Abstract
Aims
Proton pump inhibitors (PPIs) belong to the most frequently used drugs, also in patients with cirrhosis. PPIs are extensively metabolized by the liver, but practice guidance on prescribing in cirrhosis is lacking. We aim to develop practical guidance on the safe use of PPIs in patients with cirrhosis.
Methods
A systematic literature search identified studies on the safety (i.e. adverse events) and pharmacokinetics of PPIs in cirrhotic patients. This evidence and data from the product information was reviewed by an expert panel who classified drugs as safe; no additional risks known; additional risks known; unsafe; or unknown. Guidance was aimed at the oral use of PPIs and categorized by the severity of cirrhosis, using the Child–Turcotte–Pugh (CTP) classification.
Results
A total of 69 studies were included. Esomeprazole, omeprazole and rabeprazole were classified as having ‘no additional risks known’. A reduction in maximum dose of omeprazole and rabeprazole is recommended for CTP A and B patients. For patients with CTP C cirrhosis, the only PPI advised is esomeprazole at a maximum dosage of 20 mg per day. Pantoprazole and lansoprazole were classified as unsafe because of 4‐ to 8‐fold increased exposure. The use of PPIs in cirrhotic patients has been associated with the development of infections and hepatic encephalopathy and should be carefully considered.
Conclusions
We suggest using esomeprazole, omeprazole or rabeprazole in patients with CTP A or B cirrhosis and only esomeprazole in patients with CTP C. Pharmacokinetic changes are also important to consider when prescribing PPIs to vulnerable, cirrhotic patients.
Keywords: drug safety, evidence‐based medicine, hepatology, liver
What is Already Known about this Subject
Proton pump inhibitors (PPIs) are all metabolized by the liver.
Pathophysiological changes occurring in cirrhosis affect the pharmacokinetics and pharmacodynamics of drugs.
The safety of PPIs in cirrhosis has been questioned lately.
What this Study Adds
Exposure to lansoprazole and pantoprazole in patients with cirrhosis was considerably increased while esomeprazole pharmacokinetics seemed largely spared.
When used orally, a PPI without large pharmacokinetic changes is recommended in the vulnerable cirrhotic patient.
Future studies examining the safety of PPIs should also pay attention to differences between PPIs.
Introduction
http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=160 inhibitors (PPIs) are among the most frequently used medications worldwide 1. They are effective drugs in suppressing acid secretion and have a wide margin of safety. In recent years, safety issues have been raised which led the FDA to issue several warnings 2. Long‐term PPI use has been associated with increased risk of respiratory infections, bone fractures and hypomagnesaemia, especially in older people with comorbidities such as renal or liver disease 3, 4, 5. In addition, use of PPIs in patients with cirrhosis has been linked to the development of spontaneous bacterial peritonitis and hepatic encephalopathy (HE) 6, 7, 8. Intestinal bacterial overgrowth and translocation are mentioned as possible causes 9, 10. These risks are particularly relevant as patients with cirrhosis frequently use PPIs. Two recent studies suggest that more than half of cirrhotics received a PPI, often without a clear indication 6, 11.
All PPIs are metabolized by the liver. The pathophysiological changes that accompany cirrhosis affect pharmacokinetics. Portal vein shunting leads to a higher systemic availability of drugs, while synthetic insufficiency results in low levels of plasma proteins and a higher unbound fraction 12, 13. Even so, the activity of drug‐metabolizing enzymes is decreased and biliary excretion can be reduced 12, 13. These changes often result in higher plasma concentrations and increased exposure to drugs in patients with cirrhosis. For PPIs, a rise in exposure can lead to enhanced acid suppression 14, 15. This raises questions whether pharmacokinetic alterations due to cirrhosis influence the safety profile of PPIs and whether dose adjustments are needed.
Currently, there is a paucity of practice guidance for the safe use and dosing of PPIs in cirrhosis. In a previous study, a method was developed to use pharmacokinetic and safety data for evaluating drug safety in cirrhosis 16. In the current study, we use this method to develop safety and dosing practical guidance for the use of PPIs in patients with cirrhosis.
Methods
We used a combination of information from registration authorities, literature and expert opinion to develop practical guidance 16. A specific method was needed to translate the available literature and experience into an easy manageable source of information on safe prescribing aimed at the needs of clinical decision making. A detailed version of this method has been published before 16. All PPIs currently registered in the Netherlands were considered for evaluation. These were: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5488, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7208, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4279, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7260 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7290. We focused on developing guidance for the oral use of PPIs; the intravenous use in gastrointestinal bleeding is considered life‐saving and only used for a short period of time. The safety evaluation process consisted of several steps. Steps 1–3 were performed by a pharmacist with experience in evaluating drug safety in cirrhosis (R.W.). Critical steps were checked by a second pharmacist/epidemiologist (S.B.).
Step 1: Collection of evidence
Data collection focused on gathering all available evidence needed to evaluate the safety and pharmacokinetics of PPIs in cirrhotic patients. This included data from registration authorities (product information) and published literature. Electronic databases PubMed and Embase were searched and Web of Science was used for citation tracking. The search strategy can be found in Table 1. Articles were included if (one of) the outcome(s) was safety and/or pharmacokinetics of a PPI in patients with cirrhosis.
Table 1.
Search strategy used for electronic database search
| Pubmed | (“Liver cirrhosis”[Mesh] OR cirrho*[ti] OR “hepatic impairment”[ti] OR “liver impairment”[ti] OR “hepatic dysfunction”[ti] OR “liver dysfunction”[ti] OR “hepatic insufficiency”[ti] OR “liver insufficiency”[ti]) AND (“Esomeprazole”[Mesh] OR “Omeprazole”[Mesh] OR “Lansoprazole”[Mesh] OR “Rabeprazole”[Mesh] OR “pantoprazole”[Supplementary Concept] OR “Proton Pump Inhibitors”[Mesh] OR “Esomeprazole”[tiab] OR “Omeprazole”[tiab] OR “Lansoprazole”[tiab] OR “Rabeprazole”[tiab] OR “pantoprazole”[tiab] OR “proton pump inhibitor”[tiab] OR “proton pump inhibitors”[tiab]) |
| Embase | ‘liver cirrhosis’/exp OR cirrho*:ti OR ‘hepatic impairment’:ti OR ‘liver impairment’:ti OR ‘hepatic dysfunction’:ti OR ‘liver dysfunction’:ti OR ‘hepatic insufficiency’:ti OR ‘liver insufficiency’:ti AND (‘omeprazole’/exp OR ‘pantoprazole’/exp OR ‘esomeprazole’/exp OR ‘rabeprazole’/exp OR ‘lansoprazole’/exp OR ‘omeprazole’:ab,ti OR ‘pantoprazole’:ab,ti OR ‘esomeprazole’:ab,ti OR ‘rabeprazole’:ab,ti OR ‘lansoprazole’:ab,ti) AND [humans]/lim |
Step 2: Data extraction and presentation
Pharmacokinetic and safety data were extracted from the American and European authorized product information of each PPI and presented in a table. If no European product information was available, the Dutch product information was used. From the included literature, the study design, number and characteristics of patients and controls (e.g. severity of cirrhosis) and details on the intervention were retrieved. The following data were extracted on the outcome(s):
Pharmacokinetics: pharmacokinetic parameters of the PPI [e.g. maximum plasma concentration (C max) and area under the curve (AUC)].
Safety: the number of adverse events (AEs) observed during PPI use and data on discontinuation due to these events.
Results were reported in summary tables for each outcome and sorted by level of evidence. The evidence level of each study was assessed using the treatment harms criteria from the Oxford Centre for Evidence‐Base Medicine 17.
Step 3: Classification and dose suggestion
Based on the collected data, an initial safety classification and dose was suggested for each PPI, if applicable sorted by severity of cirrhosis. The severity was expressed using the Child–Turcotte–Pugh (CTP) classification 18. The safety classification could be: safe, no additional risks known, additional risks known, unsafe, or unknown. Table 2 provides an overview of the safety classification and the actions advised for health care professionals. Pharmacokinetic data were used to judge whether a dose adjustment was necessary.
Table 2.
