Abstract
Bacillus cereus strain Jdm1 was isolated and tested for activity as a biocontrol agent to suppress Meloidogyne incognita. Petri dish test results indicated that Jdm1 culture supernatant significantly inhibited the second-stage juvenile (J2) activity and egg hatching, and also decreased the number of galls on tomato roots in the pot test. Control efficiency reached 43%, with improved growth compared to control plants. In field tests, control efficacies were greater than 50% 30 day post-inoculation, before decreasing. Furthermore, when avermectins were included to manage M. incognita, the yield of tomatoes was increased significantly. The effect of Jdm1 on the bacterial community in the tomato rhizosphere soil was monitored using PCR-denaturing gradient gel electrophoresis (PCR-DGGE) on field plants. DGGE band patterns and principal component analysis showed that application of Jdm1 did not permanently imperil the bacterial community, which recovered soon after inoculation, despite being impacted initially. The plant growth stage had a much greater influence on the bacterial community in tomato rhizosphere soil.
Keywords: Bacillus cereus strain Jdm1, Biocontrol agent, Meloidogyne incognita, PCR-DGGE, Principal component analysis, Bacterial community
Introduction
Plant parasitic nematodes including root-knot nematodes (RKN) cause global losses to crop plants of up to 125 billion US dollars per year in the tropics (Chitwood 2003; Palomares-Rius et al. 2017). Meloidogyne incognita was the dominant nematode species and the primary destructive force, causing more than 10% losses in the yields of the major tomato production regions in China and worldwide (Topp et al. 1998; Xu et al. 2004; Li et al. 2018). Soil fumigants, organophosphate, and carbamate nematicides have been reported to effectively control RKN (Noling and Becker 1994). However, with an increased appreciation of the harmful physiological effects of chemical pesticides and the changing public attitude towards environmental pollution, alternative management strategies for protecting crops from RKN infestations are currently being investigated (Oka et al. 2000). Using biocontrol agents (BCA) such as antagonistic microorganisms is one potential alternative to chemical nematicides.
Bacteria have been used extensively as BCAs due to their rapid replication and metabolic productivity compared with fungi. Recently, rhizobacteria such as Bacillus cereus group isolates, including B. cereus and B. thuringiensis, from diverse soils were reported to exhibit extreme virulence to RKN. Gokte and Swarup (1988) earlier reported that B. subtilis, B. pumilis, B. cereus, and two species of Pseudomonas were larvicidal on cyst and RKN. B. cereus could inhibit root penetration of Meloidogyne spp. by efficiently colonizing the rhizosphere soil and the root surfaces of tomato. As a result, the number of galls, egg masses, and eggs on roots was significantly decreased (Oka et al. 1993; Xiao et al. 2012). Recent research found that B. cereus increased the levels of certain defense compounds (Siahpous et al. 2011) or stimulated induced systemic resistance (ISR) (Niu et al. 2012) in plants to control RKN and other pathogens. Some B. cereus strains have also been found to promote plant growth (Jetiyanon et al. 2008).
Besides their use as BCAs, inoculation of Bacillus spp. into the soil can cause changes in the composition and structure of the microbial communities, which may negatively impact the efficiency of bio-fertilizers applied to plant roots (Ramos et al. 2003). Fingerprints produced by PCR-denaturing gradient gel electrophoresis (PCR-DGGE) allow spatial and temporal comparisons of soil microbial communities within and between locations or among treatments (Nakatsu 2007). Bacteria are particularly abundant in the root zone, and are known to be influenced by a range of biotic and abiotic factors (Buckley and Schmidt 2002).
The objectives of this study were: (1) to evaluate the efficacy of B. cereus strain Jdm1 as a BCA against M. incognita and (2) to assess the impact of Jdm1 on the indigenous bacterial communities in the rhizosphere soil of field-grown tomato.
