Abstract
Functional connectivity between the amygdala and the prefrontal cortex is critical for socioemotional processing, particularly during face processing. Though processing others’ emotions is important for a myriad of complex social behaviors, more research is needed to understand how different types of emotional expressions differentially elicit connectivity of the amygdala with widespread neural regions. Moreover, though prior studies have reported cross-sectional associations between altered amygdala-prefrontal cortex functional connectivity and internalizing symptoms (e.g., depression, anxiety), few studies have examined whether amygdala functional connectivity is prospectively related to changes in these symptoms, with little work focusing on low-income men living in stressful contexts. The current study used psychophysiological interaction analyses at the within-subjects level to examine how amygdala connectivity differed while participants viewed fearful, angry, and neutral faces. We used structural equation modeling at the between-subjects level, using extracted parameter estimates, to test whether amygdala connectivity during face processing predicted increases in internalizing psychopathology over time, controlling for earlier symptoms. An urban sample of 167 young men from low-income families was employed. Results indicated that negative connectivity between the amygdala and prefrontal regions was modulated by emotional face type. Neuronal activity in the cingulate and frontal cortices was connected to amygdala reactivity during fearful and neutral, but not angry, face processing. Moreover, weaker left amygdala–left middle frontal gyrus negative connectivity when viewing fearful faces and stronger right amygdala–left inferior frontal gyrus negative connectivity when viewing neutral faces at age 20 both predicted increases in internalizing behaviors from age 20 to age 22. Our findings show that amygdala-prefrontal cortex connectivity can predict the persistence of internalizing symptoms among high-risk participants over time but suggest that these patterns may differ depending on the emotional stimuli examined.
Keywords: functional connectivity, emotional processing, amygdala, internalizing
Socioemotional processing, including attention to and appraisal of others’ emotions, is critical for interpersonal functioning. Facial expressions are robust indicators of others’ emotions, and humans have evolved to specifically detect facial expressions that signal threat (i.e., anger), distress (i.e., fear), and ambiguity (i.e., neutral faces) (Ekman & Friesen, 1976; Oatley & Johnson-Laird, 1987; Whalen & Phelps, 2009). Though neural processes enable humans to attend and respond to affectively-laden facial expressions, variability in how the brain processes socioemotional information is associated with serious psychiatric disorders, including anxiety and depression (Kim et al., 2011; Price & Drevets, 2010). To extend this knowledge base, the current study investigated how multiple emotional facial expressions differentially elicited neural patterns in a racially diverse sample of young men living in low-income urban contexts, and whether specific patterns of neural connectivity during socioemotional processing predicted changes in psychopathology over time.
The corticolimbic circuit, which includes the amygdala and regions of the prefrontal cortex (PFC), is critical to socioemotional processing (LeDoux, 2000; Ochsner, Silvers, & Buhle, 2012; Whalen & Phelps, 2009). The amygdala is highly sensitive to facial expressions that signal threat, uncertainty, or other salient information in the environment, and is robustly activated during functional magnetic resonance imaging (fMRI) tasks where participants either implicitly or explicitly view emotional faces (Fusar-Poli, Placentino, Carletti, Landi, & Abbamonte, 2009; Shi, Wang, & Yao, 2013). Medial and lateral regions of the PFC, as well as the anterior cingulate cortex (ACC), also support socioemotional processing by integrating affective valuations from the amygdala with inputs from other neural regions, including the brainstem and thalamus (Egner, Etkin, Gale, & Hirsch, 2007; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Fuster, 2001; Ochsner et al., 2012). Functional parcellation of the medial frontal lobe suggests a rostral-ventral distinction in emotion processing where the dorsal ACC, dorsal-medial PFC (dmPFC), and dorsal-lateral PFC (dlPFC) support the cognitive components of emotion processing (e.g., appraisal), while the ventral ACC (including the rostral and subgenual components) and the ventro-medial PFC (vmPFC) and ventro-lateral PFC (vlPFC) support emotion regulation (Etkin, Egner, & Kalisch, 2011; Fuster, 2001).
The mPFC is structurally and functionally connected to the amygdala (Kim et al., 2011). Bi-directional functional connections support bottom-up signaling from the amygdala to represent salient information about emotional stimuli and top-down signaling from the PFC to regulate responses to emotional stimuli (Ochsner & Gross, 2005; Quirk, Garcia, & González-Lima, 2006; Stein et al., 2007). Compared to healthy controls, patients with vmPFC lesions show persistent amygdala reactivity to aversive images (Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015), highlighting the important regulatory role of the mPFC. Though not as extensively or directly structurally connected to the amygdala as the mPFC (Ghashghaei & Barbas, 2002), lateral regions of the PFC (particularly the dlPFC) also have been shown to be functionally connected to the amygdala in resting-state and task-based connectivity studies (Lu et al., 2012; Sato, Kochiyama, Uono, Yoshikawa, & Toichi, 2016; Stein et al., 2007). Thus, the reciprocal functional connections between the amygdala and areas of the PFC suggest the need to investigate these brain regions as part of one “corticolimbic circuit” (Hariri, 2015). A circuit-based approach is particularly relevant for studies exploring complex socioemotional behavior, including internalizing symptoms such as anxiety and depression (Sporns, Chialvo, Kaiser, & Hilgetag, 2004; van den Heuvel & Hulshoff Pol, 2010).
A recent meta-analysis of 49 task-based connectivity studies (Di, Huang, & Biswal, 2017) showed that the amygdala is more strongly functionally connected to a host of regions during face processing (particularly fear) when compared to baseline, including the medial frontal gyrus (Brodmann Area [BA] 10), anterior cingulate gyrus (BA 32, 24), and inferior frontal gyrus (BA 47). However, this meta-analysis also highlighted several limitations of existing research examining amygdala connectivity patterns during emotion processing. Notably, many studies of task-based amygdala connectivity have only focused on fearful face processing. However, the amygdala plays a broad role in processing threat, danger, and salience (LeDoux, 2000; Whalen et al., 2001), which have also been probed with angry facial expressions and ambiguous neutral faces (Marusak, Zundel, Brown, Rabinak, & Thomason, 2016; Neta & Whalen, 2010; Pollak & Sinha, 2002). Each facial expression is thought to represent a different type or degree of threat or salience. For example, whereas angry facial expressions with directed eye gaze may represent a clear and direct threat, fearful facial expressions with directed eye gaze may represent a more ambiguous threat, as the source of threat is unclear (Adams, Gordon, Baird, Ambady, & Kleck, 2003). Neutral faces are ambiguous and may also be interpreted negatively (Blasi et al., 2009), particularly for individuals who have grown up in adverse environments (Marusak et al., 2016; Pollak & Sinha, 2002). Thus, delineating how connectivity between the amygdala and widespread neural regions varies by emotional face type may help to elucidate how threat and ambiguity are processed in the brain.
Though processing emotional facial expressions enables individuals to interact with their social environments, individuals who present with internalizing symptoms, including anxiety and depression, show exaggerated attention to threatening stimuli (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). This greater attention to emotion is evident even at the neural level, with studies showing aberrant patterns of functional connectivity (i.e., in both task-based paradigms and at rest) within the corticolimbic circuit among individuals with elevated internalizing symptoms or disorders (Etkin, Prater, Hoeft, Menon, & Schatzberg, 2010; Hardee et al., 2013; Kim et al., 2011; Lu et al., 2012; Prater, Hosanagar, Klumpp, Angstadt, & Phan, 2013). Specifically, a failure of the PFC to downregulate amygdala hyper-activation to threatening facial expressions is thought to increase risk for anxiety and depression (Kim et al., 2011; Rive et al., 2013). Yet much of this prior research has been cross-sectional. Prospective, longitudinal studies are needed to examine temporal relations between neural and behavioral markers of psychopathology, accounting for current levels of internalizing symptoms. Such a longitudinal design could examine specifically whether weakened corticolimbic connectivity is prospectively related to changes in internalizing symptomatology over time. Greater amygdala reactivity to emotional (and neutral) facial expressions has been associated with increases in negative affect in children (Gaffrey, Barch, & Luby, 2016), poorer antidepressant treatment response in adults (Goldstein-Piekarski et al., 2016), and increases in depressive symptoms in both the current sample (Mattson, Hyde, Shaw, Forbes, & Monk, 2016) and others (Swartz, Knodt, Radtke, & Hariri, 2015). However, with two exceptions (Connolly et al., 2017; Scheuer et al., 2017), relatively little work has examined whether amygdala functional connectivity predicts changes in internalizing symptoms over time. As emerging research emphasizes that coordinated neural networks are more likely to explain complex behavior than activity in single regions of interest (Woo, Chang, Lindquist, & Wager, 2017), research is needed to understand how connectivity within the corticolimbic circuitry is prospectively related to complex behavior across time.