Safety classification of drugs used in cirrhosis
| Description | Action | |
|---|---|---|
| Safe | The drug has been evaluated in patients with cirrhosis, and no increase in harm was found. The safety of the drug is supported by pharmacokinetic studies and/or safety studies over a long period. It might be necessary to use an adjusted dose. | This drug can be used by patients with cirrhosis. |
| No additional risks known | Limited data suggest that this drug does not increase harm in patients with cirrhosis in comparison with persons without cirrhosis. It might be necessary to use an adjusted dose. | The drug can be used in patients with cirrhosis. Adverse events need to be monitored. |
| Additional risks known | Limited data suggest an increase in patient harm in patients with cirrhosis compared to persons without cirrhosis. However, the number of studies is limited and/or the studies show contradicting results about the safety in patients with cirrhosis. | This drug should preferably not be used in patients with cirrhosis if there is a safer alternative available. Adverse events need to be monitored. |
| Unsafe | Data indicate this drug is not safe in patients with cirrhosis. | This drug should be avoided in patients with cirrhosis. |
| Unknown | For this drug, insufficient data are available to evaluate the safety in patients with cirrhosis. | This drug should preferably not be used in patients with cirrhosis if there is a safer alternative available. Individual judgement of therapeutic need vs. additional risks in patients with cirrhosis. Adverse events need to be monitored. |
Adapted from: Weersink et al. 16
Step 4: Discussion and conclusion by an expert panel
An expert panel was composed consisting of ten members with specific expertise in the treatment of patients with cirrhosis, in clinical pharmacology and/or in evidence‐based medicine. These included gastroenterologists, a general practitioner and hospital and community pharmacists. The expert panel evaluated data extraction and presentation (Steps 1 and 2) and endorsed conclusions derived from the evidence (Step 3). Likewise, the validity and clinical relevance of the proposed safety classification and suggested dose were discussed by the expert panel during a meeting. The final advice was based on evidence and clinical experience of the expert panel and concluded by consensus. All conflicts of interest of the members of the expert panel were identified, disclosed and published 16. The chair of the expert panel (S.B.) declared no conflicts of interest.
Step 5: Implementation
Practical guidance was incorporated in the two national drug databases in the Netherlands (Pharmabase and G‐standard) and on a free website. Health care professionals will get specific alerts when prescribing PPIs in cirrhosis and are referred to the website for more information.
Step 6: Continuity
To keep the advice up‐to‐date, literature searches will be checked yearly and relevant studies will be discussed with the expert panel. Once every five years, a complete update is planned.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 19, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 20.
Results
The developed practical guidance is based on information from the product information (Table 3) 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 and data extracted from 69 articles included in the literature review (Figure 1) 6, 7, 8, 11, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95. Twelve of the included studies focused on pharmacokinetics (Table 4), 51 on safety, and six studied both safety and pharmacokinetics of PPIs. Of the safety studies, 20 specifically investigated the safety of an individual PPI (Table 5), while 37 studied safety issues of PPIs as a group (Table S1).
Table 3.
Special warnings of the European and US product information regarding the use of PPIs in patients with cirrhosis
| PPI | SmPC a | FDA label |
|---|---|---|
| Esomeprazole [ 21 , 22 ] | In patients with mild or moderate hepatic impairment, the metabolism of esomeprazole could be decreased. In patients with severe hepatic impairment, the metabolism of esomeprazole is decreased leading to a doubling of the AUC. Therefore, do not exceed the maximum dose of 20 mg in patients with severe hepatic impairment. Esomeprazole and main metabolites do not tend to accumulate with once daily dosing. | The steady state pharmacokinetics of esomeprazole obtained after administration of 40 mg once daily to four patients each with mild (Child–Pugh A), moderate (Child–Pugh Class B), and severe (Child–Pugh Class C) liver insufficiency were compared to those obtained in 36 male and female Gastro‐oesophageal Reflux Disease patients with normal liver function. In patients with mild and moderate hepatic insufficiency, the AUCs were within the range that could be expected in patients with normal liver function. In patients with severe hepatic insufficiency, the AUCs were 2–3 times higher than in the patients with normal liver function. No dosage adjustment is recommended for patients with mild to moderate hepatic insufficiency (Child–Pugh Classes A and B). However, in patients with severe hepatic insufficiency (Child–Pugh Class C), a dose of 20 mg once daily should not be exceeded. |
| Lansoprazole [ 23 , 24 ] | The exposure to lansoprazole is doubled in patients with mild hepatic impairment and much more increased in patients with moderate to severe hepatic impairment. Patients with moderate to severe hepatic impairment should be kept under regular supervision and a 50% reduction of the daily dose is recommended. | In patients with various degrees of chronic hepatic impairment, the mean plasma half‐life of lansoprazole was prolonged from 1.5 h to 3.2–7.2 h. An increase in the mean AUC of up to 500% was observed at steady state in hepatically‐impaired patients compared to healthy subjects. Consider dose reduction in patients with severe hepatic impairment. |
| Omeprazole [ 25 , 26 ] | In patients with hepatic impairment, the metabolism of omeprazole is decreased causing a higher AUC. The once daily dosing of omeprazole has no tendency to accumulate. For patients with hepatic impairment, a daily dose of 10–20 mg may be sufficient. | In patients with chronic hepatic disease, the bioavailability increased to approximately 100% compared with an IV dose, reflecting decreased first‐pass effect, and the plasma half‐life of the drug increased to nearly 3 h compared with the half‐life in normal subjects of 0.5–1 h. Plasma clearance averaged 70 ml min−1, compared with a value of 500–600 ml min−1 in normal subjects. Dose reduction, particularly where maintenance of healing of erosive esophagitis is indicated, for the hepatically impaired should be considered. |
| Pantoprazole [ 27 , 28 ] | Although for patients with liver cirrhosis (Child–Pugh A and B) the half‐life increased to 7–9 h, and the AUC increased by a factor 5–7, the maximum serum concentration only increased by a factor of 1.5 compared to healthy individuals. In patients with severe hepatic impairment, a daily dose of 20 mg of pantoprazole may not be exceeded. Pantoprazole 40 mg should not be used in combination therapy for the eradication of H. pylori in patients with moderate to severe hepatic impairment, since no data are available on the efficacy and safety. Liver enzymes in patients with severe hepatic impairment should be monitored regularly. If there is an increase in liver enzyme values, the treatment should be stopped | In patients with mild to severe hepatic impairment (Child–Pugh A–C cirrhosis), maximum pantoprazole concentrations increased only slightly (1.5‐fold) relative to healthy subjects. Although serum half‐life values increased to 7–9 h and AUC values increased by five‐ to sevenfold in hepatic‐impaired patients, these increases were no greater than those observed in CYP2C19 poor metabolizers, where no dosage adjustment is warranted. These pharmacokinetic changes in hepatic‐impaired patients result in minimal drug accumulation following once‐daily, multiple‐dose administration. No dosage adjustment is needed in patients with mild to severe hepatic impairment. Doses higher than 40 mg day−1 have not been studied in hepatically impaired patients. |
| Rabeprazole [ 29 , 30 ] | In patients with mild to moderate hepatic impairment, the AUC doubled compared to healthy volunteers after administration of a single dose of 20 mg rabeprazole, and there was a two‐ to three‐fold increase in the half‐life of rabeprazole. After a daily dose of 20 mg for 7 days, however, the AUC was increased only by a factor of 1.5 and the C max only by a factor of 1.2. In patients with hepatic impairment, the half‐life of rabeprazole was 12.3 h compared to 2.1 h in healthy volunteers. The pharmacodynamic response in the two groups (determination of pH in the stomach) was clinically comparable. For patients with hepatic impairment, no dose adjustments are required. | In a single‐dose study of 10 patients with chronic mild to moderate compensated cirrhosis of the liver who were administered a 20 mg dose of rabeprazole, AUC was approximately doubled, the elimination half‐life was two‐ to threefold higher, and total body clearance was decreased to less than half compared to values in healthy men. In a multiple‐dose study of 12 patients with mild to moderate hepatic impairment administered 20 mg rabeprazole once daily for eight days, AUC and C max values increased approximately 20% compared to values in healthy age‐ and gender‐matched subjects. These increases were not statistically significant. No information exists on rabeprazole disposition in patients with severe hepatic impairment. Administration of rabeprazole to patients with mild to moderate liver impairment resulted in increased exposure and decreased elimination. Due to the lack of clinical data on rabeprazole in patients with severe hepatic impairment, caution should be exercised in those patients. |
AUC, area under the curve; C max, maximum plasma concentration; PPI, proton pump inhibitor; SmPC, summary of product characteristics.
Translated from Dutch.
Figure 1.