Materials and methods
B. cereus strain Jdm1
The B. cereus strain Jdm1 was isolated by the Nematology Lab of the Institute of Plant Protection, Jiangsu Academy of Agricultural Science (JAAS), and cultured on modified Potato Dextrose Agar (PDA) medium (200 g/l, 10 g/l glucose, 10 g/l agar, pH 7.2) as described by Wei et al. (2010). Jdm1 (Gene bank number: KF001838) was not a human pathogenic strain by comparing with other B. cereus strain in terms of the morphological and biochemical characteristics, and 16S rRNA evolutionary relationship. The strain had high of starch, glutin, and casein hydrolytic activities, and was registered in the China General Microbiological Culture Collection Center and assigned as CGMCC 7073.
Bacterial preparation
A single colony of Jdm1 was picked and cultured in modified potato dextrose (PD) broth (200 g/l, 10 g/l glucose, and pH 7.2) and incubated at 30 °C with shaking (180 rpm) for 24 h. Following centrifugation at 8000 rpm for 90 min, the supernatant was filtrated through a Millipore membrane filter (0.22 µm) and stored at 4 °C until needed. For the Petri dish test, bacterial cells were washed twice with sterile distilled water, adjusted to 107 CFU/ml, and stored at 4 °C until needed for the pot test. Jdm1 cultured in PD broth (105 CFU/ml) was used directly for field tests.
For a comparative study of the biocontrol efficacy of Jdm1 against M. incognita in field tests, avermectin B1 (North China Pharmaceutical Company Aino Co., Ltd.) with a water solubility of approximately 9 ppm was used.
Nematode preparation
Meloidogyne incognita, originally isolated from the Chinese medicinal herb Trichosanthes kirilowii, was cultured and maintained on tomatoes (Solanum lycopersicum, cultivar ‘Shanghai Cooperation 903’). Extraction and hatching of eggs were performed using a modified protocol described by Atamian et al. (2012). Suspensions containing approximately 100 J2 worms or 1000 eggs per ml were used.
Petri dish test
To determine the effects of Jdm1 filtrate on J2 activity and egg hatching, 2 ml of the culture supernatant was added to a Petri dish (4 cm in diameter) with 1 ml of J2 suspension or 1 ml of egg suspension. Dishes were incubated in the dark at room temperature. As a control, sterile-modified PD broth was used. All treatments were replicated three times. The mortality of J2 was calculated every 12 h using a stereomicroscope. The number of hatched J2 were counted after 24, 48, and 72 h of incubation, and inhibition of egg hatching was calculated according to the formula: IE (%) = (C − T)/C × 100, where IE represents the inhibition of the egg hatching, T represents the egg hatching ability in the treatment group, and C represents the egg hatching ability in the control group.
Pot test
Tomato seeds were surface-sterilized with 2% sodium hypochlorite for 2 min and rinsed three times with sterile water. Seeds were sown in a single pot in steam-sterilized sandy soil (soil:sand:organic mix in a 2:2:1 v:v:v ratio) and maintained at 22–28 °C in a greenhouse with a relative humidity of 30% and a 16 h/8 h day/night photoperiod. One week after germination, seedlings were transplanted individually into pots (15 cm in diameter, 11 cm in height) filled with sterilized sandy soil.
Two weeks after transplanting, the soil around the roots of each tomato plant was drenched with 40 ml of Jdm1 suspension (an equal volume of sterile water was applied to the controls). Two days after application of Jdm1, 600 freshly hatched J2 worms were pipetted into three holes surrounding the root zone of each tomato plant. Each treatment was repeated three times, and each replicate included 15 plants. The pots were arranged in a complete randomized design and maintained under the same conditions as described above for 7 weeks. Whole plants were then carefully removed from the pots and root systems were washed to remove soil. The lengths, fresh, and oven-dried weights (70 °C for 72 h) of the plant shoots and roots were measured. Gall indices were assessed using the 0–10 grade (Bridge and Page 1980), and disease severity and control efficacy were calculated as follows:
Field test
A field test with sandy soil was conducted in Shang-qiu (34°30′N, 115°39′E), Henan Province. Tomato seeds were sown in soil on 15th July 2012, and the tomato seedlings were kept at a density of 100 plants per m2 until transplantation. When transplanting, tomato seedlings were irrigated with either 500 ml (1.5 × 106 CFU/ml) of strain Jdm1 cultured in PD broth, avermectins solution (9 ppm), or both (250 ml of each). In the control treatment, an equal volume of water was used. Each treatment included four replicate plots, each of which contained three ridges (0.6 m × 8 m) interspersed by furrows (0.5 m). Twenty-five tomato seedlings were transplanted in each of the two parallel lines along the margins of each ridge. All plots were arranged randomly. Plant heights and gall indices of each plot were recorded at 30, 60, and 90 day post-inoculation, and the disease severity and control efficacy were calculated as described above for the pot test. The weight of tomato fruits was recorded immediately after picking, and the cumulative yields were calculated when all fruits were harvested. At 15, 30, and 60 day post-inoculation, 15 plants and soil were removed from each pot, and only soil adhered to the roots was considered to be rhizosphere soil. Rhizosphere soil was collected by shaking from the roots (Xu et al. 2009). After sieving (2 mm), each composite soil sample was immediately placed into autoclaved microcentrifuge tubes and stored at − 70 °C until needed for PCR-DGGE.
