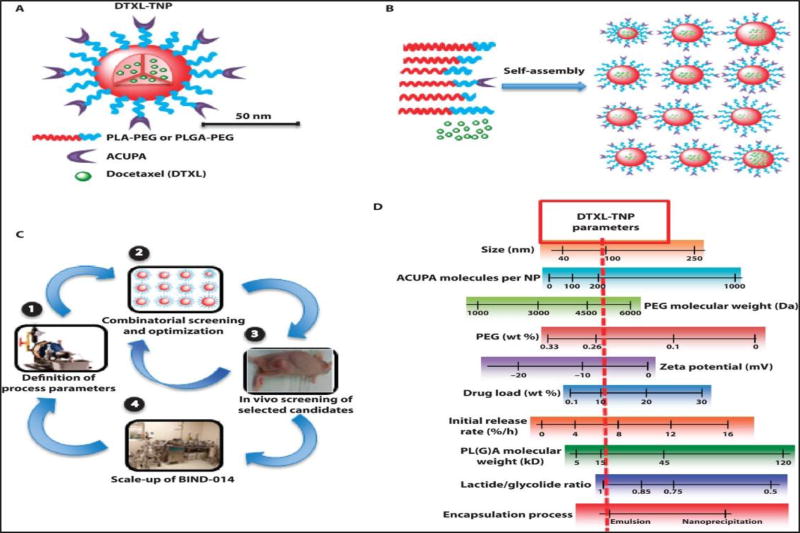
Figure 2.
Hit selection criteria and lifecycle of a targeted delivery system for cancer therapy. An example of combinatorial screening and optimization of DTXL-TNPs. (a) The PSMA targeting PLGA-PEG nanoparticle (BIND-014) encapsulated with deocetaxel and a 50 nm nanoparticle is suitable for internalizing tumor by the EPR effect. (b) Combinatorial library nanoparticle with various shape, size and drug loading. (c) The screeninig of a library at the cellular level and an animal tumor model to find a best hit for clinical tranlation. (d) The importatnt parameter and FDA requirements that need to be monitored for achieving efficient nanoparticle hits for human trials. Images reproduced, with permission, from [34].
Deciphering an undruggable target to drug resistance cancer using clinically validated lipid nanoparticles for siRNA delivery
Ras genes harbor most abundant early genetic mutation events in tumor progression. Overall, Ras mutations have been detected in 10–30% of all tumor samples [80]. The frequency and distribution of Ras gene mutations are not uniform. Among different types of Ras gene, KRas has the highest frequency of mutation (>80%) compared with the other subtypes: NRas 5–10% and HRas 3%. The frequency of the KRas mutation (mKRas) is highest in PDAC with >95%, followed by colon and rectal carcinomas (CRCs) where KRas mutation is predominant in ~80%, and lung adenocarcinoma has 30–35% [81]. Several strategies including direct mKRas inhibitors and mKRas effector protein inhibitors have been developed for more than three decades to suppress mKRas function but no potent inhibitors have entered the clinic, which led to the perception that KRas protein was undruggable [82]. Despite challenges, several studies on siRNA libraries have identified the specific sequences to knockdown mKRas formation but not wild-type KRas. Recently, a Phase I clinical study (NCT01188785) demonstrated the feasibility, safety and efficacy of siRNA (siG12D) delivery against G12D point-mutated mKRas for PDAC patients [83] and it has shown nearly 80% gene knockdown in the Panc-1 cell line [84]. Thus, there is a hope of success to inhibit mKRas activity using siRNA technology and use of multicomponent (tumor epithelial cells, stromal cells) targeting siRNA delivery systems is an excellent approach for fighting against the mKRas-positive deadliest cancers: PDAC, lung cancer and CRC. Delivery of siRNA is challenging owing to a high negative charge and molecular weight, and it showed poor transfection efficiency in vitro and in vivo. In this regard, lipid nanoparticles have many advantages for siRNA delivery – they are efficacious, biocompatible, nonimmunogenic and non-mutagenic [85]. The majority of lipid-nanoparticle-based siRNA delivery systems require cationic lipids that could interact with anionic phosphate groups in siRNA to electrostatically form a stable complex. However, the cationic nanocarriers can promote inflammation and genotoxicity, and cationic charges are inactivated by nonspecific binding to healthy organs and proteins that limit gene expression in vivo. Thus, they are incompatible in the clinical set up [86]. A pH-sensitive, non-cationic, ionizable lipid such as 1,2-dioleoyl-3-dimethylammonium-propane (DODAP), as well as its derivatives, is considered as a much safer nanocarrier for siRNA delivery, and several research outcomes, as well as clinical trial data, support this [87,88]. The pH-responsive amine head group of DODAP becomes protonated in the acidic endosome of cancer cells, thus enhancing siRNA transfection efficiency and facilitating siRNA escape from the endosome to the cytosol [85]. Along with DODAP, liposomes could be co-formulated with phosphatidylcholine (PC) or sphingomyelin (SM) with a variable twin carbon chain (C16–18) and cholesterol for the stability of the liposome bilayer, regulating fluidity of the lipid bilayer [89]. It is reported that SMs are more stable than PCs in the in vivo circulation owing to the presence of an amide linker, whereas PCs have an ester linker [89]. This azide (N3) and PEGylated lipid (DSPE-Peg2000-N3) could be used for a reagent-free click reaction with DBCO-linked targeting ligands (Figure 3). PEGylation will help the nanoformulations escape from the mononuclear phagocytic system (MPS) and mediate nonspecific liver and spleen uptake in mice [49,62,68].
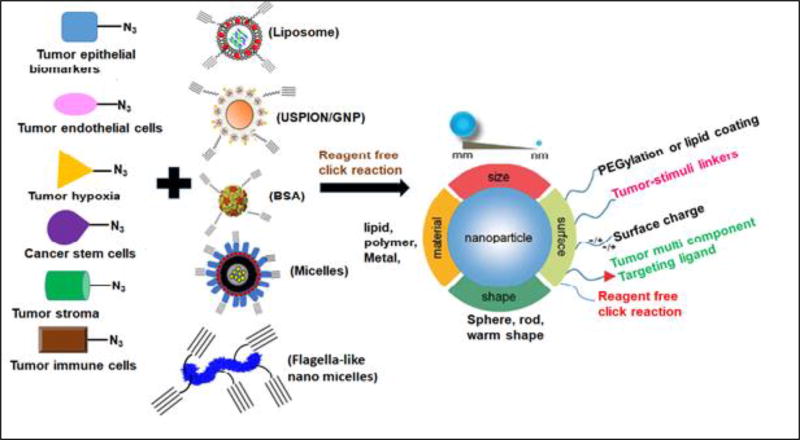
Figure 3.
Library synthesis of tumor multicomponent targeting ligands and various types of drug and gene delivery systems, coupling them using reagent-free click reaction (azide and DBCO) for selective cancer therapy and imaging. Different size-, shape- and material-based nanoparticles will be used so that they can be multimodal agents, for example flagella-like nanomicelles will be used for lowering macrophage-associated liver and spleen uptake. Thus, the need is obvious for the development of an approach to design universal nanothernaostic delivery systems that aim at particular receptor targets on the cancer cells and cancer-associated cells containing drug, dye and a cargo carrier and, hence, include the most important aspects of any therapy into one system: diagnosis, targeting and drug delivery.