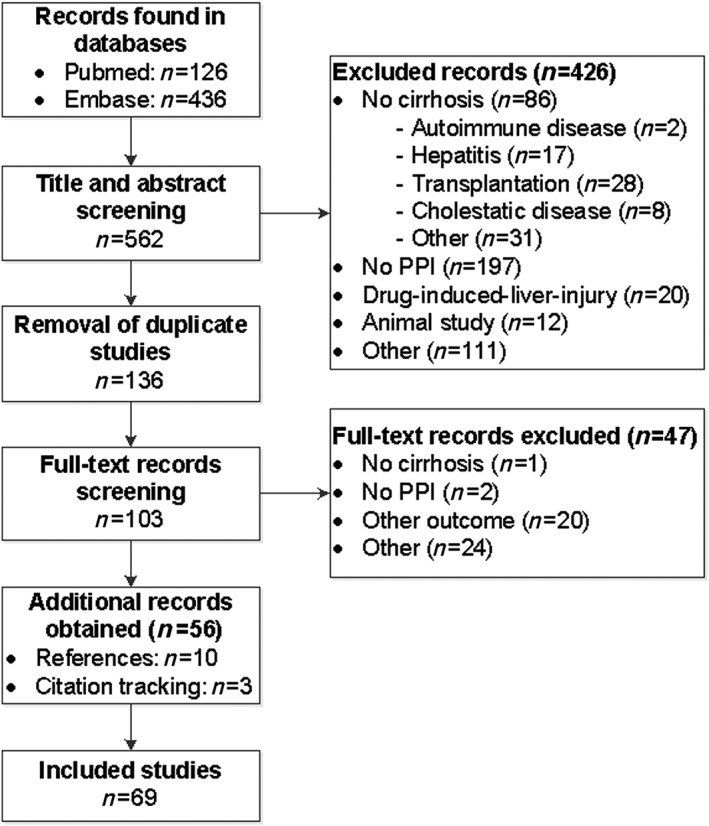
Flowchart of study selection process
Table 4.
Summary table of pharmacokinetic studies of PPIs in patients with cirrhosis, sorted by Child–Pugh class 18
| Ref. | Evidence level | Intervention | Results (expressed as ratioa ) | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Controls | Cirrhotic patients | |||||
| CTP A | CTP B | CTP C | |||||
| [31] | 4 | Esomeprazole (40 mg day−1 for 5 days) | n = 36 (literature) | n = 4 | n = 4 | n = 4 | |
| C max | 1 | 1.38 | 1.15 | 1.36 | |||
| AUC t | 1 | 1.42 | 1.77 | 2.34 | |||
| [32] | 3 | Lansoprazole (single dose of 30 mg) | n = 18 | n = 8 (compensated) | n = 8 (decompensated) | ||
| C max | 1 | 1.39 | 1.10 | ||||
| AUC 0–48 h | 1 | 4.38 | 4.01 | ||||
| [33] | 4 | Lansoprazole (PK modelling) | AUC total | 1 | 2.94 | 4.13 | 7.56 |
| AUC unbound | 1 | 3.19 | 5.41 | 12.73 | |||
| [34] | 3 | Omeprazole (single dose of 20 mg) | n = 10 | n = 10 | n = 10 | n = 10 | |
| C max | 1 | 0.95 | 1.15 | 1.32 | |||
| AUC ∞ | 1 | 1.69 | 2.71 | 2.79 | |||
| [35] | 3 | Omeprazole (single dose of 20 mg) | n = 8 | n = 8 (CTP unknown) | |||
| C max | 1 | 2.55 | |||||
| AUC ∞ | 1 | 8.38 | |||||
| [36] | 4 | Omeprazole (80 mg bolus + 8 mg h−1 continuous infusion for 47.5 h; total 460 mg) | n = 12 | n = 5 | n = 4 | n = 3 | |
| C max | 1 | 1.49 | |||||
| AUC 0–48 h | 1 | 1.59 | 1.85 | 2.14 | |||
| [37] | 4 | Omeprazole (single dose of 40 mg) | n = 18 (literature) | n = 3 | n = 4 | n = 1 | |
| C max | 1 | 2.57 | |||||
| AUC ∞ | 1 | 7.3 | |||||
| [33] | 4 | Omeprazole (PK modelling) | AUC total | 1 | 2.65 | 3.61 | 6.96 |
| AUC unbound | 1 | 3.23 | 5.04 | 10.74 | |||
| [38] | 3 | Pantoprazole (40 mg day−1 for 7 days) | n = 12 | n = 12 (CTP A+B) | |||
| C max | 1 | 1.55 | |||||
| AUC 0–24 h | 1 | 6.77 (5.3–7.8) | |||||
| Pantoprazole (30 mg day−1 IV for 5 days) | n = 8 | n = 12 (CTP A+B) | |||||
| C max | 1 | 1.66 | |||||
| AUC 0–24 h | 1 | 5.03 | |||||
| [39] | 3 | Pantoprazole (40 mg day−1 for 7 days) | n = 12 (CTP unknown) | ||||
| C max | 1 | 1.44 | |||||
| AUC 0–24 h | 1 | 6.6 | |||||
| Pantoprazole (30 mg day−1 IV for 5 days) | C max | 1 | 1.62 | ||||
| AUC 0–24 h | 1 | 5.5 | |||||
| [33] | 4 | Pantoprazole (PK modelling) | AUC total | 1 | 2.49 | 2.90 | 3.80 |
| AUC unbound | 1 | 2.70 | 3.79 | 6.35 | |||
| [40] | 3 | Rabeprazole (single dose of 20 mg) | n = 13 | n = 10 (compensated) | |||
| C max | 1 | 1.58 | |||||
| AUC 0–24 h | 1 | 2.20 | |||||
| [33] | 4 | Rabeprazole (PK modelling) | AUC total | 1 | 1.98 | 2.34 | 3.09 |
| AUC unbound | 1 | 2.42 | 3.29 | 5.15 | |||
Presented are studies that determined the AUC for patients with cirrhosis and compared it to healthy controls. Studies determining other pharmacokinetic parameters are presented in the text. AUC, area under the curve; C max, peak plasma concentration; CTP, Child–Turcotte–Pugh class; IV, intravenous; PK, pharmacokinetic; Ref, reference.
Ratio: value for C max or AUC divided by the value of the control group.
Table 5.
Summary table of studies on the safety of individual PPIs in cirrhosis
| Ref. | Evidence level | Study design | Patients | Intervention (n; CTP A/B/C) | Control (n; CTP A/B/C) | Patients with AEs | AEs reported with PPI intervention | Discontinuation | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| [41] | 2 | Randomized controlled trial | Cirrhosis + bleeding oesophageal varices | OME or PANT 40 mg day−1 IV for 5 days ➔ PANT 40 mg PO for 14 days (n = 58; 15/24/19) |
Somatostatin 250 μg h−1 or terlipressin 1 mg/6 h for 5 days IV (n = 60; 18/32/10) |
• I: n = 3 (5.2%) • C: n = 33 (55.0%) |
• Fever (n = 2) and oesophageal ulcer bleeding (n = 1) |
• I: 0/58 • C: 0/60 |
|
| [42] | 2 | Randomized controlled trial | Cirrhosis + oesophageal varices + previous EVL | EVL, followed by RAB 10 mg OD for 2 years (n = 21; 17/4/0) |
Only EVL (n = 22; 16/6/0) |
• I: n = 9 (43%) • C: n = 11 (50%) |
• Mild dysphagia (n = 4), ascites (n = 4), and haemorrhoid bleeding (n = 1). |
• I: 0/21 • C: NA |
|
| [43] | 2 | Randomized controlled trial | Cirrhosis + history of bleeding oesophageal varices | EVL and 40 mg PANT IV + 40 mg PO for 9 days (n = 22; 10/8/4) |
EVL and IV saline + placebo for 9 days (n = 22; 9/10/3) |
• I: n = 0 • C: n = 4 (18%) |
‐ |
• I: 0/22 • C: 2/22 |
|
| [44] | 3 | Clinical trial | Cirrhosis | OME 40 mg OD for 14 days (n = 15; 15/0/0) | Age‐matched healthy controls receiving the same treatment (n = 15) |
• I: n = 0 • C: n = 0 |
‐ | ‐ | |
| [45] | 3 | Open‐label study for 8 weeks | Cirrhosis + peptic lesions | RABE 10 mg day−1 or 20 mg day−1 (n = 70; 30 compensated cirrhosis) | ‐ | • I: n = 9 (13%) |
• Mild: purpura, eosinophilia, loose stools (all n = 2), increased AP + γ‐GT (n = 3) • Severe: dyslalia, tremor and HE (n = 1), elevated bilirubin (n = 1) |
• I: 2/70 | • Most received 10 mg dose (all who suffered AEs) |
| [46] | 3 | Clinical trial | Cirrhosis + oesophageal ulcers | 40 mg OME BID for 4 weeks (n = 14) | ‐ | • I: n = 0 | ‐ | ‐ | • Severity of cirrhosis unknown |
| [34] | 3 | Open‐label PK study | Cirrhosis | Single dose of 20 mg OME (n = 30; 10/10/10) | Healthy controls receiving same treatment (n = 10) |
• I: n = 0 • C: n = 0 |
‐ | ‐ | |
| [47] | 3 | Open‐label PK study | Cirrhosis |
10 mg OME IV (day 1) + PO (day 8–14) (n = 10; 2/4/4) |
‐ | • I: n = 0 | ‐ | ‐ | |
| [36] | 3 | Open‐label PK study | Cirrhosis | Continuous infusion of 460 mg OME over 47.5 h (n = 12; 5/4/3) | Healthy controls receiving same treatment (n = 12) |
• I: n = 3 • C: n = 0 |
• Epigastric pain (n = 1), arthralgia (n = 1), HE (n = 1) | • I: 0/12 | |
| [40] | 3 | Open‐label PK study | Cirrhosis | Single dose of 20 mg RAB (n = 10; compensated cirrhosis) | Healthy controls receiving same treatment (n = 13) |
• I: n = 0 • C: n = 3 |
‐ | ‐ | |
| [48] | 3 | Prospective cohort | Cirrhosis + peptic ulcer | 2 weeks BID: 20 mg OME, 1 g amoxicillin and 500 mg clarithromycin + 3 weeks 20 mg OME (n = 19) | 20 mg OME for 4 weeks (n = 11) |
• I: n = 11 (58%) • C: n = 0 |
• Bitterness of taste (n = 7), abdominal fullness (n = 2), headache (n = 1), diarrhoea (n = 1) |
• I: 0/19 • C: 0/11 |
• Severity of cirrhosis unknown • AEs not specific for PPI |
| [49] | 3 | Clinical trial | Cirrhosis + H. pylori infection | 2 weeks: 40 mg OME OD + 500 mg clarithromycin TID (n = 20) | ‐ | • I: n = 6 (30%) | • Dyspepsia (n = 3), metallic taste (n = 1), tongue numbness (n = 1), headache (n = 1) | • I: 6/20 |
