Abstract
Despite decades of research on depression, the underlying pathophysiology of depression remains incompletely understood. Emerging evidence from task-based studies suggests that the abnormal reward-related processing contribute to the development of depression. It is unclear about the function pattern of reward-related circuit during resting state in depressive patients. In present study, seed-based functional connectivity was used to evaluate the functional pattern of reward-related circuit during resting state. Selected seeds were two key nodes in reward processing, medial orbitofrontal cortex (mOFC) and nucleus accumbens (NAcc). Fifty depressive patients and 57 healthy participants were included in present study. Clinical severity of participants was assessed with Hamilton depression scale and Hamilton anxiety scale. We found that compared with healthy participants, depressive patients showed decreased connectivity of right mOFC with left temporal pole (TP_L), right insula extending to superior temporal gyrus (INS_R/STG) and increased connectivity of right mOFC with left precuneus. Similarly, decreased connectivity of left mOFC with TP_L and increased connectivity with cuneus were found in depressive patients. There is also decreased connectivity of right NAcc with bilateral temporal pole, as well as decreased connectivity of left NAcc with INS_R/STG. In addition, the functional connectivity of right nucleus accumbens with right temporal pole (TP_R) was negatively correlated with clinical severity. Our results emphasize the role of communication deficits between reward systems and paralimbic cortex in the pathophysiology of depression.
Keywords: depression, reward system, orbitofrontal cortex, nucleus accumbens, temporal pole
Introduction
Depression is among common psychiatric disorders and the leading causes of disability worldwide (Ferrari et al., 2013). Despite decades of research on depression, the pathological neural mechanisms of depression remains incompletely understood. As a heterogenous psychiatric disorder, diverse symptoms of depression may attribute to distinct pathophysiology (Drysdale et al., 2016; Guo et al., 2016). Increasingly, anhedonia is regarded as a cardinal feature of depression (Pizzagalli, 2014) and associated with increased risk for suicide (Ducasse et al., 2018) and poor treatment outcome (Vrieze et al., 2014). Anhedonia is defined as reduced interest or pleasure previously rewarding activities, part of a spectrum of reward circuit abnormalities (Der-Avakian and Markou, 2012). Convergent studies implicated the dysfunction of reward brain system in the neurobiology of anhedonia (Auerbach et al., 2017; Karcher et al., 2017). Indeed, behavioral studies have revealed the abnormality during reward-related processing in depression (Rizvi et al., 2018), displayed several types such as reward response bias, impaired reward learning ability and increased risk avoidance (Smoski et al., 2009).
The orbitofrontal cortex (OFC) is a key node in processing salience and magnitude of rewards (O’Doherty et al., 2001; Gottfried et al., 2003; Schoenbaum et al., 2011). Besides, OFC also plays a critical role in integrating reward information based on its strong anatomical connection with reward-related regions sensory, limbic and ventral striatal cortex (Kahnt et al., 2012). Ventral striatum is another core region in reward-related processing (Bartra et al., 2013; Sescousse et al., 2013). As a part of the ventral striatum, the nucleus accumbens (NAcc) is an important component of the reward circuit in the brain (Misaki et al., 2016), mainly responsible for mediating hedonic perception of rewards. In addition to perception of rewards, NAcc also takes on as a modulator in motivation-related behavior, which can influence several symptoms in depression, such as lack of motivation, anergia, or psychomotor slowing (Salamone et al., 2005).
Indeed, evidence from task-based neuroimaging studies has validated the maladaptive neural response of OFC and NAcc in reward-related processing in patients with depression. Blunted activity in OFC and NAcc for reward outcomes was consistently identified in depression (Tremblay et al., 2005; Pizzagalli et al., 2009). Depressive patients also exhibit hypoactivation of medial OFC in the neural coding for reward prediction (Rothkirch et al., 2017), as well as the decreased activity in NAcc (Ubl et al., 2015). In addition, the aberrancy of OFC and NAcc in depression is also supported by evidence from structural findings (Kempton et al., 2011; Lu et al., 2017).