Targeting tumor epithelial cells
Cancer is a feature of complicated subclonal heterogenous structures with dynamic evolution. Precision medicine strategies are a rational approach for an individual cancer. Thus, correct treatments are provided to patients based on the role of tumor-specific biomarkers. Any targeted precision medicine strategy should be a roadmap for constant treatment against all major components of the tumor microenvironment, resulting in fewer side effects and prevention of tumor metastasis. This dynamic approach explicitly considers tumor heterogeneity, drug resistance and evolutionary dynamics. The majority of solid tumors are of epithelial origin. Tumor epithelial cells overexpressed various types of receptors. For example, FR-α, a cell surface receptor, is highly overexpressed in a wide range of cancer types such as ovarian, cervical, endometrial, pancreatic, bladder and squamous cancers with limited expression in healthy organs [90]. Folic acid (FA), also known as vitamin B9, is a high-affinity agonist of FR-α with very high binding affinity (Kd ~10 nM) [52,91]. Owing to tumor epithelial cell selective overexpression, FR-α has been explored as a clinically useful biomarker for targeted cancer therapy and imaging. Currently, >20 clinical trials have been performed using FA-conjugated small-molecule drug or diagnostic agents, and some of them are in Phase III trials (NCT03180307) for targeted-imaging-guided cancer surgery. Besides the success, several FA-drug small-molecule conjugates have failed in clinical trials (NCT01953536, NCT01688791, NCT02049281) owing to short half-life and nonspecific toxicity. Asialoglycoprotein receptors (ASGPRs) are lectin receptors that were found to be overexpressed in several human neoplastic hepatocytes [92] and hepatocellular carcinoma (HCC) owing to its high binding affinity [93,94]. ASGPRs selectively bind and internalize different molecules exposing carbohydrate residues such as galactose, galactosamine or N-acetylgalactosamine and internalize various cell nutrients through clathrin-mediated endocytosis. Galactosamine was proven to achieve ASGPR-mediated efficient liver tumor targeting in a Phase I clinical trial of poly[N-(2-hydroxypropyl)methacrylamide] copolymer carrying doxorubicin [95,96]. Galactosamine-conjugated delivery systems have been very promising in achieving more-effective HCC therapy, with better accumulation of therapeutic agents in the tumor tissue and fewer side effects. This is the advantage in developing a combinatorial library of targeting ligands with variable tumor-stimuli-responsive linkers and formulation of a wide variety of lipid-, polymer- and lipid–polymer-based nanoparticles [6,62,97,98]. Further, targeting ligands and nanoparticles can be conjugated using a reagent-free click reaction. The click-reaction-based nanoparticles will solve the shorter plasma half-life and nonspecific cytotoxicity of a potent drug without compromising the receptor-targeting ability. Moreover, these clinically failed potent drugs will be repurposed as cancer nanomedicines.
GPCRs are the largest family of cell-surface receptors involved in signal transmission, and are cellular sensors in various types of diversified organisms [99]. Recently, they have emerged as crucial factors in tumor growth and metastasis. Tumor cells often hijack physiological functions of GPCRs for their survival, proliferation, immune system evasion, angiogenesis and invasion and metastasis to other organs. Several GPCRs, such as luteinizing hormone receptor (LHR) and follicle-stimulating hormone receptor (FSHR), somatostatin receptor (SST), insulin-like growth factor (IGF)-1, sphingosine-1-phosphate (S1P), epidermal growth factor (EGF), hepatocyte growth factor (HGF) and other receptor tyrosine kinase (RTK) receptors, have been recognized as valuable for targeting cancer and some of the targeting receptors are in clinical trials [99]. Many small-molecule and peptide agonists have been identified as having high binding affinity to GPCRs. Developing click-chemistry-based synthesis of a targeting library and coupling this with drug- or gene-encapsulated nanoparticles for tumor-selective payload delivery can be a rewarding strategy. Similarly, hyaluronic acid (HA) can be conjugated with lipid to target CD44-overexpressing triple-negative breast cancer (TNBC) or pancreatic cancer. Two high affinity (Kd ~10 nM) octa-peptides: YLFFVFER (H6) and KLRLEWNR (H10), isolated from a phage display library can be utilized for targeting HER-2 receptor in breast cancer [100]. This ligand can be chemically functionalized with N3, or such activating groups, and the resulting product will be ready for a click reaction with DBCO-functionalized lipid or lipid–polymer hybrid and USPION nanoparticles. The nanoparticles can further be encapsulated with tumor-specific drugs.
Targeting tumor hypoxia
It has been recognized that hypoxia is an important factor in cancer drug resistance and has a role in metastasis. Different proteins are responsible for cancer progression and they are selectively overexpressed in hypoxic tumors, including clear cell renal cell carcinoma (ccRCC) and colorectal cancer. Hypoxia-inducing factor (HIF)-1α is a crucial transcription factor in regulating pH of the tumor microenvironment under hypoxic conditions. It also supports tumor cell survival and invasion in hypoxic microenvironments [101]. Because of these reasons and lack of treatment options, hypoxia targting and HIF-1α have become of growing interest for targeted cancer therapy and diagnosis. To this end, several studies have been developed for finding small-molecule and antibody-based inhibitors to target hypoxia. Among them, sulfonamide-based small molecules, such as acetazolamide (an FDA-approved drug used to prevent and reduce the symptoms of altitude sickness and open-angle glaucoma), have been repurposed for studying tumor hypoxia inhibition. Several groups, including ours, have been working on antibody-based hypoxia-targeting ligands for tumor hypoxia targeting and therapy [102]. Nitro-imidazole derivatives (18F-HX4, 18F-FAZA, 18F-FMISO) and the thiazol derivative 18F-VM4037 have been studied in the early stages of clinical trials (NCT00884520, NCT01075399) as positron emission tomography (PET) and computed tomography (CT) imaging agents for various types of cancers including lung, squamous, head and neck and hepatic. All these data indicate that the hypoxia-targeted proteins are very important and clinically validated cell surface receptors for targeting tumor drug resistance hypoxia components. Along with these successes, several hypoxia protein inhibitors have been terminated or withdrawn from clinical study, owing to dose-limiting toxicity and low to medium tumor/background for PET/CT imaging (NCT01712685, NCT02216669). Efforts should be made in developing multitasking theranostic nanoparticles like ultra-small lipid–polymer hybrid nanoparticles and USPIONs with surface-functionalized hypoxia-targeted proteins targeting ligand using a copper-free click reaction. This lipid–polymer nanoparticle will be engineered to conjugate with a near-infrared (NIR) dye (S0456) for fluorescent imaging [79]. By contrast, USPIONs have high-contrast magnetic resonance properties and are preclinically used for tumor MRI. They can also be chemically functionalized with NIR dyes, so that USPIONs can be repurposed for dual MRI and NIR imaging toward hypoxic tumor detection [52]. The majority of hypoxia tumors harbor several oncogenic features, such as genetic mutations – KRas mutation for pancreatic cancer and von Hippel Lindau (VHL) mutation for renal cell carcinoma. Tumorigenic transcription factor HIF-1α is crucial for maintaining tumor hypoxia and hypoxia-dependent protein overexpression. Further, nanosized liposomes for co-encapsulation of HIF-1α inhibitors, such as roxadustat, along with mutant siRNA of VHL for renal cancer and KRas gene for pancreatic cancer, can be developed [103]. This is a proven strategy for tumor targeting as is evident from the preliminary data that strongly support the combination siRNA and HIF-1α and thus can be a powerful approach for synergistic hypoxic tumor therapy [104–106].