• Severity of cirrhosis not specified for treated patients • AEs not specific for PPI |
| [50] | 3 | Clinical trial | Cirrhosis + H. pylori infection | 2 weeks OD: 30 mg LANS + 500 mg metronidazole + 400 mg clarithromycin (n = 30; 9/12/9) | Peptic ulcer patients receiving same intervention (n = 88) |
• I: n = 4 (13%) • C: n = 9 (10%) |
• Mild diarrhoea (n = 3), taste disturbances (n = 1) | • I: 0/30 | • AEs not specific for PPI |
| [51] | 3 | Randomized trial | Cirrhosis + H. pylori infection | 2 weeks BID: 20 mg OME + 1 g amoxicillin (n = 41; 22/11/8) | 1 week BID: 20 mg OME + 500 mg tetracycline + 250 mg clarithromycin (n = 42; 20/16/6) |
• I: n = 5 (12%) • C: n = 6 (14%) |
• Mild diarrhoea (n = 3;4 (I;C)), abdominal pain (n = 2;2), mouth burning (n = 1;0) |
• I: 0/41 • C: 0/42 |
• No randomization in dosing of omeprazole (−) • AEs not specific for PPI |
| [52] | 4 | Retrospective data analysis | Cirrhosis + H. pylori infection | 1 or 2 weeks BID: standard dose PPI + 1 g amoxicillin + 500 mg clarithromycin (n = 104; 70/28/6) | ‐ | • I: n = 13 (12.5%) | • Bitter taste, loose stool and abdominal discomfort (no. ns) | • NS |
• Type of PPI unknown • AEs not specific for PPI |
| [53] | 4 | Case report | Cirrhosis | Switch from 20 mg ESO for 1 month to LANS PO (n = 1) | ‐ | • I: n = 1 | • Anaphylactic reaction | • I: 1/1 | • No dose described of LANS |
| [54] | 4 | Case report | Cirrhosis | LANS (n = 1) | ‐ | • I: n = 1 | • DRESS syndrome | Patient died |
• Abstract No dose described |
| [55] | 4 | Case report | Cirrhosis | First: LANS 30 mg day−1, Second: OME (n = 1; CTP B) | ‐ | • I: n = 1 | • Tremors, confusion (with both PPIs, also after rechallenge) | • I: 1/1 | • No dose described of OME |
| [31] | 4 | Historically controlled PK study | Cirrhosis | 40 mg day−1 ESOM OD for 5 days (n = 12; 4/4/4) | Literature controls receiving same treatment (n = 36) | • I: n = 3 (25%) | • Constipation (n = 1), diarrhoea (n = 1), HE (n = 1) | • I: 1/12 (HE) | • No safety data of controls |
| [56] | 4 | Historically controlled PK study | Cirrhosis | 40 mg day−1 PANT for 4 days, followed by dosing on 2 alternate days (n = 21; 0/13/9) | Slow CYP2C19 metabolizers receiving same treatment (n = 17) | • I: n = 7 (33%) (CTP B/C 4/3) |
• CTP B: headache (n = 2), accidental injury, peripheral oedema, upper respiratory infection and skin disorder (all n = 1) • CTP C: ascites, vomiting, weight loss, joint disorder, HE (all n = 1) |
• I: 2/21 (both CTP C) | • No safety data of controls |
AE, adverse event; AP, alkaline phosphatase; BID, twice daily; C, control; CTP, Child–Pugh classification; ESO, esomeprazole; EVL, endoscopic variceal ligation; HE, hepatic encephalopathy; I, intervention; IV, intravenously; LANS, lansoprazole; NA, not applicable; NS, not specified; OD, once daily; OME, omeprazole; PANT, pantoprazole; PK, pharmacokinetic; PO, per os; PPI, proton pump inhibitor; RAB, rabeprazole; Ref, reference; TID, three times daily; γ‐GT, γ‐glutamyl transpeptidase.
Esomeprazole
In a multiple‐dose pharmacokinetic study (level 4) exposure to esomeprazole in eight cirrhotic patients with CTP A and B was comparable with healthy controls, while it more than doubled in four CTP C patients (Table 4) 31. This study was also mentioned in the product information, where a maximum dosage of 20 mg is advised in CTP C patients (Table 3) 21, 22. Regarding safety, in one case report esomeprazole was tolerated well (Table 5) 53. In the pharmacokinetic study, 25% of 12 patients suffered an adverse event (i.e. constipation, diarrhoea and HE) when using 40 mg per day for five days. The patient with HE had severe cirrhosis.
Expert judgement
Based on these limited data, esomeprazole was classified as ‘no additional risks known’. In CTP C patients, the evidence is very thin (one study in four subjects). Because of a doubling in exposure in CTP C patients, the recommendations of the product information are adopted to use no more than 20 mg per day in CTP C patients.
Omeprazole
In ten studies (level 3 and 4) with a total of 140 patients, the pharmacokinetics of omeprazole were explored (Table 4) 33, 34, 35, 36, 37, 47, 57, 58, 59, 60. Two articles showed higher exposure with increasing severity of cirrhosis, and a modelling study predicted the same 33, 34, 36. In CTP A, the AUC was slightly higher in comparison with healthy controls, in CTP B it was doubled, and exposure was more than doubled in CTP C patients. Two other single‐dose studies found a higher increase in exposure (seven‐ to eightfold), but the severity of cirrhosis was not described 35, 37. In healthy persons, omeprazole has an elimination half‐life of less than 1 h, prolonging in patients with cirrhosis to 2–4 h 47, 57, 60. Elimination half‐life seems to increase with severity of cirrhosis 34.
The safety of omeprazole has been described in ten articles (level 2, 3 and 4) with 220 cirrhotic subjects (Table 5) 34, 36, 41, 44, 46, 47, 48, 49, 51, 55. In eight of these studies only mild AEs occurred with omeprazole treatment, even when treatment lasted for more than four weeks. More severe adverse events (epigastric pain, arthralgia and worsening of HE) were seen in a study where patients received a continuous infusion for two days 36. Furthermore, in a case report, a patient with decompensated cirrhosis developed neurological adverse events (tremor, disbalance and confusion) while being on omeprazole treatment 55.
Expert judgement
In the clinical studies where patients were sorted by CTP class, exposure increased with severity of cirrhosis to an almost threefold higher exposure in CTP C compared to healthy controls. Two studies measured a seven‐ and eightfold increase in exposure in cirrhotics with unknown severity. In the literature about safety, omeprazole was mostly well tolerated. However, neurological AEs were reported in patients who received a high intravenous dose and in a patient with severe cirrhosis. In CTP A and B patients, omeprazole is classified as ‘no additional risks known’ if a maximum dose of 20 mg per day is used. In CTP C, omeprazole is classified as ‘unsafe’ based on the significant pharmacokinetic alterations and it is advised to avoid its usage.
