Abstract
Introduction: Skin graft is the standard therapeutic technique in patients with deep ulcers, but like every surgical procedure, it may present some complications. Although several modern dressings are available to enhance comfort of donor site, the use of techniques that accelerate wound healing may enhance patient’s satisfaction. Low level laser therapy (LLLT) has been used in several medical fields, especially for wound healing, but it may take several months for large ulcers treated with laser to heal completely.
Methods: Nine patients with bilateral similar grade 3 burn ulcers in both hands or both feet were selected as candidates for split-thickness skin graft (STSG). One side was selected for laser irradiation and the other side as control, randomly. Laser was irradiated every day for 7 days with red 655 nm light, 150 mW, 2 J/cm2 at the bed of the ulcer and with infra-red 808 nm light, 200 mW for the margins.
Results: The rate of wound dehiscence after skin graft surgery was significantly lower in laser treated group in comparison to control group which received only classic dressing (P=0.019).
Conclusion: The results showed LLLT to be a safe effective method which improves graft survival and wound healing process and decreases the rate of wound dehiscence in patients with deep burn ulcers.
Keywords: Low level laser therapy, Skin transplantation, Wound healing, Regenerative medicine, Wound dehiscence
Introduction
Split-thickness skin graft (STSG) is the standard tool for covering skin defects in deep burn ulcers.1 Successful graft transplantation needs 3 phases: (1) Capillary revascularization, (2) Lymphatic revascularization, (3) Re-innervation. Failure in each step may result in graft failure.2 Using therapeutic methods for improving these steps may increase the success in skin grafting and decrease the rate of repeated surgery.3
Low level laser therapy (LLLT) as an effective wound healing method may affect these steps and accelerate healing. Several reviews and meta-analysis introduce this technique as a safe and effective therapeutic modality.4-6 It seems that laser therapy induces neovascularization and increases tissue perfusion.5,7 Several molecular mechanisms have been described for low level lasers effects that lead to accelerating tissue perfusion and wound healing, including: reactive oxygen species formation,8 activation of ATPases,9 stimulation of Ca-influx and mitosis rate,10 increased mRNA and protein synthesis and cellular proliferation,11,12 increased vascular endothelial growth factor and neovascularization.13
Clinical studies report significant effects of LLLT in the healing of different kind of ulcers, including venous, pressure and diabetic foot ulcers.14,15 The efficacy of this method in burn ulcers is studied only on animals.16-19 The only report using LLLT in burn patients is our previous study, using LLLT along with skin graft surgery in grade 3 burn of diabetic ulcer.20
In the present study, we are the first to evaluate the effect of LLLT on the healing of STSG in patients with grade 3 burn ulcer as a randomized clinical trial.
Methods
The study was conducted in Motahari specialized burn hospital. Nine patients with bilateral full thickness burns on both hands or both feet were selected for the study. Early excision and grafting was done for all patients within 3-4 days of admission. When the patients were resuscitated and became stable, they were prepared for STSG. To harvest donor sites, electric dermatome (Humaca Instruments, Poland) was set at 0.4 mm. One side (one hand or one foot) was selected as laser group (A) and one as control (B). Photographs were taken from both sides using iPhone 5S camera. After excision of burned skin, only side A was laser irradiated while STSG was done for both sides. Similar dressing (vaseline gauze) was done for both sides. After 3 days, when dressing was changed routinely, laser irradiation was done for side A and photography was done. Laser irradiation was continued every day for 7 days and again photography was done. The situation for all photographs was the same and a ruler within the photograph was used for calibration. Wound surface area was evaluated by PictZar software. Figure 1 illustrates the consort diagram.
Figure 1.
Consort Diagram.
