Abstract
Aims
The objective of the present study was to investigate the current situation concerning, and risk factors for, vancomycin (VAN)‐induced acute kidney injury (VI‐AKI) in elderly Chinese patients, to assess outcomes and risk factors in patients who have developed VI‐AKI, in order to provide suggestions for improving the prevention and treatment of this condition in these patients.
Method
We retrospectively identified elderly older inpatients who had received four or more doses of VAN treatment. We compared patients with VI‐AKI with those who received VAN treatment and had not developed AKI (NO‐AKI). We defined VI‐AKI as developing AKI during VAN therapy or within 3 days after withdrawal of VAN.
Results
A total of 647 out of 862 elderly inpatients were included in the study. Among those excluded, in 89.3% of cases (192/215) this was because of lack of data on serum creatinine (SCr). Among included patients, 32.5% (210/647) of patients received therapeutic drug monitoring (TDM) during VAN therapy. In 66.9% of cases (424/634), there was insufficient TDM, and in 3.9% (25/634) this was appropriate. A total of 102 patients had confirmed VI‐AKI, with an incidence of 15.8% (102/647). Multiple logistic regression analysis revealed that hyperuricaemia [odds ratio (OR) = 3.045; P = 0.000)], mechanical ventilation (OR = 1.906; P = 0.022) and concomitant vasopressor therapy (OR = 1.919; P = 0.027) were independent risk factors for VI‐AKI; higher serum albumin (OR = 0.885; P = 0.000) was determined to be an independent protective factor for VI‐AKI.
Conclusions
For the elderly Chinese patients treated with VAN, there was insufficient monitoring of SCr, too little use of VAN TDM, and lower rate of patients whose VAN though serum concentrations were not obtained at the correct time. We recommend that hospital managers increase investment in clinical pharmacists, to strengthen professional management. Patients with concomitant hyperuricaemia and on mechanical ventilation and vasopressor therapy should be paid more attention, and a higher serum albumin was determined to be an independent protective factor for VI‐AKI.
Keywords: acute kidney injury, older patients, risk factors, vancomycin
What is Already Known about this Subject
Acute kidney injury (AKI) is the main serious adverse drug reaction associated with vancomycin (VAN) treatment and can lead to renal insufficiency or even death.
A large number of studies have shown that older people, especially the elderly, due to their specific physiological status, are at higher risk for nephrotoxicity, and race may be a risk factor for VAN nephrotoxicity.
However, the current data for VAN‐induced (VI) AKI in elderly Chinese patients are very limit.
What this Study Adds
For the elderly Chinese patients treated with VAN, there was insufficient monitoring of serum creatinine, too little use of VAN TDM, and lower rate of patients whose VAN though serum concentrations were obtained at the correct time.
102 patients had confirmed VI‐AKI, with an incidence of 15.8% (102/647). Multiple logistic regression analysis revealed that concomitant hyperuricaemia, use of mechanical ventilation and concomitant vasopressor therapy were independent risk factors for VI‐AKI; in addition, higher serum albumin was determined to be an independent protective factor for VI‐AKI.
We recommend that hospital managers increase investment in clinical pharmacists, to strengthen professional management. Patients with concomitant hyperuricaemia and on mechanical ventilation and vasopressor therapy should be paid more attention, and a higher serum albumin was determined to be an independent protective factor for VI‐AKI.
Introduction
Vancomycin (VAN) is the important drug of choice for methicillin‐resistant Staphylococcus aureus (MRSA) 1. To prevent resistance and ensure clinical efficacy, the clinical guidelines recommend always keeping trough levels above 10 mg l−1, and a trough level of 15–20 mg l−1 is recommended in more serious infections 2. However, acute kidney injury (AKI) is still the main serious adverse drug reaction (ADR) experienced by patients receiving VAN treatment, especially in septic patients admitted to the intensive care unit (ICU). The concomitance of other factors and the use of VAN makes it difficult to establish whether or not AKI is caused by VAN‐induced nephrotoxicity. Thus, some authors have questioned whether high serum levels of VAN are the cause or a consequence of AKI 3, 4, 5. Many factors are known to affect AKI development, although findings have not been consistent among studies 5. The research of Bosso et al. showed that black race was a risk factor for VAN‐induced nephrotoxicity (VIN) 6. A large number of studies have shown that older people, especially the elderly, due to their specific physiological status, are at higher risk for nephrotoxicity 4, 7, 8. With the increasing ageing of Chinese society, VAN‐induced AKI (VI‐AKI) in the elderly deserves more attention 9. However, there have been few studies in elderly Chinese patients, and little is known about the risk factors for VI‐AKI among Chinese individuals. A recent national survey on AKI in China revealed a serious issue with drug safety in the AKI population, as up to 70% of AKI patients had been exposed to potentially nephrotoxic drugs before or during their kidney injury, which is much higher than in developed countries (20–50%) 10. The use of concomitant drugs has an important influence on VI‐AKI. The individual agents that have been studied most extensively are aminoglycosides and piperacillin–tazobactam, but research on other potential toxins, such as Chinese patent medicines, is still needed 4. The combination of multiple illnesses and use of complicated medications in elderly patients may make the issue of VI‐AKI more serious. Meanwhile, data on of the outcome of elderly patients who have developed VI‐AKI and on their risk factors are also limited, and deserving of further study. The objective of the present study was to assess the outcomes and identify the risk factors associated with VI‐AKI in elderly Chinese patients, in order to provide suggestions for improving prevention and treatment in this population.
Methods
Study design and population
This was a single‐centre retrospective study performed at ZhongShan Hospital, FuDan University. We recruited all elderly inpatients treated with VAN at our hospital from January 2016 to June 2017. The study was retrospective, and data were collected from the medical records of discharged patients. We did not need to obtain written informed consent from the patients whose records were used, as the study had received a Retrospective Clinical Application from ZhongShan Hospital. The study had received a Retrospective Clinical Application from ZhongShan Hospital. Patient data were anonymized prior to analysis. Another pharmacist, who was not participating in the study, was responsible for anonymizing these data.
The VI‐AKI survey was designed to include three steps (see Figure 1). First, we screened the patients treated with VAN at our hospital; the inclusion criteria were: (i) ≥65 years of age; (ii) receiving four or more doses of VAN during treatment period. Patients were excluded if: (i) there was incomplete case information; (ii) they had received a kidney transplant; (iii) they had stage 5 chronic kidney disease (CKD) or were receiving regular dialysis; (iv) there were no serum creatinine (SCr) measurements available.