Lansoprazole
Pharmacokinetics of lansoprazole were explored in four articles (level 3 and 4) with a total of 38 cirrhotic patients 32, 33, 60, 61. In a single‐dose study, the AUC was more than fourfold higher in compensated and in decompensated cirrhotics compared to healthy controls (Table 4) 32. A modelling study also predicted increased exposure, especially in CTP C patients 33. The FDA label 24 described an increment in the AUC of up to 500% in patients with various degrees of hepatic impairment, while the Dutch product information 23 mentioned a doubling in AUC in mild hepatic impairment and a higher increase in moderate to severe hepatic impairment (Table 3). The FDA label and three studies describe a prolongation of the half‐life from 1.5 h in healthy subjects to 6–7 h in cirrhotics 24, 32, 60, 61.
In three case reports and one other study (level 3 and 4) the safety of lansoprazole was explored in a total of 33 cirrhotic patients (Table 5) 50, 53, 54, 55. In the case reports severe AEs happened that were probably caused by lansoprazole (i.e. DRESS syndrome, anaphylactic reaction and neurological adverse events) 53, 54, 55. In the fourth study, only mild AEs occurred during two weeks of treatment 50.
Expert judgement
For all CTP classes lansoprazole is classified as ‘unsafe’, based on the marked increase in exposure compared to healthy controls and the availability of PPIs without these pharmacokinetic changes. It is recommended to avoid the use of lansoprazole in patients with cirrhosis.
Pantoprazole
We identified six pharmacokinetic studies (level 3 and 4) with pantoprazole in 77 cirrhotic patients (Table 4) 33, 38, 39, 56, 60, 62. In two multiple‐dose studies, the AUC was five‐ to sevenfold higher in patients with cirrhosis compared to healthy controls after oral and intravenous dosing. The same increase is described in the product information of pantoprazole (Table 3) 27, 28. Another article found a similar exposure to pantoprazole for patients with CTP B and CTP C cirrhosis and controls who were slow CYP2C19 metabolizers 56. When comparing these data with healthy controls, the AUC was five times higher in the cirrhotic patients. A modelling study predicted the same increases in exposure 33. In healthy persons, pantoprazole has an elimination half‐life of approximately 1 h. Five studies found an elimination half‐life of 7–9 h in patients with cirrhosis 38, 39, 56, 60, 62.
Three articles (level 2, 3 and 4) studied the safety of pantoprazole in 101 patients with cirrhosis (Table 5) 41, 43, 56. Pantoprazole was mostly well tolerated. In one study, a CTP C patient developed HE and in a randomized trial two patients suffered from fever possibly related to PPI use 41, 56.
Expert judgement
For all CTP classes, pantoprazole is classified as ‘unsafe’, based on the marked increase in exposure and prolonged half‐life, which cannot be corrected by dose reduction. Since there are alternatives without these large increases in exposure, we would recommend avoiding the use of pantoprazole in cirrhotic patients.
Rabeprazole
Two pharmacokinetic studies (level 3 and 4) were retrieved including 10 cirrhotic patients (Table 4) 33, 40. Exposure to rabeprazole more than doubled in patients with compensated cirrhosis compared to healthy controls 40. In a modelling study this was also predicted for CTP A cirrhosis, while exposure increased more than threefold in CTP B and fivefold in CTP C cirrhosis 33. In an article described in the product information (Table 3), there was no accumulation of rabeprazole after multiple doses in patients with CTP A and B 29, 30. The intragastric pH was comparable between cirrhotics and healthy controls. Rabeprazole has an elimination half‐life of 1 h, prolonging to almost 4 h in cirrhotics after a single dose and to 12 h after multiple dosing 40.
Three articles (level 2 and 3) studied the safety of rabeprazole in 101 cirrhotics (Table 5) 40, 42, 45. In two, rabeprazole was well tolerated with only mild adverse events 40, 42. In a post‐marketing surveillance study, nine of 70 patients with cirrhosis (13%) suffered an AE 45. These were severe in two (one HE and one serious elevation in bilirubin), both recovered after discontinuation.
Expert judgement
For CTP A and B patients, rabeprazole is classified as ‘no additional risks known’ and a starting dose of 10 mg is recommended, based on the doubled exposure. In CTP B patients, maintaining the 10 mg dose level is advised. As there are no clinical data from CTP C patients and a modelling study predicted an increase in AUC of more than fivefold, it is, again, advised to use a PPI without these large changes and rabeprazole is classified as ‘unsafe’ in CTP C patients.
Safety of PPIs as group
Thirty‐seven articles studied the safety of PPIs as a group in patients with cirrhosis (Table S1) 6, 7, 8, 11, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95. These studies mostly focused on the risk of spontaneous bacterial peritonitis or infections in general. A few recent ones also examined the risk of HE.
Risk of spontaneous bacterial peritonitis
Twenty‐four observational studies (level 3 and 4) 6, 69, 70, 71, 72, 73, 75, 76, 78, 79, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 92, 93, 94, 95 and seven systematic reviews (level 2) 7, 63, 64, 65, 66, 67, 68 explored the risk of spontaneous bacterial peritonitis with PPI use in cirrhotics. All meta‐analyses detected a significant association between PPI use and the development of spontaneous bacterial peritonitis 7, 63, 64, 65, 66, 67. These meta‐analyses included at least four studies 67 and at most 17 65. Heterogeneity was high in some meta‐analyses, the meta‐analysis of Trikudanathan et al. 67 had the lowest heterogeneity (22%) and found an odds ratio of 2.77 [95% confidence interval (CI) 1.82–4.23]. In the meta‐analysis of Yu and colleagues, a sub‐analysis of only the cohort studies retrieved an odds ratio of 1.18 (95% CI 0.99–1.41) without heterogeneity (0%) 64.
No meta‐analysis incorporated dose or duration of PPI therapy in their risk calculation. Of the observational studies, four specifically investigated the duration of therapy. In three, a longer duration of PPI use was linked to a higher risk of spontaneous bacterial peritonitis 71, 82, 90, while in the other, no such relation was found 72. Four additional studies specified the dose used by cirrhotics 78, 84, 92, 93. One found a higher risk with twice daily dosing versus once daily dosing 93, while two others did not 84, 92. The fourth study compared the risk between a half PPI dose and a full dose and did not find a difference 78.
Risk of infections
In 11 observational studies (level 3 and 4) and two systematic reviews (level 2) the risk of bacterial infection with PPI use in cirrhotics was determined 11, 65, 68, 72, 73, 74, 75, 77, 79, 80, 81, 87, 91. In the meta‐analysis of Xu et al. 65, the odds ratio for bacterial infection was 1.98 (95% CI 1.36–2.87, heterogeneity 0%). Two studies also calculated the dose‐dependent risk of infections 81, 87. One of these did not find differences in dose between patients who developed an infection and patients who did not 81. The other noted more AEs with an inadequate dosed PPI (too high or contra‐indicated) 87.
Risk of HE
Six observational studies (level 3, 4) looked at the risk of HE with the use of PPIs 6, 8, 69, 70, 71, 72. Four found an increased risk of HE with PPI use in cirrhotics 6, 8, 69, 70, while two did not 71, 72. The case–control study of Tsai and colleagues 8 provided sub‐analyses per PPI and per dose and duration of treatment. They found a positive relationship between HE risk and cumulative defined daily doses. The highest risk was found for pantoprazole, followed by lansoprazole, omeprazole and esomeprazole. The risk of HE with rabeprazole was not statistically significant but had the largest confidence interval due to a low number of users.
Expert judgement
There is conflicting data for all outcomes of interest. Only one study provided sub‐analyses for the risk of HE per PPI. Based on these results, it is possible that pharmacokinetic alterations contributed to an increased risk. We advise to cautiously use PPIs in cirrhotics and monitor for these AEs during treatment.
Implementation and continuity
The practical guidance on PPIs has been implemented in the two national drug databases in the Netherlands in 2017. The first update is planned for 2022.
Discussion
We developed practical guidance for the safe use of PPIs in patients with cirrhosis based on the product information, literature and expert opinion. Our results show that relevant changes in pharmacokinetics occur due to cirrhosis. Based on the available evidence, we recommend esomeprazole, omeprazole and rabeprazole for use in patients with CTP A and B cirrhosis. In CTP C cirrhosis, we recommend to prescribe only esomeprazole whereas the use of lansoprazole and pantoprazole in all patients with cirrhosis is discouraged because of increased exposure compared to non‐cirrhotics.
