Abstract
Objectives
the purpose of this study was to determine whether a commercial formulation of hypochlorous acid hygiene solution (0.01%), Avenova™, can destroy existing biofilms formed by ocular clinical bacterial isolates, including blepharitis isolates of Staphylococcus aureus and coagulase-negative staphylococci (CNS), and a keratitis isolate of Pseudomonas aeruginosa.
Methods
biofilms grown in bacterial growth media on disposable contact lens cases were challenged with hypochlorous acid hygiene solution. At various time points, surviving bacteria were quantified by serial dilution and colony counts. S. aureus biofilms formed on glass were challenged using a hypochlorous acid hygiene solution, and imaged using vital staining and confocal laser scanning microscopy.
Results
bactericidal activity (≥3 Log10; 99.9%) was observed for all tested bacterial species after a 30-minute exposure. S. aureus biofilms had a bactericidal level of killing by 10 minutes (p<0.01), S. capitis by 5 minutes (p<0.001), S. epidermidis by 30 minutes (p<0.001), and P. aeruginosa by 10 minutes (p<0.01). Confocal microscopy and crystal violet staining analysis of bacterial biofilms treated with hypochlorous acid solution both demonstrated that biofilm bacteria were readily killed, but biofilm structure was largely maintained.
Conclusions
hypochlorous acid (0.01%) hygiene solution was able to achieve bactericidal levels of killing of bacteria in biofilms, but did not disrupt biofilm structures. Susceptibility of tested staphylococcal blepharitis isolates varied by species, with S. capitis being the most susceptible and S. epidermidis being the least susceptible.
Keywords: hypochlorous acid, biofilm, antibiotic, blepharitis, keratitis, ocular infection, contact lens, contact lens case
Introduction
Blepharitis, or inflammation of the eyelids, is a chronic ocular surface disease1–2. Patients present with significant discomfort and symptoms that include redness, itching, burning, foreign body sensations, and blurred vision2. Blepharitis is quite common1–2; one study estimated that 37–47% of patients seen by ophthalmologists and optometrists have blepharitis1. Though bacteria may or may not initiate blepharitis, the oily and inflamed eyelid margins of blepharitis patients are frequently colonized by bacteria, predominantly by Gram-positive bacteria including species of the Staphylococcus, Propronibacterium, and Corynebacterium genera3,4–5. A bioinformatic study that used 16S rRNA analysis on eyelash and tear samples from blepharitis versus normal patients (n = 7 and 4 respectively) came to a largely consistent conclusion, and recorded a relative increase in bacteria from the Staphylococcus and Corynebacterium genera from the eyelids and tears of blepharitis patients6. Another recent study of 36 patients used MALDI-TOF and 16s rRNA analysis to identify bacteria from the eyelid skin of blepharitis patients7; 14 genera of bacteria were isolated with Staphylococcus species being the most prevalent.
It is hypothesized that lid colonization leads to local inflammation8–9. Chronic recurrent inflammation and antibiotic tolerance are hallmarks of biofilm-associated infections. Because of this and the increased bacterial burdens of lids and lashes of blepharitis patients, it has been hypothesized that infectious blepharitis is a biofilm-associated disease10–11. Current treatment routines include cleaning the eyelids, the use of warm compresses, and often the use of topical antibiotics2. However, many individuals with blepharitis require long-term therapy as blepharitis is chronic and relief of symptoms may be temporary. A new therapeutic approach for the treatment of blepharitis is the use of stabilized hypochlorous acid (HOCl). Avenova™, an FDA cleared device previously known as I-Lid Cleanser, has a proprietary pure formulation of 0.01% HOCl as the active ingredient7. In nature HOCI is formed by polymorphonuclear neutrophils to kill microorganisms12, and commercial formulations have been shown to be useful in treatment of non-healing wounds that are also associated with chronic infection by staphylococci and other microbes13. Previous studies indicate that HOCl can kill bacteria in biofilms14–17; however, clinical blepharitis isolates were not tested. The goal of this study was to test whether a clinical formulation of HOCl is effective in killing bacteria in biofilms formed by ocular clinical isolates.