Moreover, much of the research examining associations between corticolimbic connectivity and internalizing symptoms has relied on resting state functional connectivity (Connolly et al., 2017; Scheuer et al., 2017), which does not allow for the assessment of task-based changes in connectivity in relation to specific types of stimuli. As internalizing disorders (and symptoms) are associated with variability in socioemotional processing (e.g., individuals with anxiety show neural hypersensitivity to threatening stimuli) (Monk et al., 2008; Price & Drevets, 2010), studies are needed that examine neural correlates of changes in internalizing symptoms using task-based connectivity analyses. One method that can complement resting state approaches is psychological-physiological interaction (PPI) analysis, which can identify whether functional coupling between two regions is moderated by task condition (Friston et al., 1997). PPI analysis is critical for studying emotional face processing because it quantifies both the direction and strength of connectivity. Thus, PPI analysis allows inferences to be drawn about how connectivity between two regions changes while participants view one face type (e.g., fear) versus another condition, and how these patterns vary by contrast (e.g., fearful face > baseline versus neutral face > baseline).
Examining the hierarchical structure of internalizing behaviors using structural equation modeling (SEM) represents a useful method to explore how neural activity within different contrasts (e.g., fearful face > baseline) are related to internalizing psychopathology. In particular, anxiety and depression are highly comorbid phenotypically, with both marked by heightened negative affect and oversensitivity to threat cues (Etkin, 2012; Krueger & Markon, 2006; Price & Drevets, 2010). Moreover, symptoms of anxiety and depression often load onto a broad internalizing latent construct within SEM frameworks (Hankin et al., 2016; Krueger & Markon, 2006; Lahey et al., 2012). However, the majority of neuroimaging studies have focused separately on anxiety or depression (e.g., Etkin et al., 2010; Prater et al., 2013; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). As evidence suggests that psychopathology is both hierarchically-organized and dimensional in nature (Krueger & Markon, 2006), studies are needed to explore whether weakened corticolimbic connectivity represents a risk factor for the overlap (i.e., shared variance) of these two disorders (Zald & Lahey, 2017) using dimensional measures.
Additionally, studies examining associations between functional connectivity and internalizing symptoms have often focused on female-only samples (e.g., Kaiser et al., 2017; Laeger et al., 2012). Although women are more likely to be diagnosed with internalizing disorders than men (Kessler et al., 1994), the lifetime prevalence for internalizing disorders in men is still substantial, with some estimates as high as 19% (Kessler et al., 1994). Thus, more research is needed to understand the biological mechanisms of internalizing behaviors in male samples. Moreover, as many connectivity studies have used samples of convenience (e.g., ethnic majority samples, relatively high SES) we know little about how these processes unfold in individuals living in lower SES contexts and in ethnic minority populations, who are exposed to high levels of stress and threat and show concomitant high rates of mental health problems (Falk et al., 2013; Hyde, 2015; Robins & Regier, 1991). Finally, the transition to adulthood between ages 19–23 is a developmental stage marked by changing roles, increasing independence, and increased risk for psychopathology (Arnett, 2000; Obradovic, Burt, & Masten, 2006; Schulenberg, Sameroff, & Cicchetti, 2004). Thus, outside of the late-childhood and adolescent period, young adulthood represents a critical window for understanding the persistence and escalation in psychopathology.
The purpose of the current study was to (1) characterize the connectivity of the amygdala during emotional face processing (i.e., in response to viewing fearful, angry, and neutral faces), at the within-subjects level, in a racially diverse sample of low-income, urban males, and (2) examine prospective, longitudinal associations between amygdala functional connectivity and changes in internalizing symptoms in young adulthood from ages 20–22, at the between-subjects level.
Consistent with existing meta-analytic evidence (Di et al., 2017; Robinson, Laird, Glahn, Lovallo, & Fox, 2009) and prior research using PPI models in face processing tasks (Cremers et al., 2010; Iidaka et al., 2001; Monk et al., 2008), we hypothesized that the left and right amygdala would exhibit stronger positive connectivity with visual processing regions and stronger negative connectivity with the PFC (particularly the vmPFC and ventral ACC) during face processing versus a non-face control condition, at the within-subjects level. At the between-subjects level, we hypothesized that weaker negative corticolimbic connectivity would predict increases in internalizing psychopathology, reflecting an impairment of the PFC to downregulate heightened threat-related amygdala reactivity (Myers-Schulz & Koenigs, 2012). We did not have hypotheses about the specificity of these effects for connectivity in response to the different facial expressions examined (fearful, angry, and neutral) based on the relative lack of research in this area to date.
Materials and Methods
Participants
Participants are part of the Pitt Mother & Child Project (PMCP), a longitudinal study of 310 low-income boys and their families recruited in 1991 and 1992 from Allegheny County Women, Infant and Children (WIC) Nutritional Supplement Clinics when boys were 6–17 months old (Shaw, Hyde, & Brennan, 2012). The sample is low income and ethnically diverse (52% European-American, 38% African-American, 7% biracial, 3% other of those included at age 20). Children and mothers were seen almost yearly from age 1.5–23 in the laboratory and/or home with assessments that included questionnaires, psychiatric interviews, and at age 20, an MRI scanning session. fMRI data were available for 167 men (see Supplemental Methods and S1) consistent with other publications from this sample using this task (Gard et al., 2017; Hyde et al., 2015; Mattson et al., 2016). The sample was originally recruited to study the onset and progression of conduct problems over time, hence the focus only on boys. However, as these boys were raised in low-income, urban environments, they were exposed to a myriad of stressors and heightened risk for a variety of maladaptive outcomes, including internalizing symptoms and disorders. These aforementioned factors make this high-risk community sample useful for the study of internalizing psychopathology (Mattson et al., 2016; Morgan et al., 2016; Morgan, Shaw, & Forbes, 2013). Participants were reimbursed for their time at the end of each assessment and all procedures were approved by the institutional review board of the University of Pittsburgh.
fMRI task
Participants performed an implicit emotional face processing task (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002), which consists of four blocks of perceptual face processing interleaved with five blocks of sensorimotor control (see also Hyde et al., 2015; Manuck, Brown, Forbes, & Hariri, 2007) (see S2). Participants viewed a trio of faces and selected one of two faces (bottom) identical to a target face. Each face processing block consisted of six images, balanced for sex, all derived from a standard set of pictures of facial affect (Ekman & Friesen, 1976). Each of the four face processing blocks consisted of a different emotional facial expression (i.e., anger, fear, surprise, neutral), and participants were randomly assigned to one of four different orders of block presentation. During the sensorimotor control blocks, participants viewed a trio of simple geometric shapes (circles, vertical and horizontal ellipses) and selected one of two shapes (bottom) identical to a target shape (top). All blocks were preceded by brief instructions (“Match Faces’’ or “Match Shapes’’) lasting 2 s. In the face processing blocks, each of the six face trios was presented for 4s with a variable interstimulus interval (ISI) of 2—6s (mean = 4 s) for a total block length of 48s. A variable ISI was used to minimize expectancy effects and resulting habituation, as well as to maximize amygdala reactivity throughout the paradigm. In the sensorimotor control blocks, each of the six shape trios was presented for 4s with a fixed ISI of 2s for a total block length of 36s. Total task time was 390s.