Besides task-based and structural methods, another functional neuroimaging tool, resting-state functional magnetic resonance imaging (RS-fMRI), enables the detection of spontaneous brain activity to identify brain dysfunction in diseases (Biswal et al., 1995; Zhang and Raichle, 2010; Ji et al., 2017). With the non-invasive and task-independent feature, RS-fMRI has been increasingly applied in several mental diseases, especially in depressive disorder (Jalbrzikowski et al., 2017; Anhoj et al., 2018; Chen et al., 2018). Dysfunction within reward circuit among depressive individuals has been recognized using RS-fMRI (Cheng et al., 2016; Gong et al., 2017). Seed-based functional connectivity is one of technique to measure multi-regional cooperation within a special network. With the seed of medial and lateral OFC, Cheng et al. (2016) found that depressive patients exhibit reduced functional connectivity of medial OFC (mOFC) with temporal gyrus but increased functional connectivity of lateral OFC with precuneus and angular gyrus. Accordingly, they concluded medial reward and lateral non-reward orbitofrontal cortex circuits in depression. Consistent with this view, Gong et al. (2017) found decreased connectivity of NAcc with OFC, dorsomedial prefrontal cortex, superior temporal gyrus and insular lobe in depression. However, there are also studies reported against this view (Avery et al., 2014; Rzepa and McCabe, 2016). Diversity of seeds selection in different studies may explain these discrepancies. There is few work used diverse seeds within reward network in a single study to validate the reward-network abnormality in depression.
In present study, we investigated the functional coupling of depressive patients within reward network with diverse seeds (mOFC and NAcc). We hypothesized that there are similar alteration of coupling pattern between the NAcc-based and mOFC-based reward circuit in depressive individuals. In addition, we also investigated the neural alteration of reward-circuits coupling and clinical severity.
Materials and Methods
Participants
Fifty patients with depressive episode from Anhui Mental Health Center were included in present study. All patients diagnosed with depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Patients were excluded if they met the following exclusion criteria: (1) a history of ECT in the last 3 months; (2) age > 65 years; (3) diagnosed with substance misuse, schizoaffective disorder, or schizophrenia; (4) past or current neurological illness; (5) head motion exceeding 2 mm in translation or 2° in rotation during fMRI scanning; (6) other contraindications of MRI scan. Clinical severity of patients was assessed with Hamilton depression scale (HAMD) and Hamilton anxiety scale (HAMA). We also recruited 57 healthy participants who met the same exclusion criteria as depressive patients except the diagnosis of depressive disorder. This study was carried out in accordance with the recommendations of Human Brain Imaging Collection, Anhui Medical University Ethics Committee. The protocol was approved by the Anhui Medical University Ethics Committee. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
MRI Data Acquisition
Resting-state and structural images of participants were acquired at the First Affiliated Hospital of Anhui Medical University. Participants were instructed to keep their eyes closed and move and think as little as possible during the MRI scanning. Resting-state MRI scans were conducted under a 3.0 T MRI scanner (Signa HDxt 3.0 T, GE Healthcare, Buckinghamshire, United Kingdom) composed of 240 echo-planar imaging volumes with the following parameters: TR = 2000 ms; TE = 22.5 ms; flip angle = 30°; matrix size = 64 × 64, field of view = 220 mm × 220 mm; slice thickness = 4 mm; 33 continuous slices (one voxel = 3.4 mm × 3.4 mm × 4.6 mm). High resolution three-dimensional brain volume imaging (3D BRAVO) for each participant was also acquired as an anatomical reference with following parameters: TR = 8.676 ms; TE = 3.184 ms; inversion time = 800 ms; flip angle = 8°; field of view = 256 mm × 256 mm; slice thickness = 1 mm; voxel size = 1 mm × 1 mm × 1 mm.
Functional Data Preprocessing
Functional data pre-processing was conducted with the Data Processing Assistant for Resting-State Functional MR Imaging toolkit (DPARSF), a software package based on Statistical Parametric Mapping software (SPM81) and Resting State Functional MR Imaging Toolkit (REST2). The first 10 volumes were discarded to exclude the influence of unstable longitudinal magnetization. The remaining volumes were processed using the following steps: slice timing correction; realignment; coregistering to respective structural images; smoothed with a Gaussian kernel of 6 mm × 6 mm × 6 mm. The resulting images were regressed out nuisance signals, including global mean, white matter, cerebrospinal fluid signals, and 24 head-motion parameters. Finally, the images were filtered with a temporal band-pass of 0.01–0.1 Hz.
Functional Connectivity of Reward Circuits
The bilateral masks of NAcc and mOFC were defined according to the Human Brainnetome Atlas (Fan et al., 2016), a new brain atlas build upon connectional architecture. The mOFC masks consist of three subregions, labeled as ID 41, 47, 49 (left mOFC) and ID 42, 48, 50 (right mOFC) in Human Brainnetome Atlas. All seeds were showed in Figure 1. For each seeds, the functional connectivity was acquired by Pearson correlation coefficients between the mean time series of seed region and all brain voxels (defined by the binary gray matter mask in SPM). A Fisher’s Z transformation was applied to improve the normality of correlation coefficient values. Finally, two-sample t-tests were applied to map group difference of connectivity map for each seed between depressive and healthy participants.