CSC targeting
A small group of cancer cells: tumor-initiating or cancer stem cells (CSCs), lead to drug resistance, metastasis and relapse of cancers, significantly affecting anticancer therapy. Tumor drug resistance seems to be closely related to many intrinsic or acquired properties of CSCs, such as DNA repair ability, quiescence, specific morphology and overexpression of antiapoptotic proteins and drug efflux transporters. This CSC microenvironment (also called the niche) provides additional protection of the tumor against antitumor therapy and tumor hypoxia accompanies maintenance of CSCs. CSCs maintain the tumor mesenchymal phenotype through expression of CD44 on the cell surface [107]. IHC analysis showed that CD44 expression is abundant in a wide variety of solid and hematological cancers [108]. HA, a natural glycosaminoglycan polymer, has a high affinity for CD44 receptor and is widely used for drug delivery [10,53,65,104,106,109,110]. Recently, it has been shown that the HA-linked copoly(styrene maleic acid) (HA-SMA) nanomicelles can selectively target CD44+ stem-like pancreatic cancer [53]. Therefore, HA–polymer represents a valuable tool for CD44-mediated drug delivery to CSCs. Because CSCs reside at the core of the tumor, the majority of drugs cannot reach the deep tissues through penetration of the dense tumor stromal barrier. To enhance the tumor penetration, clinically used stroma-penetrating enzymes, such as PEGylated hyaluronidase, can be applied [111]. Further, the majority of chemotherapeutic drugs are poorly water soluble and thus present challenges for intravenous administration to patients. One way to address this issue is to encapsulate these poorly aqueous-soluble drugs in nanoformulations, such as micelles, lipids and USPION nanoparticles, that are surface-decorated with the stroma-penetrating enzymes like HA or hyaluronidase using a reagent-free click reaction to target the CSCs. The highly water-soluble chemotherapeutics, such as doxorubicin and cisplatin, have the limitation of faster clearance from the body which limits efficacy. To solve this challenge, the strategy of developing a pro-drug approach, such as lipid-tail-modified doxorubicin and cisplatin functionalized with HA-based macrostructures, can be adopted because the lipid-modified HA will facilitate self-assembly in aqueous media resulting in improvement of plasma circulation time with CD44-targeted therapy.
Tumor vasculature targeting
The growth of solid tumors is dependent on the formation of new blood vessels that are responsible for the supply of nutrients through blood – a process called angiogenesis. The targeted inhibition and destruction of new blood vessels has become an exciting therapeutic opportunity for tumor measurement [112]. Many efforts have been directed toward the development of antiangiogenic agents that disrupt tumor neo-blood vessel formation. Thus, the strategy of utilizing the clinically validated tumor angiogenic biomarkers, such as VEGFR-2, PSMA and TNF-α, for selective nanotherapeutic delivery could prove to be very beneficial. VEGFR-1 and VEGFR-2 represent a family of tyrosine kinase receptors that promote angiogenesis, vascular permeability and endothelial cell survival in blood and lymphatic vessels of the tumor microenvironment. VEGF, in binding with VEGFR-1 and −2, activates a tumorigenic signaling cascade, thus making the inhibition of VEGF a rational approach for tumor therapy. The humanized VEGF monoclonal antibody inhibitor bevacizumab (Avastin®) was the first-line cancer treatment for various solid tumors, such as colon, lung, glioblastoma and renal cell carcinoma. The major disadvantage of antibody therapy is nonspecific liver and spleen uptake, owing to the high abundance of protein scavenger receptors and macrophages that cause undesired side effects. To solve the problem of nonspecific liver uptake, the novel technique of developing flagella-like nanomicelles with surface functionalization of small PEGylated peptides, such as CT-322, a Phase II clinically trialed VEGFR-2 inhibitor [41,42], was developed. The advantage of flagella-like micelles is that they can easily avoid the mononuclear phagocytosis system (MPS) of the plasma, thus increasing efficient drug delivery to the tumor.
Prostate-specific membrane antigen (PSMA) is a membrane glycoprotein and is predominantly overexpressed in prostate cancer [115]. Elevation of prostate-specific antigen (PSA) in serum concentrations is often considered as an initial symptom of prostate cancer and commonly used in the clinic for early detection of prostate cancer [116]. However, several researchers have reported tumor-associated PSMA overexpression in the neovasculature of patient-derived solid tumors [117], for example 74% for primary breast cancers, 100% for breast metastatic cancers and brain, whereas expression was limited in the normal vasculature [118]. Low molecular weight urea-based derivatives have very high binding affinity for PSMA of cancer cells, and some of them are already in clinical trials that involve radioimaging of renal cell carcinoma (NCT02687139) and tubulysin therapy of castration-resistant prostate cancer (NCT02202447) through targeted inhibition of angiogenic blood vessels. Because tumor blood vessels are leaky in nature and have PSMA overexpression, the nanoparticles have dual advantages: (i) active tumor targeting through PSMA receptor; and (ii) passive targeting via the EPR effect. Hence, the highly chemotherapeutic drug tubulysin-encapsulated vitamin E (TPGS)-styrene maleic anhydride (SMA)-based nanomicelles for PSMA-targeted antiangiogenic therapy [119] is a reasonable approach. For imaging of tumor angiogenic blood vessels, nanoengineered TPGS–SMA nanomicelles with a NIR dye could be used as a reagent-free click chemistry approach.
Tumor stroma targeting
The tumor microenvironment is a dynamic composite that can be broadly categorized into immune and non-immune cells within a scaffold of fibroblast-rich extracellular matrix, where tumor cells flourish. Among the various non-immune cell types, such as cancer stromal cells, fibroblasts have emerged as crucial players that promote tumor progression, immune evasion, angiogenesis and metastasis [120]. Stroma associated with a tumor commonly contributes a significant portion of the mass of many malignancies, and it can account for >90% of the tumor mass in carcinomas characterized by a desmoplastic reaction, for example breast, colon and pancreatic carcinomas. The presence of stromal tissue creates a dense layer surrounding the tumor epithelial cells, resulting in the appearance of drug resistance and a tolerogenic environment. Recently, it was demonstrated that the activation of FAP-expressing tumor stromal tissue was primarily responsible for tumor growth and drug resistance. Thus, FAP-targeted therapy represents a paradigm-shifting approach for treating human cancer. This includes the FAP-targeting nanoparticle-mediated payload delivery for tumor stroma penetration, inhibition for therapy and imaging for imaging-guided surgery, exploiting FAP targeting using single-chain Fv-antibody-decorated lipid nanoparticles encapsulated with drug and siRNA [42,121].