Data acquisition
Each participant was scanned with a research-dedicated Siemens 3T TIM Trio. Blood oxygenation level–dependent (BOLD) functional images were acquired with a gradient-echo echoplanar imaging (EPI) sequence (TR/TE=2000/29 milliseconds, FOV=200x200), which covered 34 interleaved axial slices (3-mm slice thickness). BOLD functional images were aligned with the AC-PC plane and encompassed the entire cerebrum and most of the cerebellum to maximum coverage of limbic structures. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before collecting fMRI data for each participant, a reference echoplanar imaging scan was acquired and visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume of acquisition. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each participant to minimize field inhomogeneities.
Imaging data pre-processing
Functional data were analyzed in SPM8 (Statistical Parametric Mapping, Wellcome Trust Centre, London, United Kingdom). Images for each participant were segmented, realigned to the mean volume in the time series, unwarped to correct for head motion, co-registered to high resolution structural scans (MPRAGE), spatially normalized into a standard stereotactic space (MNI template) using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 6 mm FWHM. Voxelwise signal intensities were ratio-normalized to the whole-brain global mean. After preprocessing, the Artifact detection Tools (ART) software package (http://www.nitrc.org/projects/artifact_detect/) was used to detect global mean intensity and translation or rotational motion outliers (> 4.5 SD from the mean global brain activation, >2mm movement or 2° translation in any direction); nuisance covariates were created for all volumes satisfying one of these criteria. Additionally, because of the relatively extensive signal loss typically observed in the amygdala, single-subject BOLD fMRI data were only included in subsequent analyses if there was a minimum of 90% signal coverage in the amygdala bilaterally, defined using the Automated Anatomical Labeling (AAL) atlas in the WFU PickAtlas Tool, version 1.04 (Maldjian, Laurienti, Kraft, & Burdette, 2003). The accuracy criterion for the task was ≥75%; 19 participants were removed from the imaging sample because of motion, signal coverage, and behavioral quality control criteria (S1).
Functional connectivity analysis
Psychological-physiological interaction (PPI) analyses in the generalized PPI toolbox (McLaren, Ries, Xu, & Johnson, 2012) in SPM8 were used to measure context-dependent connectivity of the left and right amygdala. In a PPI analysis, a design matrix is constructed at the level of the individual with the following columns of variables: (a) a physiological variable that represents the time course of the seed region (e.g., left or right amygdala) across the task, (b) a psychological variable indicating the experimental variable (e.g., onset times for an experimental condition), and (c) a product term of the interaction between the physiological and psychological variables. At the individual level, the regression coefficient for the interaction term represents the change in activity between the seed and identified “target region” across different conditions, such as emotional face types (i.e., the seed-target functional coupling is context-dependent). The gPPI toolbox developed by McLaren and colleagues (2012) allows for the simultaneous specification of all task conditions and interactions with the seed region time series in the same individual-level model (Friston et al., 1997). This is advantageous because it reduces the number of specified models and the overall type I error rate. Moreover, compared to correlation methods (Rissman, Gazzaley, & D’esposito, 2004), gPPI methods have been found to be more powerful for detecting functional connectivity during block designs (Cisler, Bush, & Steele, 2014).
As we were interested in reporting amygdala connectivity during the emotional faces matching paradigm (i.e., the group-level PPI effects) across the entire brain, we defined the left and right amygdala as the seed regions (AAL definition using WFU PickAtlas, version 1.04; Maldjian et al., 2003). Two general linear models at the individual level were constructed (i.e., one for the left amygdala seed and one for the right amygdala seed). Using the gPPI toolbox, the time series of the left or right amygdala seed was entered as the physiological variable in the design matrix, the explanatory variables for each of the five conditions in our task (i.e., facial expressions of fear, anger, surprise, and neutral faces, and shapes) were entered as psychological variables, and the five product terms between the amygdala seed and conditions were entered as the interaction terms. As we were interested in whether functional coupling of the left or right amygdala and target regions varied as a function of emotional face type, we specified three primary contrasts at the individual level: fearful faces interaction term > shapes interaction term, angry faces interaction term > shapes interaction term, and neutral faces interaction term > shapes interaction term. However, as the amygdala plays a role in broad salience detection and emotion processing above and beyond threat processing specifically (Fitzgerald, Angstadt, Jelsone, Nathan, & Phan, 2006; Whalen & Phelps, 2009), we also examined connectivity of the left and right amygdala in the all faces > shapes contrast. Contrasts from the individual level models were then used in random effects, group-level models (i.e., there were 8 total group-level models: left and right amygdala X 4 contrasts). In our analysis, a significant fear > shapes PPI at the group level would suggest increased positive covariation of activity in the seed (i.e., left or right amygdala) and the target region (indicated by a positive regression weight) while participants are viewing fearful facial expressions than at shapes. Conversely, a significant fear < shapes PPI (i.e., indicated by a negative regression weight) would suggest increased negative covariation of activity in the seed and the target region while participants are looking at fearful facial expressions than at shapes.
To identify target regions that were more strongly positively or negatively connected with the left or right amygdala, we followed an unbiased whole brain approach. That is, we did not restrict our target regions to previously-defined regions of interest (ROI), a method which is limited by masking of potential target regions that have not yet been identified by previous literature. We report the extent thresholds (i.e., peaks) and cluster sizes of target regions that met a Family-Wise-Error (FWE) correction threshold of p<.05 for multiple comparisons across the entire brain, using SPMs built-in correction procedure. To examine the associations between amygdala functional connectivity and behavioral outcomes, we extracted all significant group-level PPI effects (i.e., as the principal eigenvariate of each target cluster across subjects) by contrast (e.g., rostral ACC target region in the fearful faces versus shapes contrast).
Behavioral measures and data analysis
Symptoms of internalizing psychopathology were modeled at both ages 20 and 22 as a latent variable within a structural equation modeling (SEM) framework using full information maximum-likelihood (FIML) estimation in Mplus version 7.2 (Muthén & Muthén, 2006). Validated self-reported and clinician-rated measures of anxiety and/or depression were used as indicators of the latent internalizing measure, with the same measures used at ages 20 and 22: (1) self-reported total score on the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) (2) self-reported total score on the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988), (3) clinician-rated symptom count of current Major Depressive Disorder using DSM-IV criteria on the Structured Clinical Interview (SCID) of the Diagnostic and Statistical Manual (DSM) IV Axis I disorders (First, Spitzer, Gibbon, Williams, & others, 1995), and (4) clinician-rated symptom count of current social phobia using DSM-IV criteria on the SCID (First et al., 1995). Symptoms of social phobia, rather than symptoms of generalized anxiety disorder (GAD), were included as indicators of latent internalizing because more participants reported social phobia symptoms (e.g., n = 39 at age 22) than symptoms of GAD (e.g., n = 12 at age 22), resulting in more variation in the latent factor and better model fit. Descriptive statistics, reliability of self-report depression and anxiety, and zero-order correlations between indicators of the internalizing factor are reported in S3. Confirmatory factor analysis indicated good model fit (X2([18] = 13.29, p=.77, RMSEA=.001 90% CI (.001, .05), CFI=1.00, TLI=1.02, SRMR=.04) (Kline, 1998) and all factor loadings were greater than 0.3 and significant at the 95% confidence interval, suggesting that the underlying latent construct of internalizing explained a significant portion of the variance in each of the observed indicators (see S4).