DNA extraction and PCR-DGGE
Total soil DNA was extracted from approximately 500 mg of rhizosphere soil with a Fast DNA SPIN Kit for Soil (Qbiogene, Carlsbad, CA, USA) following the manufacturer’s protocol. PCR amplification of partial bacterial 16S rRNA was performed with primer pair F984-GC/R1378 (Heuer et al. 1997) according to the methods previously described (Marschner et al. 2001). DGGE was performed using an 8% (w/v) polyacrylamide gel with 35–55% denaturant gradient run in a 1 × TAE (Tris–acetate–EDTA) buffer on a DCode universal mutation detection system (Bio-Rad, USA). Electrophoresis was initiated by pre-running at a voltage of 200 V for 10 min and then at a constant voltage of 80 V for 13 h at 60 °C. After electrophoresis, gels were silver-stained according to the method of Sanguinetti et al. (1994).
Data analysis
Data were analyzed using ANOVA with SAS statistical software (SAS Institute, USA). The significance of differences within treatments was determined using the least significant difference (LSD) test (p < 0.05). DGGE band patterns were analyzed using Quantity One software (version 4.62, Bio-Rad), and band position and intensity were determined automatically. The density of each band was divided by the average band density of the lane to minimize the influence of differences in the amount of DNA loaded. Normalized data were used for principal component analysis (PCA) using SPSS software (version 20, IBM).
Results
Jdm1 nematicidal activity and inhibition of egg hatching
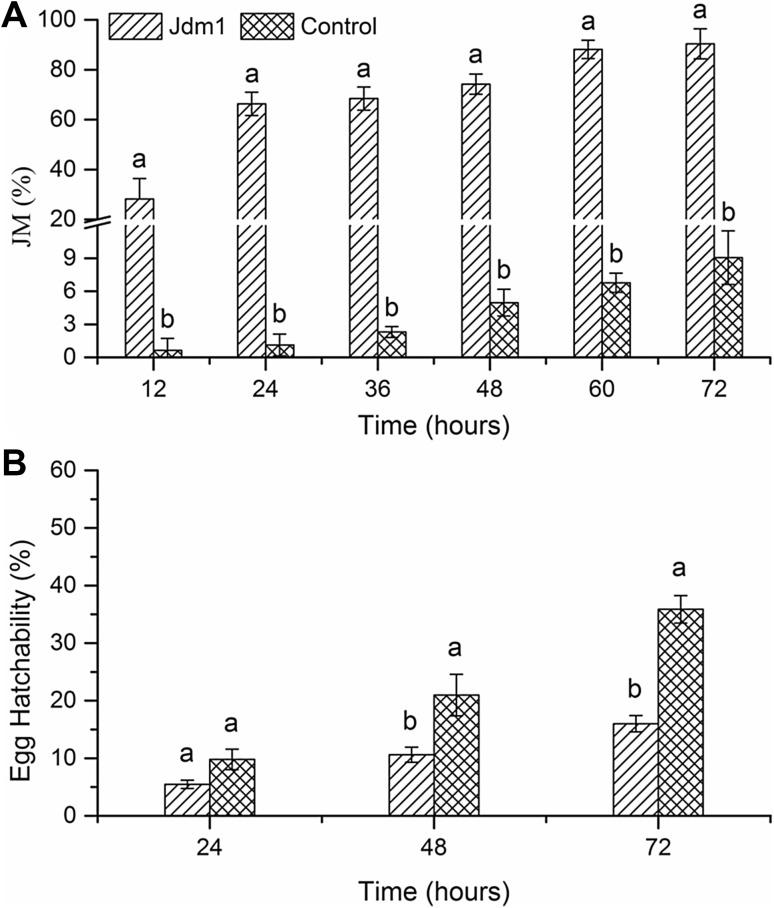
The Jdm1 cell-free supernatant was tested for the inhibition of J2 M. incognita activity and egg hatching. Metabolites produced by Jdm1 were clearly lethal for the nematodes as they did not move, even when probed with a fine needle. J2 worms became less and less active over time, and J2 mortality was greater than 90% after 60 h of incubation, in contrast to that of the controls (less than 7%; Fig. 1a). The metabolites also strongly inhibited egg hatching (Fig. 1b), which also increased with incubation time. At 72 h after incubation, egg hatching was 16% for Jdm1 treated samples, while the controls had a hatching rate of 36% (Fig. 1b).