Tumor immune cell targeting
Macrophage activation is a crucial factor for tumor immune modulation that is directly dependent on cytokine secretion. Activated macrophages have been functionally grouped according to their response: M1-macrpophages – tumor-suppressing proinflammatory; and M2-macrophages – tumor helping anti-inflammatory [122]. FR-α is a marker for M2-macrophages and its expression correlates with increased folate uptake ability, tumor growth, immune evasion and metastasis [123]. In agreement with the research data, FR-α also has elevated expression in TAMs, which exhibit a M2-macrophage-like oncogenic functional profile and play a major immunosuppressive part in the tumor microenvironment. Thus, targeting FR-α with FA-conjugated nanoparticles is a valuable immunotherapy approach to deliver chemotherapeutic and tumorigenic signaling pathway inhibitors to M2-macrophages and the TAM-rich tumor environment.
Another example is dendritic cell (DC)-based vaccination where nanoparticles can deliver TAAs and adjuvant to DCs at the same time and further incite a strong immune response, this can also increase the cellular uptake of the antigen, improvising immunogenicity [124]. It is known that the nanotechnology delivery systems will be key players in overcoming the challenges that face the application of DC-based vaccination for gastrointestinal (GIT) malignancy such as compromised immune system, immune tolerance and short half-life of antigen presentation on the surface of DCs. Nanoparticle-based delivery systems can target the antigen to DCs with sustained release which eventually leads to production of long-acting DCs and frequent T cell stimulation (Figure 4). Also, the application of nanodecorated DC vaccines after a therapeutic plan will remove the residual malignancy and prevent tumor relapse [125]. For instance, one of the liposomal-based DC vaccines that has passed Phase I clinical trials is DepoVax™ (DPX-0907) which is composed of novel carrier for TAAs and adjuvant. This nanoformulation is considered liposome-in-oil design and it consists of a blend of peptide epitopes for cytotoxic and helper T cells, in addition to adjuvant. It was found that this type of vaccination, when tested on advanced cases of breast, ovarian and prostate cancer, showed efficient trapping of DCs to the site of injection. Also, it was smoothly delivered and presented by antigen-presenting cells, enhancing antigen presentation to cytotoxic T cells [69]. The DPX-0907 vaccine was indicated to be safe and well tolerated, with injection-site reactions shown to be the most commonly seen adverse event. Also, vaccinated cancer patients displayed a 61% immune response rate, with higher response rates in the breast and ovarian cancer patients [126].
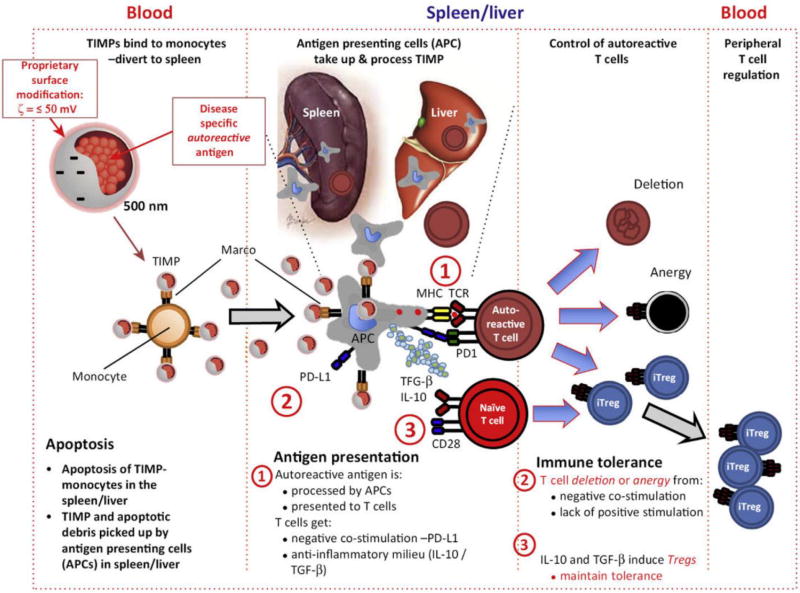
Figure 4.
The functional outcome of nanoparticle (NP) immune modulation depends on numerous factors that are intrinsic to NPs, such as composition, size and charge, as well as extrinsic factors such as route of administration. These concepts and how they relate to manipulating immune responses are described in this figure. Reproduced, with permission, from [140].
Delivery through the EPR effect
The EPR effect utilizes the anatomical and physiological abnormalities of tumor tissue in general and the tumor vasculature in particular [5,127]. It is commonly displayed in most solid tumors, either primary or metastatic in nature. It is modulated or mediated by different factors caused by tumor cells, infiltrating leukocytes or even tumor-surrounding normal cells [51,98,128,129]. Clearly, several vascular mediators, such as BK, PGs and NO, also affect normal blood vessels surrounding tumor tissue. In addition, vascular density and microanatomical defects demonstrate crucial functions in this phenomenon. The EPR effect will present most macromolecular and nanoparticle delivery systems with specific tumor-targeting characteristics and, if delivered to the tumor microenvironment, these macromolecular drugs will remain in the tumor tissues for long periods of time. Even targeting antibodies conjugated to drugs will depend on access to tumor tissue, mediated by the EPR effect (Figure 5). Following EPR-driven tumor delivery, the targeted molecules will be accumulated in tumor cells, after release of drugs from their carriers [4,54]. Intracellular availability of drugs to molecular targets is frequently challenged by the release rate or uptake rate of macromolecules by either endocytosis or by receptor-mediated uptake into the cancer cells. Thus far, the EPR effect is one of the most common properties that distinguish a tumor from normal healthy tissue. A smart choice of drug delivery system, which can take advantage of the EPR effect, and targeting ligand, which can guide the drugs into the cells, will be an excellent combination for efficacious therapy [5].
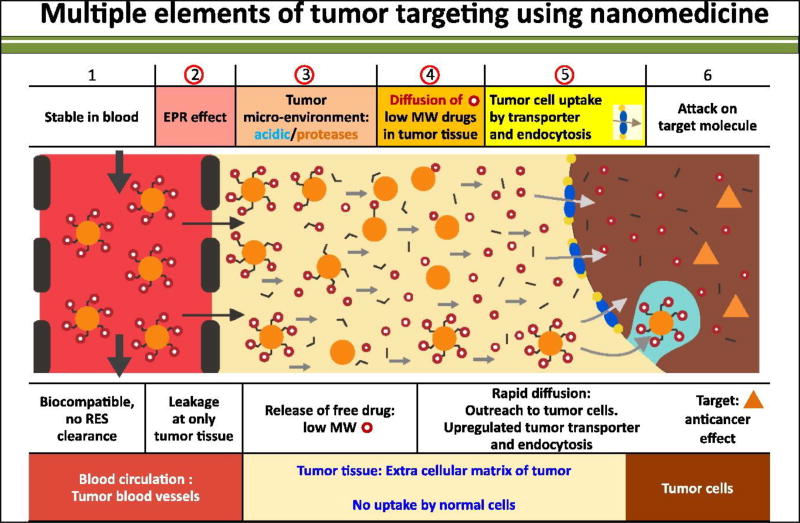
Figure 5.
The enhanced permeability retention effect (EPR) in primary and metastatic tumors is described. The mechanistic pathway indicates that nanoparticles diffuse from leaky blood vessels to the distal area of the tumor. The EPR effect depends on molecular weight of the nanoparticles, pH of the tumor environment, expression of transporter proteins and cellular endocytosis. Reproduced, with permission, from [3].