We constructed SEM models to test whether amygdala connectivity at age 20 predicted changes in the latent construct of internalizing psychopathology from ages 20 to 22. FIML estimation uses the covariance matrix of all available data to produce unbiased estimates and standard errors in the context of missing data (McCartney, Burchinal, & Bub, 2006). Thus, our sample size for our SEM models was 167 (i.e., the number of young men with valid imaging data), despite 10 young men missing some behavioral data at age 22 (see Supplemental Methods). To test our hypothesis that negative connectivity between the left and right amygdala and the mPFC during socioemotional processing would predict increases in internalizing behaviors, we tested two models. Model 1 included all (i.e., across all neural contrasts) negative group-level PPI effects with prefrontal target regions as predictors of changes in internalizing psychopathology from ages 20–22 (5 predictors). Model 2 tested a more stringent model that included all identified negative group-level PPI effects (i.e., including target regions outside of the prefrontal cortex) in the same model (12 predictors). In both models, we accounted for the covariance in the predictors so that brain-behavior pathways represented unique effects. In all models linking neural function to behavior, we also controlled for the effects of participant monthly income at ages 20 and 22 (i.e., a mean across the two ages) as an index of SES, which has been shown to influence neural function (Javanbakht et al., 2015) and internalizing psychopathology (Robins & Regier, 1991). Note that our results did not change when we accounted for educational attainment as an additional measure of SES. We also accounted for participant race and total score on the self-report of delinquency (SRD; Elliott, Huizinga, & Ageton, 1985) at age 20 in analyses, based on the comorbidity of externalizing and internalizing psychopathology and the original goal of studying externalizing disorders in this sample (Kessler, Chiu, Demler, & Walters, 2005; Shaw et al., 2012).
Results
Positive connectivity of the left and right amygdala during face versus shapes processing
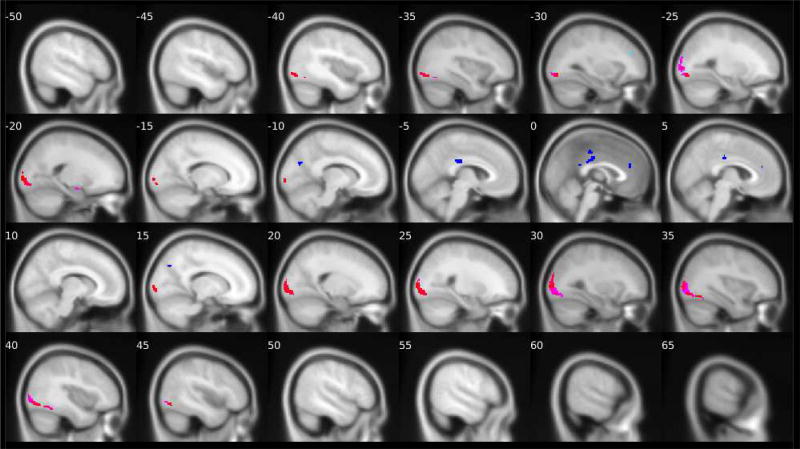
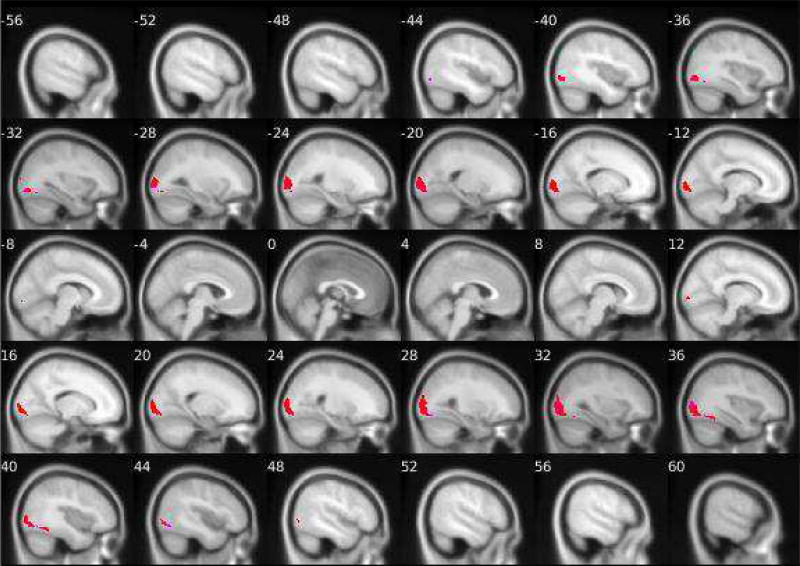
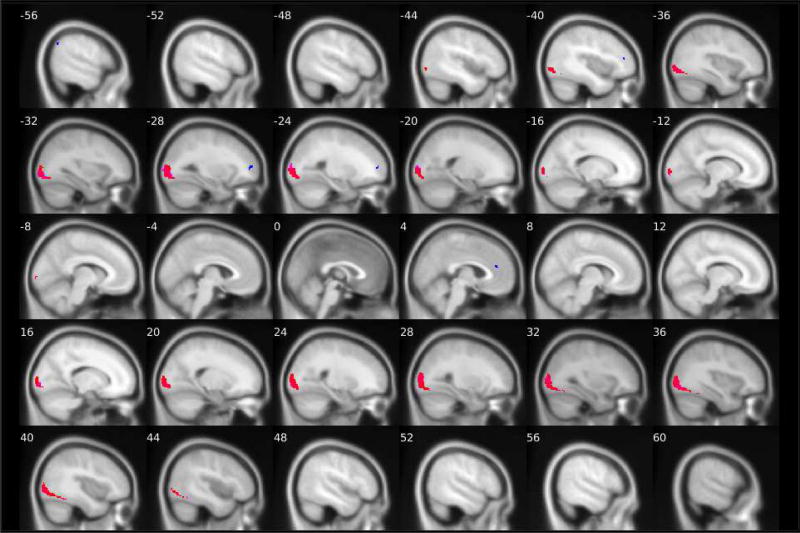
Consistent with the facial stimuli being more visually complex than the shapes stimuli, across all contrasts we examined, the task elicited significantly greater positive connectivity between the left and right amygdala and visual processing regions (Table 1) while participants viewed the facial stimuli versus the shapes stimuli. More specifically, activity in both the left and right amygdala was more strongly positively connected with activity in the bilateral inferior occipital gyrus and the fusiform gyrus while participants viewed fearful facial expressions than when participants viewed shapes stimuli (Figure 1). Second, activity in both the left and right amygdala was more strongly positively connected with bilateral activation in the middle occipital gyrus and the fusiform gyrus while participants viewed angry facial expressions as compared to shapes (Figure 2). Finally, the task elicited greater positive connectivity between the left amygdala and the right lingual gyrus, left middle occipital gyrus, and right fusiform gyrus. There was also greater positive connectivity between the right amygdala and the right middle and inferior occipital gyri and the left fusiform gyrus while participants viewed neutral faces versus shapes stimuli (Figure 3). Similar target regions were also identified for the all faces versus shapes contrast (Table 1).
Table 1.
Target neural regions exhibiting greater positive connectivity with the amygdala during face versus shapes stimuli processing, by facial emotion type
| Contrast | Amygdala Seed Region |
Target Region | (x,y,z), t extent threshold, k cluster size | BA |
|---|---|---|---|---|
| Fear > Shapes | left | left inferior occipital gyrus | (-24,-88,-10) t=7.87***, k=284*** | - |
| left | right inferior occipital gyrus | (34,-84,-8) t=6.97***, k=640*** | 18 | |
| left | right fusiform gyrus | (38,-52,-18) t=6.57***, k=44*** | 37 | |
| left | left fusiform gyrus | (-34,-64,-14) t=5.43**, k=17** | - | |
| left | left calcarine sulcus | (-14,-100,-2) t=5.30*, k=13** | - | |
| right | right inferior occipital gyrus | (34,-82,-10) t=6.63***, k=611*** | 18 | |
| right | left inferior occipital gyrus | (-26,-86,-10) t=6.51***, k=260*** | - | |
| right | left fusiform gyrus | (-38,-66,-12) t=5.36**, k=6** | - | |
| right | left middle occipital gyrus | (-22,-98,14) t=5.30*, k=55* | 18 | |
|
| ||||
| Anger > Shapes | left | right middle occipital gyrus | (32,-88,8) t=6.93***, k=614*** | - |
| left | left middle occipital gyrus | (-20,-100,6) t=6.93***, k=404*** | 18 | |
| left | left fusiform gyrus | (-36,-68,-12) t=5.91**, k=10** | - | |
| right | left middle occipital gyrus | (-18,-98,4) t=7.43***, k=611*** | 18 | |
| right | right middle occipital gyrus | (32,-92,12) t=7.05***, k=833*** | - | |
| right | right fusiform gyrus | (36,-60,-14), t=6.61***, k=57*** | - | |
|
| ||||
| Neutral > Shapes | left | right lingual gyrus | (24,-90,-2) t=7.13***, k=592*** | - |
| left | left middle occipital gyrus | (-30,-92,-4) t=6.91***, k=528*** | - | |
| left | right fusiform gyrus | (42,-56,-16), t=5.70**, k=18** | - | |
| right | right middle occipital gyrus | (32,-92,6), t=7.64***, k=857*** | - | |
| right | left inferior occipital gyrus | (-24,-88,-10) t=7.37***, k=571*** | - | |
| right | left fusiform gyrus | (-38,-68,-12), t=5.49**, k=5* | - | |
|
| ||||
| All faces > Shapes | left | right middle occipital gyrus | (34,-90,8) t=10.17***, k=1522*** | - |
| left | left inferior occipital gyrus | (-24,-88,-10) t=9.93***, k=1178*** | - | |
| right | right middle occipital gyrus | (34,-90,6) t=10.64***, k=1661*** | - | |
| right | left middle occipital gyrus | (-40,-86,-6) t=9.86***, k=1321*** | - | |
Note: N=167. No target regions at the whole brain correction threshold exhibited positive connectivity with the amygdala seed regions for the Fear>Neutral or Anger>Neutral contrasts. Coordinates are in Montreal Neurological Institute (MNI) space. Target regions that did not overlap with defined Brodmann’s Areas (BA) are indicated by a dash in the right-hand column of the table.