Figure 1.
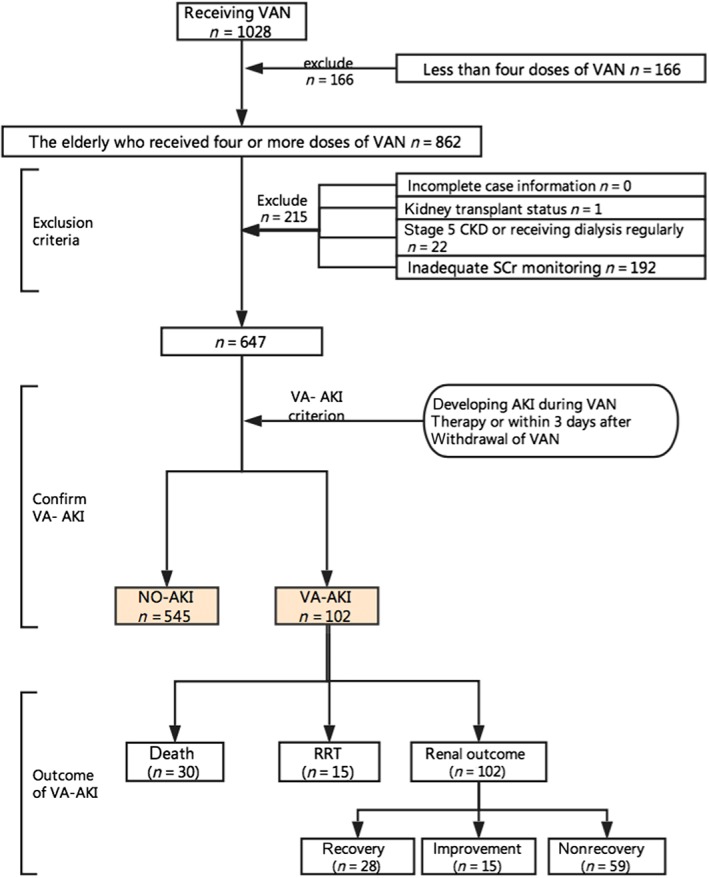
Study design. AKI, acute kidney injury; CKD, chronic kidney disease; NO‐AKI, no development of AKI; RRT, renal replacement therapy; SCr, serum creatinine; VI‐AKI, vancomycin‐induced acute kidney injury; VAN, vancomycin
Secondly, we recorded the SCr of the included patients and separated the latter into two groups: those who had not developed AKI (NO‐AKI) and the VI‐AKI group. The definition of VI‐AKI is the development of AKI during VAN therapy or within 3 days after the withdrawal of VAN. We used the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition of AKI as the major screening criterion 11: an increase in SCr by ≥0.3 mg dl−1 (≥26.5 μmol l−1) within 48 h or an increase in SCr to ≥1.5 times baseline which is known or presumed to have occurred within the prior 7 days.
Thirdly, for the patients who developed AKI, we further analysed the development, severity and outcome of this condition. AKI severity was classified by the highest stage of AKI (1, 2, or 3) observed in the patient, according to the KDIGO criteria 11. AKI outcome was examined using three variables: all‐cause in‐hospital death, receipt of renal replacement therapy (RRT) and renal outcome at discharge. Renal outcome was categorized into three levels: recovery, improvement and nonrecovery. Recovery was defined as restoration of the SCr level to baseline during hospitalization; improvement was defined as a decrease of at least 25% in the SCr level during hospitalization from the beginning of AKI onset; and nonrecovery was defined as a lack of improvement in the SCr level at discharge. We combined the recovery and improvement groups into one group for examination of the risk factors related to the renal outcome of VI‐AKI.
Data collection
We collected data on the following variables: demographic characteristics (gender, age, weight), payment methods (basic national medical insurance, self‐financing), inpatient department (medical, surgical, ICU), concomitant diseases [hypertension, diabetes, coronary heart disease (CHD), CKD and chronic lung disease (CLD), and chronic obstructive pulmonary disease (COPD)], laboratory variables [baseline estimated glomerular filtration rate (eGFR), serum albumin valley, presence or not of hyperuricaemia, peak serum lactic acid level), hospitalization‐related factors [length of stay (LOS); ICU admittance; the presence or not of cancer, sepsis, hypotension and shock; the sequential organ failure assessment (SOFA); whether the patient had undergone surgery, whether the patient was on mechanical ventilation; whether death occurred]. The following variables concerning VAN therapy and the use of concomitant drugs were also collected as comprehensively as possible: indication for VAN therapy (prophylactic, local infection, bacteraemia); variety of VAN (Wen kexin, Lai kexin); length of therapy (LOT); therapeutic drug monitoring (TDM); VAN dose adjustment; daily dose (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 g); use of vasoactive drugs (nitrates, vasopressors, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, dihydropyridines); and use of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7170, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4836, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2875, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4839, mannitol, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7150, statins, sulphonylurea, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6841, antibiotics (piperacillin–tazobactam, cephalosporin, carbapenems, aminoglycosides, quinolones, macrolides, compound sulfamethoxazole, metronidazole/ornidazole), azole antifungal agents, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4829, contrast medium, nonsteroidal anti‐inflammatory drugs (NSAIDs), steroids, calcineurin inhibitors, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6831, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7154, polyene phosphatidylcholine, edaravone, glutathione, acetylcysteine, phosphocreatine, levocarnitine, Chinese patent medicines (the root of red‐rooted salvia, liquorice, kanglaite injection, xingnaojing injection).
