Summary
To study the prevalence of anti‐nuclear antibodies (ANA) in breast cancer patients and its association with tumour characteristics. Ninety‐one patients with breast mass detected by image studies and assigned to conduct diagnostic biopsy and eventual surgical treatment were studied for demographical, tumour data and presence of ANA. Serum of positive ANA patients was screened for the extractable nuclear antigen (ENA) profile. As comparison, 91 healthy individuals matched for age and from the same geographical area were included. In this sample 72 of 91 (79·1%) had malignant lesions (83% ductal infiltrative carcinoma). ANA was positive in 44·4% of patients with malignant tumour and in 15·7% of those with benign lesions (malignant versus benign with P = 0·03). Controls had ANA positivity in 5·4%, and when compared with tumour samples showed P < 0·0001. The most common immunofluorescence pattern was a fine dense speckled pattern. In the ANA‐positive patients with malignant lesions, seven had positivity for ENA profile (three for anti‐RNP and anti‐Sm, one for just anti‐RNP, two for anti‐Ro and anti‐La e two for just anti‐La). It was not possible to associate ANA positivity with tumour histological characteristics or staging or with patient's age. A negative association of ANA with hormonal (oestrogen or oestrogen plus progesterone) receptor status was found (P = 0·01). In this sample, there was a high prevalence of ANA positivity in breast cancer patients with a negative association with the presence of hormonal receptors. More studies are needed to understand the real value of this finding.
Keywords: autoantibodies, autoimmunity, cancer
Introduction
Anti‐nuclear antibodies (ANA) are autoantibodies considered the immune biomarkers of systemic autoimmune diseases 1. However, this autoantibody may be found in some situations where its meaning is not completely clear and this may represent a diagnostic challenge, mainly when the test is persistently positive 1. Autoimmune diseases such as systemic lupus (SLE) or rheumatoid arthritis (RA) may have positivity for autoantibodies several years before its appearance 2. A positive ANA in this context may represent a state of pre‐autoimmunity 2. However, it may also be a marker of some other diseases that, although not considered to be autoimmune, are capable of offering enough antigenic stimulation for its appearance.
Neoplastic diseases may cause positive ANA. Some authors have described that ANA is found in the sera from lung, breast, head and neck cancer patients as frequently as in RA and SLE 3, 4, 5. Chapman et al. 6 has suggested that in breast cancer they may be used as an aid to early diagnosis.
ANAs are autoantibodies to nuclear cell components that are formed when the cell nuclear content is exposed to the extracellular milieu as the cell dies by apoptosis or necrosis 7, so tumour cell death may be the source of antigen stimulation for ANA formation in neoplastic diseases. In this context, its presence could be simply epiphenomena. However, it could also represent an immune response to restrain tumour spreading. Heegaard et al. 8, who studied the presence of ANA in ovarian cancer patients, found that the presence of this autoantibody is associated with a poor prognosis. Zou et al. 9 found that the finding of ANA in lymphoma patients helps in defining the prognosis of this disease.
Breast cancer is a worldwide public health problem and is currently one of the most common tumours 10; the risk of having this tumour rises with age 11. Despite its incidence having increased considerably over the last 10 years, mortality rates are falling 11. This is credited to a combination of earlier recognition and better treatment regimens. Finding a positive ANA without any sign of autoimmune disorder may draw attention to the possibility of this disease and may be a clue to an early diagnosis 6.
In this study, we examined a cohort of newly diagnosed breast cancer patients to establish the ANA prevalence in this group, to study its immunofluorescence pattern and to associate ANA presence with tumour characteristics.
Methods
This study was approved by the local Committee of Ethics in Research, and all participants provided signed consent. A total of 91 patients from two Oncology Services from the same geographical area (Curitiba, Brazil) with breast mass detected by image studies and assigned to conduct diagnostic biopsy and eventual surgical treatment were studied for demographical data and presence of ANA. None of the patients had received any treatment at time of inclusion.
The sample obtained was a convenience sample that included all patients who agreed to participate in the study from 2015 to 2016. We excluded patients with a previous diagnosis of connective tissue diseases, tumours, those using biological drugs and pregnant women.