Development of targeted nanotheranostic systems through click chemistry
It is the goal of every delivery system to address the challenges discussed so far by developing a reagent-free click chemistry process. Recently, there have been several studies and methods developed to establish such chemical reactions that are easily performed, quick and yield high amounts of the desired products with nontoxic byproducts. Several researchers now opine that click chemistry is a modular synthetic approach toward the assembly of new molecular entities. This powerful strategy relies mainly upon the construction of carbon–heteroatom bonds using spring-loaded reactants. The growing number of applications is seen in nearly all areas of modern chemistry from drug discovery to materials science [130]. These kind of click chemistry reactions broadly include cycloaddition of unsaturated species (1,3-dipolar cycloaddition), cycloaddition of unsaturated species {[4+2]-cycloaddition (Diels–Alder)}, nucleophilic substitution/ring-opening reactions, carbonyl reactions of the non-aldol type and addition to carbon–carbon multiple bonds [131,132]. Owing to the obvious advantages of such chemical reactions, click chemistry has been gaining popularity in drug discovery, drug delivery and bioconjugation reactions [133]. Its application is used in developing libraries of such targeted drug delivery systems.
Strategical administration of the targeted nanotheranostic systems
Finally, it is understood that there has to be a logical approach to the administration of the developed targeted nanotheranostics delivery systems in vivo [71,134]. The primary goal of any drug delivery research should be to design novel strategies for delivering therapeutic systems to improve the efficacy of the formulation and reduce the cytotoxicity. This should be planned based on the need of the disease condition, target chosen and the requirements of the delivery systems to maximize the specificity of the formulation. In short, it would be good practice to develop a library of such targeted nanotheranostic systems that can be used off-the-shelf for various targets and disease conditions on an as-needed basis and in suitable combinations. For administering these nanotheranostic systems, there should also be a simple, easy administration strategy such as using a single-step administration method or a multistep administration method [135]. Single-step administration involves a delivery system that has the therapeutic agent, imaging agent as well as the cargo carrier and the targeting ligand (Figure 6). In the multistep administration strategy, the idea is to administer the targeting ligand, cargo carrier and the therapeutic agent first and allow time for circulation and accumulation in the target area; followed by a subsequent delivery of the imaging agent using the same targeting ligand to that specific area [136].
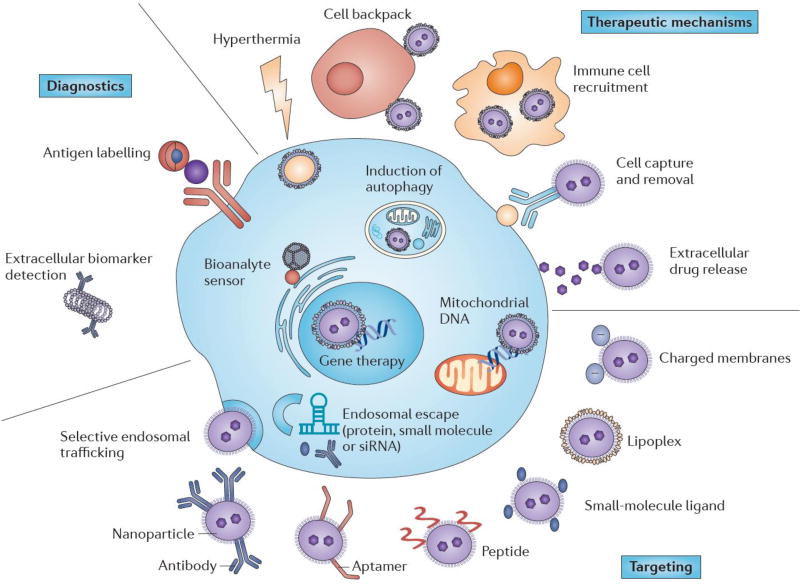
Figure 6.
The multi-pronged activity of nanoparticles to target cancer cells and regulate their uptake into tumor-specific compartments. Nanoparticles serve as therapeutic agents by modulating tumor-associated immune cells, inducing toxicity locally and blocking the tumor survival pathway. The naoparticles contained with an imaging agent can be engineered with tumor-biomarker-specific antibody and agonist to detect the tumor early for imaging and diagnostics. For receptor-mediated tumor targeting, nanoparticles are tailored with a wide variety of ligads, such as peptides, lipids and antibodies. Reproduced, with permission, from [141].
Concluding remarks
Cancer managent requires an innovative approach for successful therapy. The present delivery systems are expected to diagnose and treat the patient. In addition, the methods of synthesis are also expected to be quick, safe and efficient. An off-the-shelf ligand and carrier library is a very efficient method for diagnosing and treating cancer quickly, while the purpose of personalized medicine is served [137–139]. Click chemistry is a novel method to achieve this goal. Further, the delivery systems have to be targeted specifically to the tumor cells so that the healthy cells are not overly effected and there are minimum consequent side effects. As discussed, the prime objective for modern research in cancer therapy should aim to develop a universal approach to target all the major components of the tumor microenvironment for early detection and therapy. Several of the recent advances in the science of drug delivery have been highlighted in this review as the potential means and promises of targeting cancer using a universal approach.
Highlights.
Nanoparticle-based immunotherapy is an emerging platform for cancer
Tumor multicomponent targeting is a rational approach for achieving clinical success
Copper-free click chemistry is highly versatile for engineering multifunctional nanoparticles
Acknowledgments
K.T. would like to acknowledge Graduate ProfessionalScholarship and AGRADE scholarship from the Wayne State University Graduate School to pursue MS Studies in the Iyer Lab, Department of Pharmaceutical Sciences, Wayne State University (WSU). H.A. acknowledges the scholarship support from the College of Pharmacy at Taif University and Saudi Arabian Cultural Mission (SACM). S.K.K. would like to acknowledge the University Grants Commission (UGC, Government of India) for a Raman Postdocotroal Fellowship to undertake postdoctoral research in the Iyer Lab. The authors wish to acknowledge the funding support from US National Institutes of Health, National Cancer Institute (NIH/NCI) grant R21CA179652, American Cancer Society grant (ACS-IRG#449355) and Wayne State University start-up funding (A.K.I.).
Biographies
Samaresh Sau

Dr Samaresh Sau is a Research Associate in the Department of Pharmaceutical Sciences, Wayne State University, USA. He completed his PhD at CSIR-Indian Institute of Chemical Technology, India. He worked as a post-doc at Purdue University, USA, developing clinically translatable drug formulation with Endocyte for cancer and arthritis. He is now developing nanoparticles and small molecules for delivery of drugs, imaging agents, Crispr/Cas9 and CAR-T cells to cancer and superbugs. He is an author in 20 journals and a topic associate editor of Frontiers in Pharmacology. His goal is to develop therapeutic and diagnostic tools for clinical translation.
Sushil K. Kashaw

Dr Sushil Kashaw is an Assistant Professor in the Department of Pharmaceutical Sciences, Dr. Harisingh Gour University, (A Central University), Sagar (M.P.), India. He recently completed his Raman Postdoctoral Research at the Department of Pharmaceutical Sciences, Wayne State University, Detroit, USA. He has 85 research papers, three books and one patent published. Dr Kashaw has supervised three PhD and 37 MPharm students. To his credit, he has completed four major research projects. His area of research is medicinal chemistry and nanotechnology.