Data analysis
Normally distributed continuous variables were expressed as the mean ± standard deviation (SD), and groups were compared using the independent Student's t‐test. Non‐normally distributed continuous variables were presented as the median [interquartile range (IQR)], and groups were compared using the rank‐sum test. In addition, categorical variables were expressed as numbers (percentages) and analysed using the chi‐squared test or Fisher's exact test. Further, logistic regression models were used to assess independent risk factors for VI‐AKI incidence and outcome, and death. Multiple logistic regression models were used to identify variables with a P value of less than 0.2 in descriptive analysis; these variables were further examined in multivariate analysis to identify independent risk factors. The covariates included in multiple logistic regression analysis of VI‐AKI incidence included: age (years); baseline SCr (mg dl−1); serum albumin valley (g l−1); hyperuricaemia (yes or no); LOS (days); ICU admittance (yes or no); SOFA (yes or no); shock (yes or no), mechanical ventilation (yes or no); variety of VAN (Wen kexin or Lai kexin); TDM (yes or no); VAN dose adjustment (yes or no); and use of nitrates (yes or no), vasopressors (yes or no), β‐blockers (yes or no), spironolactone (yes or no), furosemide (yes or no), carbapenems (yes or no), compound sulfamethoxazole (yes or no), metronidazole/ornidazole (yes or no), azole antifungal agents (yes or no), steroids (yes or no), glutathione (yes or no), acetylcysteine (yes or no) and phosphocreatine(yes or no). A forward logistic model was used for the selection of variables. We used the same method to assess the independent risk factors for death and VI‐AKI outcome. All P values were two‐sided, and a P value of less than 0.05 was deemed significant. Statistical analyses were conducted using Statistical Package for Social Sciences version 23.0 (IBM, 187 Chicago, IL, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 12.
Results
Patients excluded and included
Of the 862 elderly inpatients who received four or more doses of VAN during treatment period, 647 were included in the study. The most common reason for patient exclusion was inadequate SCr monitoring to detect AKI development (89.3%; 192/215) (see Figure 2). Of the 647 patients included, 62.1% (402/647) were male, the median age was 71 years (IQR = 10) and 50.5% (327/647) were covered by basic national medical insurance. In addition, 31.2% (202/647) of patients also had hypertension, and the proportions of those with concomitant diabetes, CHD, CKD, CLD and COPD were 16.5% (107/647), 14.4% (93/647), 2.6% (17/647), 5.7% (37/647) and 5.4% (35/647), respectively. The average LOS was 24.1 (SD 25.9) days (see Table 1).
Figure 2.
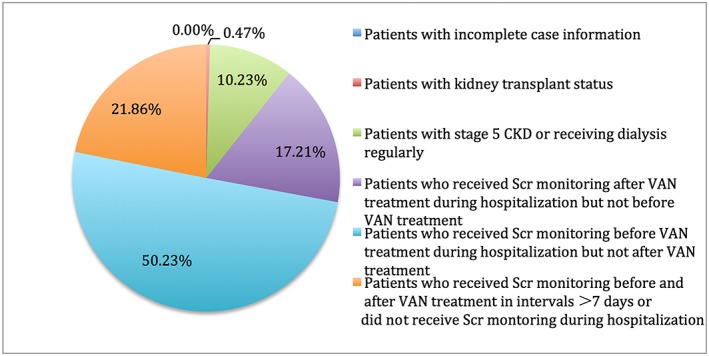
Frequency distribution of excluded patients. CKD, chronic kidney disease; SCr, serum creatinine; VAN, vancomycin
Table 1.
Comparison of clinical characteristics between patients with vancomycin‐induced acute kidney injury (VI‐AKI) and those without (NO‐AKI)
| Total (647) | NO‐AKI (545) | VI‐AKI (102) | P | |
|---|---|---|---|---|
| Demographic factors: | ||||
| Male, n (%) | 402 (62.1) | 343 (62.9) | 59 (57.8) | 0.330 |
| Age, median (IQR) | 71 (10) | 71 (9) | 72 (10) | 0.198 |
| Payment methods: | 0.443 | |||
| Basic national medical insurance, n (%) | 327 (50.5) | 279 (47.1) | 48 (51.2) | |
| Self‐paying, n (%) | 320 (49.5) | 266 (48.8) | 54 (52.9) | |
| In‐patient department: | 0.875 | |||
| Medical n (%) | 241 (37.4) | 204 (36.3) | 37 (37.6) | |
| Surgical n (%) | 378 (58.6) | 318 (58.6) | 60 (58.8) | |
| ICU, n (%) | 26 (4.0) | 21 (4.9) | 5 (3.9) | |
| Concomitant diseases: | ||||
| Hypertension, n (%) | 202 (31.2) | 165 (30.3) | 37 (36.3) | 0.23 |
| Diabetes, n (%) | 107 (16.5) | 87 (16.0) | 20 (19.6) | 0.363 |
| CHD, n (%) | 93 (14.4) | 78 (14.3) | 15 (14.7) | 0.917 |
| CKD, n (%) | 17 (2.6) | 15 (2.8) | 2 (0.3) | 0.646 |
| CLD, n (%) | 37 (5.7) | 30 (5.5) | 7 (6.9) | 0.588 |
| COPD, n (%) | 35 (5.4) | 29 (5.3) | 6 (5.9) | 0.818 |
| Laboratory variables: | ||||
| Baseline SCr, mg dl−1 | 71.0 (34.0) | 70.0 (33.0) | 82.5 (59.5) | 0.001 |
| Baseline eGFR, ml min−1 1.73 m−2 | 97.8 ± 56.5 | 99.9 ± 57.2 | 83.0 ± 58.1 | 0.000 |
| Serum albumin, g l−1 | 28.7 ± 4.88 | 29.3 ± 4.79 | 26.6 ± 4.87 | 0.000 |
| Hyperuricaemia, n (%) | 304 (47.0) | 229 (42.0) | 75 (73.5) | 0.000 |
| Peak serum lactic acid, mmol l−1 | 3.20 (4.43) | 3.05 (3.30) | 3.30 (5.00) | 0.403 |
| Hospitalization‐related factors: | ||||
| LOS (days), median (IQR) | 24.1 (25.9) | 23.3(24.5) | 27.1 (28.4) | 0.064 |
| ICU admittance, n (%) | 178 (27.5) | 143 (26.2) | 35 (34.3) | 0.094 |
| Cancer, n (%) | 151 (23.3) | 127 (23.3) | 24 (23.5) | 0.96 |
| Sepsis n (%) | 34 (5.3) | 23 (4.2) | 11 (10.8) | 0.006 |
| Hypotension, n (%) | 28 (4.3) | 20 (3.7) | 8 (7.8) | 0.102a |
| SOFA, n (%) | 40 (6.2) | 27 (5.0) | 13 (12.7) | 0.003 |
| Shock, n (%) | 75 (11.6) | 51 (9.4) | 24 (23.5) | 0.000 |
| Surgery, n (%) | 386 (59.7) | 324 (59.4) | 62 (60.8) | 0.801 |
| Mechanical ventilation, n (%) | 405 (62.6) | 325 (59.6) | 80 (78.4) | 0.000 |
| Death, n (%) | 95 (14.7) | 65 (11.9) | 30 (29.4) | 0.000 |
CHD, coronary heart disease; CKD, chronic kidney disease; CLD, chronic lung disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LOS, length of stay; SOFA, sequential organ failure assessment; SCr, serum creatinine
Continuity correction
The VAN used in our hospital was obtained from two sources: Wen kexin [trade name: Wen kexin; generic name: vancomycin hydrochloride for injection; manufacturer: Vianex Sa (Plantc), Athens, Greece; specification: 500 mg bottle−1; 66.0% (427/647) of patients were treated with VAN from this source) and Lai kexin (trade name: Lai kexin; generic name: vancomycin hydrochloride for injection; manufacturers: Zhejiang Medicine Co., Ltd and Xinchang Pharmaceutical Factory, XinChang City, ZheJiang Province, China; specification: 500 mg bottle−1). The reasons for VAN treatment were mainly local infection and bacteraemia, corresponding to 69.9% (452/647) and 25.0% (162/647), respectively. The median LOT was 5.6 days (IQR = 6.2), and the most frequently used daily doses were 1.0 g, 1.5 g and 2.0 g, with 27.6% (178/647), 26.0% (168/647) and 38.7% (250/647) of patients, respectively using these doses. We also analysed 41 types of drugs used concomitantly by the patients receiving VAN treatment (see Table 2).