Whole brain FWE-corrected:
p<.05,
p<.01,
p<.001
Figure 1. Group-level PPI effects of left and right amygdala connectivity while looking at fearful facial expressions versus shapes.
N = 167. Positive connectivity group-level PPI effects of the right amygdala are shown in red and of the left amygdala are shown in pink. Negative connectivity group-level PPI effects of the right amygdala are shown in dark blue and of the left amygdala are shown in light blue/turquoise. Details about the target regions (e.g., MNI coordinates, cluster size and extent) are reported in Tables 1 and 2. The fMRI task paradigm is pictured in Supplemental Material S2.
Figure 2. Group-level PPI effects of left and right amygdala connectivity while looking at angry facial expressions versus shapes.
N = 167. Positive connectivity group-level PPI effects of the right amygdala are shown in red and of the left amygdala are shown in pink. Negative connectivity group-level PPI effects of the right amygdala are shown in dark blue of the left amygdala are shown in light blue/turquoise. Details about the target regions (e.g., MNI coordinates, cluster size and extent) are reported in Tables 1 and 2. The fMRI task paradigm is pictured in Supplemental Material S2
Figure 3. Group-level PPI effects of left and right amygdala connectivity while looking at neutral faces versus shapes.
N = 167. Positive connectivity group-level PPI effects of the right amygdala are shown in red and of the left amygdala are shown in pink. Negative connectivity group-level PPI effects of the right amygdala are shown in dark blue and of the left amygdala are shown in light blue/turquoise. Details about the target regions (e.g., MNI coordinates, cluster size and extent) are reported in Tables 1 and 2. The fMRI task paradigm is pictured in Supplemental Figure 1.
Negative connectivity of the left and right amygdala during face versus shapes processing
Consistent with our hypotheses, the amygdala was more negatively connected to multiple regions within the PFC and cingulate cortex (Table 2) during face processing than during shapes processing. For fearful facial stimuli versus shapes, activity in the left amygdala was more negatively connected with activity in the left middle frontal gyrus (MFG) (corresponding to the left dlPFC [see S5 for methods]) and the left posterior cingulate cortex (PCC) (BA 23). Activity in the right amygdala was more negatively connected with activity in the bilateral middle cingulate gyrus (MCC) (BA 23), the left rostral ACC, the left PCC (BA 23), and the bilateral cuneus (Figure 1). For angry facial expressions versus shapes, the left amygdala was more negatively connected to the left middle occipital gyrus. For neutral faces versus shapes, the right amygdala was more negatively connected to the left MFG (BA 10), the right rostral ACC (BA 32), and the left inferior frontal gyrus (IFG) (corresponding to the left dlPFC [see S5 for methods]) (Figure 3). Finally, activity in the bilateral amygdala showed stronger negative connectivity with multiple target regions while participants viewed all faces versus shapes, including the left MFG (BA 10), right rostral ACC (BA 32), right medial orbitofrontal cortex (BA 32) (corresponding to the vmPFC), bilateral cuneus, right supramarginal gyrus (BA 40), and right superior frontal gyrus (BA 10) (Table 2). Thus, we found multiple target regions in both the prefrontal cortex and the cingulate (posterior, middle, and anterior) that were more strongly negatively connected with the amygdala during face processing versus shapes processing, but these patterns differed by the face type examined.
Table 2.
Target neural regions exhibiting greater negative connectivity during face versus shapes stimuli processing
| Contrast | Amygdala Seed Region |
AAL-Defined Target Region | (x,y,z), t extent threshold, k cluster size |
BA |
|---|---|---|---|---|
| Fear > Shapes | left | left posterior cingulate | (-2,-40,24) t=5.28*, k=13** | 23 |
| left | left middle frontal gyrus/dlPFC | (-32,38,28) t=5.05*, k=5* | - | |
| right | left middle cingulate | (-4,-28,30) t=6.21***, k=109*** | 23 | |
| right | left middle cingulate | (0,-26,46) t=5.48**, k=23** | - | |
| right | left anterior cingulate/rACC | (2,40,22) t=5.21*, k=20** | - | |
| right | right cuneus | (14,-72,38) t=5.28*, k=12* | - | |
| right | left cuneus | (-10,-74,28) t=5.20*, k=12** | - | |
| right | left posterior cingulate | (-2,-46,24) t=5.19*, k=11** | 23 | |
|
| ||||
| Anger > Shapes | left | left middle occipital gyrus | (-36,-84,36), t=5.21*, t=7** | 19 |
|
| ||||
| Neutral > Shapes | right | left middle frontal gyrus | (-26,52,6) t=5.46**, k=28*** | 10 |
| right | right anterior cingulate/rACC | (4,40,20) t=4.98*, k=7** | 32 | |
| right | left inferior frontal gyrus/dlPFC | (-40,42,12) t=5.15*, k=5* | - | |
|
| ||||
| All Faces > Shapes | left | - | (-2,-24,30) t=6.67***, k=613*** | 23 |
| left | left middle frontal gyrus/dlPFC | (-30,38,28) t=6.00***, k=77*** | - | |
| left | right anterior cingulate/rACC | (2,40,16) t=5.97**, k=143*** | 32 | |
| left | right medial orbitofrontal cortex/vmPFC | (2,46,-4) t=5.91**, k=65*** | 32 | |
| left | left precuneus | (-12,-68,30) t=5.91**, k=66*** | 7 | |
| left | left middle occipital gyrus | (-36,-84,36) t=5.79**, k=11** | 19 | |
| right | - | (-4,-26,28) t=7.06***, k=467*** | 23 | |
| right | right cuneus | (16,-72,38) t=6.39***, k=277*** | - | |
| right | left cuneus | (-10,-70,28) t=6.02***, k=210*** | - | |
| right | right precuneus | (2,-42,58) t=5.82**, k=23** | 5 | |
| right | right anterior cingulate/rACC | (4,42,18) t=5.54**, k=64*** | - | |
| right | right supramarginal gyrus | (62,-46,36) t=5.52**, k=34*** | 40 | |
| right | right superior frontal gyrus | (26,60,16) t=5.46**, k=20** | 10 | |
| right | left middle frontal gyrus/dlPFC | (-40,42,16) t=5.25*, k=16** | 10 | |
| right | right middle cingulate | (14,-36,42) t=5.08*, k=8** | - | |
N=167. BA = Brodmann Area; dlPFC = dorsolateral prefrontal cortex; rACC = rostral anterior cingulate cortext; Coordinates are in Montreal Neurological Institute (MNI) space. Target regions that did not overlap with defined Brodmann’s Areas (BA) are indicated by a dash in the right-hand column of the table. No target regions at the whole brain correction threshold exhibited negative connectivity with the amygdala seed regions for the Fear>Neutral or Anger>Neutral contrasts. Estimates highlighted in boldface font were the clusters that were used in behavioral prediction models (Figure 4)
Whole brain FWE-corrected:
p<.05,
p<.01,
p<.001
Neuroprediction model of internalizing behaviors in young adulthood
We next examined whether negative connectivity between the amygdala and target regions within the PFC (i.e., ACC, MFG, IFG) across all potential neural contrasts predicted increases in internalizing behaviors from age 20 to 22 within a SEM framework. Extracted group-level PPI effects showed only modest-to-moderate inter-correlations in zero-order associations (i.e., .001 < r < .40) (see S6), suggesting that these extracted estimates of amygdala connectivity were tapping unique aspects of amygdala functional connectivity, justifying their inclusion in a single model.