Fig. 1.
Effects of B. cereus strain Jdm1 supernatant on M. incognita J2 worm mortality and egg hatching. JM mortality of J2; IE inhibition of egg hatching. Data are mean ± SD of three replicates. Letters above bars indicate significant differences between treatments and controls (p < 0.05)
Control efficacy in the pot test
Results from the pot test indicated that Jdm1 reduced gall formation and promoted tomato plant growth (Fig. 2). Disease severity was significantly lower compared to controls (p < 0.05), and the control efficacy was approximately 43% (Table 1). Furthermore, Jdm1 increased the length of both roots and shoots, as well as the dry and fresh weights of these, respectively (Table 1). RKN produces galls on roots, which increases the root weight, and Jdm1 decreased the number of galls, which effectively decreased the fresh weight of the roots (p < 0.05).
Fig. 2.
Effects of B. cereus strain Jdm1 on tomato plant growth in the pot test
Table 1.
Effects of B. cereus strain Jdm1 on M. incognita and tomato plant growth in the pot test
| Treatment | Root | Shoot | |||||
|---|---|---|---|---|---|---|---|
| Disease severity (%) | Length (cm) | Fresh weight (g) | Dry weight (g) | Length (cm) | Fresh weight (g) | Dry weight (g) | |
| Jdm1 | 42.86 ± 4.12b | 22.95 ± 1.13a | 9.32 ± 0.58b | 0.47 ± 0.04b | 74.00 ± 1.83a | 56.12 ± 1.32a | 6.53 ± 0.39a |
| Control | 75.00 ± 2.92a | 19.21 ± 0.78b | 13.61 ± 0.88a | 1.12 ± 0.08a | 65.65 ± 2.70b | 46.95 ± 1.68b | 4.41 ± 0.37b |
Data are mean ± SD of three replicates. Letters following numbers indicate significant differences (p < 0.05)
Control efficacy of Jdm1 in the field test
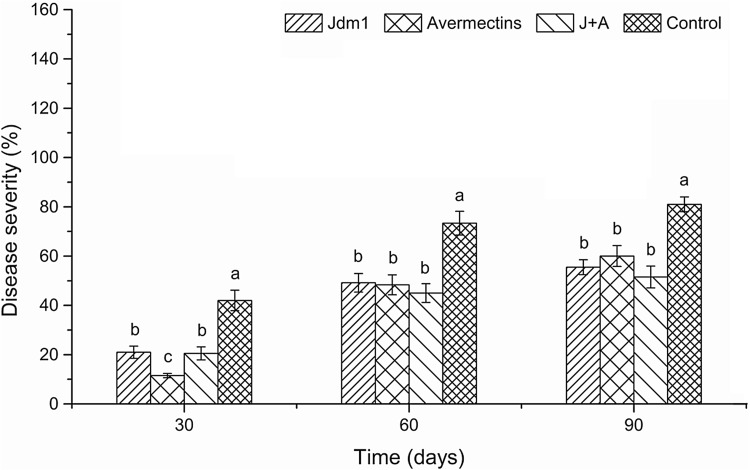
Tomato plants grown in the RKN-infested field test experienced extensive galls in the roots. Avermectin treatment had decreased the disease severity dramatically 30 day post-inoculation, and the control efficacy was as high as 72.6%. Significant reductions in disease severity were also noticeable in plots treated with Jdm1, as well as those treated with both avermectins and Jdm1, with control efficacies of 50.0 and 51.2%, respectively. The efficacies of all treatments subsequently decreased over time at different rates. At 60 and 90 day post-inoculation, the levels of root-knot gall formation had reached the same levels for all treatments, and all were significantly lower than the levels of control plants (p < 0.05; Fig. 3).