Our advice is based on evidence from both the pharmacokinetic and safety literatures. We found no studies that combined pharmacokinetic data with pharmacodynamic data. Literature shows that the AUC is the best pharmacokinetic parameter predicting gastric acid suppression 14, 15. The main question is whether increased acid suppression is a safety risk for patients with cirrhosis, an issue that is virtually not covered in the product information. In the included studies, most AEs were mild, but there were cases of HE that were attributed to PPI use. However, the causality is unclear since HE is a central feature of advanced cirrhosis. Almost all of these events occurred in patients on a relatively high dose or in patients with advanced cirrhosis. Some articles examining the safety of PPIs as a group also assessed dose‐dependent safety 8, 78, 81, 84, 87, 92, 93. Results were conflicting. One study performed a sub‐analysis for assessing the risk of HE per PPI 8. They found the highest risk of HE with pantoprazole and no significant risk with rabeprazole. The risk of HE for the remaining PPIs was comparable. An important consideration for the expert panel was not to expose cirrhotic patients to unnecessary risks. Highly increased exposure was considered a safety risk when used in non‐acute settings; hence for daily practice we discourage the oral use of lansoprazole and pantoprazole in cirrhosis and recommend the use of PPIs without these large increases, such as esomeprazole.
Our results demonstrate major pharmacokinetic alterations in patients with cirrhosis compared to healthy controls. Although maximum plasma concentrations were often comparable between cirrhotics and healthy controls, the exposure (AUC) and elimination half‐life differed to a great extent between the two groups. All PPIs are metabolized by CYP2C19 and to a lesser extent by CYP3A4. CYP2C19 is very sensitive to impairment of liver function 96. Reduced activity of CYP2C19 is probably the most important cause of the observed pharmacokinetic changes. There were also significant differences found between PPIs. The changes in pharmacokinetics were largest for lansoprazole and pantoprazole. Both have a low hepatic extraction ratio, while the other PPIs have an intermediate hepatic extraction ratio 13, 31. In contrast to drugs with an intermediate hepatic extraction ratio, hepatic clearance of drugs with a low hepatic extraction ratio is mostly dependent on intrinsic metabolic clearance (i.e. activity of metabolizing enzymes) and on protein binding. Drugs with a low hepatic extraction ratio are therefore most vulnerable to changes in the activity of hepatic metabolizing enzymes and in protein binding 13. Esomeprazole pharmacokinetics seemed to be least influenced by cirrhosis. It is remarkable that results of esomeprazole and omeprazole differ. This can be explained by differences in metabolism between the S‐enantiomer and the R‐enantiomer of omeprazole, as the S‐enantiomer (esomeprazole) is metabolized to a lesser extent by CYP2C19 than the R‐enantiomer 97. Pharmacogenetic studies with PPIs in healthy volunteers also showed that exposure of esomeprazole is least affected by CYP2C19 polymorphisms compared to other PPIs 98, 99.
The literature search identified many studies that determine the risk of HE, spontaneous bacterial peritonitis and/or infections in patients with cirrhosis using PPIs. Most of these were observational and cross‐sectional by design and provide conflicting results. The nature and quality of the data do not allow a formal meta‐analysis, which precluded a direct comparison. Cautious use of PPIs in these patients is recommended by most authors. Of note is that only one of the 37 studies examined safety risks for each individual PPI, while eight did investigate whether safety risks were dose‐dependent. In our opinion, a sub‐analysis on the dose‐dependency of the risk of HE or infections cannot be calculated in the absence of pharmacokinetic data. For further studies, determining the risk of spontaneous bacterial peritonitis, HE or infections for each PPI would be advisable.
A strength of our study is that we are not only reviewing the literature, but also developing practical guidance for health care professionals by combining literature and registration information with expert opinion. For some outcomes (e.g. pharmacokinetics in CTP C patients), our recommendations are limited by the few studies available. Therefore, continuous update of data and advice is warranted. Another strength is that the method used for combining all these data has been peer‐reviewed and published 16. A limitation of this method is that a number of steps were performed by a single author (R.W.). The most critical steps (i.e. data synthesis, advice formulation) were however, double checked by a second person (S.B.). Interpretation of the findings, discussion and conclusion was in all cases done by a multi‐disciplinary expert panel.
We provided safety and dosing guidance for the oral use of PPIs in patients with cirrhosis which can be applied in daily practice. The pharmacokinetic properties of PPIs are affected by the presence of cirrhosis. The combination of pharmacokinetic and safety data used in this study is unique and sheds new light on the current discussion about the safety of PPIs in patients with cirrhosis.
Competing Interests
There are no competing interests to declare.
This study was funded by ZonMw, the Dutch national organization for health research and healthcare innovation, grant number 836044009.
Contributors
R.W. drafted the manuscript. M.B., D.B., J.D., F.H.I., N.H., H.M., M.M.S., S.V.P. and S.B. participated in data analysis and interpretation and critically revised the manuscript. Supervision was done by K.T. and S.B. All authors approved the final version of the manuscript.
Supporting information
Table S1 Summary of studies on the safety of proton pump inhibitors as a group
Weersink, R. A. , Bouma, M. , Burger, D. M. , Drenth, J. P. H. , Harkes‐Idzinga, S. F. , Hunfeld, N. G. M. , Metselaar, H. J. , Monster‐Simons, M. H. , van Putten, S. A. W. , Taxis, K. , and Borgsteede, S. D. (2018) Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol, 84: 1806–1820. 10.1111/bcp.13615.
References
- 1. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336: 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration . Safety information proton pump inhibitors [online]. Available at https://www.fda.gov/Drugs/DrugSafety/ucm199082.htm (last accessed 28 February 2017).
- 3. Zhou B, Huang Y, Li H, Sun W, Liu J. Proton‐pump inhibitors and risk of fractures: an update meta‐analysis. Osteoporosis Int 2016; 27: 339–347. [DOI] [PubMed] [Google Scholar]
- 4. Laheij RJ, Sturkenboom MC, Hassing R, Dieleman J, Stricker BH, Jansen JB. Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA 2004; 292: 1955–1960. [DOI] [PubMed] [Google Scholar]
- 5. Hess MW, Hoenderop JGJ, Bindels RJM, Drenth JPH. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012; 36: 405–413. [DOI] [PubMed] [Google Scholar]
- 6. Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther 2016; 44: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande A, Pasupuleti V, Thota P, Pant C, Mapara S, Hassan S, et al Acid‐suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta‐analysis. J Gastroenterol Hepatol 2013; 28: 235–242. [DOI] [PubMed] [Google Scholar]
- 8. Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, et al Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology 2017; 152: 134–141. [DOI] [PubMed] [Google Scholar]
- 9. Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al Proton pump inhibitors affect the gut microbiome. Gut 2016; 65: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo W, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta‐analysis. Clin Gastroenterol Hepatol 2013; 11: 483–490. [DOI] [PubMed] [Google Scholar]
- 11. Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther 2015; 41: 459–466. [DOI] [PubMed] [Google Scholar]
- 12. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 2008; 64: 1147–1161. [DOI] [PubMed] [Google Scholar]
- 13. Delco F, Tchambaz L, Schlienger R, Drewe J, Krahenbuhl S. Dose adjustment in patients with liver disease. Drug Saf 2005; 28: 529–545. [DOI] [PubMed] [Google Scholar]
- 14. Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 2006; 23 (Suppl 2): 2–8. [DOI] [PubMed] [Google Scholar]
- 15. Shi S. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol 2008; 64: 935–951. [DOI] [PubMed] [Google Scholar]
- 16. Weersink RA, Bouma M, Burger DM, Drenth JPH, Hunfeld NGM, Kranenborg M, et al Evaluating the safety and dosing of drugs in patients with liver cirrhosis by literature review and expert opinion. BMJ Open 2016; 6: e012991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. OCEBM Levels of Evidence Working Group . The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence‐Based Medicine [online]. Available at http://www.cebm.net/index.aspx?o=5653 (last accessed 25 January 2016).
- 18. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 19. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The concise guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medicines Evaluation Board . Product information: Nexium 20 mg tablets [online]. Available at http://db.cbg-meb.nl/IB-teksten/h25387.pdf (last accessed 24 May 2017).
- 22. US Food and Drug Administration . Product information nexium esomeprazole magnesium granule [online]. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f4853677-1622-4037-688b-fdf533a11d96 (last accessed 27 November 2017).
- 23. Medicines Evaluation Board . Product information prezal 15 mg capsules [online]. Available at http://db.cbg-meb.nl/IB-teksten/h15420.pdf (last accessed 6 June 2017).
- 24. US Food and Drug Administration . Product information lansoprazole capsules Actavis [online]. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb034790-ccc0-4df4-ac12-b7715cc0d42b (last accessed 24 May 2017).
- 25. Medicines Evaluation Board . Product information losec 10 mg capsules [online]. Available at http://db.cbg-meb.nl/IB-teksten/h12438.pdf (last accessed 6 June 2017).