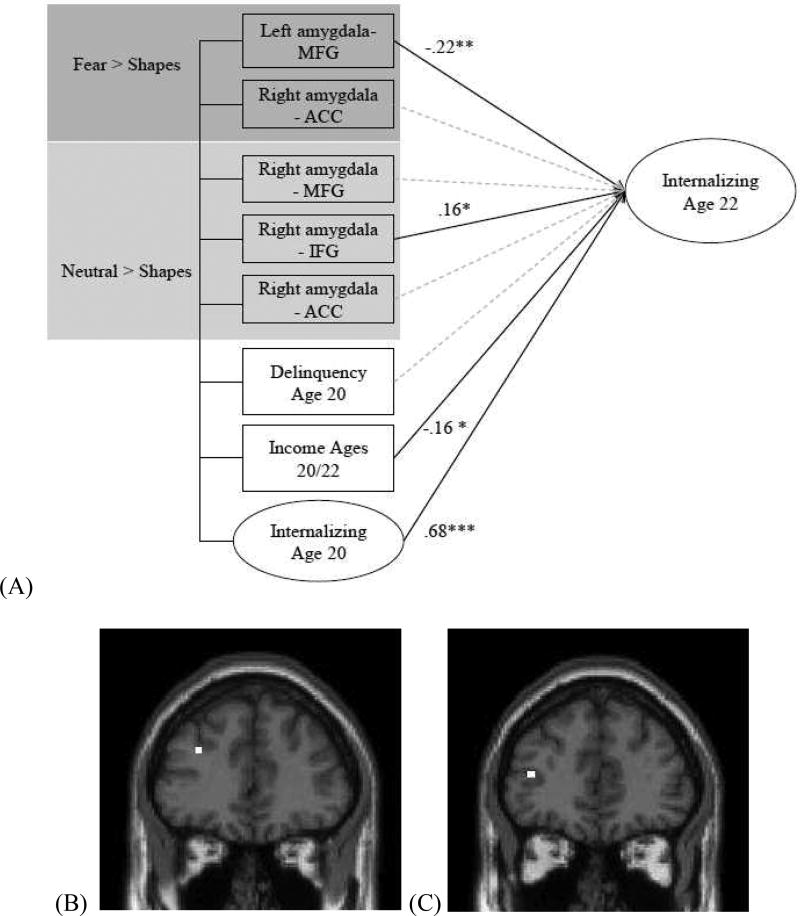
We found that weaker negative connectivity between the left amygdala and left MFG during fear processing and stronger negative connectivity between the right amygdala and left IFG during neutral face processing – each uniquely predicted increases in internalizing behaviors from age 20 to age 22 (Figure 4). These associations remained significant in a more stringent model that additionally accounted for symptoms of antisocial personality disorder, alcohol and drug use and abuse, attention-deficit hyperactivity disorder, and family income during infancy. This stringent model was examined to confirm the specificity of the group-level PPI effects on internalizing behaviors (see Supplemental Methods for descriptions of the additional behavioral controls). In a second model, we included all the negative group-level PPI estimates that we did not originally hypothesize to be related to internalizing symptoms (e.g., amygdala-MCC and amygdala-PCC negative group-level PPI estimates during fear processing). In this model, we found that the prospective relations between amygdala-MFG connectivity during fear processing and amygdala-IFG connectivity during neutral faces processing and later internalizing symptoms did not change in direction or statistical significance (see S7). By examining all relationships within a single SEM, we were able to establish unique effects and controlled for multiple comparisons. Notably, in a third model that included all negative and positive group-level PPI estimates for all contrasts examined, none of the positive group-level PPI estimates predicted internalizing (results available upon request). Thus, results were specific to negative connectivity.
Figure 4. Negative functional connectivity between the amygdala seed region and the middle frontal and inferior frontal gyri during face processing predicts increases in internalizing symptoms from age 20 to 22.
N=167. MFG, middle frontal gyrus; ACC, anterior cingulate cortex; IFG, inferior frontal gyrus. (A) Path model with all group-level PPI negative connectivity effects between the amygdala seed regions and prefrontal target regions at age 20 predicting a latent factor of internalizing symptoms (see Figure S4) at age 22. Standardized estimates are depicted. Covariates include internalizing symptoms and self-reported delinquency and income at age 20. Covariances between the predictor variables are also modeled. Model fit: X2(74)=68.24, p=.67. RMSEA=.001 90% CI (.001,.04). CFI=1.00, TLI=1.02, SRMR=.05. Standardized estimates between internalizing symptoms at age 22 and (a) left amygdala-MFG connectivity to Fear>Shapes and, (b) right amygdala-IFG connectivity to Neutral>Shapes when controlling for the following additional covariates: family income at 18 months (a) β= −.23** (b) β=.16*, symptoms of alcohol and drug use and dependence at age 20 (a) β= −.21** (b) β=.16*, symptoms of attention-deficit hyperactivity disorder at age 17 (a) β= −.28*** (b) β= .20*. (B) Group-level PPI negative connectivity between the left amygdala and left MFG during fear processing. (C) Group-level PPI negative connectivity between the right amygdala and left IFG during neutral face processing. * p<.05, ** p<.01, *** p<.001
Finally, to examine whether significant brain-behavior relations were specific to a broad latent internalizing construct (i.e., variance shared by depression and anxiety) rather than individual symptom domains, we examined a final model with separate measures of anxiety and depression as the outcome variables while accounting for their covariance. As shown in S8, weaker negative amygdala-MFG connectivity during fearful face processing predicted increased vulnerability to both depression and anxiety, supporting its longitudinal effects on general internalizing psychopathology. However, stronger negative amygdala-IFG connectivity during neutral face processing predicted increased risk for anxiety symptoms only (S8).
Discussion
In a sample of racially diverse young adult men from low-income urban families, we examined the connectivity of the left and right amygdala during emotional face processing. Using gPPI methods, we established that positive and negative connectivity between the amygdala and target regions was modulated by task condition (i.e., neutral, fearful, or angry faces versus shapes). Importantly, we also found that weaker negative amygdala-MFG connectivity to fearful faces and stronger negative amygdala-IFG connectivity to neutral faces predicted increases in internalizing symptomatology from age 20 to age 22.
The target regions that were negatively functionally connected to the amygdala varied by task condition, suggesting that connectivity of the amygdala is modulated by exposure to different types of emotional facial expressions. During neutral and fearful face processing, activity in the amygdala was negatively connected with activity in the MFG and rostral ACC, consistent with prior research suggesting a role for the rostral mPFC in emotion regulation (Etkin et al., 2006; Kim et al., 2011). However, the IFG was only negatively connected to the amygdala for the neutral > shapes contrast. Prior research suggests that the IFG is especially important in processing ambiguous stimuli like neutral faces. For example, Nomura et al., (2003) reported relatively greater IFG activation while participants viewed emotionally ambiguous versus emotionally unambiguous facial stimuli. In addition, only the PCC (BA 23) was negatively connected to the amygdala during fear processing. The PCC is involved in orientation towards and interpretation of environmental cues (Vogt, Finch, & Olson, 1992), and is part of the default mode network (Raichle et al., 2001). Our findings suggest that the PCC may play a regulatory role in modulating amygdala response to fearful stimuli specifically.