FIGURE 1.
Medial orbitofrontal cortex (mOFC) (right and left, green) and nucleus accumbens (NAcc) (right and left, purple) seed ROIs. The underlying brain template is from MNI152 standard-space structural image. The x, y, z values are in MNI coordinates.
Statistical Analysis
For group-level structural and functional connectivity analyses, we performed two-sample t-tests for comparisons between the depressive patients and healthy controls for connectivity map of each seed. All statistical maps were thresholded using the Gaussian random field (GRF) correction with a voxel-level threshold of P < 0.001 and a cluster-level threshold of P < 0.05. We also compared changes in functional connectivity with changes in clinical symptoms among depressed individual using SPSS. The statistical level with P < 0.05 was considered as significant of correlation analysis with no correction.
Results
Demographic and Clinical Characteristic
Present study included 50 patients with current depressive episode and 57 healthy controls. Demographic characteristic of the two groups are shown in Table 1. There was no significant difference between two groups in terms of age or gender. Compared with the healthy controls, depressive individuals presented greater HAMD and HAMA scores.
Table 1.
Demographic and clinical characteristic.
| Depressive individuals | Healthy controls | t or χ2 | P-value | |
|---|---|---|---|---|
| Age (mean ±SD) | 38.68 ± 11.33 | 36.68 ± 8.76 | 1.03a | 0.31 |
| Gender (M/F) | 17/33 | 22/35 | 0.24b | 0.62 |
| HAMD (mean ±SD) | 22.78 ± 3.96 | 2.93 ± 1.51 | 35.10a | <0.001 |
| HAMA (mean ±SD) | 15.10 ± 6.81 | 2.26 ± 1.34 | 33.42a | <0.001 |
at-Value; bchi-square; HAMD, Hamilton depression scale; HAMA, Hamilton anxiety scale; SD, standard deviation; M, male; F, female.
Group-Level Comparison of Functional Connectivity of the Bilateral mOFC
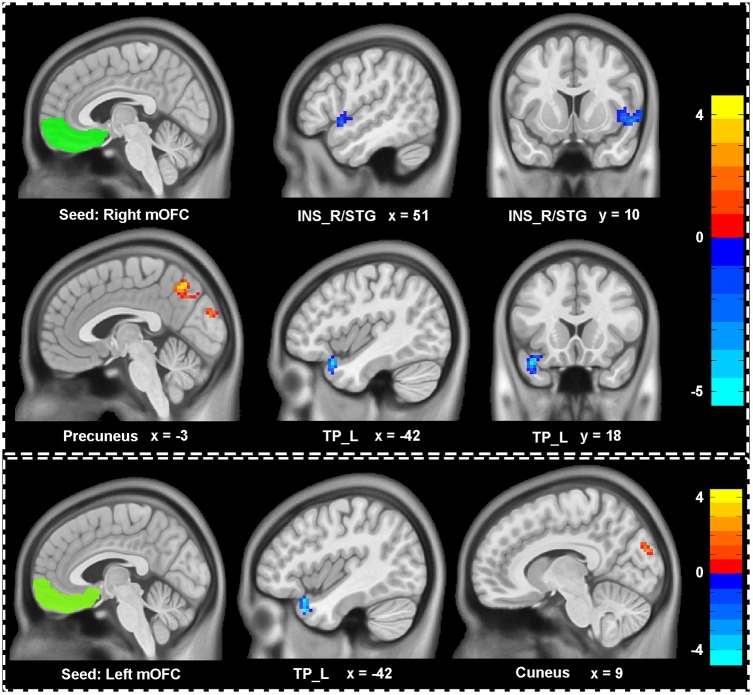
We explored the difference of functional connectivity between the two groups based on the seed of bilateral mOFC (shown in Figure 2 and Table 2). Compared with healthy controls, depressive individuals presented decreased connectivity of right mOFC with left temporal pole (TP_L) and right insula extending to superior temporal gyrus (INS_R/STG), as well as increased connectivity with left precuneus. For the left mOFC, depressive individuals presented decreased connectivity in TP_L and increased connectivity in right cuneus.
FIGURE 2.
Aberrant functional connectivity of mOFC in depressive patients compared with healthy controls. Depressive patients showed decreased connectivity of right mOFC with left temporal pole (TP_L), right insula extending to superior temporal gyrus (INS_R/STG) and increased connectivity of right mOFC with left precuneus. There were also decreased connectivity of left mOFC with LTP and increased with cuneus. All statistical maps were corrected with Gaussian random field (GRF) method at threshold of voxel P < 0.001, cluster P < 0.05. The t score bars are shown at right. The x, y values are in MNI coordinates.