- 26. US Food and Drug Administration . Product information omeprazole capsules Northwind Pharmaceuticals [online]. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2c40d64b-5e7b-4381-9ebf-df3a4bf0edfe (last accessed 24 May 2017).
- 27. Medicines Evaluation Board . Product information pantozol 40 mg tablets [online]. Available at http://db.cbg-meb.nl/IB-teksten/h18300.pdf (last accessed 6 June 2017).
- 28. US Food and Drug Administration . Product information pantoprazole sodium tablets [online]. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eda64039-771b-41d7-b9c4-970cfbf5fbb9 (last accessed 27 November 2017).
- 29. Medicines Evaluation Board . Product information pariet 10 mg tablets [online]. Available at http://db.cbg-meb.nl/IB-teksten/h23210.pdf (last accessed 27 November 2017).
- 30. US Food and Drug Administration . Product information aciphex rabeprazole sodium tablets [online]. Available at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=52459f70-e1f5-41bb-a9f4-e68ef5f5dcf5 (last accessed 24 May 2017).
- 31. Sjovall H, Bjornsson E, Holmberg J, Hasselgren G, Rohss K, Hassan‐Alin M. Pharmacokinetic study of esomeprazole in patients with hepatic impairment. Eur J Gastroenterol Hepatol 2002; 14: 491–496. [DOI] [PubMed] [Google Scholar]
- 32. Delhotal‐Landes B, Flouvat B, Duchier J, Molinie P, Dellatolas F, Lemaire M. Pharmacokinetics of lansoprazole in patients with renal or liver disease of varying severity. Eur J Clin Pharmacol 1993; 45: 367–371. [DOI] [PubMed] [Google Scholar]
- 33. Steelandt J, Jean‐Bart E, Goutelle S, Tod M. A prediction model of drug exposure in cirrhotic patients according to Child–Pugh classification. Clin Pharmacokinet 2015; 54: 1245–1258. [DOI] [PubMed] [Google Scholar]
- 34. Kumar R, Chawla YK, Garg SK, Dixit RK, Satapathy SK, Dhiman RK, et al Pharmacokinetics of omeprazole in patients with liver cirrhosis and extrahepatic portal venous obstruction. Methods Find Exp Clin Pharmacol 2003; 25: 625–630. [DOI] [PubMed] [Google Scholar]
- 35. Rinetti M, Regazzi MB, Villani P, Tizzoni M, Sivelli R. Pharmacokinetics of omeprazole in cirrhotic patients. Arzneimittelforschung 1991; 41: 420–422. [PubMed] [Google Scholar]
- 36. Piqué JM, Feu F, de Prada G, Röhss K, Hasselgren G. Pharmacokinetics of omeprazole given by continuous intravenous infusion to patients with varying degrees of hepatic dysfunction. Clin Pharmacokinet 2002; 41: 999–1004. [DOI] [PubMed] [Google Scholar]
- 37. Andersson T, Olsson R, Regårdh C, Skånberg I. Pharmacokinetics of [14C] omeprazole in patients with liver cirrhosis. Clin Pharmacokinet 1993; 24: 71–78. [DOI] [PubMed] [Google Scholar]
- 38. Huber R, Hartmann M, Bliesath H, Luhmann R, Steinijans VW, Zech K. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther 1996; 34: 185–194. [PubMed] [Google Scholar]
- 39. Brunner G, Chang J, Hartmann M, Huber R, Bliesath H, Luhmann R. Pharmakokinetik von Pantoprazole bei Patienten mit Leberzirrhose. Med Klin 1994; 89 (Suppl 1): 189. [Google Scholar]
- 40. Hoyumpa AM, Trevino‐Alanis H, Grimes I, Humphries TJ. Rabeprazole: pharmacokinetics in patients with stable, compensated cirrhosis. Clin Ther 1999; 21: 691–701. [DOI] [PubMed] [Google Scholar]
- 41. Lo G, Perng D, Chang C, Tai C, Wang H, Lin H. Controlled trial of ligation plus vasoconstrictor versus proton pump inhibitor in the control of acute esophageal variceal bleeding. J Gastroenterol Hepatol 2013; 28: 684–689. [DOI] [PubMed] [Google Scholar]
- 42. Hidaka H, Nakazawa T, Wang G, Kokubu S, Minamino T, Takada J, et al Long‐term administration of PPI reduces treatment failures after esophageal variceal band ligation: a randomized, controlled trial. J Gastroenterol 2012; 47: 118–126. [DOI] [PubMed] [Google Scholar]
- 43. Shaheen NJ, Stuart E, Schmitz SM, Mitchell KL, Fried MW, Zacks S, et al Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005; 41: 588–594. [DOI] [PubMed] [Google Scholar]
- 44. Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, et al Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014; 307: G951–G957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Makino I, Nakamura K, Sato Y, Sato Y, Sezai S, Ikeda Y, et al Postmarketing surveillance of rabeprazole in upper gastrointestinal peptic lesions in Japanese patients with coexisting hepatic disorders. Curr Ther Res Clin Exp 2006; 67: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jaspersen D, Korner T, Schorr W, Hammar CH. Omeprazole in the management of sclerotherapy‐induced esophageal ulcers resistant to H2 blocker treatment. J Gastroenterol 1995; 30: 128–130. [DOI] [PubMed] [Google Scholar]
- 47. McKee R, MacGilchrist A, Garden O, Forrest J, Carter D. The anti‐secretory effect and pharmacokinetics of omeprazole in chronic liver disease. Aliment Pharmacol Ther 1988; 2: 429–437. [DOI] [PubMed] [Google Scholar]
- 48. Tzathas C, Triantafyllou K, Mallas E, Triantafyllou G, Ladas SD. Effect of Helicobacter pylori eradication and antisecretory maintenance therapy on peptic ulcer recurrence in cirrhotic patients: a prospective, cohort 2‐year follow‐up study. J Clin Gastroenterol 2008; 42: 744–749. [DOI] [PubMed] [Google Scholar]
- 49. Scotiniotis IA, Lucey MR, Metz DC. Helicobacter pylori infection is not associated with subclinical hepatic encephalopathy in stable cirrhotic patients. Dig Dis Sci 2001; 46: 2744–2751. [DOI] [PubMed] [Google Scholar]
- 50. Azuma T, Ito S, Suto H, Ito Y, Miyaji H, Yamazaki Y, et al Pharmacokinetics of clarithromycin in Helicobacter pylori eradication therapy in patients with liver cirrhosis. Aliment Pharmacol Ther 2000; 14 (Suppl 1): 216–222. [DOI] [PubMed] [Google Scholar]
- 51. Zullo A, Rinaldi V, Meddi P, Winn S, Moscatelli R, Attili AF. Helicobacter pylori eradication with dual and low‐dose triple therapy in patients with liver cirrhosis. Ital J Gastroenterol Hepatol 31: 831–835. [PubMed] [Google Scholar]
- 52. Jung SW, Lee SW, Hyun JJ, Kim DI, Koo JS, Yim HJ, et al Efficacy of Helicobacter pylori eradication therapy in chronic liver disease. Dig Liver Dis 2009; 41: 134–140. [DOI] [PubMed] [Google Scholar]
- 53. Choi SW, Han JM, Bae YJ, Lee YS, Cho YS, Moon HB, et al Lessons from two cases of anaphylaxis to proton pump inhibitors. J Clin Pharm Ther 2012; 37: 614–616. [DOI] [PubMed] [Google Scholar]
- 54. Uppalapati A, Gogineni S, Koneru S, Kamel G. Are proton pump inhibitors (PPI) naive? A case of drug reaction with eosinophilia and systemic symptom (DRESS) secondary to lansoprazole. J Allergy Clin Immunol 2016; 133: AB273. [Google Scholar]
- 55. Prignet J, Chauveau E, Duval J, Kunkel D. Unexpected neurological adverse effects of proton pump inhibition therapy in a patient with cirrhosis of the liver. Semaine des Hôpitaux 1996; 72: 619–621. [Google Scholar]
- 56. Ferron GM, Preston RA, Noveck RJ, Pockros P, Mayer P, Getsy J, et al Pharmacokinetics of pantoprazole in patients with moderate and severe hepatic dysfunction. Clin Ther 2001; 23: 1180–1192. [DOI] [PubMed] [Google Scholar]
- 57. Sauvet P, Schouler L. Oméprazole et fonctions hépatiques. Rev Med Interne 1992; 13: 359–363. [DOI] [PubMed] [Google Scholar]
- 58. Walker S, Klotz U, Sarem‐Aslani A, Treiber G, Bode J. Effect of omeprazole on nocturnal intragastric pH in cirrhotics with inadequate antisecretory response to ranitidine. Digestion 1991; 48: 179–184. [DOI] [PubMed] [Google Scholar]
- 59. Caulin C, Gouerou H, Bretagne J, Ebrard F. Tolérance de l'oméprazole chez l'insuffisant hépatique. Etude ouverte chez 24 cirrhotiques. Gastroenterol Clin Biol 1987; 42A: 11. [Google Scholar]
- 60. DanzNeeff H, Brunner G. Comparative kinetic studies with the three proton pump inhibitors omeprazole, lansoprazole and pantoprazole in patients with complete liver cirrhosis. Gastroenterology 1996; 110: A90. [Google Scholar]