Table 2.
Comparison of concomitant drugs used between patients with vancomycin‐induced acute kidney injury (VI‐AKI) and those without (NO‐AKI)
| Total (n = 647) | NO‐AKI (n = 545) | VI‐AKI (n = 102) | P | |
|---|---|---|---|---|
| Indication for VAN therapy, n (%) #1: | 0.190 | |||
| Prophylactic, n (%) | 33 (5.1) | 29 (5.3) | 4 (3.9) | |
| Local infection, n (%) | 452 (69.9) | 373 (68.4) | 79 (77.5) | |
| Bacteraemia, n (%) | 162 (25) | 143 (26.2) | 19 (18.6) | |
| Variety of VAN: | 0.015 | |||
| Wen kexin, n (%) | 427 (66.0) | 349 (64.0) | 78 (76.5) | |
| Lai kexin, n (%) | 220 (34.0) | 196 (36.0) | 24 (23.5) | |
| LOT, median (IQR) | 5.6 (6.2) | 5.8 (6.0) | 5.5 (7.5) | 0.468 |
| TDM, n (%) | 210 (32.5) | 161 (29.6) | 49 (48.0) | 0.000 |
| Daily dose: | 0.274 | |||
| 0.5 g, n (%) | 45 (7.0) | 32 (5.9) | 13 (12.7) | |
| 1.0 g, n (%) | 178 (27.6) | 151 (27.8) | 27 (26.5) | |
| 1.5 g, n (%) | 168 (26.0) | 142 (26.1) | 26 (25.5) | |
| 2.0 g, n (%) | 250 (38.7) | 215 (39.5) | 35 (34.3) | |
| 2.5 g, n (%) | 1 (0.2) | 1 (0.2) | 0 (0) | |
| 3.0 g, n (%) | 1 (0.2) | 1 (0.2) | 0 (0) | |
| 5.0 g, n (%) | 3 (0.5) | 2 (0.4) | 1 (1.0) | |
| Concomitant drugs: | ||||
| Nitrate, n (%) | 304 (47.0) | 249 (45.7) | 55 (53.9) | 0.126 |
| Vasopressor, n (%) | 96 (14.8) | 66 (12.1) | 30 (29.4) | 0.000 |
| Doxazosin, n (%) | 8 (1.2) | 5 (0.9) | 3 (2.9) | 0.227a |
| ACEI, n (%) | 84 (13.0) | 67 (12.3) | 17 (16.7) | 0.228 |
| ARB, n (%) | 72 (11.1) | 61 (11.2) | 11 (10.8) | 0.904 |
| β‐Blocker, n (%) | 349 (53.9) | 288 (52.8) | 61 (59.8) | 0.196 |
| DHP, n (%) | 183 (28.3) | 154 (28.3) | 29 (28.4) | 0.971 |
| Hydrochlorothiazide, n (%) | 41 (6.3) | 34 (6.2) | 7 (6.9) | 0.812 |
| Spironolactone, n (%) | 332 (51.3) | 272 (49.9) | 60 (58.8) | 0.098 |
| Furosemide, n (%) | 411 (63.5) | 332 (60.9) | 79 (77.5) | 0.001 |
| Mannitol, n (%) | 44 (6.8) | 35 (6.4) | 9 (8.8) | 0.377 |
| Clopidogrel, n (%) | 96 (14.8) | 85 (15.6) | 11 (10.8) | 0.210 |
| Statin, n (%) | 164 (25.3) | 136 (25.0) | 28 (27.5) | 0.595 |
| Sulphonylurea, n (%) | 27 (4.2) | 23 (4.2) | 4 (3.9) | 1.000a |
| Repaglinide, n (%) | 9 (1.4) | 8 (1.5) | 1 (1.0) | 1.000b |
| Piperacillin–tazobactam, n (%) | 35 (5.4) | 27 (5.0) | 8 (7.8) | 0.236 |
| Cephalosporin, n (%) | 536 (82.8) | 449 (82.4) | 87 (85.3) | 0.474 |
| First‐generation cephalosporin, n (%) | 105 (16.2) | 91 (16.7) | 14 (13.7) | 0.445 |
| Second‐generation cephalosporin, n (%) | 299 (46.2) | 251 (46.1) | 48 (47.1) | 0.852 |
| Third‐generation cephalosporin, n (%) | 323 (49.9) | 267 (49.0) | 56 (54.9) | 0.273 |
| Fourth‐generation cephalosporin, n (%) | 73 (11.3) | 61 (11.2) | 12 (11.8) | 0.867 |
| Carbapenem n (%) | 525 (81.1) | 430 (78.9) | 95 (93.1) | 0.001 |
| Aminoglycoside, n (%) | 48 (7.4) | 40 (7.3) | 8 (7.8) | 0.859 |
| Quinolone, n (%) | 292 (45.1) | 242 (44.4) | 50 (49.0) | 0.390 |
| Macrolide, n (%) | 27(4.2) | 25(4.6) | 2(2.0) | 0.343a |
| Compound sulfamethoxazole, n (%) | 31 (4.8) | 20 (3.7) | 11 (10.8) | 0.002 |
| Metronidazole/ornidazole, n (%) | 91 (14.1) | 71 (13.0) | 20 (19.6) | 0.079 |
| Azole antifungal agent, n (%) | 132 (20.4) | 102 (18.7) | 30 (29.4) | 0.022 |
| Acyclovir, n (%) | 24 (3.7) | 19 (3.5) | 5 (4.9) | 0.683a |
| Contrast medium, n (%) | 222 (34.3) | 190 (34.9) | 32 (31.4) | 0.496 |
| NSAID, n (%) | 238 (36.8) | 197 (36.1) | 41 (40.2) | 0.436 |
| Steroid, n (%) | 177 (27.4) | 138 (25.3) | 39 (38.2) | 0.007 |
| Calcineurin inhibitor, n (%) | 13 (2.0) | 10 (1.8) | 3 (23.1) | 0.729a |
| Mycophenolate mofetil, n (%) | 9 (1.4) | 6 (1.1) | 4 (2.9) | 0.319a |
| Cyclophosphamide, n (%) | 16 (2.5) | 13 (2.4) | 3 (2.9) | 1.000a |
| Polyene phosphatidylcholine, n (%) | 30 (4.6) | 26 (4.8) | 4 (3.9) | 0.906a |
| Edaravone, n (%) | 17 (2.6) | 0 (0) | 17 (3.1) | 0.09b |
| Glutathione, n (%) | 309 (47.8) | 249 (45.7) | 60 (58.8) | 0.015 |
| Acetylcysteine, n (%) | 177 (27.4) | 140 (25.7) | 37 (36.3) | 0.028 |
| Phosphocreatine, n (%) | 60 (9.3) | 44 (8.1) | 16 (15.7) | 0.015 |
| Levocarnitine, n (%) | 48 ((7.4) | 41 (7.5) | 7 (6.9) | 0.815 |
| The root of red‐rooted salvia, n (%) | 27 (4.2) | 21 (3.9) | 6 (5.9) | 0.502a |
| Liquorice, n (%) | 102 (15.8) | 68 (15.2) | 34 (16.9) | 0.590 |
| Kanglaite injection, n (%) | 23 (3.6) | 20 (3.7) | 3 (2.9) | 0.941a |