Table 2.
Brain regions showing significant difference of functional connectivity with the reward network.
| Seeds | Abnormal regions | Number of voxels | Peak intensity | Peak coordinates (x, y, z)a |
|---|---|---|---|---|
| Right mOFC | TP_L | 45 | -5.47 | -42, 18, -24 |
| INS_R/STG | 80 | -4.46 | 54, 9, -6 | |
| Precuneus | 161 | 4.65 | -3, -63, 48 | |
| Left mOFC | TP_L | 50 | -4.83 | -42, 18, -33 |
| Cuneus | 63 | 4.11 | 6, -87, 24 | |
| Right NAcc | TP_L | 36 | -4.59 | -42, 18, -33 |
| TP_R | 32 | -4.38 | 33, 6, -36 | |
| Left NAcc | INS_R/STG | 35 | -4.36 | 33, 6, -6 |
aThe x, y, and z values are in Montreal Neurological Institute (MNI) coordinates. mOFC, medial orbitofrontal cortex; NAcc, nucleus accumbens; TP_L, left temporal pole; INS_R/STG, right insula extending to superior temporal gyrus; TP_R, right temporal pole.
Group-Level Comparison of Functional Connectivity of the Bilateral NAcc
Figure 3 and Table 2 showed the difference of functional connectivity based on bilateral NAcc between depressive individuals and healthy controls. Compared with healthy controls, depressive individuals presented decreased connectivity of right NAcc with right temporal pole (TP_R) and TP_L. There was also decreased connectivity of left NAcc with INS_R/STG.
FIGURE 3.
Aberrant functional connectivity of NAcc in depressive patients compared with healthy controls. Depressive patients showed decreased connectivity of right NAcc with left and right temporal pole (TP_L, TP_R), as well as decreased connectivity of left NAcc with right insula extending to superior temporal gyrus (INS_R/STG). All statistical maps were corrected with GRF method at threshold of voxel P < 0.001, cluster P < 0.05. The t score bars are shown at right. The x, y values are in MNI coordinates.
The Relationship Between Functional Connectivity and the Clinical Severity of Depressive Individuals
A negative relationship (r = -0.393, P = 0.005) existed between right NAcc-TP_R connectivity and depressive symptomatology among depressive individuals as shown in Figure 4. There was also a negative relationship (r = -0.305, P = 0.031) between right NAcc-TP_R connectivity and anxiety severity among depressive individuals.
FIGURE 4.
Scatter plots of the negative relationship between clinical severity and functional connectivity of right NAcc with right temporal pole (TP_R). The clinical severity were assessed with Hamilton depression scale (HAMD) and Hamilton anxiety scale (HAMA).
Discussion
In the present study, we investigated the function-pattern alterations of reward-related circuit during resting state in depressive patients with two key nodes in reward network, mOFC and NAcc. Compared with healthy participants, with both seeds of mOFC and NAcc, depressive individuals displayed decreased connectivity of reward network with paralimbic cortex, including temporal pole (TP), insula, and superior temporal gyrus. Furthermore, decreased connectivity between reward network and paralimbic cortex was significantly associated with clinical severity in depressive individuals.
Consistent with previous task-related neuroimaging studies, our findings with RS-fMRI also revealed the disrupted reward circuits in depressive individuals, specially, a reduction on the connectivity of mOFC and NAcc with paralimbic cortex. It is well-established that mOFC and NAcc play a crucial role in the representation of value-based information (Bartra et al., 2013). Blunt neural responses of mOFC and NAcc toward reward-related stimulus were frequently reported in depressive individuals (Tremblay et al., 2005; Redlich et al., 2015). Reduced activities of mOFC and NAcc during reward processing are associated with clinical characteristic in depression, such as depressive severity, anhedonia severity, and suicide in depression (Misaki et al., 2016; Kim et al., 2017; Rothkirch et al., 2017). Recently, findings from resting-state studies also validated the abnormal coupling of mOFC or NAcc with other brain regions in depressive individuals (Baeken et al., 2017; Gong et al., 2017). Gong et al. (2017) found decreased connectivity of NAcc with OFC, dorsomedial prefrontal cortex, superior temporal gyrus, and insular lobe in depression. Similarly, another study with brain-wide voxel-level resting state neuroimaging analysis revealed reduced functional connectivity of mOFC with temporal gyrus in depression (Cheng et al., 2016). In line with previous studies, based on both the seeds of mOFC and NAcc, we found reduced connectivity of reward system with paralimbic cortex (temporal pole and insular lobe) in depression. Significantly, the connectivity between mOFC and TP is negatively associated with clinical severity.