Materials and Methods
Bacterial culture, biofilm formation, and susceptibility analysis
Bacterial isolates were confirmed clinical samples obtained at the Charles T. Campbell Ophthalmic Microbiology Laboratory at the UPMC Eye Center. Bacterial strains were from blepharitis18 or keratitis patients19. Bacteria were maintained in frozen glycerol stocks at −80°C, streaked out onto blood agar plates (tryptic-soy agar with 5% sheep erythrocytes – Remel, Lenexa, KS). Bacterial single colonies were used to inoculate 5 ml cultures of brain heart infusion broth (BHI) for staphylococci or lysogeny broth (LB) for P. aeruginosa. Bacteria were diluted in BHI + glucose (0.2% v/v) for staphylococci or LB for P. aeruginosa to an OD600 = 0.01. Bacteria (0.4 ml) were introduced into contact lens cases and incubated at 37°C for 24 hours. Contact lens cases were Deepwell lens cases obtained from Beitler McKee Optical Company, Pittsburgh, PA. Non-biofilm bacteria were removed by aspiration and the remaining biofilms were washed twice with 0.5 ml of with phosphate buffered saline (PBS, pH 8). Biofilms were challenged with 0.5 ml of Avenova™ or azithromycin (1%) submerging the biofilms for 1, 5, 10, or 30 minutes. Biofilms were washed with 0.5 ml of PBS to remove HOCl or azithromycin, and colony forming units (CFU) were determined by serial dilution. Avenova™ ophthalmic hygiene solution was provided by NovaBay Pharmaceuticals, Inc. Emeryville CA. Avenova™ consists of HOCl-preserved (0.01%) saline solution, referred to here as HOCl solution. As a negative control, biofilms were incubated with 0.5 ml PBS for 30 minutes. Bacteria were removed from the CL cases using Dacron swabs and CFU were determined by colony counts on LB agar plates. Experiments were performed with 5 or more independent replicates for each time point and isolate on three or more separate occasions. CFU were enumerated and Log10 transformed; the absence of bacteria was recorded at the limit of detection rather than zero, as noted in the figure legend. Experiments were performed on several different days and the means of the CFU were compared using One Way ANOVA with Tukey’s post-test.
Alternatively, biofilms were stained and fixed with crystal violet (0.1% v/v) for 10 minutes, air dried, and imaged by using a digital scanner, or evaluated by confocal laser scanning microscopy (noted below).
Microscopy
S. aureus B1412 biofilms were formed on glass bottomed Mattek plates in BHI + glucose medium as described above. Planktonic bacteria were removed by washing biofilms twice with PBS and biofilms were challenged with HOCl solution or PBS as a non-toxic control for 30 minutes and washed twice with PBS. Biofilms were stained with LIVE/DEAD BacLight bacterial viability kit (ThermoFisher, Pittsburgh, PA) following the manufacturer’s recommended concentrations for 30 minutes. Biofilms were imaged with a 60× oil immersion objective using an Olympus IX-81 inverted microscope with an FV-1000 laser scanning confocal system (Olympus) and FluoView imaging software. Experiments were performed twice (n=4 per group).