Epidemiological data were collected by chart revision. Ten ml of venous blood were drawn, aliquoted and preserved at −80°C until ANA and extractible nuclear antigen (ENA) tests were performed. All the samples were screened to ANA by indirect immunofluorescence on human epithelial type 2 (HEp‐2) cells, using the commercially available kit ANA HEp‐2 (Hemagen Diagnostics, Columbia, MD, USA), as recommended by the manufacturer. A titre of 1 : 80 or higher was considered to indicate ANA positivity. The fluorescence patterns were interpreted as fine‐speckled, coarse‐speckled, homogeneous, peripheral, centromeric, nucleolar and cytoplasmic. Only samples with positive ANA tests were assessed by enzyme‐linked immunosorbent assay (ELISA) for antibodies against ENA antigens [single‐strand (SS)‐A/Ro, SS‐B/La, Smith (Sm), ribonucleoprotein (RNP), histidyl‐sRNA synthetase (Jo‐1) and scleroderma 70 (Scl‐70)], using individual ENA kits (Orgentec®, Mainz, Germany) for detection and confirmation of the test. The cut‐off level was set at 10 U/ml, as recommended by the manufacturer.
After the surgical procedure, patients were divided into two groups: those with benign tumours and those with malignant tumours to compare the prevalence of ANA. Those with malignant disease were studied for histological characteristics, staging, presence of hormone receptor (oestrogen and progesterone) and human epidermal growth factor 2 (HER2). In the malignant disease group, ANA‐positive individuals were compared with ANA‐negative patients.
As controls, we included 91 healthy women from medical staff matched for age.
The data obtained were collected in frequency and contingency tables. Comparison studies were performed using χ2 and Fisher's tests (nominal data) and by unpaired t‐test (numeric data). The adopted significance was 5%.
Results
In the group of breast lesion patients, 90 of 91 (98·9%) were women with a mean age of 53·92 ± 14·51 years; in the control group, 90 of 91 (98·9%) were women (P = 1·00) with a mean age of 51·5 ± 11·28 years (P = 0·22). In the breast lesion group, 19 of 91 (20·8%) had a benign lesion and 72 of 91 (79·1%) had malignant lesion. The main characteristics of the malignant disease patients are shown in Table 1.
Table 1.
Main characteristics of the studied population with malignant breast tumours (n = 72)
| n (%) | ||
|---|---|---|
| Histological classification | Ductal in situ | 2/72 (2·7) |
| Infiltrative ductal | 60/72 (83·3) | |
| Infiltrative lobular | 4/72 (5·5) | |
| Infiltrative ductolobular | 4/72 (5·5) | |
| Phyloid tumour | 1/72 (1·3) | |
| Infiltrative papillary | 1/72 (1·3) | |
| Staging | In situ | 7/57 (12·2) |
| I | 8/57 (14·0) | |
| II | 13/57 (22·8) | |
| III | 8/57 (14·0) | |
| IV | 21/57 (36·8) | |
| Oestrogen receptor‐positive | 42/62 (67·7) | |
| Progesterone receptor‐positive | 39/62 (62·9) | |
| Luminal A | 12/55 (21·8) | |
| Luminal B | 26/55 (47·2) | |
| Hormonal receptor‐positive* | 42/65 (64·2) | |
| HER2‐positive | 7/55 (12·7) | |
| Triple‐negative | 10/55 (18·1) | |
| Smokers | 6/45 (13·3) | |
| Mean body mass index | 27·42 ± 4·87 kg/m2 | |
*Hormonal receptor‐positive = to oestrogen or oestrogen + progesterone receptor‐positive. HER2 = human epidermal growth factor receptor 2.
Comparison of ANA prevalence in the three groups (controls, benign lesions and malignant lesions) is shown in Fig. 1.
Figure 1.
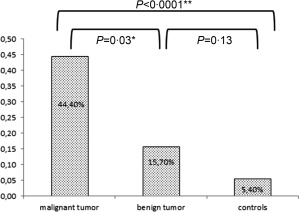
Prevalence of anti‐nuclear antibodies (ANA) in patients with breast tumours and controls. *Malignant versus benign: odds ratio (OR) = 4·26; 95% confidence interval (CI) = 1·14–15·92. **Malignant versus controls: OR = 13·76; 95% CI = 4·98–37·95.