The TP is a node of paralimbic system with strong connectivity with orbitofrontal cortex, striatum, insula, amygdala, and other emotion-related regions (Fan et al., 2014). As an association cortex, the TP enable multisensory integration and plays key roles in cognitive and socioemotional processing, such as memory, face processing and theory of mind (Olson et al., 2007). The disturbance of these processing is constantly correlated with depression (Zobel et al., 2010; Bistricky et al., 2011). Structural and functional abnormities of the TP have been detected in depression (Beauregard et al., 2006; Peng et al., 2011). Coupled with our findings, decreased mOFC-TP connectivity in depressive individuals has been demonstrated by prior works (Cheng et al., 2016; Rolls et al., 2018). Cheng et al. (2016) concluded that reduced functional connectivity between brain areas involved in pleasant feelings and rewards with memory systems, and that this may be part of the mechanism of depression. This hypothesis is strengthened by the significant relation between the depressive severity and the reduced connection between the mOFC and temporal lobe, revealed by both present and previous studies.
Along with the TP, the insula is another part of paralimbic system and involved in the evaluation of emotional or motivational salience of external and internal stimuli (Damasio et al., 2013). Specially, the insula participate in the representation of subjective value and act as a modulator for neural responses to losses and gains, which contribute to computing the costs and benefits in mixed valence scenarios (Bartra et al., 2013). Abnormal neural responses in insula during reward-related processes were closely related with depressive symptoms (Engelmann et al., 2017). Further, our study found impaired connection between insula and acknowledged reward-related regions, OFC and ventral striatum, in depressive individuals. Indeed, there are strong structural connectivity between insula and acknowledged reward-related regions (Leong et al., 2016; Ghaziri et al., 2017). In line with our finding, previous neuroimaging study suggested that high risk for depression is related with the abnormal connection between OFC and insula during reward-related task (DelDonno et al., 2017). Based on the crucial role of insula in representation of emotional awareness and interoceptive signals, this connectional abnormity was interpreted that the impaired integration of the value of loss with the emotional and interoceptive awareness is correlated with the occurrence of depression (Rolls, 2016).
It is worthy of note that, besides decreased connectivity, increased connection of reward network among depressive individuals was also found within precuneus and cuneus. The precuneus is a key node of default mode network and involved in the sense of self and agency (Cavanna and Trimble, 2006). Increased functional connectivity during reward task between reward network and precuneus has been reported among patients with reward-related disease (Weiland et al., 2013). Studies with resting-state tool found the enhanced connectivity between precuneus and lateral OFC (defined as a non-reward/punishment system in depression) (Cheng et al., 2016). Along with precuneus, the cuneus is another prominent functional hub in the neural model of depression (Tomasi and Volkow, 2011). The cuneus is involved in the perception of facial emotion (Fusar-Poli et al., 2009), which is important for social interaction. Resting neuroimaging study in depression has suggested increased connectivity of reward network with the cuneus and that the enhanced connection was correlated with increased anhedonia severity (Yang et al., 2017). It is suggested be related to the explicit affectively negative sense of the self and increased anhedonia in depression (Rolls, 2016).
It is generally considered that there is high rate of suicide in patients with depression. Patients with suicidal behavior or ideation also presented aberrant reward processing and responses (Dombrovski et al., 2013; Silvers et al., 2016). The decreased connection between reward network and paralimbic cortex in depression may also attribute to the effect of suicide. Besides suicide, as a heterogeneous disease, depression manifest as diverse symptoms, such as anhedonia, low mood, anxiety, and somatic complaints. These symptoms have been associated with impaired reward-related processing (Avinun et al., 2017; Porreca and Navratilova, 2017; Nelson et al., 2018). For example, the increased anxiety symptoms were associated with decreased cortical activity while reward processing in both healthy participants and depressive patients (Nelson et al., 2018). Consistent with this finding, our results also implied the significant correlation between NAcc-TP connection and anxiety severity. In addition, different symptoms may have distinct effects on reward-based processing (Harle et al., 2017). Unfortunately, our study did not include the information about the specific symptoms of depressed patients. The absence of these informations restrains our attendance to understand what particular aspects of depression are associated with abnormal reward-network function. Further examinations are needed to explore the effect of specific symptoms on the reward network in depression.