Results
Impact of HOCl solution on biofilms formed by blepharitis isolates of Staphylococcus aureus and coagulase negative staphylococci
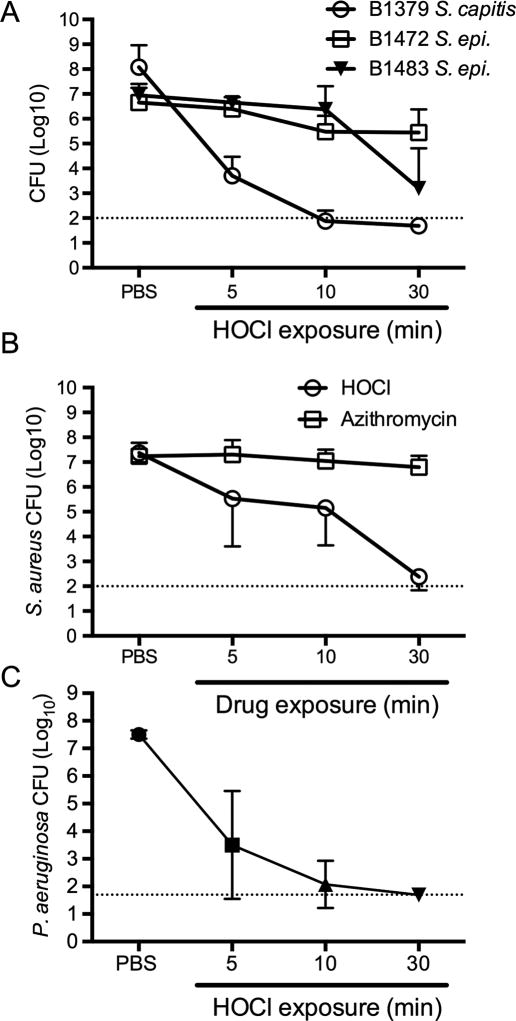
Blepharitis is associated with colonization of eyelashes and eyelids by bacteria of the Staphylococcus genus2. Biofilms were formed on contact lens cases for 24 hours and then challenged with HOCl at 5, 10, and 30 minutes; biofilms were washed and surviving CFU were measured. PBS was used in lieu of HOCl solution as a negative control. Coagulase negative staphylococci were initially tested. S. capitus strain B1379 biofilms were highly susceptible to HOCl solution with a bactericidal (≥3-Log10 killing) effect observed as early as 5 minutes (Figure 1A). S. epidermidis strain B1483 biofilms were less susceptible to HOCl solution, but were still significantly reduced by over 3 Log10 units at 30 minutes (Figure 1A). S. epidermidis strain B1472 also showed limited susceptibility with a significant (p<0.05) 1.2 Log10 reduction in viable CFU detected by 30 minutes (Figure 1A).
Figure 1. Effect of HOCl acid hygiene solution on the viability of bacteria in biofilms.
A. Surviving coagulase negative staphylococci following challenge with HOCl or PBS (negative control) for the times indicated. B. Surviving bacteria from S. aureus strain B1412 biofilms that were challenged with either HOCl (n=8) or azithromycin (1% w/v, n≥6). C. Surviving bacteria from HOCl challenged biofilms of P. aeruginosa strain K900 (n≥5). For all charts, means and standard deviations are shown. Dotted lines indicate the limit of detection.
We then tested whether a shorter HOCl challenge could reduce the viability of bacteria in biofilms using the most susceptible species tested above, S. capitis B1379 and P. aeruginosa K900. A significant 1.5 Log10 reduction in biofilm CFU (p < 0.0001, Student’s T-test) was measured from S. capitis biofilms treated with HOCl compared to the PBS control after a 60 second exposure (Figure 2A).
Figure 2. Antimicrobial effect of a 60 second exposure of HOCl acid hygiene solution on biofilm bacteria viability.
A. Surviving S. capitis following challenge with HOCl or PBS (negative control) for 60 seconds (n=8). B. Surviving P. aeruginosa following challenge with HOCl or PBS (negative control) for 60 seconds (n=14). Means and standard deviations are shown.
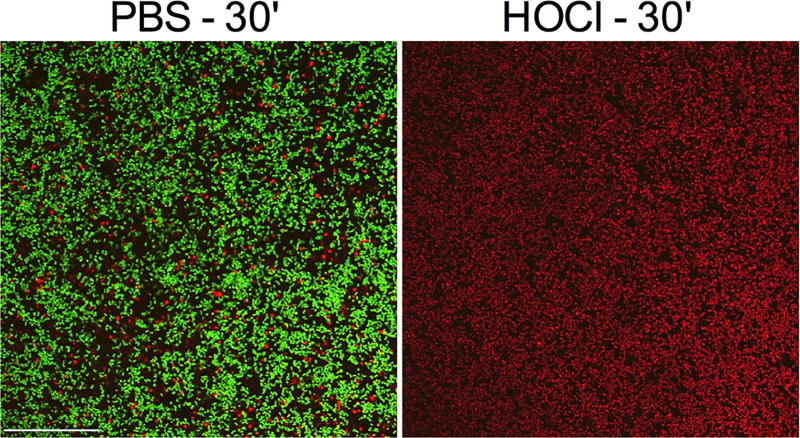
S. aureus strain B1412 biofilms were significantly reduced by HOCl solution by as early as 5 minutes of exposure, with a bactericidal effect measured at 30 minutes (Figure 1B). Confocal laser scanning microscopy was used to image HOCl solution and PBS treated S. aureus B1412 biofilms (Figure 3). Live-dead staining of the biofilm supports the CFU analysis, with the HOCl solution treated cells strongly taking up propidium iodide, whereas the PBS treated control biofilms remained viable with minimal propidium iodide staining (Figure 3). The same strain was similarly tested with azithromycin (1%), since macrolides are commonly used to treat blepharitis. B1412 was previously characterized as susceptible to azithromycin with an MIC of 1 µg/ml18. Whereas azithromycin is effective against strain B1412 in a planktonic state18, it was unable to effectively kill S. aureus B1412 biofilm bacteria in vitro, with less than a 0.5 Log10 reduction measured after 30 minutes of exposure (Figure 1B).