Laser Irradiation
Portable laser probe (PLP), 650 nm red laser; 150 mW, radiation area: 0.25 cm2, power density: 0.6 W/cm2, contact, continuous mode, 2 J/cm2, (Canadian Optic Laser Center, COL laser, Canada) was used for irradiation of the bed of the ulcers and portable 3 L, 808 nm infra-red, radiation area: 1 cm2, contact, continuous mode, 6 J/cm2 (Iran), was used to irradiate the margins. This is a suggested protocol for wound treatment in most studies.14,21,22
Sterile transparent cover was used for contact laser irradiation. Output laser power after passing through the cover was calculated using dosimeter.
Results
Patients parameters are illustrated in Table 1. Data were analyzed with SPSS (Statistical package for the social sciences) software version 21. Nine patients were recruited for the study (Age [mean ± SD]: 40.11 ± 9.07 years, with burn surface percentage [mean ± SD]: 34.80 ±18.30) (Table 2). Normality of continuous data was assessed by Shapiro-Wilks test. The mean of burn ulcers before and after treatment and in control and laser groups were compared by independent t test (Figure 2).
Table 1. Patients’ Parameters of Nine Participants .
| Age | Burn Surface |
Control Pre-treatment
Ulcer Surface/cm 2 |
Control Post-treatment Ulcer Surface/cm 2 | Laser Pre-treatment | Laser Post-treatment |
| 40 | 45% | 83.477 | 18.343 | 70.564 | 2.993 |
| 53 | 10% | 70.543 | 8.718 | 81.857 | 0 |
| 41 | 40% | 86.337 | 14.594 | 64.286 | 3.031 |
| 50 | 11% | 74.438 | 0 | 124.910 | 0 |
| 35 | 50% | 124.523 | 14.527 | 68.256 | 0 |
| 30 | 55% | 126.553 | 4.457 | 130.352 | 3.1 |
| 32 | 45% | 93.175 | 81.0 | 167.105 | 14.833 |
| 30 | 45% | 76.893 | 14.414 | 99.165 | 4.684 |
| 50 | 12% | 30.169 | 6.595 | 49.396 | 0 |
Figure 2.
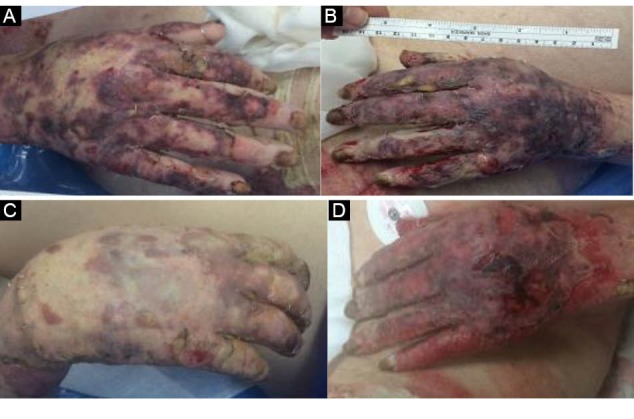
(A,B) Skin Graft for Bilateral Grade 3 Burn Ulcers. (C): After 10 Session of LLLT. (D) Control Side.
Table 2. Mean ± SD (cm2) of Ulcer Surfaces (Dehiscence Area) in 2 Study Groups Before and After Treatment .
| Laser Group | Control Group | P Value | |
| Before | 95.10 ± 38.52 | 85.12 ± 29.05 | 0.931 |
| After | 3.18 ± 4.72 | 18.07 ± 24.31 | 0.019 |
Discussion
In the present study, we were the first to evaluate the effects of LLLT on the healing process after skin graft surgery in patients with deep burn ulcers. Local irradiation of red and infra-red laser light significantly reduced the rate of dehiscence of grafted area (P = 0.019).