Arun K. Iyer

Dr Iyer is the Director of U-BiND Systems Laboratory and Assistant Professor of Pharmaceutical Sciences at Wayne State University, Detroit, Michigan, USA. Dr Iyer received his PhD at Sojo University, Japan, under Professor Hiroshi Maeda (2016 Nobel Prize in Chemistry Nominee). In 2012, Dr Iyer received the prestigious CRS T. Nagai Research Achievement award. Dr Iyer has authored >100 publications in peer-reviewed international journals and books and has wide expertise in biomaterials and nanomedicine for treating diseases such as infection, inflammation and cancer. His laboratory is funded by agencies such as NIH, American Cancer Society and private foundations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no affiliations with or involvement in any organization or entity with any financial interest or nonfinancial interest in the subject matter or materials discussed in this manuscript.
References
- 1.CDC Centers Dis. Control Prev. Expected New Cancer Cases and Deaths in 2020. 2016 Available at: https://www.cdc.gov/cancer/dcpc/research/articles/cancer_2020.htm.
- 2.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 3.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Bhise K, et al. Nanomedicine for cancer diagnosis and therapy: advancement, success and structure--activity relationship. Ther. Deliv. 2017;8:1003–1018. doi: 10.4155/tde-2017-0062. [DOI] [PubMed] [Google Scholar]
- 5.Iyer AK, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Tatiparti K, et al. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials. 2017;7:77. doi: 10.3390/nano7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer AK, et al. High-loading nanosized micelles of copoly(styrene-maleic acid)-zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials. 2007;28:1871–1881. doi: 10.1016/j.biomaterials.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 8.Iyer A, et al. 111In-labeled immunoliposomes targeting both epithelioid and sarcomatoid mesothelioma. J. Nucl. Med. 2010;51:396. [Google Scholar]
- 9.Amiji MM, Iyer AK. Multifunctional Self-assembling Polymeric Nanosystems. 9,173,840 US Pat No. 2015
- 10.Ganesh S, et al. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abeylath SC, et al. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc. Chem. Res. 2011;44:1009–1017. doi: 10.1021/ar2000106. [DOI] [PubMed] [Google Scholar]
- 12.Iyer AK, et al. Image-guided nanosystems for targeted delivery in cancer therapy. Curr. Med. Chem. 2012;19:3230–3240. doi: 10.2174/092986712800784685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amjad MW, et al. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017;64:154–181. [Google Scholar]
- 14.Ganesh S, et al. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release. 2013;172:699–706. doi: 10.1016/j.jconrel.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer AK, et al. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013;65:1784–1802. doi: 10.1016/j.addr.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, et al. Multifunctional nanosystems for cancer therapy. 2013;2013:387–413. [Google Scholar]
- 17.Iyer A, et al. Polymeric nanoparticles as target-specific delivery systems. 2010 doi: 10.4032/9789814267588. [DOI] [Google Scholar]
- 18.Jain S, et al. Multifunctional nanoparticles for targeting cancer and inflammatory diseases. J. Drug Target. 2013;21:888–903. doi: 10.3109/1061186X.2013.832769. [DOI] [PubMed] [Google Scholar]
- 19.Iyer AK, et al. Nanodelivery systems for nucleic acid therapeutics in drug resistant tumors. Mol. Pharm. 2014;11:2511–2526. doi: 10.1021/mp500024p. [DOI] [PubMed] [Google Scholar]
- 20.Abeylath SC, et al. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeutic delivery. Acc. Chem. Res. 2011;44:1009–1017. doi: 10.1021/ar2000106. [DOI] [PubMed] [Google Scholar]
- 21.Kesharwani P, Iyer AK. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today. 2015;20:536–547. doi: 10.1016/j.drudis.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganesh S, et al. Combination of siRNA-directed gene silencing with cisplatin reverses drug resistance in human non-small cell lung cancer. Mol. Ther. Nucleic Acids. 2013 doi: 10.1038/mtna.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsaab H, et al. Folate decorated nanomicelles loaded with a potent curcumin analogue for targeting retinoblastoma. Pharmaceutics. 2017;9:15. doi: 10.3390/pharmaceutics9020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhury H, et al. Recent advances in TPGS-based nanoparticles of docetaxel for improved chemotherapy. Int. J. Pharm. 2017;529:506–522. doi: 10.1016/j.ijpharm.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Cheriyan VT, et al. A CARP-1 functional mimetic loaded vitamin E-TPGS micellar nano-formulation for inhibition of renal cell carcinoma. Oncotarget. 2017;8:104928–104945. doi: 10.18632/oncotarget.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickens JM, et al. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today. 2017;22:665–680. doi: 10.1016/j.drudis.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luong D, et al. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surfaces B Biointerfaces. 2017;157:490–502. doi: 10.1016/j.colsurfb.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luong D, et al. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules. 2017;18:1197–1209. doi: 10.1021/acs.biomac.6b01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer AK, et al. Novel human single chain antibody fragments that are rapidly internalizing effectively target epithelioid and sarcomatoid mesotheliomas. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, et al. Cluster of differentiation 44 targeted hyaluronic acid based nanoparticles for MDR1 siRNA delivery to overcome drug resistance in ovarian cancer. Pharm. Res. 2015;32:2097–2109. doi: 10.1007/s11095-014-1602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amjad MW, et al. In vivo antitumor activity of folate-conjugated cholic acid-polyethylenimine micelles for the codelivery of doxorubicin and siRNA to colorectal adenocarcinomas. Mol. Pharm. 2015;12:4247–4258. doi: 10.1021/acs.molpharmaceut.5b00827. [DOI] [PubMed] [Google Scholar]
- 32.Kesharwani P, et al. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells. Biomacromolecules. 2015;16:3042–3053. doi: 10.1021/acs.biomac.5b00941. [DOI] [PubMed] [Google Scholar]
- 33.Kesharwani P, et al. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surfaces B Biointerfaces. 2015;136:413–423. doi: 10.1016/j.colsurfb.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 34.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 35.Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010;39:1272. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almansour AI, et al. Design, synthesis and antiproliferative activity of decarbonyl luotonin analogues. Eur. J. Med. Chem. 2017;138:932–941. doi: 10.1016/j.ejmech.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Anjibabu R, et al. Heteropoly acid catalyzed synthesis of 8-methyl-2-aryl/alkyl-3-oxabicyclo[3.3.1]non-7-ene derivatives through (3,5)-oxonium-ene reaction. Tetrahedron Lett. 2013;54:7160–7163. [Google Scholar]
- 38.Sau S, Banerjee R. Cationic lipid-conjugated dexamethasone as a selective antitumor agent. Eur. J. Med. Chem. 2014;83:433–447. doi: 10.1016/j.ejmech.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alsaab HO, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017;8:1–15. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, et al. Targeting prostate cancer cells in vivo using a rapidly internalizing novel human single-chain antibody fragment. J. Nucl. Med. 2010;51:427–432. doi: 10.2967/jnumed.109.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer AK, et al. Novel human single chain antibody fragments that are rapidly internalizing effectively target epithelioid and sarcomatoid mesotheliomas. Cancer Res. 2011;71:2428–2432. doi: 10.1158/0008-5472.CAN-10-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer AK, He J. Radiolabeled oligonucleotides for antisense imaging. Curr. Org. Synth. 2011;8:604–614. doi: 10.2174/157017911796117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He J, et al. Targeting prostate cancer cells in vivo using a rapidly internalizing novel human single-chain antibody fragment. J. Nucl. Med. 2010;51:427–432. doi: 10.2967/jnumed.109.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheriyan VT, et al. A CARP-1 functional mimetic loaded vitamin E-TPGS micellar nano-formulation for inhibition of renal cell carcinoma. Oncotarget. 2017;8:104928–104945. doi: 10.18632/oncotarget.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amiji MM, Iyer AK. Multifunctional Self-assembling Polymeric Nanosystems. 9173840 US. 2015