Figure 3. S. aureus B1412 biofilms are killed by HOCl acid hygiene solution.
Biofilms exposed to PBS as a negative control or HOCl for 30 minutes were stained with a LIVE/DEAD stain consisting of propidium idodide and Syto 9. Biofilms were imaged by confocal laser scanning microscopy. Representative images show the clear loss of viability following HOCl treatment. Bar = 50 µm.
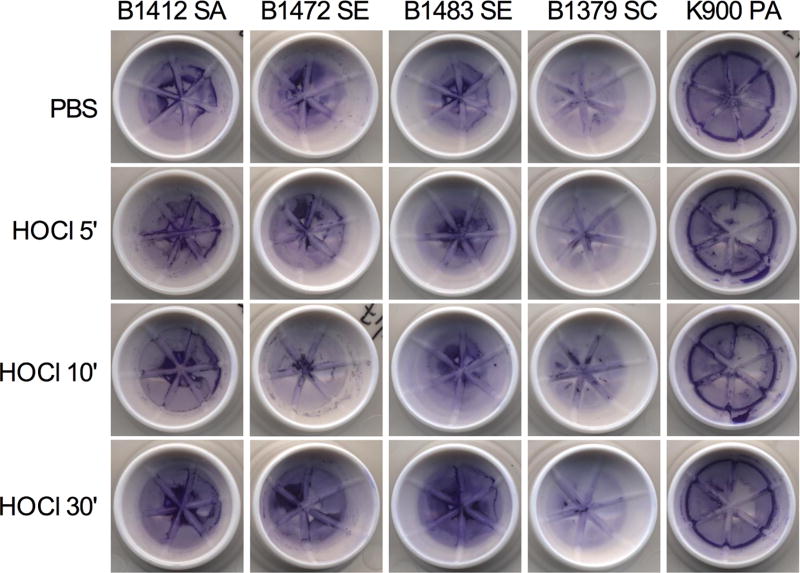
Crystal violet staining of biofilms can be used to measure biofilm biomass. Crystal violet staining of Strain B1412 biofilms reveal that while the bacteria within the biofilm are killed, 30 minutes of HOCl solution did not disrupt gross biofilm structure (Figure 4). Similar results were observed with tested coagulase negative staphylococcal strains (Figure 1).
Figure 4. HOCl acid hygiene solution does not disrupt biofilms formed by clinical ocular isolates.
Crystal violet stained biofilms were similar with and without HOCl treatment for up to 30 minutes (30'); PBS served as a negative control. Representative biofilms from the blepharitis isolates and one P. aeruginosa keratitis isolate (K900) used in this study on the contact lens cases used in this study. SA = S. aureus, SE = S. epidermidis, SC = S. capitus, PA = P. aeruginosa.
Impact of HOCl solution on biofilms formed by a keratitis isolate of Pseudomonas aeruginosa
Whereas P. aeruginosa is frequently associated with contact lens-associated keratitis, it can infrequently cause blepharitis and blepharoconjunctivitis20–21. Moreover, P. aeruginosa biofilms are well known to be highly resistant to antimicrobial therapy22. P. aeruginosa keratitis isolate K900 biofilms were challenged by HOCl solution in vitro and were found to be highly susceptible to HOCl solution killing, with a 4 Log10 CFU reduction after 5 minutes of treatment, and the elimination of any detectable viable cells by 30 minutes (Figure 1C). P. aeruginosa K900 biofilms treated with HOCl were reduced 2.8 Log10 compared to biofilms treated with the PBS control after a 60 second exposure (p < 0.0001, Student’s T-test) (Figure 2B). Like what was observed with S. aureus strain B1412, P. aeruginosa K900 biofilms were not structurally destroyed by HOCl solution (Figure 4).