- 61. Coste T, Logeais C, Delhotal‐Landes B, Dellatolas F, Lemaire M, Rautureau J, et al Pharmacokinetics of lansoprazole after repeated administration in cirrhosis patients. Gastroenterology 1993; 104 (Suppl): A59. [Google Scholar]
- 62. Benet LZ, Zeck K. Pharmacokinetics: a relevant factor for the choice of a drug? Aliment Pharmacol Ther 1994; 8 (Suppl 1): 25–32. [DOI] [PubMed] [Google Scholar]
- 63. Dong H, Luo S, Dong Y, Feng W, Wei Y. The use of PPI is associated with spontaneous bacterial peritonitis in cirrhotic patients of different ethnic groups: a meta‐analysis. Int J Clin Exp Med 2016; 9: 1227–1235. [Google Scholar]
- 64. Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: a meta‐analysis. Dig Liver Dis 2016; 48: 353–359. [DOI] [PubMed] [Google Scholar]
- 65. Xu H, Wang H, Li C, Ye S, Dong MS, Xia QJ, et al Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta‐analysis. Genet Mol Res 2015; 14: 7490–7501. [DOI] [PubMed] [Google Scholar]
- 66. Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta‐analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2015; 27: 1327–1336. [DOI] [PubMed] [Google Scholar]
- 67. Trikudanathan G, Israel J, Cappa J, O'Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients – a systematic review and meta‐analysis. Int J Clin Pract 2011; 65: 674–678. [DOI] [PubMed] [Google Scholar]
- 68. Lo EA, Wilby KJ, Ensom MH. Use of proton pump inhibitors in the management of gastroesophageal varices: a systematic review. Ann Pharmacother 2015; 49: 207–219. [DOI] [PubMed] [Google Scholar]
- 69. Schiavon LL, Silva TE, Fischer J, Narciso‐Schiavon JL. Letter: proton pump inhibitors and prognosis of cirrhosis – searching for the balance point. Aliment Pharmacol Ther 2017; 45: 378–379. [DOI] [PubMed] [Google Scholar]
- 70. Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016; 64: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 71. Huang K, Kuan Y, Luo J, Lin C, Liang J, Kao C. Impact of long‐term gastric acid suppression on spontaneous bacterial peritonitis in patients with advanced decompensated liver cirrhosis. Eur J Intern Med 2016; 32: 91–95. [DOI] [PubMed] [Google Scholar]
- 72. Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, et al Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015; 62: 1056–1060. [DOI] [PubMed] [Google Scholar]
- 73. O'Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, et al Long‐term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2015; 13: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sargenti K, Prytz H, Strand A, Nilsson E, Kalaitzakis E. Healthcare‐associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int 2015; 35: 391–400. [DOI] [PubMed] [Google Scholar]
- 75. Merli M, Lucidi C, Di Gregorio V, Giannelli V, Giusto M, Ceccarelli G, et al The chronic use of beta‐blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int 2015; 35: 362–369. [DOI] [PubMed] [Google Scholar]
- 76. Miozzo SA, Tovo CV, John JA, de Mattos AA. Proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhosis: an undesirable association? J Hepatol 2015; 63: 529–530. [DOI] [PubMed] [Google Scholar]
- 77. Nahon P, Lescat M, Layese R, Bourcier V, Talmat N, Allam S, et al Bacterial infection in compensated viral cirrhosis impairs 5‐year survival (ANRS CO12 CirVir prospective cohort). Gut 2017; 66: 330–341. [DOI] [PubMed] [Google Scholar]
- 78. Min Y, Lim K, Min B, Gwak GY, Paik YH, Choi MS, et al Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014; 40: 695–704. [DOI] [PubMed] [Google Scholar]
- 79. Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Summereder C, et al Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PloS One 2014; 9: e110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bajaj JS, Ratliff SM, Heuman DM, Lapane KL. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther 2012; 36: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van Vlerken LG, Huisman EJ, van Hoek B, Renooij W, de Rooij FW, Siersema PD, et al Bacterial infections in cirrhosis: role of proton pump inhibitors and intestinal permeability. Eur J Clin Invest 2012; 42: 760–767. [DOI] [PubMed] [Google Scholar]
- 82. Chang SS, Lai CC, Lee MT, Lee YC, Tsai YW, Hsu WT, et al Risk of spontaneous bacterial peritonitis associated with gastric acid suppression. Medicine (Baltimore) 2015; 94: e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kwon JH, Koh S, Kim W, Jung YJ, Kim JW, Kim BG, et al Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol 2014; 29: 775–781. [DOI] [PubMed] [Google Scholar]
- 84. Ratelle M, Perreault S, Villeneuve J, Tremblay L. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol 2014; 28: 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miura K, Tanaka A, Yamamoto T, Adachi M, Takikawa H. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med 2014; 53: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 86. Vos M, Vroey B, Garcia BG, Roy C, Kidd F, Henrion J, et al Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int 2013; 33: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 87. Franz CC, Hildbrand C, Born C, Egger S, Bravo AER, Krähenbühl S. Dose adjustment in patients with liver cirrhosis: impact on adverse drug reactions and hospitalizations. Eur J Clin Pharmacol 2013; 69: 1565–1573. [DOI] [PubMed] [Google Scholar]
- 88. Goel GA, Deshpande A, Lopez R, Hall GS, Van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol 2012; 10: 422–427. [DOI] [PubMed] [Google Scholar]
- 89. Aditi A, Crippin JS, Abhishek A. Role of proton pump inhibitors in the development of spontaneous bacterial peritonitis amongst cirrhotics; a retrospective cohort study. Gastroenterology 2012; 142: S946. [Google Scholar]
- 90. Choi EJ, Lee HJ, Kim KO, Lee SH, Eun JR, Bang BI, et al Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol 2011; 46: 616–620. [DOI] [PubMed] [Google Scholar]
- 91. Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, et al Clostridium difficile is associated with poor outcomes in patients with cirrhosis: a national and tertiary center perspective. Am J Gastroenterol 2010; 105: 106–113. [DOI] [PubMed] [Google Scholar]
- 92. Bajaj JS, Zadvornova Y, Heuman DM, Hafeezullah M, Hoffmann RG, Sanyal AL, et al Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 93. Bulsiewicz WJ, Scherer JR, Feinglass JM, Howden CW, Flamm SL. Proton pump inhibitor (PPI) use is independently associated with spontaneous bacterial peritonitis (SBP) in cirrhotics with ascites. Gastroenterology 2009; 136: A11. [Google Scholar]
- 94. Campbell MS, Obstein K, Reddy KR, Yang Y. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53: 394–398. [DOI] [PubMed] [Google Scholar]
- 95. Northup PG, Argo CK, Berg CL. Chronic proton pump inhibitor use is strongly associated with hepatorenal syndrome and spontaneous bacterial peritonitis in cirrhosis patients. Hepatology 2008; 48: 325A. [Google Scholar]
- 96. Rodighiero V. Effects of liver disease on pharmacokinetics. Clin Pharmacokinet 1999; 37: 399–431. [DOI] [PubMed] [Google Scholar]
- 97. Andersson T, Weidolf L. Stereoselective disposition of proton pump inhibitors. Clin Drug Investig 2008; 28: 263–279. [DOI] [PubMed] [Google Scholar]
- 98. Hunfeld NGM, de Goede AL, Grandia L, Kuipers EJ, Touw DJ. Systematic review: the influence of CYP2C19 polymorphism on the acid‐inhibitory effects of proton pump inhibitors. In: Hunfeld NGM. Clinical effects of proton pump inhibitors [thesis]. The Hague: Erasmus University Rotterdam, 2010: 115–127.
- 99. Schwab M, Klotz U, Hofmann U, Schaeffeler E, Leodolter A, Malfertheiner P, et al Esomeprazole‐induced healing of gastroesophageal reflux disease is unrelated to the genotype of CYP2C19: evidence from clinical and pharmacokinetic data. Clin Pharmacol Ther 2005; 78: 627–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of studies on the safety of proton pump inhibitors as a group