- 47.Susa M, et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010 doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain S, et al. Multifunctional nanoparticles for targeting cancer and inflammatory diseases. J. Drug Target. 2013;21:888–903. doi: 10.3109/1061186X.2013.832769. [DOI] [PubMed] [Google Scholar]
- 49.Luong D, et al. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016;2016:1–16. doi: 10.1016/j.actbio.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Luong D, et al. Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J. Colloid Interface Sci. 2016;484:33–43. doi: 10.1016/j.jcis.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 51.Gawde KA, et al. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J. Colloid Interface Sci. 2017;496:290–299. doi: 10.1016/j.jcis.2017.01.092. [DOI] [PubMed] [Google Scholar]
- 52.Luong D, et al. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules. 2017;18:1197–1209. doi: 10.1021/acs.biomac.6b01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kesharwani P, et al. Hyaluronic acid engineered nanomicelles loaded with 3,4-difluorobenzylidene curcumin for targeted killing of CD44+ stem-like pancreatic cancer cells. Biomacromolecules. 2015;16:3042–3053. doi: 10.1021/acs.biomac.5b00941. [DOI] [PubMed] [Google Scholar]
- 54.Cheriyan VT, et al. A CARP-1 functional mimetic loaded vitamin E-TPGS micellar nano-formulation for inhibition of renal cell carcinoma. OncoTarget. 2017 doi: 10.18632/oncotarget.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel R, et al. Polymeric microspheres: a delivery system for osteogenic differentiation. Polym. Adv. Technol. 2017 doi: 10.1002/pat.4084. [DOI] [Google Scholar]
- 56.Iyer AK, et al. High-loading nanosized micelles of copoly(styrene-maleic acid)-zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials. 2007;28:1871–1881. doi: 10.1016/j.biomaterials.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 57.Iyer AK, et al. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007;15:496–506. doi: 10.1080/10611860701498252. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, et al. Polymeric nanoparticle-based delivery of microRNA-199a-3p inhibits proliferation and growth of osteosarcoma cells. Int. J. Nanomedicine. 2015;10 doi: 10.2147/IJN.S79143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Susa M, et al. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amjad MW, et al. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017;64:154–181. [Google Scholar]
- 61.Thirumurugan P, et al. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 62.Luong D, et al. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surfaces B Biointerfaces. 2017;152:490–502. doi: 10.1016/j.colsurfb.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee Y-H, Chang D-S. Fabrication, characterization, and biological evaluation of anti-HER2 indocyanine green-doxorubicin-encapsulated PEG-b-PLGA copolymeric nanoparticles for targeted photochemotherapy of breast cancer cells. Sci. Rep. 2017;7:46688. doi: 10.1038/srep46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kubota T, et al. Novel HER2-targeted gold nanoparticles; integration of antibody therapy and nanotechnology 2016 [Google Scholar]
- 65.Wickens JM, et al. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today. 2017;22:665–680. doi: 10.1016/j.drudis.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu BO, et al. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010;27:286–298. doi: 10.3109/09687688.2010.521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koide H, et al. A polymer nanoparticle with engineered affinity for a vascular endothelial growth factor (VEGF165) Nat. Chem. 2017;9:715–722. doi: 10.1038/nchem.2749. [DOI] [PubMed] [Google Scholar]
- 68.Libutti SK, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alsaab HO, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017 doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohaegbulam KC, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhise K, et al. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: quality by design (QbD) approach. Int. J. Pharm. 2017;526:506–515. doi: 10.1016/j.ijpharm.2017.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kesharwani P, et al. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surfaces B Biointerfaces. 2015;132:138–145. doi: 10.1016/j.colsurfb.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Fujiwara Y, et al. Imaging mass spectrometry for the precise design of antibody-drug conjugates. Sci. Rep. 2016;6:24954. doi: 10.1038/srep24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wayua C, et al. Selective tumor targeting of desacetyl vinblastine hydrazide and tubulysin B via conjugation to a cholecystokinin 2 receptor (CCK2R) ligand. Mol. Pharm. 2015;12:2477–2483. doi: 10.1021/acs.molpharmaceut.5b00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie D, Xie K. Pancreatic cancer stromal biology and therapy. Genes Dis. 2015;2:133–143. doi: 10.1016/j.gendis.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ting DT, et al. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science. 2011;331:593–596. doi: 10.1126/science.1200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang D, et al. Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int. J. Cancer. 2013;132:993–1003. doi: 10.1002/ijc.27715. [DOI] [PubMed] [Google Scholar]
- 78.Li M, et al. Comparison of two ultrasmall superparamagnetic iron oxides on cytotoxicity and MR imaging of tumors. Theranostics. 2012;2:76–85. doi: 10.7150/thno.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dehaini D, et al. Ultra-small lipid-polymer hybrid nanoparticles for tumor-penetrating drug delivery. Nanoscale. 2016;8:14411–14419. doi: 10.1039/c6nr04091h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nunez MI, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J. Thorac. Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bournet B, et al. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: hopes and realities. Eur. J. Cancer. 2016;54:75–83. doi: 10.1016/j.ejca.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Cox AD, et al. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khvalevsky EZ, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golan T, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. OncoTarget. 2015;6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo P, et al. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics. 2016;6:1–13. doi: 10.7150/thno.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knudsen KB, et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine Nanotechnology Biol. Med. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Jayaraman M, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chemie Int. Ed. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 2015;16:543–552. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thuy T, et al. The stability of lipid rafts-like micro-domains is dependent on the available amount of cholesterol. J. Biophys. Chem. 2016;2016:74–85. [Google Scholar]
- 90.Kelemen LE. The role of folate receptor α in cancer development, progression and treatment: cause, consequence or innocent bystander? Int. J. Cancer. 2006;119:243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 91.Amjad MW, et al. In vivo antitumor activity of folate-conjugated cholic acid-polyethylenimine micelles for the codelivery of doxorubicin and siRNA to colorectal adenocarcinomas. Mol. Pharm. 2015;12:4247–4258. doi: 10.1021/acs.molpharmaceut.5b00827. [DOI] [PubMed] [Google Scholar]
- 92.Trerè D, et al. The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells. Br. J. Cancer. 1999;81:404–408. doi: 10.1038/sj.bjc.6690708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng D-J, et al. Inhibition of hepatocarcinoma by systemic delivery of apoptin gene via the hepatic asialoglycoprotein receptor. Cancer Gene Ther. 2007;14:66–73. doi: 10.1038/sj.cgt.7700985. [DOI] [PubMed] [Google Scholar]