In a similar study we used LLLT along with skin graft surgery in diabetic patients with grade 3 burn ulcers who were candidates for amputation. The results showed significant effect of LLLT on the prognosis of surgery and all patients who were candidates for amputation, healed completely.20 Our previous findings showed the significant effects of laser therapy on growth factors involved in wound healing including fibroblast growth factor (FGF),12 on wound healing process and neuropathy of diabetic patients,14,23 pressure ulcer,15 after skin graft surgery in diabetic patients20 and post cesarean surgery.24
Bossini et al studied the effect of 670 nm laser light on viability of skin graft in 100 rats. They compared different doses: 3, 6, 12 and 24 J/cm2 with control group. They reported that all of these therapeutic doses increased the survival of the skin flap and higher doses had better results.25
Prado et al used 830 nm laser light with 36 J/cm2 energy density for cutaneous flap survival in rats. They reported that this regimen increases flap viability.26 Pinfildi et al evaluated the effects of 670 nm laser light on mast cells and survival of transverse rectus abdominis musculocutaneous flap. They suggested that laser irradiation reduced necrotic area, stimulated mast cells growth, increased tissue perfusion and finally increased flap viability.27 Cury et al reported that LLLT increases angiogenesis by effecting on VEGF, MMP-2 and HIF-1 in rat skin flap.28
Several in vitro and in vivo studies demonstrate that laser therapy accelerates wound healing by enhancing epithelialization, fibroblasts activity, revascularization, increasing perfusion, and improving the tensile strength of scars.19,29-31 In a review by Schindl et al, they reported laser therapy as a valuable adjuvant treatment in wound healing.5 In another review by Chukuka et al, they mentioned LLLT as a highly effective treatment for accelerating tissue repair and pain management.32 Although LLLT has been used successfully for treatment of different kinds of ulcers including post-surgery,24 diabetic5,14 and pressure ulcers,33 the efficacy of this technique on burn ulcers is studied only on animal models. Bayat et al reported that LLLT decreased the rate of infection with Staphylococcus aureus and Staphylococcus epidermis in deep burn of rats.17 Mester et al concluded that laser therapy accelerates epithelial formation in burned mice.34 Ezzati et al reported that LLLT improves healing of third degree burn ulcer in rats.18 Dantas et al suggested that using low level laser and sodium alginate/chitosan film accelerates neovascularization, epithelialization, and collagen formation of burn ulcers in mice.16
Several techniques are accompanied by STSG for improving the surgery prognosis. Scherer et al used vacuum assisted closure device for improving graft survival. They reported that this technique is a safe and effective method for securing STSG,3 but this technique is an expensive therapy in comparison with LLLT. Modern dressings including alginate dressings are also used to accelerate wound healing. Steenfos and Agren studied the efficacy of these dressings after STSG in a randomized controlled study. They reported that although these dressings increased initial blood absorption and faster homeostasis, but they did not find significant effect on epithelialization, in comparison with conventional dressing.35
Conclusion
In the present study, for the first time in a randomized clinical trial, we used LLLT to improve the prognosis of skin graft surgery and for prevention of dehiscence in patients with grade 3 burn ulcers. Our results showed that using LLLT significantly decreased the rate of dehiscence in these patients.
Conflict of Interests
None.
Ethical Considerations
The protocols and informed consent were reviewed according to Medical Ethics Board of Shahid Beheshti University of Medical Sciences (IR.SBMU.REC.1394.363) and Iranian Registry of Clinical Trials (IRCT2016020226069N2).
Acknowledgments
We extend our gratitude to the staff of Motahari Burn Center for their assistance.
Please cite this article as follows: Kazemikhoo N, Vaghardoost R, Dahmardehei M, et al. Evaluation of the effects of low level laser therapy on the healing process after skin graft surgery in burned patients: a randomized clinical trial. J Lasers Med Sci. 2018;9(2):139-143. doi:10.15171/jlms.2018.26.