It is must be acknowledged that there are several additional limitations in our study. On one hand, patients included in current study were given antidepressive medications. Future studies with drug-naïve patients to exclude the effects of medication are necessary. On the other hand, depressive patients enrolled into our study consisted of patients with both unipolar and bipolar depression. Given the different neural pattern of reward networks between unipolar and bipolar depression (Redlich et al., 2015), our findings mixed in factor of diagnostic types. Hence, our results should be interpreted with caution, and future investigations are needed divide participants into subgroups to clarify the distinct mechanisms underlying the specific subtypes of depressive individuals.
Conclusion
It compared with healthy participants, the depressive individuals showed decreased connectivity of reward network with paralimbic cortex, including TP, insula. The findings were validated with two key seeds of reward network, mOFC and NAcc. Significantly, the decreased connectivity between mOFC and TP was associated with depressive severity. Our study demonstrated reward-network abnormalities among depressive patients in resting-state functional pattern that underlies the pathogenesis of depression. These findings might also imply a potential biomarker for clinical applications.
Author Contributions
TB and MZ performed the analysis and wrote the paper. YC, WX, CC, and QW helped to collect behavioral and imaging data. G-JJ help for the analysis of resting-state imaging data. YT and KW designed and supervised the present study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participants for their cooperation during this study. We also thank G-JJ for his assistance during our analysis of resting-state data.
Funding. This work was supported by funding from the National Nature Science Foundation of China (Grant Nos. 81471117, 81671354, 81601187, 91432301, and 91732303), the National Basic Research Program of China (Grant No. 2015CB856400), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2015BAI13B01), and the Anhui Provincial Science Fund for Distinguished Young Scholars (Grant No. 1808085J23).
References
- Anhoj S., Odegaard Nielsen M., Jensen M. H., Ford K., Fagerlund B., Williamson P., et al. (2018). Alterations of intrinsic connectivity networks in antipsychotic-naive first-episode schizophrenia. Schizophr. Bull. 10.1093/schbul/sbx171 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R. P., Pisoni A., Bondy E., Kumar P., Stewart J. G., Yendiki A., et al. (2017). Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology 42 2087–2095. 10.1038/npp.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J. A., Drevets W. C., Moseman S. E., Bodurka J., Barcalow J. C., Simmons W. K. (2014). Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatry 76 258–266. 10.1016/j.biopsych.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avinun R., Nevo A., Knodt A. R., Elliott M. L. (2017). Reward-related ventral striatum activity buffers against the experience of depressive symptoms associated with sleep disturbances. 37 9724–9729. 10.1523/jneurosci.1734-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeken C., Duprat R., Wu G. R., De Raedt R., van Heeringen K. (2017). Subgenual anterior cingulate-medial orbitofrontal functional connectivity in medication-resistant major depression: a neurobiological marker for accelerated intermittent theta burst stimulation treatment?. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 556–565. 10.1016/j.bpsc.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Bartra O., McGuire J. T., Kable J. W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M., Paquette V., Levesque J. (2006). Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport 17 843–846. 10.1097/01.wnr.0000220132.32091.9f [DOI] [PubMed] [Google Scholar]
- Bistricky S. L., Ingram R. E., Atchley R. A. (2011). Facial affect processing and depression susceptibility: cognitive biases and cognitive neuroscience. Psychol. Bull. 137 998–1028. 10.1037/a0025348 [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Cavanna A. E., Trimble M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Chen X., Ji G. J., Zhu C., Bai X., Wang L., He K., et al. (2018). Neural correlates of auditory verbal hallucinations in schizophrenia and the therapeutic response to Theta-Burst transcranial magnetic stimulation. Schizophrenia. Bull. 10.1093/schbul/sby054 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E. T., Qiu J., Liu W., Tang Y., Huang C. C., et al. (2016). Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 139 3296–3309. 10.1093/brain/aww255 [DOI] [PubMed] [Google Scholar]