The ANA immunofluorescence patterns and titres in the three groups are shown in Table 2.
Table 2.
Anti‐nuclear antibodies (ANA) immunofluorescence pattern and titre in breast lesion patients and controls (n = 182)
| Immunofluorescence pattern | Malignant tumours n = 32/72 | Benign tumours n = 3/19 | Controls n = 5/91 |
|---|---|---|---|
| Speckled (fine dense) | 10/32 (31·2%) | 2/3 (66·6%) | 2/5 (40·0%) |
| Speckled (fine) | 9/32 (28·1%) | 0 | 0 |
| Speckled (coarse) | 8/32 (25·0%) | 1/3 (33·4%) | 1/5 (20%) |
| Nucleolar | 3/32 (9·4%) | 0 | 0 |
| Homogeneous | 2/32 (6·2%) | 0 | 0 |
| Cytoplasmatic | 0 | 0 | 2 (40·0%) |
| ANA titre | 1/80–12/32 (37·5%) | All = 1 : 80 | 1:80–3/5 (60%) |
| 1/160–13/32 (40·6%) | 1/160–1/5 (20%) | ||
| 1/320–5/32 (15·6%) | 1/320–1/5 (20%) | ||
| 1/640–2/32 (6·2%) |
Table 3 shows the comparison between ANA‐positive and ‐negative patients from the malignant breast tumour group. In this table it is possible to see that hormonal receptor‐positive patients had a lower prevalence of ANA.
Table 3.
Comparison of malignant breast lesions characteristics according to positivity of anti‐nuclear antibody (ANA)
| Positive ANA n = 32 | Negative ANA n = 40 | P | |
|---|---|---|---|
| Ethnic background | Caucasians = 32/32, 100% |
Caucasians = 38/40, 95% African descendants = 2/40, 5% |
0·49 |
| Female gender | 32/32, 100% | 39/40, 97·5% | 1·00 |
| Mean age (years) | 53·1 ± 14·74 | 55·10 ± 14·44 | 0·57 |
| Histology |
Ductal invasive = 26/32, 81·2% Others = 6/32, 18·7% |
Ductal invasive = 34/40, 85% Others = 6/40, 15% |
0·30 |
| Stage IV | 10/27, 37·0% | 11/30, 36·6% | 0·97 |
| Luminal A | 5/25, 20% | 7/30, 23·3% | 0·76 |
| Luminal B | 10/25, 40% | 16/30, 53·3% | 0·32 |
| HER‐2‐positive | 4/25, 16% | 3/30, 10% | 0·68 |
| Triple‐negative | 6/25, 24% | 4/30, 13·3% | 0·48 |
| Hormonal receptor‐positive | 16/28, 57·1% | 26/30, 86·6% | 0·01* |
| Smoking | 2/16, 12·5% | 2/26, 7·6% | 0·62 |
| Body mass index (kg/m2) | 29·18 ± 6·13 | 25·99 ± 3·28 | 0·09 |
*Odds ratio = 4·8 (95% confidence interval = 1·33–17·7).
All patients with positive ANA were tested for an ENA profile. Among these, seven of 32 (21·9%) had at least one positive test in the ENA profile: three of 32 (9·4%) were positive for anti‐Sm, four of 32 (12·5%) for anti‐RNP, two of 32 (6·3%) for anti‐Ro and four of 32 (12·5%) for anti‐La. All patients with anti‐Sm had also anti‐RNP; all positive for anti‐Ro also had anti‐La. None of these patients had known rheumatic disease. All patients with a positive ENA profile had malignant lesions. The histological pattern in six of seven patients was ductal infiltrating and was ducto‐lobular infiltrating in one.
Discussion
Our results showed that breast cancer patients have a high prevalence of positivity for ANA that is significantly higher than in patients with benign lesions and controls. It was not possible to associate the ANA presence with any of tumour characteristics except by a negative connection with hormonal receptor.