| Xingnaojing injection, n (%) | 46 (7.1) | 38 (7.0) | 8 (7.8) | 0.917a |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; DHP, dihydropyridine; IQR, interquartile range; LOT, length of therapy; NSAID, nonsteroidal anti‐inflammatory drug; TDM, therapeutic drug monitoring; VAN, vancomycin
Continuity correction
Fisher's exact test
TDM of included patients
A total of 32.5% (210/647) of the patients received TDM during VAN therapy. The judgement as to whether a patient required TDM was based on a consensus review of the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists 13 and a consensus of Chinese experts 14; 634 patients met the requirements of the former, and all patients met the requirements of the latter. The proportion of patients in whom TDM was inadequate was 66.9% (424/634) for the American consensus and 67.5% (437/647) for the Chinese consensus. We further analysed the time of trough samples obtained for TDM and found that this was appropriate in 3.9% of cases (25/634) for the American consensus, and 8.2% (53/647) for the Chinese consensus (see Table 3).
Table 3.
TDM situation according the American and Chinese consensuses
| P received TDM (n) | TDM rate (%) | P required TDM (n) | TDM R of reaching consensus (%) | Inadequate TDM R (%) | Correct TDM time (n) | R of correct TDM (%) | |
|---|---|---|---|---|---|---|---|
| America | 210 | 32.5 | 634 | 33.1 | 66.9 | 25 | 3.9 |
| China | 210 | 32.5 | 647 | 32.5 | 67.5 | 53 | 8.2 |
Correct TDM time: American consensus for TDM: Trough samples should be obtained just before the fourth dose in patients with normal renal function, to ensure that target concentrations are attained. TDM Chinese consensus: Trough samples should be obtained just before the third dose of VAN therapy or before the fourth dose of VAN therapy. P, patients; R, rate; TDM, therapeutic drug monitoring; VAN, vancomycin
Clinical characteristics of VI‐AKI
A total of 102 patients developed VI‐AKI, corresponding to an incidence of 15.8% (102/647); 57.8% (59/102) of these were male and the median age was 72 years (IQR = 10). There was no significance difference between patients with and without VI‐AKI in terms of their concomitant diseases, including hypertension, diabetes, CHD, CKD, CLD and COPD. In terms of laboratory variables, patients with VI‐AKI were more likely than those without to have a lower baseline eGFR (99.9 ± 57.2 vs. 83.0 ±58.1; P = 0.000), a lower serum albumin valley (29.3 ± 4.79 vs. 26.6 ± 4.87; P = 0.000) and hyperuricaemia (42.0% vs. 73.5%; P = 0.000). We also analysed hospitalization‐related factors. No significance difference was found between the groups in LOS, ICU admittance, the presence or not of cancer and whether or not they had undergone surgery. VI‐AKI patients were more likely have had sepsis (4.2% vs. 10.8%; P = 0.006), to have undergone a SOFA (5.0% vs. 12.7%; P = 0.003), to have gone into shock (9.4% vs. 23.5%) and to have received mechanical ventilation (59.6% vs. 78.4%; P = 0.000) (see Table 1). When we further analysed the cause of shock, the main cause was septic shock, with an incidence of 60% (45/75) (see Table S1).
Concomitant drugs taken by those with VI‐AKI
We further analysed 41 types of drug taken by patients while receiving VAN treatment. Patients with VI‐AKI were significantly more likely than those without to be taking a vasopressor (12.1% vs. 29.4%; P = 0.000). Among the diuretics (hydrochlorothiazide, spironolactone, furosemide), only furosemide use was found to be significantly higher in VI‐AKI patients (60.9% vs. 77.5%; P = 0.001) than in others. Compared with the NO‐AKI patients, significantly more VI‐AKI patients were taking carbapenems (78.9% vs. 93.1%; P = 0.001), compound sulfamethoxazole (3.7% vs. 10.8%; P = 0.002), azole antifungal agents (18.7% vs. 29.4%; P = 0.022) and steroids (25.3% vs. 38.2%; P = 0.007). VI‐AKI patients were also more likely to be taking glutathione (45.7% vs. 58.8%; P = 0.015), acetylcysteine (25.7% vs. 36.3%; P = 0.028) and phosphocreatine (8.1% vs. 15.7%, P = 0.015). Chinese patent medicines (the root of red‐rooted salvia, liquorice, kanglaite injection, xingnaojing injection) were analysed and no significantly differences were found in their use between patient groups (see Table 2).