Fig. 3.
Control efficacy of Jdm1 against M. incognita in the field test. Data are mean ± SD of four replicates. Letters above bars indicate significant differences between treatments (p < 0.05)
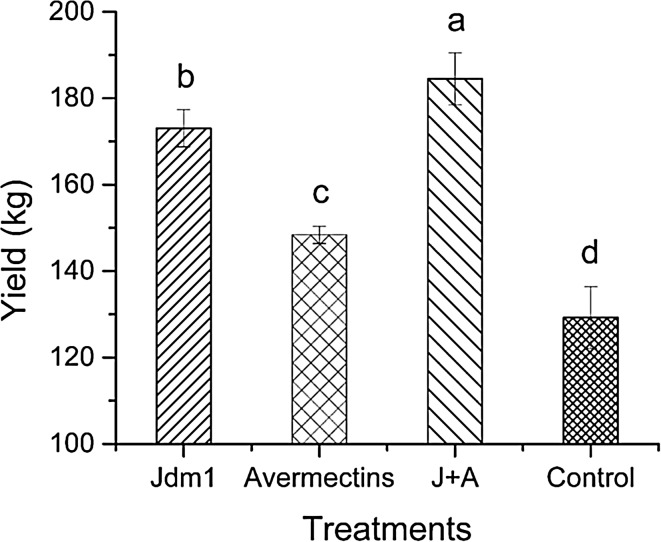
All treated plots gave significantly higher fruit yields than control plots (p < 0.05). The highest yield was observed from plots treated with the combination of Jdm1 and avermectins, indicating that combination treatment may be optimal. Treatment with avermectins alone was considerably less protective (Fig. 4).
Fig. 4.
Effects of Jdm1 on tomato yield. Letters above bars indicate significant differences between treatments (p < 0.05)
Rhizosphere bacterial community
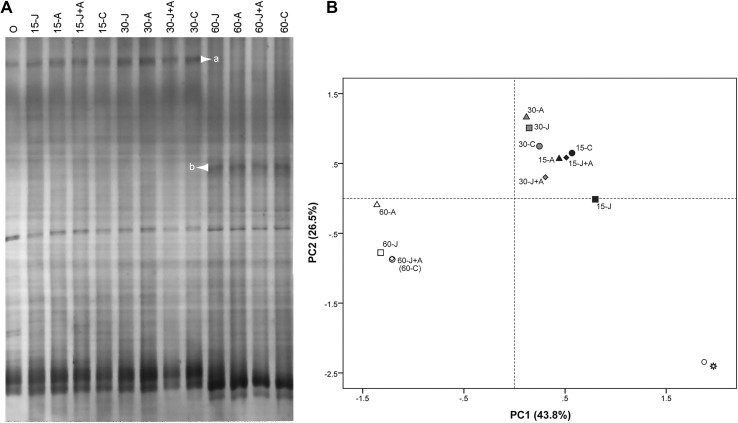
By optimizing the running conditions, we found that the DGGE profiles of the four replicate samples were highly reproducible, and we were able to run a randomly selected sample after this discovery. Multiple bands were detected in all samples (Fig. 5a). The DGGE profiles of bacterial communities from the soils sampled at the same time were very similar to each other, with very few significant differences. Band a displayed a relatively high intensity within the initial 30 days, and the intensity of band b was increased at 60 day post-inoculation.
Fig. 5.