Shiel and Jason 12 reported that, in 2·9% of all patients with ANAs and no established diagnosis referred to a rheumatologist for evaluation, a neoplasia was found. An interesting study in patients with chronic liver disease 13 whose liver cancer was detected later showed that 27% of patients were ANA‐positive prior to cancer diagnosis and in 40% the ANA titre rose just before the cancer appearance. In those who were negative, 30% converted to positive ANA when the cancer was detected. These findings show that the immune system of such patients reacts to factors involved in carcinogenesis and that ANA, as part of this response, may be of use to identify such patients.
Autoantibodies found in a cancer patient may be classified into two broad categories 14: (i) specific antibodies to antigens that are not associated directly with the tumour. In this group are found antibodies to antigens that play a role in the regulation of cell cycle and mitosis, ANA belongs to this group; and (ii) antibodies against specific tumour antigens (TAA or tumour‐associated antigens) as oncoproteins, tumour suppression genes, onconeural antigens, etc. In this context, antibodies against p53, anti‐HER2, anti‐c‐myc and anti BRCA2 are found 6. According to Tan et al. 15, the function of the immune response to TAAs is to remove precancerous lesions during the early events of carcinogenesis. However, not only TAAs but also ANAs have been associated with a protective role against tumour spread 9, 16. Experimental studies have shown that ANAs have anti‐tumour activity. Some explanations for this activity are antibody‐dependent cell‐mediated cytotoxicity, release of cytokines that enhance the immune function and that are induced by the formation of ANA immune complexes and the reduction of the inhibitory effect of extracellular chromatin on natural killer (NK) cell activity through the binding of ANAs, and extracellular nuclear chromatin released from apoptotic tumour cells 9. There is an interesting observation that the mortality rate of cancer patients with autoimmune diseases may be significantly lower than that of general cancer patients 9, 16, although not all authors agree 17.
In the present study we could not link the ANA presence with variables that indicate a poor tumour prognosis, such as triple‐negative receptors or stage IV disease. Our findings agree with those of Mohammed et al. 18 who, studying 35 newly diagnosed breast cancer patients, found that ANA was increased significantly in these patients irrespective of the grade or tumour stage. Conversely, Heegaard et al. 8, studying ovarian cancer survival, found that it was significantly shorter in ANA‐positive compared with ‐negative cancer patients.
We found a high prevalence of positive ANA (44·4%) in patients with malignant breast tumours, a result similar to those of Wasserman et al. 19, who found ANA positivity in 35% of their patients. Madrid et al. 5 found a much higher prevalence (up to 99% in invasive breast cancer), but these authors combined the HEp‐2 cells immunofluorescence technique with immunoblot of breast cancer proteins, broadening their field of investigation. Although extremely interesting from a research viewpoint, this last technique is not available in current daily clinical practice.
Some of the currently studied patients had immunofluorescence patterns that are highly valued in rheumatology clinics, such as homogeneous, nucleolar, fine‐ and coarse‐speckled and nucleolar patterns. A homogeneous pattern is often linked to anti‐dsDNA, anti‐nucleosome and anti‐histone antibodies, while fine‐speckled is linked to anti‐Ro and anti‐La antibodies; coarse‐speckled is linked to anti‐RNP and anti‐Sm antibodies 20. These autoantibodies are seen in systemic lupus, drug‐induced lupus, Sjögren's syndrome and overlap syndromes, among others. A nucleolar pattern is seen frequently in scleroderma patients and may indicate the presence of anti‐Scl‐70 (or anti‐topoisomerase‐1) 20.
The most commonly found ANA immunofluorescence pattern in this sample was the fine dense‐speckled pattern. This is an antibody with a controversial meaning 20. Despite being common and capable of reaching high titres, they lack specificity and can be found in apparently healthy individuals and in diverse non‐rheumatic inflammatory disorders 21. This antibody is considered to be directed against the dense fine‐speckled protein of 70 kDa/lens epithelium‐derived growth factor p75 (DFS70/LEDGFp75) that was presumed originally to be a lens epithelial cell growth factor. Currently there is some evidence that this autoantigen may, indeed, be a stress response protein that is expressed universally in mammalian cells and tissues and over‐expressed in tumour cells. DFS70/LEDGFp75 may be of relevance in supporting cell survival when the cell faces ambient stressors such as alcohol, ultraviolet B (UVB) irradiation, viral infections and cytotoxic drugs 22. It has been shown that LEDGF/p75 is up‐regulated in cancer cells when compared to normal cells 23, 24, 25. The fine dense‐speckled pattern is not associated with SLE, so its presence should be a reminder that cancer diagnosis is more likely than a diagnosis of lupus.