Risk factors for VI‐AKI
Multiple logistic regression analysis revealed that hyperuricaemia [odds ratio (OR) = 3.045; P = 0.000), mechanical ventilation (OR = 1.906; P = 0.022) and concomitant vasopressor therapy (OR = 1.919; P = 0.027) were independent risk factors for VI‐AKI; serum albumin valley (OR = 0.885; P = 0.000) was determined to be an independent protective factor for VI‐AKI. The goodness of fit was evaluated using the analysis of Hosmer and Lemeshow, and found to be 0.798 (see Table 4).
Table 4.
Risk factors for vancomycin‐induced acute kidney injury
| Factors | P value | OR | 95% Cl |
|---|---|---|---|
| Serum albumin | 0.000 | 0.885 | 0.843, 0.928 |
| Hyperuricaemia | 0.000 | 3.045 | 1.834, 5.057 |
| Mechanical ventilation | 0.022 | 1.906 | 1.098, 3.310 |
| Vasopressor | 0.027 | 1.919 | 1.078, 3.418 |
CI, confidence interval; OR, odds ratio
VI‐AKI severity and treatment
VI‐AKI severity was classified according to the highest stage of AKI observed in the patient. The highest disease stage was stage 1 for 61.8% (63/102) of the patients and 26.5% (27/102) were stage 3 (see Table S2). Therapy adjustments, including termination of VAN treatment or adjustment in the dose, took place in only 45.1% (46/102) of these VI‐AKI patients after the onset of AKI, and 14.7% (15/102) received RRT (see Table 5).
Table 5.
Treatment characteristics for vancomycin‐induced acute kidney injury
| Treatment | N (%) |
|---|---|
| No adjustment | 41 (40.2) |
| Termination of VAN | 24 (23.5) |
| Dose adjustment | 22 (21.6) |
| RRT | 15 (14.7) |
RRT, renal replacement therapy; VAN, vancomycin
Outcomes and risk factors for VI‐AKI
The all‐cause in‐hospital mortality rate for VI‐AKI patients was 29.4% (30/102). Multiple logistic regression analysis revealed that the independent risk factors for death were the presence of diabetes (OR = 11.178; P = 0.005), concomitant metronidazole/ornidazole (OR = 46.171; P = 0.001) and steroids (OR = 7.696; P = 0.005). Payment methods (OR = 0.026; P = 0.000) and high serum albumin levels (OR = 0.809; P = 0.011) were independent protective factors (see Table 6). We separated the renal outcomes of the VI‐AKI patients into three categories: recovery, improvement and nonrecovery. Renal function recovered in 28 patients, with a median recovery time of 7.7 days (IQR = 14.8) after receipt of VAN therapy, and renal function improved in 15 patients, with a median recovery time of 4.0 days (IQR = 3.3). Thus, 43 patients showed either recovery or improvement, representing 42.2% (43/102) of the total VI‐AKI patient group. The remaining 57.8% (59/102) of the VI‐AKI patients had renal insufficiency at the time of hospital discharge. Multiple logistic regression analysis revealed that payment methods (OR = 5.353; P = 0.006), the presence of CHD (OR = 6.197; P = 0.041), and taking contrast medium (OR = 4.326; P = 0.016) were independent risk factors for renal outcome in VI‐AKI patients (see Table 7). We performed an additional post‐estimation analysis using the Akaike score (see Table 8).
Table 6.
Risk factors for death in patients with vancomycin‐induced acute kidney injury
| Factors | P value | OR | 95% Cl |
|---|---|---|---|
| Payment methods | 0.000 | 0.026 | 0.003, 0.198 |
| Diabetes | 0.005 | 11.178 | 2.092, 59.720 |
| Serum albumin | 0.011 | 0.809 | 0.688, 0.953 |
| Metronidazole/ornidazole | 0.001 | 46.171 | 4.790, 445.052 |
| Steroid | 0.005 | 7.696 | 1.828, 32.396 |
CI, confidence interval; OR, odds ratio
Table 7.
Risk factors for renal outcome in patients with vancomycin‐induced acute kidney injury
| Factors | P value | OR | 95% Cl |
|---|---|---|---|
| Payment methods | 0.006 | 5.353 | 1.623, 17.659 |
| CHD | 0.041 | 6.197 | 1.074, 35.749 |
| Contrast medium | 0.016 | 4.326 | 1.313, 14.248 |
CHD, coronary heart disease; CI, confidence interval; OR, odds ratio
Table 8.
Akaike score
| Goodness‐of‐fit evaluation | Risk factors for VI‐AKI | Risk factors for death | Risk factors for renal outcome |
|---|---|---|---|
| Akaike score | 518.977 | 84.142 | 120.508 |
VI‐AKI, vancomycin‐induced acute kidney injury
Discussion
VAN is often associated with nephrotoxicity. Older age has been found to be significantly associated with nephrotoxicity in patients receiving VAN 4. Elderly patients are more likely to have combination of multiple illnesses and take complicated medications, which may give rise to VI‐AKI. This single‐centre retrospective study conducted at our hospital aimed to investigate the current situation concerning VI‐AKI in elderly Chinese patients and identify its risk factors, to provide some suggestions for improving the prevention and treatment of AKI.