DGGE profile (a) and PCA analysis (b) of bacterial 16S rRNA fragments. O represents soil samples taken during transplantation before inoculation; results for 15, 30, and 60 day post-inoculation are shown. J, A, J + A, and C are Jdm1, avermectins, Jdm1 + avermectins, and control (water) treatments, respectively
PCA of the DGGE band patterns also showed that the bacterial communities of soils sampled at the same time were highly similar and formed separate groups from samples taken at other times, irrespective of the different treatments. The exception was for soil treated with Jdm1 for 15 days, which was distinct from the other 15 days treatment groups (Fig. 5a). This result indicated that Jdm1 altered the bacterial community of the rhizosphere soil of tomato within 15 days post-inoculation, but this effect lasted less than 30 days. These results suggest that neither Jdm1 nor avermectin treatments had negative long-term effects on the rhizosphere bacterial community in tomato field tests. Indeed, the tomato plant growth stage was the major factor influencing the rhizosphere microbial community.
Discussion
The results of the Petri dish test indicated that application of the cell-free supernatant of a B. cereus culture significantly increased the mortality of J2 worms and decreased egg hatching. This suggests that extracellular nematicidal metabolites were present in the supernatant. The previous studies have shown that extracellular proteins from culture supernatants of various bacteria (Huang et al. 2009; Tian et al. 2006) were active against RKN, although the mode of action of extracellular substances from different microorganisms may be different. The B. cereus strain Jdm1 has strong protease, chitinase, and siderophore activities (data not shown), which probably acted as important virulence factors in the infectious process against M. incognita J2 worms or eggs. Further studies are needed to identify which Jdm1 extracellular proteins and/or other factors are involved and to determine their dose–response relationship with M. incognita.
The present study demonstrated that biocontrol of RKN both in pot and field tests could be effectively achieved using Jdm1 extract (Table 1; Fig. 3). In addition to disease suppression, Jdm1 also promoted tomato plant growth, which was inversely proportional to disease severity (Table 1). Although the mechanism of action of Jdm1 was not elucidated in these experiments, we hypothesize that nematode reduction may be attributed to a direct effect of Jdm1 metabolites that inhibit M. incognita egg hatching and kill J2 worms. Simultaneously, Jdm1 in the vicinity of the roots may also have increased the levels of defense compounds such as hydrogen peroxide (H2O2) in the tomato plants, or induced the plants own systemic resistance and host defenses, which restrained nematode invasion and prevented infection. Siahpous et al. (2011) found that the chemical inducer salicylic acid, in combination with B. cereus, stimulated plant defense systems and increased H2O2 levels. Niu et al. (2012) reported B. cereus AR156 ISR to Pseudomonas syringae pv. tomato DC300 in tomato plants. The growth promotion mechanism was shown to be dependent on the substances released from bacteria and/or efficient nutrient absorption (Glick et al. 1999).
In this study, we also compared the biocontrol efficacy of Jdm1 in combination with avermectins in the field test. Avermectins offer an outstanding alternative to any of the available synthetic nematicides because of their novel mode of action and high potency, and their specific physico-chemical properties prevent rapid degradation in soil and avoid leaching (Reddy 2013). After 30 day post-inoculation, Jdm1 treatment displayed increased biocontrol efficacy compared with avermectin treatment. However, no significant differences were observed on days 60 and 90. When we inoculated the tomato rhizosphere with a combination of Jdm1 and avermectins, there was not a significant difference in control efficacy from treatment with Jdm1 alone (Fig. 3). However, Jdm1- and avermectin-treated plants produced significantly increased tomato fruit yields (Fig. 4).
The presence of introduced rhizobacteria could significantly modify the rhizosphere environment, altering the soil microbial diversity and affecting the potential for biological invasion. DGGE and PCA analysis (Fig. 5) showed that introducing Jdm1 had a very strong effect on the soil bacterial community by day 15 post-inoculation, but this effect lasted less than 30 days, by which time that the community diversity had returned to pre-treatment conditions. Other studies also showed only transient effects on soil microbial communities following inoculation with biocontrol agents such as Pseudomonas fluorescens (Gao et al. 2012), Streptomyces melanosporofaciens (Prévost et al. 2006), and Corynebacterium glutamicum (Vahjen et al. 1995).