Discussion
This study demonstrates that bacteria within biofilms formed by blepharitis isolates of the Staphylococcus genus are killed by HOCl solution. The effect varied by species, with S. capitis and S. aureus being more susceptible than S. epidermidis. As S. epidermidis is part of the healthy skin flora and S. aureus is a more pathogenic species that transiently colonizes humans, the ability of HOCl solution to selectively kill S. aureus while maintaining S. epidermidis may be beneficial. P. aeruginosa keratitis isolate K900 biofilm bacteria were particularly susceptible HOCl solution, with a 2.8 Log10 reduction of biofilm CFU with a 60 second exposure.
Bacterial biofilms are notoriously recalcitrant to antimicrobial therapy and this is due to differential expression of genes in biofilms, different microenvironments within biofilms that reduce the activity of antibiotics, the presence of persister cells, and a large number of non-dividing bacteria in a low metabolic state10, 22–23. This phenomenon of biofilm antimicrobial tolerance was exemplified in this study where the S. aureus strain B1412 that is susceptible to azithromycin (MIC of 1 µg/ml for planktonic cells)18, was almost completely resistant to azithromycin at 10,000 µg/ml when in a biofilm. This may not be particularly surprising as azithromycin is bacteriostatic for susceptible S. aureus and 30 minutes is not a long time for an antibiotic to work, but the differences between biofilm susceptibility to an anti-infective such as HOCI and an antibiotic were clearly demonstrated in Figure 1B.
In this study contact lens cases were used. While the primary focus was as a proof of principle test as to whether biofilms formed by blepharitis isolates on a given surface are susceptible to HOCl, data suggest that there is a potential application to kill bacteria on contact lenses using this disinfectant, where a 30 minute or longer application is recommended. This study demonstrates that bacteria in biofilms were killed by HOCl solution; however, bacterial biofilms formed by blepharitis isolates remained structurally intact as demonstrated by confocal microscopy and crystal violet staining. Therefore, mechanical measures, such as washing or scrubbing, are likely required to remove the killed bacterial cells from surfaces. For example, eyelid scrubs are a typically prescribed intervention for blepharitis patients2 and should be effective in the mechanical removal of killed bacteria.
Beyond blepharitis, it is possible that hypochlorous acid could be used for prophylaxis prior to ocular surgeries and as an alternative or in addition to other anti-infective agents such as povidone-iodine. Additional experimentation to evaluate the use of HOCI to prevent endophthalmitis is warranted.
In summary, this study indicates that bacteria in biofilms formed by tested ocular pathogens, particularly those from blepharitis patients, can be killed by HOCI if left in contact for sufficient time.
Acknowledgments
Sources of financial support: NovaBay Pharmaceuticals, Inc., Research to Prevent Blindness, Inc., New York, New York; Eye and Ear Foundation of Pittsburgh, NIH grants EY024785 and EY08098;
Footnotes
Material Disclosures: Avenova™ Daily Lid & Lash Hygiene Solution was kindly donated by NovaBay, Pharmaceuticals, Inc; None of the authors has a financial or proprietary interest in any material or method mentioned.