We have found also that, in our sample, seven patients had positivity for the ENA profile, the most common being anti‐RNP and anti‐La, followed by anti‐Sm and anti‐Ro; all these patients had tumour‐invasive forms. As these autoantibodies are found classically in autoimmune rheumatic diseases, such results may reinforce diagnosis and delay the tumour discovery.
An interesting finding of the present research was that the hormonal receptor‐positive (oestrogen or oestrogen plus progesterone receptor) patients had less ANA than negative patients [odds ratio (OR) = 4·8; 95% confidence interval (CI) = 1·33–17·7). Corroborating this result, Gadalla et al. 26 found that women with SLE might be at reduced risk for oestrogen receptor‐negative tumours. In addition, Chan et al. 27 found a high proportion of triple‐negative breast cancers in SLE women. Oestrogen receptors have dual localization in the cell: intracellular and plasma membrane. Immune cell such as T and B and NK lymphocytes express intracellular oestrogen receptors 14. However, the oestrogen effect on mature immune cells is complex: in high levels such as those seen in the periovulatory period and pregnancy states, it inhibits proinflammatory cytokines such as tumour necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6 and NK cell activity and activates anti‐inflammatory pathways such as IL‐4 and IL‐10 14. In lower concentrations it has an opposite action. It is also known that oestrogens enhance the number and function of regulatory T cells (Treg), suggesting a potential interaction between this hormone and immune regulatory mechanisms 14. In this context it is interesting to remember the curious observation that systemic lupus patients had fewer breast cancers than the general population 28. Therefore, the inverse relationship between ANA and oestrogen receptor presence, although interesting, is not clear at present, but certainly deserves further studies.
Despite the fact that almost 60% of our patients had ANA titres ≥ 1/160, it should be noted that we considered those with titres ≥ 1/80 as ANA‐positive and are not considered valuable in autoimmune rheumatic diseases. We ignore how these titres should be considered in malignant diseases.
In conclusion, the authors highlight that ANA prevalence in patients with breast cancer is high. More studies are needed to understand the real value of ANA testing in this context.
Disclosure
None.
References
- 1. Pisetsky DS. Antinuclear antibody testing – misunderstood or misbegotten? Nat Rev Rheumatol 2017; 13:495–502. [DOI] [PubMed] [Google Scholar]
- 2. Robertson JM, James JA. Preclinical systemic lupus erythematosus. Rheum Dis Clin North Am 2014; 40:621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. 3. Fernández‐Madrid F, VandeVord PJ, Yang X et al Antinuclear antibodies as potential markers of lung cancer. Clin Cancer Res 1999; 5:1393–400. [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández Madrid F, Karvonen RL, Ensley J et al Spectra of antinuclear antibodies in patients with squamous cell carcinoma of the lung and of the head and neck. Cancer Detect Prev 2005; 29:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madrid FF, Maroun MC, Olivero OA et al Autoantibodies in breast cancer sera are not epiphenomena and may participate in carcinogenesis. BMC Cancer 2015; 15:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman C, Murray A, Chakrabarti J et al Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 2007; 18:868–73. [DOI] [PubMed] [Google Scholar]
- 7. Ramírez‐Sandoval R, Sánchez‐Rodríguez SH, Herrera‐van Oostdam D, Avalos‐Díaz E, Herrera‐Esparza R. Antinuclear antibodies recognize cellular autoantigens driven by apoptosis. Joint Bone Spine 2003; 70:187–94. [DOI] [PubMed] [Google Scholar]