- Damasio A., Damasio H., Tranel D. (2013). Persistence of feelings and sentience after bilateral damage of the insula. Cereb. Cortex 23 833–846. 10.1093/cercor/bhs077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelDonno S. R., Jenkins L. M., Crane N. A., Nusslock R., Ryan K. A., Shankman S. A., et al. (2017). Affective traits and history of depression are related to ventral striatum connectivity. J. Affect. Disord. 221 72–80. 10.1016/j.jad.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Markou A. (2012). The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35 68–77. 10.1016/j.tins.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski A. Y., Szanto K., Clark L., Reynolds C. F., Siegle G. J. (2013). Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry 70:1. 10.1001/jamapsychiatry.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale A. T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., et al. (2016). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducasse D., Loas G., Dassa D., Gramaglia C., Zeppegno P., Guillaume S., et al. (2018). Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress. Anxiety 35 382–392. 10.1002/da.22709 [DOI] [PubMed] [Google Scholar]
- Engelmann J. B., Berns G. S., Dunlop B. W. (2017). Hyper-responsivity to losses in the anterior insula during economic choice scales with depression severity. Psychol. Med. 47 2879–2891. 10.1017/s0033291717001428 [DOI] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L., et al. (2016). The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex. 26 3508–3526. 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Wang J., Zhang Y., Han W., Yu C., Jiang T. (2014). Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb. Cortex 24 3365–3378. 10.1093/cercor/bht196 [DOI] [PubMed] [Google Scholar]
- Ferrari A. J., Charlson F. J., Norman R. E., Patten S. B., Freedman G., Murray C. J., et al. (2013). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10:e1001547. 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 34 418–432. [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J., Tucholka A., Girard G., Houde J. C., Boucher O., Gilbert G., et al. (2017). The corticocortical structural connectivity of the human insula. Cereb. Cortex 27 1216–1228. 10.1093/cercor/bhv308 [DOI] [PubMed] [Google Scholar]
- Gong L., Yin Y., He C., Ye Q., Bai F., Yuan Y., et al. (2017). Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J. Psychiatr. Res. 84 9–17. 10.1016/j.jpsychires.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Gottfried J. A., O’Doherty J., Dolan R. J. (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301 1104–1107. 10.1126/science.1087919 [DOI] [PubMed] [Google Scholar]
- Guo C. C., Hyett M. P., Nguyen V. T., Parker G. B., Breakspear M. J. (2016). Distinct neurobiological signatures of brain connectivity in depression subtypes during natural viewing of emotionally salient films. Psychol. Med. 46 1535–1545. 10.1017/S0033291716000179 [DOI] [PubMed] [Google Scholar]
- Harle K. M., Guo D., Zhang S., Paulus M. P., Yu A. J. (2017). Anhedonia and anxiety underlying depressive symptomatology have distinct effects on reward-based decision-making. PLoS One 12:e0186473. 10.1371/journal.pone.0186473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Larsen B., Hallquist M. N., Foran W., Calabro F., Luna B. (2017). Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry 82 511–521. 10.1016/j.biopsych.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G. J., Liao W., Chen F. F., Zhang L., Wang K. (2017). Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: more than just noise. Sci. Bull. 62 656–657. 10.1016/j.scib.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Kahnt T., Chang L. J., Park S. Q., Heinzle J., Haynes J. D. (2012). Connectivity-based parcellation of the human orbitofrontal cortex. J. Neurosci. 32 6240–6250. 10.1523/jneurosci.0257-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher N. R., Bartholow B. D., Martin E. A., Kerns J. G. (2017). Associations between electrophysiological evidence of reward and punishment-based learning and psychotic experiences and social anhedonia in at-risk groups. Neuropsychopharmacology 42 925–932. 10.1038/npp.2016.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton M. J., Salvador Z., Munafo M. R., Geddes J. R., Simmons A., Frangou S., et al. (2011). Structural neuroimaging studies in major depressive disorder. meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry 68 675–690. 10.1001/archgenpsychiatry.2011.60 [DOI] [PubMed] [Google Scholar]
- Kim K., Kim S. W., Myung W., Han C. E., Fava M., Mischoulon D., et al. (2017). Reduced orbitofrontal-thalamic functional connectivity related to suicidal ideation in patients with major depressive disorder. Sci. Rep. 7;15772. 10.1038/s41598-017-15926-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. K., Pestilli F., Wu C. C., Samanez-Larkin G. R., Knutson B. (2016). White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron 89 63–69. 10.1016/j.neuron.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Shen Z., Cheng Y., Yang H., He B., Xie Y., et al. (2017). Alternations of white matter structural networks in first episode untreated major depressive disorder with short duration. Front. Psychiatry 8:205. 10.3389/fpsyt.2017.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Suzuki H., Savitz J., Drevets W. C., Bodurka J. (2016). Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Sci. Rep. 6:21227. 10.1038/srep21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. D., Kessel E. M., Klein D. N., Shankman S. A. (2018). Depression symptom dimensions and asymmetrical frontal cortical activity while anticipating reward. Psychophysiology 55:e12892. 10.1111/psyp.12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J., Kringelbach M. L., Rolls E. T., Hornak J., Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- Olson I. R., Plotzker A., Ezzyat Y. (2007). The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130 1718–1731. 10.1093/brain/awm052 [DOI] [PubMed] [Google Scholar]