We conducted a three step‐survey to identify the VI‐AKI population. In the first step, 89.3% patients were excluded because of inadequate SCr monitoring, which highlighted the fact that clinicians may need to pay more attention to patients' renal function after receiving VAN treatment, and the possibility of a missed diagnosis of VI‐AKI. The study by Van Hal et al. also demonstrated that clinicians should closely monitor the renal function of patients receiving VAN 15. We then investigated the TDM situation of the patients included. According to the American consensus for TDM, the proportion of patients in whom TDM was inadequate was 66.9% (compared with 67.5% for the Chinese consensus). The main reasons for the relatively low TDM rate were that, firstly, TDM for VAN was first used in our hospital in November 2015; prior to this, patients receiving VAN did not undergo TDM. Clinicians adjust the VAN dose based on patients' therapeutic effect (such as body temperature, routine blood test results, bacterial cultures, etc.) and adverse reactions (such as the SCr value). As TDM for VAN had been in use for only 1.5 years, clinicians may have not have developed a familiarity with VAN TDM in accordance with the guidelines. In this regard, it is necessary for clinicians to develop an understanding of the importance of VAN concentration monitoring. Secondly, China's clinical pharmacy system is still in its initial and exploratory stage. Although our hospital is one of the most developed in this field in China, clinical pharmacists can still only cover key clinical departments, such as the departments of infectious diseases, respiratory medicine, cardiology, neurology and ICU, to optimize clinical drug use, and because of their work, the TDM rate is higher in these departments. However, in surgical departments, surgeons focus more on surgical rather drug therapy issues, so the appropriate use of VAN and monitoring for adverse drug reactions would have been weaker. Both of the two reasons are likely to have contributed to the low TDM rate. The TDM correction was also a problem, only 3.9% patients' trough samples of VAN for TDM were obtained at the right time (compared with 8.2% for the Chinese consensus). The low proportion of cases in which TDM of VAN was carried out correctly was mainly the result of clinicians' lack awareness of the guidelines for the therapeutic monitoring of VAN, and clinical pharmacists' poor management of the appropriate use of this agent. Given the recommendations to increase serum VAN concentrations to 15–20 mg l−1 to combat rising minimal inhibitory concentrations (MICs), this information reinforces the valuable role that TDM plays in optimizing the safe use of VAN 16. In addition, the serum VAN concentration is one of the most important bases for dose adjustment. However, Moffett et al. 17 and Otto et al. 18 found that, even with close monitoring of the serum concentration, levels above the upper limit of the target level could occur, which aggravated this problem. The results of TDM and dose adjustment should be analysed by clinical pharmacists for the results of TDM and dose adjustment is also very important. Panwar et al. 19 conducted a study in patients with CKD and suggested frequent drug level monitoring subsequently, to minimize VIN risk. In elderly patients, who have a certain degree of renal dysfunction, this calls for more attention. A meta‐analysis showed that TDM not only decreased the rate of nephrotoxicity significantly, but also could increase clinical efficacy in patients treated with VAN 20. Katikaneni et al. 21 found that regular monitoring (preferably twice weekly) of SCr and VAN trough levels was advisable for minimizing VAN‐associated AKI. Smith et al. 22 conducted a pharmacist‐initiated VAN monitoring programme and evaluated its effect. The overall incidence of AKI decreased from 16.3% to 4.7% (P = 0.013). Implementation of pharmacist‐driven VAN monitoring significantly improved compliance with monitoring VAN serum levels and kidney function, and also reduced the incidence of AKI in the long‐term care setting 22. In our hospital, the main reasons for the low levels of TDM and lower rate of patients whose VAN though serum concentrations were obtained at the correct time were mainly because of clinicians' lack awareness of the guidelines for the therapeutic monitoring of VAN and clinical pharmacists' poor management of the appropriate use of this agent. Compared with clinicians, clinical pharmacists have a wealth of expertise in drug safety and appropriate use. We suggest that hospital managers increase the input of clinical pharmacists, not only to improve the management of VAN use, but also to free up more of clinicians’ time to focus on their diagnosis of the disease. Physician–pharmacist collaboration is also cost‐effective, as has been demonstrated in many other cases of disease management 23, 24, 25, 26.
A major feature of elderly patients is the combined incidence of multiple diseases. In a further analysis comparing NO‐AKI with VI‐AKI patients, our study incorporated as many pathophysiological states as possible. There was no significance difference between patients with and without VI‐AKI in terms of their concomitant diseases, but VI‐AKI patients were more likely also to have had sepsis, to have undergone a SOFA, to have gone into shock and to have undergone surgery. More concomitant risk factors may have contributed to their higher risk of AKI development. Multivariate regression analysis showed that mechanical ventilation was an independent risk factor for VI‐AKI. This was the first demonstration of mechanical ventilation use as an independent risk factor for VI‐AKI in elderly Chinese patients. Liu et al. 8 previously showed that mechanical ventilation is a risk factor for AKI and has a statistically significant interaction with age. The explanation for this may be that mechanical ventilation use is one of the variables of illness severity.
Our study included several laboratory data on the factors reflecting the clinical conditions. VI‐AKI patients were more likely to have a lower baseline eGFR, lower serum albumin valley and hyperuricaemia. Multivariate regression found that hyperuricaemia was an independent risk factor and that serum albumin valley was an independent protective factor. Hyperuricaemia has rarely been analysed in previous studies. Liu Y et al. 27 published a case report on acute renal failure induced by primary hyperuricaemia in children. In their review, Ejaz et al. 28 proposed that hyperuricaemia might contribute pathogenetically to renal vasoconstriction as well as to endothelial dysfunction, an inflammatory response, oxidative stress and the disturbances in autoregulation that occur with acute kidney failure, and affect renal outcomes adversely. In another study, these authors suggested that the measurement of serum uric acid levels offers the potential to predict AKI at any perioperative time point 29. This was the first time that hyperuricaemia was demonstrated to be an independent factor for VI‐AKI. These results should act as a reminder to clinicians to pay more attention to patients with hyperuricaemia when considering administering VAN to them. When giving VAN, an intervention on uric acid may be superior, but this needs to be established in further, well‐designed studies. Our study also demonstrated that the serum albumin valley was an independent protective factor. The serum albumin valley in patients with VI‐AKI was significantly lower than in those without (P = 0.000). It was first to demonstrate that a lower serum albumin valley is significantly associated with VI‐AKI. In the study by Burgess et al. 30, hypoalbuminaemia was found to be one of the factors predisposing patients to drug‐induced AKI. Wiedermann et al. 31, in a meta‐analysis, also found that hypoalbuminaemia was a significant independent predictor of AKI, and that the renoprotective action of albumin was mediated by its ability to scavenge reactive oxygen species, preventing oxidative damage, and binding and delivering protective lysophosphatidic acid. It is advisable for clinicians to be aware of patients' serum albumin levels, to avoid hypoalbuminaemia, when giving VAN therapy.