PCA analysis showed that the bacterial communities in the soil were grouped with others taken at the same time. This suggests that the plant growth stage is the most influential factor determining the composition of the microbial community, as was shown previously for potato-associated bacterial communities (van Overbeek and van Elsas 2008; Xu et al. 2009).
In summary, B. cereus strain Jdm1 can significantly suppress M. incognita infection in tomato roots, while simultaneously promoting plant growth, and the bacterial community of tomato rhizosphere was not disrupted in the long term.
Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities (2017CXNL04) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Compliance with ethical standards
Conflict of interest
The authors have declared that no competing interests exist.
References
- Atamian HS, Roberts PA, Kaloshian I. High and low throughput screens with root-knot nematodes Meloidogyne spp. J Visu Exp. 2012;61:e3629. doi: 10.3791/3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J, Page SLJ. Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop Pest Manag. 1980;26:296–298. doi: 10.1080/09670878009414416. [DOI] [Google Scholar]
- Buckley DH, Schmidt TM. Exploring the biodiversity of soil—a microbial rain forest. In: Staley JT, Reysenbach AL, editors. Biodiversity of microbial life. New York: Wiley-Liss, Inc; 2002. [Google Scholar]
- Chitwood DJ. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture, Agricultural Research Service. Pest Manag Sci. 2003;59:748–753. doi: 10.1002/ps.684. [DOI] [PubMed] [Google Scholar]
- Gao GP, Yin DH, Chen SJ, Xia F, Yang J, Li Q, Wang W. Effect of biocontrol agent Pseudomonas fluorescens 2P24 on soil fungal community in cucumber rhizosphere using T-RFLP and DGGE. PLoS One. 2012;7:e31806. doi: 10.1371/journal.pone.0031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR, Patten CL, Penrose DM. Biochemical and genetic mechanisms used by plant growth promoting bacteria. London: Imperial College Press; 1999. [Google Scholar]
- Gokte N, Swarup G. On the potential of some bacterial biocides against root-knot and cyst nematodes. Indian J Nematol. 1988;18:152–153. [Google Scholar]
- Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol. 1997;63:3233–3241. doi: 10.1128/aem.63.8.3233-3241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XW, Liu JW, Ding JM, He QS, Xiong R, Zhang KQ. The investigation of nematocidal activity in Stenotrophomonas maltophilia G2 and characterization of a novel virulence serine protease. Can J Microbiol. 2009;55:934–942. doi: 10.1139/W09-045. [DOI] [PubMed] [Google Scholar]
- Jetiyanon K, Wittaya-Areekul S, Plianbangchang P. Film coating of seeds with Bacillus cereus RS87 spores for early plant growth enhancement. Can J Microbiol. 2008;54:861–867. doi: 10.1139/W08-079. [DOI] [PubMed] [Google Scholar]
- Li BX, Ren YP, Zhang DX, Xu SY, Mu W, Liu F. Modifying the formulation of abamectin to promote its efficacy on southern root-knot nematode (Meloidogyne incognita) under blending-of-soil and root-irrigation conditions. J Agric Food Chem. 2018;66:799–805. doi: 10.1021/acs.jafc.7b04146. [DOI] [PubMed] [Google Scholar]
- Marschner P, Yang CH, Lieberei R, Crowley DE. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem. 2001;33:1437–1445. doi: 10.1016/S0038-0717(01)00052-9. [DOI] [Google Scholar]
- Nakatsu CH. Soil microbial community analysis using denaturing gradient gel electrophoresis. Soil Sci Soc Am J. 2007;71:562–571. doi: 10.2136/sssaj2006.0080. [DOI] [Google Scholar]
- Niu DD, Wang CJ, Guo YH, Jiang CH, Zhang WZ, Wang YP, Guo JH. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces resistance in tomato with induction and priming of defence response. Biocontrol Sci Technol. 2012;22:991–1004. doi: 10.1080/09583157.2012.706595. [DOI] [Google Scholar]