- 8. Heegaard NH, West‐Nørager M, Tanassi JT et al Circulating antinuclear antibodies in patients with pelvic masses are associated with malignancy and decreased survival. PLOS ONE 2012; 7:e30997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zou HY, Gu X, Yu WZ, Wang Z, Jiao M. Detection of serum antinuclear antibodies in lymphoma patients. Genet Mol Res 2015; 14:16546–52. [DOI] [PubMed] [Google Scholar]
- 10. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res 2017; 50:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 11. Griffiths C, Brock A. Twentieth century mortality trends in England and Wales. Office for National Statistics. Health Stat Q 2003; 18:5–17. [Google Scholar]
- 12. 12. Shiel WC, Jason M. The diagnostic associations of patients with antinuclear antibodies referred to a community rheumatologist. J Rheumatol 1989; 16:782–5. [PubMed] [Google Scholar]
- 13. Imai H, Nakano Y, Kiyosawa K, Tan EM. Increasing titers and changing specificities of antinuclear antibodies in patients with chronic liver disease who develop hepatocellular carcinoma. Cancer 1993; 71:26–35. [DOI] [PubMed] [Google Scholar]
- 14. Ortona E, Pierdominici M, Berstein L. Autoantibodies to estrogen receptors and their involvement in autoimmune diseases and cancer. J Steroid Biochem Mol Biol 2014; 144(Pt B):260–7. [DOI] [PubMed] [Google Scholar]
- 15. 15. Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J 2009; 276:6880–904. [DOI] [PubMed] [Google Scholar]
- 16. Erkanli A, Taylor DD, Dean D et al Application of Bayesian modeling of autologous antibody responses against ovarian tumor‐associated antigens to cancer detection. Cancer Res 2006; 66:1792–8. [DOI] [PubMed] [Google Scholar]
- 17. Altintas A, Cil T, Pasa S et al Clinical significance of elevated antinuclear antibody test in patients with Hodgkin's and non‐Hodgkin's lymphoma: a single center experience. Minerva Med 2008; 99:7–14. [PubMed] [Google Scholar]
- 18. Mohammed ME, Abdelhafiz K. Autoantibodies in the sera of breast cancer patients: antinuclear and anti‐double stranded DNA antibodies as example. J Cancer Res Ther 2015; 11:341. [DOI] [PubMed] [Google Scholar]
- 19. Wasserman J, Glas U, Blomgren H. Autoantibodies in patients with carcinoma of the breast. Correlation with prognosis. Clin Exp Immunol 1975; 19:417–22. [PMC free article] [PubMed] [Google Scholar]
- 20. Francescantonio PL, Cruvinel WdeM, Dellavance A et al IV Brazilian guidelines for autoantibodies on HEp‐2 cells. Rev Bras Reumatol 2014; 54:44–50. [PubMed] [Google Scholar]
- 21. Basu A, Sanchez TW, Casiano CA. DFS70/LEDGFp75: an enigmatic autoantigen at the interface between autoimmunity, AIDS, and cancer. Front Immunol 2015; 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basu A, Rojas H, Banerjee H et al Expression of the stress response oncoprotein LEDGF/p75 in human cancer: a study of 21 tumor types. PLOS ONE 2012; 7:e30132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Daniels T, Molinaro C, Lilly MB, Casiano CA. Caspase cleavage of the nuclear autoantigen LEDGF/p75 abrogates its pro‐survival function: implications for autoimmunity in atopic disorders. Cell Death Differ 2002; 9:915–25. [DOI] [PubMed] [Google Scholar]
- 24. Brown‐Bryan TA, Leoh LS, Ganapathy V et al Alternative splicing and caspase‐mediated cleavage generate antagonistic variants of the stress oncoprotein LEDGF/p75. Mol Cancer Res 2008; 6:1293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daniels T, Zhang J, Gutierrez I et al Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate 2005; 62:14–26. [DOI] [PubMed] [Google Scholar]
- 26. Gadalla SM, Amr S, Langenberg P et al Breast cancer risk in elderly women with systemic autoimmune rheumatic diseases: a population‐based case–control study. Br J Cancer 2009; 100:817–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan K, Clarke AE, Ramsey‐Goldman R et al Breast cancer in systemic lupus erythematosus (SLE): receptor status and treatment. Lupus 2018; 27:120–3. [DOI] [PubMed] [Google Scholar]
- 28. Bernatsky S, Ramsey‐Goldman R, Petri M et al Breast cancer in systemic lupus. Lupus 2017; 26:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