It is surprising that we found no group-level PPI effects for the angry facial expressions versus shapes contrast, particularly given that angry faces with direct eye gaze may reflect a clear threat (Adolphs, 2002; Davis & Whalen, 2001; Ekman & Friesen, 1976). Moreover, the young men in this same sample show the expected pattern of greater amygdala reactivity to angry faces versus shapes (Gard et al., 2017; Mattson et al., 2016). Thus, the non-significant group-level PPI effects for the angry facial expressions contrasts should be interpreted with caution. Finally, amygdala seed regions exhibited expected positive connectivity with visual processing regions (e.g., fusiform gyri, middle occipital gyrus), which was similar across all face types. These findings suggest generality in functional connections within the visual processing system to emotional faces, but specificity of amygdala-PFC connectivity during different types of socioemotional processing.
We next used SEM to examine whether the negative corticolimbic group-level PPI estimates at age 20 predicted changes in internalizing symptoms at age 22, while controlling for internalizing symptoms at age 20. Findings indicated that weaker negative amygdala-MFG connectivity during fearful face processing and stronger negative amygdala-IFG connectivity during neutral face processing predicted increases in internalizing symptoms from age 20 to 22. Both the MFG and IFG target regions fell within the lateral PFC (Figure 4), a cognitive control region important for the allocation of attentional resources (Fuster, 2001). Based on its role in supporting goal-directed behavior (Ochsner & Gross, 2005), the lateral (particularly the dorsolateral) PFC is important for the regulation of mood and emotion (Pessoa, 2008). Neuroimaging studies suggest that individuals with depression, which is marked by cognitive dysfunctions such as impaired working memory and attentional processing, show hypoactivation in the dlPFC (Koenigs & Grafman, 2009; Ochsner & Gross, 2005). However, much of this research has focused on cognitive tasks (e.g., working memory) or resting state connectivity, rather than emotional face processing. Critically, these studies have not examined prospective associations between amygdala-lateral PFC connectivity and the development of internalizing symptoms. Our results suggest that weaker negative amygdala-lateral PFC connectivity during fearful face processing, likely reflecting decreased prefrontal regulation, may contribute to increased vulnerability for increasing internalizing psychopathology during young adulthood. Moreover, this connectivity estimate predicted both the broad internalizing construct and depression and anxiety symptoms when examined separately, suggesting that this a risk factor for broad internalizing psychopathology.
In contrast to expectations, it was found that stronger negative amygdala-IFG functional connectivity during neutral face processing predicted increases in internalizing symptoms (and specifically anxiety symptoms) two years later. Connectivity estimates reflect bottom-up signaling from the amygdala to PFC regions and/or top-down regulatory signaling from the PFC to the amygdala. Thus, speculatively, it is possible that the ambiguous nature of neutral faces elicits stronger bottom-up signaling from the amygdala to the IFG during neutral face viewing and stronger top-down PFC input, particularly for participants with anxiety symptoms. Anxious participants relative to controls show greater amygdala reactivity to neutral faces (Somerville, Kim, Johnstone, Alexander, & Whalen, 2004), and are thought to interpret ambiguous stimuli as threatening (Monk, 2008). Future studies capable of evaluating participant-reported valence of neutral faces, and studies that include other less ambiguous/non-threatening faces (e.g., calm) are needed to empirically test this explanation.
There were many strengths to this study, including the relatively large sample size for a fMRI study, the focus on a racially diverse and low-income population of men that has been understudied in prior research (Henrich, Heine, & Norenzayan, 2010), the characterization of amygdala functional connectivity during the processing of multiple face types using gPPI, and the examination of a prospective neuroprediction pathway to internalizing symptomatology modeled as a latent construct using SEM. However, several limitations warrant consideration. First, the baseline condition in our paradigm was a non-face shapes stimulus. Thus, our connectivity results could be identifying target regions that were more strongly functionally connected to the amygdala during general face processing rather than processing of a specific emotion. At the same time, we found that connectivity of the amygdala was moderated by condition: different target regions were engaged while participants were exposed to fearful, neutral, or angry faces, suggesting that even our contrasts comparing an emotional face type to a non-face shapes stimulus highlight differences based on emotional facial expression. Second, we did not collect participant ratings of the valence or emotional content of the facial stimuli. Though fearful and angry facial expressions are ecologically-valid stimuli thought to convey threat and/or distress (Adolphs, 2002; Davis & Whalen, 2001; Ekman & Friesen, 1976; Wager, Phan, Liberzon, & Taylor, 2003), and recent studies find that participants view ambiguous neutral faces as threatening in some contexts (Davis, Neta, Kim, Moran, & Whalen, 2016; Marusak et al., 2016), we cannot be certain of our participants’ interpretation of these emotional facial stimuli. Third, PPI analyses do not establish directionality, as these analyses do not provide information about temporal ordering for either the positive or negative connectivity estimates. Hence, we cannot conclusively determine whether the amygdala-PFC connectivity observed here reflects bottom-up signaling from the amygdala to PFC or top-down signaling from the PFC to the amygdala. Fourth, we focused only on low-income males, meaning that our results may not be generalizable to other populations (e.g., rural samples, women, more socioeconomically-advantaged segments of the population). This issue of generalizability is particularly relevant based on our non-significant negative group-level PPI effects during angry face processing. At the same time, our study contributes to recent efforts in the neuroimaging literature to include more diverse participants (Falk et al., 2013). Finally, although we found that corticolimbic connectivity was associated with increases in internalizing psychopathology from ages 20 to 22, future studies beginning in middle-childhood could help to establish whether the same disrupted patterns of task-based neural connectivity precede the onset of internalizing disorders earlier in development.
In sum, we present connectivity patterns of the left and right amygdala during face processing in a racially diverse sample of young men raised in low-income, urban, environments. Our results stress the importance of examining multiple face types within one analysis, as it appears that prospective associations between corticolimbic connectivity and the development of internalizing symptoms depends on the type of facial expression examined. Therefore, these different face types may tap various aspects of socioemotional processing related to threat and ambiguity that differentially predict future vulnerability for internalizing psychopathology. Our findings highlight the utility of an analytic strategy that combines functional connectivity analyses with SEM to predict how multiple patterns of neural function uniquely predict changes in psychopathology, which we modeled as a latent construct to account for comorbidities in internalizing symptoms. Our prediction model implicates amygdala functional connectivity as a premorbid vulnerability for later psychopathology, which could eventually be used as a method for identifying individuals at risk for mental health problems.