- Noling JW, Becker JO. The challenge of research and extension to define and implement alternatives to methyl bromide. J Nematol. 1994;26:573–586. [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Chet I, Spiegel Y. Control of the rootknot nematode Meloidogyne javanica by Bacillus cereus. Biocontrol Sci Technol. 1993;3:115–126. doi: 10.1080/09583159309355267. [DOI] [Google Scholar]
- Oka Y, Koltai H, Bar-Eyal M, Mor M, Sharon E, Chet I, Spiegel Y. New strategies for the control of plant-parasitic nematodes. Pest Manag Sci. 2000;56:983–988. doi: 10.1002/1526-4998(200011)56:11<983::AID-PS233>3.0.CO;2-X. [DOI] [Google Scholar]
- Palomares-Rius JE, Escobar C, Cabrera J, Vovlas A, Castillo P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front Plant Sci. 2017;8:1987. doi: 10.3389/fpls.2017.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost K, Couture G, Shipley B, Brzezinski R, Beaulieu C. Effect of chitosan and a biocontrol streptomycete on field and potato tuber bacterial communities. BioControl. 2006;51:533–546. doi: 10.1007/s10526-005-4240-z. [DOI] [Google Scholar]
- Ramos B, García JAL, Probanza A, Barrientos ML, Gutierrez Mañero FJ. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis. Environ Exp Bot. 2003;49:61–68. doi: 10.1016/S0098-8472(02)00059-X. [DOI] [Google Scholar]
- Reddy PP. Recent advances in crop protection: avermectins. New Delhi: Springer; 2013. [Google Scholar]
- Sanguinetti CJ, Dias Neto E, Simpson AJ. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques. 1994;17:914–921. [PubMed] [Google Scholar]
- Siahpous S, Sahebani N, Aminian H. Change of some defense compounds of cucumber treated with Bacillus cereus and salicylic acid against Meloidogyne javanica. Afr J Plant Sci. 2011;5:829–834. [Google Scholar]
- Tian BY, Li N, Lian LH, Liu JW, Yang JK, Zhang KQ. Cloning, expression and deletion of the cuticle-degrading protease BLG4 from nematophagous bacterium Brevibacillus laterosporus G4. Arch Microbiol. 2006;186:297–305. doi: 10.1007/s00203-006-0145-1. [DOI] [PubMed] [Google Scholar]
- Topp E, Millar S, Bork H, Welsh M. Effects of marigold (Tagetes sp.) roots on soil microorganisms. Biol Fertil Soils. 1998;27:149–154. doi: 10.1007/s003740050413. [DOI] [Google Scholar]
- Vahjen W, Munch JC, Tebbe CC. Carbon source utilization of soil extracted microorganisms as a tool to detect the effects of soil supplemented with genetically engineered and non-engineered Corynebacterium glutamicum and a recombinant peptide at the community level. FEMS Microbiol Ecol. 1995;18:317–328. doi: 10.1111/j.1574-6941.1995.tb00188.x. [DOI] [Google Scholar]
- van Overbeek L, van Elsas JD. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.) FEMS Microbiol Ecol. 2008;64:283–296. doi: 10.1111/j.1574-6941.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- Wei LH, Xu QY, Wei BQ, Wang YM, Li SM, Chen LF, Guo JH. Screening of antagonistic bacterial strains against Meloidogyne incognita using protease activity. Biocontrol Sci Technol. 2010;20:739–750. doi: 10.1080/09583151003714109. [DOI] [Google Scholar]
- Xiao TJ, Tan SY, Shen QR, Ran W. Bacillus cereus X5 suppresses root-knot nematode of tomato by colonizing in roots and soil. Afr J Microbiol Res. 2012;6:2321–2327. doi: 10.5897/AJMR12.688. [DOI] [Google Scholar]
- Xu JH, Liu PL, Meng QP, Long H. Characterisation of Meloidogyne species from China using isozyme phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. Eur J Plant Pathol. 2004;110:309–315. doi: 10.1023/B:EJPP.0000019800.47389.31. [DOI] [Google Scholar]
- Xu YX, Wang GG, Jin J, Liu JJ, Zhang QY, Liu XB. Bacterial communities in soybean rhizosphere in response to soil type, soybean genotype, and their growth stage. Soil Biol Biochem. 2009;41:919–925. doi: 10.1016/j.soilbio.2008.10.027. [DOI] [Google Scholar]