- 94.Singh M, Ariatti M. Targeted gene delivery into HepG2 cells using complexes containing DNA, cationized asialoorosomucoid and activated cationic liposomes. J. Control. Release. 2003;92:383–394. doi: 10.1016/s0168-3659(03)00360-2. [DOI] [PubMed] [Google Scholar]
- 95.Vasey PA, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl) methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents--drug–polymer conjugates. Clin. Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 96.Shen Z, et al. A galactosamine-mediated drug delivery carrier for targeted liver cancer therapy. Pharmacol. Res. 2011;64:410–419. doi: 10.1016/j.phrs.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Mukherjee S, et al. Green synthesis and characterization of monodispersed gold nanoparticles: toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J. Biomed. Nanotechnol. 2016;12:165–181. doi: 10.1166/jbn.2016.2141. [DOI] [PubMed] [Google Scholar]
- 98.Sau S, et al. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale. 2014;6:6745–6754. doi: 10.1039/c4nr00974f. [DOI] [PubMed] [Google Scholar]
- 99.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, et al. Microarray based screening of peptide nano probes for HER2 positive tumor. Anal. Chem. 2015;87:8367–8372. doi: 10.1021/acs.analchem.5b01588. [DOI] [PubMed] [Google Scholar]
- 101.Lou Y, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 102.Minn I, et al. [64Cu] XYIMSR-06: a dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. OncoTarget. 2016;7:56471. doi: 10.18632/oncotarget.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burroughs SK, et al. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med. Chem. 2013;5:553–572. doi: 10.4155/fmc.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang X, et al. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep. 2015;5:8509. doi: 10.1038/srep08509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ganesh S, et al. Combination of siRNA-directed gene silencing with cisplatin reverses drug resistance in human non-small cell lung cancer. Mol. Ther. Nucleic Acids. 2013;2:e110. doi: 10.1038/mtna.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang X, et al. Cluster of differentiation 44 targeted hyaluronic acid based nanoparticles for MDR1 siRNA delivery to overcome drug resistance in ovarian cancer. Pharm. Res. 2015;32:2097–2109. doi: 10.1007/s11095-014-1602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li XP, et al. Expression of CD44 in pancreatic cancer and its significance. Int. J. Clin. Exp. Pathol. 2015;8:6724–6731. [PMC free article] [PubMed] [Google Scholar]
- 108.Chanmee T, et al. Key roles of hyaluronan and its CD44 receptor in the stemness and survival of cancer stem cells. Front. Oncol. 2015;5:180. doi: 10.3389/fonc.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang X, et al. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep. 2015;5:8509. doi: 10.1038/srep08509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kesharwani P, et al. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surfaces B Biointerfaces. 2015;136:413–423. doi: 10.1016/j.colsurfb.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 111.Hingorani SR, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 2016;22:2848–2854. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim TH, et al. Filamentous, mixed micelles of triblock copolymers enhance tumor localization of indocyanine green in a murine xenograft model. Mol. Pharm. 2011;9:135–143. doi: 10.1021/mp200381c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schiff D, et al. Phase 2 study of CT-322, a targeted biologic inhibitor of VEGFR-2 based on a domain of human fibronectin, in recurrent glioblastoma. Invest. New Drugs. 2015;33:247–253. doi: 10.1007/s10637-014-0186-2. [DOI] [PubMed] [Google Scholar]
- 114.Geng YAN, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy J, et al. DUPA conjugation of a cytotoxic indenoisoquinoline topoisomerase I inhibitor for selective prostate cancer cell targeting. J. Med. Chem. 2015;58:3094–3103. doi: 10.1021/jm5018384. [DOI] [PubMed] [Google Scholar]
- 116.Basch E, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J. Clin. Oncol. 2012;30:3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang SS, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 118.Wernicke AG, et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS. 2014;122:482–489. doi: 10.1111/apm.12195. [DOI] [PubMed] [Google Scholar]
- 119.Alsaab H, et al. Folate decorated nanomicelles loaded with a potent curcumin analogue for targeting retinoblastoma. Pharmaceutics. 2017;9:15. doi: 10.3390/pharmaceutics9020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Criscitiello C, et al. Tumor-stroma crosstalk: targeting stroma in breast cancer. Curr. Opin. Oncol. 2014;26:551–555. doi: 10.1097/CCO.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 121.Wang LC, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Shannessy DJ, et al. Expression of folate receptors alpha and beta in normal and cancerous gynecologic tissues: correlation of expression of the beta isoform with macrophage markers. J. Ovarian Res. 2015;8:29. doi: 10.1186/s13048-015-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shen J, et al. Assessment of folate receptor-β expression in human neoplastic tissues. OncoTarget. 2015;6:14700–14709. doi: 10.18632/oncotarget.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Conniot J, et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014 doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bhargava A, et al. Nanoengineered strategies to optimize dendritic cells for gastrointestinal tumor immunotherapy: from biology to translational medicine. Nanomedicine. 2014;9:2187–2202. doi: 10.2217/nnm.14.115. [DOI] [PubMed] [Google Scholar]
- 126.Karkada M, et al. Therapeutic vaccines and cancer: focus on DPX-0907. Biol. Targets Ther. 2014;8:27. doi: 10.2147/BTT.S55196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matsumura Y, Maeda HA. A new concept for macromolecular therapeutics in cancer-chemotherapy - mechanism of tumoritropic acumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 128.Sahu P, et al. Stumuli-responsive bio-hybrid nanogels: an emerging platform in medicinal arena. Global J. Nanomedicine. 2017;1:6–8. [Google Scholar]
- 129.Sahu P, et al. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: in vitro and ex vivo studies. J. Control. Release. 2017;253:122–136. doi: 10.1016/j.jconrel.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 130.Moses JE, Moorhouse AD. The growing applications of click chemistry. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 131.Nandivada H, et al. Click chemistry: versatility and control in the hands of materials scientists. Adv. Mater. 2007;19:2197–2208. [Google Scholar]
- 132.Kolb HC, et al. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 133.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 134.Sau S, et al. Combination of cationic dexamethasone derivative and STAT3 inhibitor (WP1066) for aggressive melanoma: a strategy for repurposing a Phase I clinical trial drug. Mol. Cell. Biochem. 2017;2017:1–18. doi: 10.1007/s11010-017-3084-z. [DOI] [PubMed] [Google Scholar]
- 135.Roy E, et al. Stimuli-responsive poly(N-isopropyl acrylamide)-co-tyrosine@ gadolinium: iron oxide nanoparticle-based nanotheranostic for cancer diagnosis and treatment. Colloids Surfaces B Biointerfaces. 2016;142:248–258. doi: 10.1016/j.colsurfb.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 136.Iyer AK, He J. Radiolabeled oligonucleotides for antisense imaging. Curr. Org. Synth. 2011;8:604–614. doi: 10.2174/157017911796117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jain KK. Role of nanobiotechnology in developing personalized medicine for cancer. Technol. Cancer Res. Treat. 2005;4:645–650. doi: 10.1177/153303460500400608. [DOI] [PubMed] [Google Scholar]
- 138.Hamburg MA, Collins FS. The path to personalized medicine. N. Engl. J. Med. 2010;2010:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 139.Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012;64:1394–1416. doi: 10.1016/j.addr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 140.Getts DR, et al. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015;36:419–427. doi: 10.1016/j.it.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schroeder A, et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer. 2012;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]