References
- 1.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7:S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 2.Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012:CD005556. doi: 10.1002/14651858.CD005556.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty JM, McCulley JP. Comparative bacteriology of chronic blepharitis. Br J Ophthalmol. 1984;68:524–8. doi: 10.1136/bjo.68.8.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCulley JP, Dougherty JM. Bacterial aspects of chronic blepharitis. Trans Ophthalmol Soc U K. 1986;105(Pt 3):314–8. [PubMed] [Google Scholar]
- 5.Groden LR, Murphy B, Rodnite J, Genvert GI. Lid flora in blepharitis. Cornea. 1991;10:50–3. [PubMed] [Google Scholar]
- 6.Lee SH, Oh DH, Jung JY, Kim JC, Jeon CO. Comparative ocular microbial communities in humans with and without blepharitis. Invest Ophthalmol Vis Sci. 2012;53:5585–93. doi: 10.1167/iovs.12-9922. [DOI] [PubMed] [Google Scholar]
- 7.Stroman DW, Mintun K, Epstein AB, Brimer CM, Patel CR, Branch JD, Najafi-Tagol K. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707–714. doi: 10.2147/OPTH.S132851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber-Spitzy V, Baumgartner I, Bohler-Sommeregger K, Grabner G. Blepharitis--a diagnostic and therapeutic challenge. A report on 407 consecutive cases. Graefes Arch Clin Exp Ophthalmol. 1991;229:224–7. doi: 10.1007/BF00167872. [DOI] [PubMed] [Google Scholar]
- 9.Boto-de-los-Bueis A, del Hierro Zarzuelo A, Garcia Perea A, de Pablos M, Pastora N, Noval S. Staphylococcus aureus blepharitis associated with multiple corneal stromal microabscess, Stromal Edema, and Uveitis. Ocul Immunol Inflamm. 2015;23:180–3. doi: 10.3109/09273948.2013.870214. [DOI] [PubMed] [Google Scholar]
- 10.Kowalski RP, Romanowski EG, Shanks RM. The surprising power of bacterial biofilms. Topics in Ocular Antiinfectives. 2013;44:1–4. [Google Scholar]
- 11.Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clin Ophthalmol. 2016;10:2455–2467. doi: 10.2147/OPTH.S114674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Nauseef WM. Salt, chloride, bleach, and innate host defense. J Leukoc Biol. 2015;98:163–72. doi: 10.1189/jlb.4RU0315-109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crew J, Varilla R, Rocas TA, Debabov D, Wang L, Najafi A, Rani SA, Najafi RR, Anderson M. NeutroPhase((R)) in chronic non-healing wounds. Int J Burns Trauma. 2012;2:126–34. [PMC free article] [PubMed] [Google Scholar]
- 14.Luppens SB, Reij MW, van der Heijden RW, Rombouts FM, Abee T. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl Environ Microbiol. 2002;68:4194–200. doi: 10.1128/AEM.68.9.4194-4200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandvik EL, McLeod BR, Parker AE, Stewart PS. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS One. 2013;8:e55118. doi: 10.1371/journal.pone.0055118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Atanasio N, Capezzone de Joannon A, Mangano G, Meloni M, Giarratana N, Milanese C, Tongiani S. A New Acid-oxidizing Solution: Assessment of Its Role on Methicillin-resistant Staphylococcus aureus (MRSA) Biofilm Morphological Changes. Wounds. 2015;27:265–73. [PubMed] [Google Scholar]
- 17.Almatroudi A, Gosbell IB, Hu H, Jensen SO, Espedido BA, Tahir S, Glasbey TO, Legge P, Whiteley G, Deva A, Vickery K. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: implications for infection control. J Hosp Infect. 2016;93:263–70. doi: 10.1016/j.jhin.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Wu EC, Kowalski RP, Romanowski EG, Mah FS, Gordon YJ, Shanks RM. AzaSite(R) inhibits Staphylococcus aureus and coagulase-negative Staphylococcus biofilm formation in vitro. J Ocul Pharmacol Ther. 2010;26:557–62. doi: 10.1089/jop.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalski RP, Romanowski EG, Mah FS, Shanks RM, Gordon YJ. Topical levofloxacin 1.5% overcomes in vitro resistance in rabbit keratitis models. Acta Ophthalmol. 2010;88:e120–5. doi: 10.1111/j.1755-3768.2010.01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John MA, Austin TW, Bombassaro AM. Pseudomonasaeruginosa blepharitis in a patient with vancomycin induced neutropenia. Can J Infect Dis. 1996;7:63–5. doi: 10.1155/1996/301396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenoff SH, Wolf ML, Chabner BA. Pseudomonas blepharoconjunctivitis: a complication of combination chemotherapy. Arch Ophthalmol. 1974;91:490–1. doi: 10.1001/archopht.1974.03900060504016. [DOI] [PubMed] [Google Scholar]
- 22.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 23.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]