- Peng J., Liu J., Nie B., Li Y., Shan B., Wang G., et al. (2011). Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur. J. Radiol. 80 395–399. 10.1016/j.ejrad.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 10 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D. A., Holmes A. J., Dillon D. G., Goetz E. L., Birk J. L., Bogdan R., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 166 702–710. 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F., Navratilova E. (2017). Reward, motivation, and emotion of pain and its relief. Pain 158(Suppl. 1) S43–S49. 10.1097/j.pain.0000000000000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R., Dohm K., Grotegerd D., Opel N., Zwitserlood P., Heindel W., et al. (2015). Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology 40 2623–2631. 10.1038/npp.2015.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S. J., Lambert C., Kennedy S. (2018). Presentation and neurobiology of anhedonia in mood disorders: commonalities and distinctions. Curr. Psychiatry Rep. 20:13. 10.1007/s11920-018-0877-z [DOI] [PubMed] [Google Scholar]
- Rolls E. T. (2016). A non-reward attractor theory of depression. Neurosci. Biobehav. Rev. 68 47–58. 10.1016/j.neubiorev.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Rolls E. T., Cheng W., Gilson M., Qiu J., Hu Z., Ruan H., et al. (2018). Effective connectivity in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 187–197. 10.1016/j.bpsc.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Rothkirch M., Tonn J., Kohler S., Sterzer P. (2017). Neural mechanisms of reinforcement learning in unmedicated patients with major depressive disorder. Brain 140 1147–1157. 10.1093/brain/awx025 [DOI] [PubMed] [Google Scholar]
- Rzepa E., McCabe C. (2016). Decreased anticipated pleasure correlates with increased salience network resting state functional connectivity in adolescents with depressive symptomatology. J. Psychiatr. Res. 82 40–47. 10.1016/j.jpsychires.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J. D., Correa M., Mingote S. M., Weber S. M. (2005). Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr. Opin. Pharmacol. 5 34–41. 10.1016/j.coph.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Takahashi Y., Liu T. L., McDannald M. A. (2011). Does the orbitofrontal cortex signal value? Ann. N. Y. Acad. Sci. 1239 87–99. 10.1111/j.1749-6632.2011.06210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G., Caldu X., Segura B., Dreher J. C. (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 37 681–696. 10.1016/j.neubiorev.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Silvers J. A., Hubbard A. D., Chaudhury S., Biggs E., Shu J., Grunebaum M. F., et al. (2016). Suicide attempters with borderline personality disorder show differential orbitofrontal and parietal recruitment when reflecting on aversive memories. J. Psychiatr. Res. 81 71–78. 10.1016/j.jpsychires.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski M. J., Felder J., Bizzell J., Green S. R., Ernst M., Lynch T. R., et al. (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord. 118 69–78. 10.1016/j.jad.2009.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N. D. (2011). Functional connectivity hubs in the human brain. Neuroimage 57 908–917. 10.1016/j.neuroimage.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L. K., Naranjo C. A., Graham S. J., Herrmann N., Mayberg H. S., Hevenor S., et al. (2005). Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch. Gen. Psychiatry 62 1228–1236. 10.1001/archpsyc.62.11.1228 [DOI] [PubMed] [Google Scholar]
- Ubl B., Kuehner C., Kirsch P., Ruttorf M., Diener C., Flor H. (2015). Altered neural reward and loss processing and prediction error signalling in depression. Soc. Cogn. Affect. Neurosci. 10 1102–1112. 10.1093/scan/nsu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E., Demyttenaere K., Bruffaerts R., Hermans D., Pizzagalli D. A., Sienaert P., et al. (2014). Dimensions in major depressive disorder and their relevance for treatment outcome. J. Affect. Disord. 155 35–41. 10.1016/j.jad.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland B. J., Welsh R. C., Yau W. Y., Zucker R. A., Zubieta J. K., Heitzeg M. M. (2013). Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 128 130–139. 10.1016/j.drugalcdep.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Tian K., Wang D. F., Wang Y., Cheung E. F. C., Xie G. R., et al. (2017). Anhedonia correlates with abnormal functional connectivity of the superior temporal gyrus and the caudate nucleus in patients with first-episode drug-naive major depressive disorder. J. Affect. Disord. 218 284–290. 10.1016/j.jad.2017.04.053 [DOI] [PubMed] [Google Scholar]
- Zhang D., Raichle M. E. (2010). Disease and the brain’s dark energy. Nat. Rev. Neurol. 6 15–28. 10.1038/nrneurol.2009.198 [DOI] [PubMed] [Google Scholar]
- Zobel I., Werden D., Linster H., Dykierek P., Drieling T., Berger M., et al. (2010). Theory of mind deficits in chronically depressed patients. Depress. Anxiety 27 821–828. 10.1002/da.20713 [DOI] [PubMed] [Google Scholar]