Supplementary Material
Acknowledgments
This work was support by the National Institutes of Health Grant Nos. R01 MH50907 (to DSS), R01 MH01666 (to DSS), K05 DA25630 (to DSS), R01 DA026222 (to DSS and EEF), L40 DA036468 (to LWH), T32 HD00710936 (to AMG), T32 AA007477 (to RW); and the Avielle Foundation (to LWH). JRS was supported by Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis. We are grateful to the work of the staff of the Pitt Mother & Child Project for their many years of service, and to our study families for sharing their lives with us and making this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Adams RB, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300(5625):1536–1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. doi: 10.1037//0003-066X.55.5.469. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh JK. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, Mattay VS. Preferential amygdala reactivity to the negative assessment of neutral faces. Biological Psychiatry. 2009;66(9):847–853. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Social Neuroscience. 2013;8(2):122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, Yang TT. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of Affective Disorders. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol M-J, van der Wee NJA, Roelofs K. Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010;49(1):963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Davis FC, Neta M, Kim MJ, Moran JM, Whalen PJ. Interpreting ambiguous social cues in unpredictable contexts. Social Cognitive and Affective Neuroscience. 2016;11(5):775–782. doi: 10.1093/scan/nsw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Di X, Huang J, Biswal BB. Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions. Brain Structure and Function. 2017;222(1):619–634. doi: 10.1007/s00429-016-1239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2007;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Measuring facial movement. Environmental Psychology and Nonverbal Behavior. 1976;1(1):56–75. doi: 10.1007/BF01115465. [DOI] [Google Scholar]
- Elliott DS, Huizinga D, Ageton SS. Explaining delinquency and drug use. Sage Publications; 1985. Retrieved from http://agris.fao.org/agris-search/search.do?recordID=US201300630571. [Google Scholar]
- Etkin A. Neurobiology of Anxiety: From Neural Circuits to Novel Solutions? Depression and Anxiety. 2012;29(5):355–358. doi: 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of Anterior Cingulate Activation and Connectivity With the Amygdala During Implicit Regulation of Emotional Processing in Generalized Anxiety Disorder. American Journal of Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, et al. What is a representative brain? Neuroscience meets population science. Proceedings of the National Academy of Sciences. 2013;110(44):17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, et al. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30(4):1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Abbamonte M. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience: JPN. 2009;34(6):418. [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Luby JL. Amygdala reactivity to sad faces in preschool children: An early neural marker of persistent negative affect. Developmental Cognitive Neuroscience. 2016;17:94–100. doi: 10.1016/j.dcn.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AM, Waller R, Shaw DS, Forbes EE, Hariri AR, Hyde LW. The long reach of early adversity: Parenting, stress, and neural pathways to antisocial behavior in adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017 doi: 10.1016/j.bpsc.2017.06.005. [DOI] [PMC free article] [PubMed]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/S0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Goldstein-Piekarski AN, Korgaonkar MS, Green E, Suppes T, Schatzberg AF, Hastie T, Williams LM. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proceedings of the National Academy of Sciences. 2016;113(42):11955–11960. doi: 10.1073/pnas.1606671113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Snyder HR, Gulley LD, Schweizer TH, Bijttebier P, Nelis S, Vasey MW. Understanding comorbidity among internalizing problems: Integrating latent structural models of psychopathology and risk mechanisms. Development and Psychopathology. 2016;28(4pt1):987–1012. doi: 10.1017/S0954579416000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. Looking inside the disordered brain. 1. Sunderland, MA, USA: Sinauer Associates, Incorporated; 2015. [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The Amygdala Response to Emotional Stimuli: A Comparison of Faces and Scenes. NeuroImage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behavioral and Brain Sciences. 2010;33(2–3):61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Hyde LW. Developmental psychopathology in an era of molecular genetics and neuroimaging: A developmental neurogenetics approach. Development and Psychopathology. 2015;27(02):587–613. doi: 10.1017/S0954579415000188. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, Forbes EE. Dissecting the Role of Amygdala Reactivity in Antisocial Behavior in a Sample of Young, Low-Income, Urban Men. Clinical Psychological Science. 2015:1–18. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural Interaction of the Amygdala with the Prefrontal and Temporal Cortices in the Processing of Facial Expressions as Revealed by fMRI. Journal of Cognitive Neuroscience. 2001;13(8):1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, Pizzagalli DA. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychological Medicine. 2017:1–13. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler Ronald C, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Kendler KS. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States: Results From the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York, NY: Guilford Press; 1998. [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting Comorbidity: A Model-Based Approach to Understanding and Classifying Psychopathology. Annual Review of Clinical Psychology. 2006;2(1):111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger I, Dobel C, Dannlowski U, Kugel H, Grotegerd D, Kissler J, Zwanzger P. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research. 2012;233(2):508–516. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012;121(4):971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lu Q, Li H, Luo G, Wang Y, Tang H, Han L, Yao Z. Impaired prefrontal–amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: A dynamic causal modeling study on MEG. Neuroscience Letters. 2012;523(2):125–130. doi: 10.1016/j.neulet.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. The American Journal of Psychiatry. 2007 doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed]
- Marusak HA, Zundel C, Brown S, Rabinak CA, Thomason ME. Is neutral really neutral? Converging evidence from behavior and corticolimbic connectivity in children and adolescents. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw182. Retrieved from https://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/pubmed/28008076. [DOI] [PMC free article] [PubMed]
- Mattson WI, Hyde LW, Shaw DS, Forbes EE, Monk CS. Clinical neuroprediction: Amygdala reactivity predicts depressive symptoms 2 years later. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw018. nsw018. [DOI] [PMC free article] [PubMed]
- McCartney K, Burchinal MR, Bub KL. Best practices in quantitative methods for developmentalists. Monographs of the Society for Research in Child Development. 2006 doi: 10.1111/j.1540-5834.2006.07103001.x. i–145 . [DOI] [PubMed]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20(04):1231. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Pine DS. Amygdala and Ventrolateral Prefrontal Cortex Activation to Masked Angry Faces in Children and Adolescents With Generalized Anxiety Disorder. Archives of General Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Forbes EE. Physiological and behavioral engagement in social contexts as predictors of adolescent depressive symptoms. Journal of Youth and Adolescence. 2013;42(8):1117–1127. doi: 10.1007/s10964-012-9815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Olino TM, Musselman SC, Kurapati NT, Forbes EE. History of depression and frontostriatal connectivity during reward processing in late adolescent boys. Journal of Clinical Child & Adolescent Psychology. 2016;45(1):59–68. doi: 10.1080/15374416.2015.1030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry. 2015;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus Version 7 user’s guide. Los Angeles, CA: Muthén & Muthén; 2006. [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry. 2012;17(2):132. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Whalen PJ. The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Science. 2010 doi: 10.1177/0956797610373934. Retrieved from http://pss.sagepub.com.proxy.lib.umich.edu/content/early/2010/06/08/0956797610373934.abstract. [DOI] [PMC free article] [PubMed]
- Nomura M, Iidaka T, Kakehi K, Tsukiura T, Hasegawa T, Maeda Y, Matsue Y. Frontal lobe networks for effective processing of ambiguously expressed emotions in humans. Neuroscience Letters. 2003;348(2):113–116. doi: 10.1016/S0304-3940(03)00768-7. [DOI] [PubMed] [Google Scholar]
- Oatley K, Johnson-Laird PN. Towards a cognitive theory of emotions. Cognition and Emotion. 1987;1(1):29–50. [Google Scholar]
- Obradovic J, Burt KB, Masten AS. Pathways of Adaptation from Adolescence to Young Adulthood: Antecedents and Correlates. Annals of the New York Academy of Sciences. 2006;1094(1):340–344. doi: 10.1196/annals.1376.046. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion: Functional imaging studies of emotion regulation. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38(5):784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan LK. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety. 2013;30(3):234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F. Prefrontal Mechanisms in Extinction of Conditioned Fear. Biological Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37(10):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Robins LN, Regier DA. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, NY: Free Press; 1991. [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2009 doi: 10.1002/hbm.20854. NA-NA. [DOI] [PMC free article] [PubMed]
- Sato W, Kochiyama T, Uono S, Yoshikawa S, Toichi M. Direction of Amygdala-Neocortex Interaction During Dynamic Facial Expression Processing. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw036. bhw036. [DOI] [PubMed] [Google Scholar]
- Scheuer H, Alarcón G, Demeter DV, Earl E, Fair DA, Nagel BJ. Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Research: Neuroimaging. 2017;266(Supplement C):35–41. doi: 10.1016/j.pscychresns.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Sameroff AJ, Cicchetti D. The transition to adulthood as a critical juncture in the course of psychopathology and mental health. Development and Psychopathology. 2004;16(04):799–806. doi: 10.1017/S0954579404040015. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Development and Psychopathology. 2012;24(03):871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Yao S. Comparison of activation patterns between masking and inattention tasks: a coordinate-based meta-analysis of implicit emotional face processing. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased Amygdala and Decreased Dorsolateral Prefrontal BOLD Responses in Unipolar Depression: Related and Independent Features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo D, Kaiser M, Hilgetag C. Organization, development and function of complex brain networks. Trends in Cognitive Sciences. 2004;8(9):418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA. The human amygdala. Guilford Press; 2009. Retrieved from https://books.google.com/books?hl=en&lr=&id=275mEq72pkUC&oi=fnd&pg=PA3&dq=phelps+and+whalen&ots=nbpSj5yHAM&sig=s_CW_nOM58WzxSE1h97eBVVSwbc. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nature Neuroscience. 2017;20(3):365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Lahey BB. Implications of the Hierarchical Structure of Psychopathology for Psychiatric Neuroimaging. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(4):310–317. doi: 10.1016/j.bpsc.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.