References
- 1.Voineskos SH, Ayeni OA, McKnight L, Thoma A. Systematic review of skin graft donor-site dressings. Plast Reconstr Surg. 2009;124(1):298–306. doi: 10.1097/PRS.0b013e3181a8072f. [DOI] [PubMed] [Google Scholar]
- 2.Llanos S, Danilla S, Barraza C. et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg. 2006;244(5):700–705. doi: 10.1097/01.sla.0000217745.56657.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg. 2002;137(8):930–933; discussion 933. doi: 10.1001/archsurg.137.8.930. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff LD, Bounkeo JM, Brannon WM. et al. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg. 2004;22(3):241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]
- 5.Schindl A, Schindl M, Pernerstorfer-Schon H, Schindl L. Low-intensity laser therapy: a review. J Investig Med. 2000;48(5):312–326. [PubMed] [Google Scholar]
- 6.Beckerman H, de Bie RA, Bouter LM, De Cuyper HJ, Oostendorp RA. The efficacy of laser therapy for musculoskeletal and skin disorders: a criteria-based meta-analysis of randomized clinical trials. Phys Ther. 1992;72(7):483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- 7. Dyson M. Cellular and subcellular aspects of low level laser therapy. Ohshiro T, Calderhead RD, eds. Progress in laser therapy. J Wiley & Sons;1991:221.
- 8.Karu TI. Effects of visible radiation on cultured cells. Photochem Photobiol. 1990;52(6):1089–1098. doi: 10.1111/j.1751-1097.1990.tb08450.x. [DOI] [PubMed] [Google Scholar]
- 9.Passarella S, Casamassima E, Molinari S. et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 10.Lubart R, Malik Z, Rochkind S, Fisher T. A possible mechanism of low level laser-living cell interaction. Laser Ther. 1990;2(2):65–68. doi: 10.5978/islsm.90-OR-03. [DOI] [Google Scholar]
- 11.Funk JO, Kruse A, Neustock P, Kirchner H. Helium-neon laser irradiation induces effects on cytokine production at the protein and the mRNA level. Exp Dermatol. 1993;2(2):75–83. doi: 10.1111/j.1600-0625.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 12.Khoo NK, Shokrgozar MA, Kashani IR. et al. In vitro Therapeutic Effects of Low Level Laser at mRNA Level on the Release of Skin Growth Factors from Fibroblasts in Diabetic Mice. Avicenna J Med Biotechnol. 2014;6(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 13.Kipshidze N, Nikolaychik V, Keelan MH. et al. Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med. 2001;28(4):355–364. doi: 10.1002/lsm.1062. [DOI] [PubMed] [Google Scholar]
- 14.Kazemi-Khoo N. Successful treatment of diabetic foot ulcers with low-level laser therapy. Foot. 2006;16(4):184–187. doi: 10.1016/j.foot.2006.05.004. [DOI] [Google Scholar]
- 15.Kazemikhoo N, Rahbar MR, Akrami SM. Low-level laser therapy along with intravascular laser in deep pressure ulcer resistant to conventional therapies. J Ski Stem Cell. 2015;2(4):e30686. doi: 10.17795/jssc30686. [DOI] [Google Scholar]
- 16.Dantas MD, Cavalcante DR, Araujo FE. et al. Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J Photochem Photobiol B. 2011;105(1):51–59. doi: 10.1016/j.jphotobiol.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Bayat M, Vasheghani MM, Razavi N, Taheri S, Rakhshan M. Effect of low-level laser therapy on the healing of second-degree burns in rats: a histological and microbiological study. J Photochem Photobiol B. 2005;78(2):171–177. doi: 10.1016/j.jphotobiol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Ezzati A, Bayat M, Taheri S, Mohsenifar Z. Low-level laser therapy with pulsed infrared laser accelerates third-degree burn healing process in rats. J Rehabil Res Dev. 2009;46(4):543–554. doi: 10.1682/jrrd.2008.09.0121. [DOI] [PubMed] [Google Scholar]
- 19.Jaffary F, Changizi V, Mardani H. et al. Macroscopic effect of blue light cure on wound healing in NMRI mice NMRI. Adv Biomed Res. 2014;3:106. doi: 10.4103/2277-9175.129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahmardehei M, Kazemikhoo N, Vaghardoost R. et al. Effects of low level laser therapy on the prognosis of split-thickness skin graft in type 3 burn of diabetic patients: a case series. Lasers Med Sci. 2016;31(3):497–502. doi: 10.1007/s10103-016-1896-9. [DOI] [PubMed] [Google Scholar]
- 21.Nilforoushzadeh MA, Jaffary F, Ansari N, Siadat AH, Heidari A, Adibi N. Treatment of recalcitrant diabetic ulcers using trichloroacetic acid. J Res Med Sci. 2012;17(2):S286–S290. [Google Scholar]
- 22.Al-Watban FA, Zhang XY, Andres BL, Al-Anize A. Visible lasers were better than invisible lasers in accelerating burn healing on diabetic rats. Photomed Laser Surg. 2009;27(2):269–272. doi: 10.1089/pho.2008.2310. [DOI] [PubMed] [Google Scholar]
- 23.Khamseh ME, Kazemikho N, Aghili R. et al. Diabetic distal symmetric polyneuropathy: effect of low-intensity laser therapy. Lasers Med Sci. 2011;26(6):831–835. doi: 10.1007/s10103-011-0977-z. [DOI] [PubMed] [Google Scholar]
- 24.Mokmeli S, Khazemikho N, Niromanesh S, Vatankhah Z. The application of low-level laser therapy after cesarean section does not compromise blood prolactin levels and lactation status. Photomed Laser Surg. 2009;27(3):509–512. doi: 10.1089/pho.2008.2314. [DOI] [PubMed] [Google Scholar]
- 25.Bossini PS, Fangel R, Habenschus RM. et al. Low-level laser therapy (670 nm) on viability of random skin flap in rats. Lasers Med Sci. 2009;24(2):209–213. doi: 10.1007/s10103-008-0551-5. [DOI] [PubMed] [Google Scholar]
- 26.Prado RP, Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM. Effect of application site of low-level laser therapy in random cutaneous flap viability in rats. Photomed Laser Surg. 2009;27(3):411–416. doi: 10.1089/pho.2008.2320. [DOI] [PubMed] [Google Scholar]
- 27.Pinfildi CE, Liebano RE, Hochman BS. et al. Effect of low-level laser therapy on mast cells in viability of the transverse rectus abdominis musculocutaneous flap. Photomed Laser Surg. 2009;27(2):337–343. doi: 10.1089/pho.2008.2295. [DOI] [PubMed] [Google Scholar]
- 28.Cury V, Moretti AI, Assis L. et al. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1alpha and MMP-2. J Photochem Photobiol B. 2013;125:164–170. doi: 10.1016/j.jphotobiol.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kana JS, Hutschenreiter G, Haina D, Waidelich W. Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg. 1981;116(3):293–296. doi: 10.1001/archsurg.1981.01380150021005. [DOI] [PubMed] [Google Scholar]
- 30.Lyons RF, Abergel RP, White RA, Dwyer RM, Castel JC, Uitto J. Biostimulation of wound healing in vivo by a helium-neon laser. Ann Plast Surg. 1987;18(1):47–50. doi: 10.1097/00000637-198701000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Cambier DC, Vanderstraeten GG, Mussen MJ, van der Spank JT. Low-power laser and healing of burns: a preliminary assay. Plast Reconstr Surg. 1996;97(3):555–558; discussion 559. doi: 10.1097/00006534-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg. 2004;22(4):323–329. doi: 10.1089/pho.2004.22.323. [DOI] [PubMed] [Google Scholar]
- 33.Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess. 2001;5(9):1–221. doi: 10.3310/hta5090. [DOI] [PubMed] [Google Scholar]
- 34.Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122(4):532–535. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- 35.Steenfos HH, Agren MS. A fibre-free alginate dressing in the treatment of split thickness skin graft donor sites. J Eur Acad Dermatol Venereol. 1998;11(3):252–256. [PubMed] [Google Scholar]