The use of complicated medications is another feature of elderly patients. We included 41 different types of drug taken by patients alongside VAN treatment that may have had an effect on VI‐AKI. Compared with the vast majority of studies, the classification of drugs was more detailed and the variety of drugs more comprehensive in our study, and we also included Chinese patent medicines. Even though diuretics present a risk factor for the development of VI‐AKI, most studies have included only loop diuretics or diuretics 7, 32. We specifically classified diuretics into hydrochlorothiazide, spironolactone or furosemide. Our study showed that furosemide was the only type of diuretic that was significantly more likely to be taken by VI‐AKI patients concomitantly with VAN. This was similar to the results of studies in younger adults 4. The selection of diuretics in patients receiving VAN may be an important consideration. The effect of administering Chinese patent drugs concomitantly with VAN on the development of VI‐AKI was demonstrated in a study in a Chinese adult population 33. The present study was the first to explore the influence of Chinese patent drugs on VI‐AKI in elderly Chinese patients being treated with VAN. Our study showed no significant difference between the groups taking and not taking Chinese patent drugs. This might have been because our study population took only small amounts of Chinese patent drugs. We also analysed the effect in this patient group of taking concomitant antioxidants. Many studies have suggested that oxidative stress is one of the main pathogenic mechanisms in VIN, and that the use of antioxidants decreases the severity of VIN 34. In various experimental models, numerous antioxidants have been shown to be protective against VIN 4. We included drugs with antioxidant activity (polyene phosphatidylcholine, edaravone, glutathione, acetylcysteine, phosphocreatine, levocarnitine). Of these, we found that VI‐AKI patients were significantly more likely to be taking glutathione, acetylcysteine and phosphocreatine, which was not consistent with the results of previous studies. This may have been because of the complicated concomitant diseases and medications associated with elderly patients. The individual agents most extensively studied in the previous studies include aminoglycosides and piperacillin–tazobactam; both were independent risk factors and significantly associated with a higher level of occurrence of VI‐AKI. However, we did not find any difference in the level of occurrence of VI‐AKI between the groups taking and not taking these agents. This is likely to have been because elderly patients are at high risk of VI‐AKI, and clinicians will avoid combined therapy if possible. Multivariate regression analysis showed that taking a vasopressor was an independent risk factor for VI‐AKI, which was consistent with previous findings. Vasopressors are capable of affecting kidney function, and patients receiving these agents are more likely to be in a critically ill state 4, 16.
We further analysed the outcomes of elderly patients with VI‐AKI, which has rarely been performed in previous studies. In our study, 61.8% of elderly patients with VI‐AKI had stage 1 disease and 14.7% received RRT. A prospective observational study of AKI in an elderly population showed that the severity of the disease and the need for dialysis were significantly associated with mortality 35. Once AKI has developed, there are few effective means of treating it. Dialysis is a final resort for treating AKI. Petronijevic et al. 36 conducted a study to measure the effect of treatment on short‐term outcomes in elderly patients with AKI and found no significant difference in survival during the follow‐up period up to 90 days between the haemodialysis‐ and conservatively treated group. Another study showed that high‐dose and low‐dose haemofiltration produce similar outcomes in AKI patients 37. The most important factor in improving patient outcomes and reducing medical expenses is to avoid the risk factors for VI‐AKI. When VI‐AKI occurs, targeted treatment and avoidance of risk factors are recommended. In our study, 40.2% of elderly VI‐AKI patients did not receive any targeted treatment after the occurrence of this condition, which suggests that clinicians need to be more vigilant about the development of VI‐AKI.
Our study analysed the risk factors for death and renal function outcomes in elderly VI‐AKI patients. It showed that payment methods (basic national medical insurance or self‐financing) were independent protective factors for death, but independent risk factors for renal outcome in VI‐AKI patients. Basic national medical insurance is a national health insurance policy in China, and patients covered by Medicare can be reimbursed for part of their medical expenses. Medical expenses are an important factor in patient outcome. An increased investment in health insurance will be conducive to the patient recovery. However, in our study, payment methods showed the opposite influence on death and renal outcome in VI‐AKI patients. This may have been due to the small sample size, and further studies, with larger sample sizes, should be carried out to confirm this finding. The presence of diabetes and serum albumin level reflected patients’ physical condition, and were independent risk factors for death. This suggests that more attention should be paid to patients with diabetes, and that low serum albumin levels should be avoided. CHD was an independent risk factor for renal outcome in VI‐AKI patients, which also warrants attention. Concomitant drugs also had an effect on the outcome of AKI; concomitant metronidazole/ornidazole or steroids were independent risk factors for death and concomitant contrast medium was an independent risk factor for renal outcome. Therefore, when AKI occurs, alternative drugs should be chosen and the patient should be monitored carefully. However, as our sample size was relatively small and the specific mechanisms for these associations with outcomes for VI‐AKI patients are unclear, there is clearly a need for a larger prospective study, and also further mechanistic studies.
The strengths of the present study were as follows. First, it examined a large population of elderly Chinese patients, and there have been few studies on risk factors for AKI in this population previously. Secondly, our survey analysed not only the condition of AKI and its associated risk factors, but also its treatment and outcomes. We identified risk factors for VI‐AKI, to support its prevention and management. Thirdly, our study analysed 41 types of drugs used concomitantly with VAN treatment, which is a large number; the diversity of drugs examined was greater than that in most previous studies, and this was the first study to examine the concomitant use of Chinese patent drugs in elderly Chinese patients. However, the study also had several limitations. First, this was a single‐centre retrospective study. Secondly, we lacked VAN therapeutic concentrations due to the low TDM rate and lower rate of patients whose VAN though serum concentrations were obtained at the correct time in clinical practice. Thirdly, our study did not analyse plasma VAN concentrations as a risk factor for AKI. Finally, our study did not analyse the financial costs associated with AKI.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. This research did not receive any specific grant from any funding agency.
Contributors
P.K., L.X. and L.Q. designed the research. P.K and W.Y. analysed the data. P.K. wrote the manuscript. P.K., C.Z., X.J., C.L., X.Q., W.W. and D.P. gave final approval of the version to be published.
Supporting information
Table S1 Cause of shock
Table S2 The severity of vancomycin‐induced acute kidney injury
Pan, K. , Wu, Y. , Chen, C. , Chen, Z. , Xu, J. , Cao, L. , Xu, Q. , Wu, W. , Dai, P. , Li, X. , and Lv, Q. (2018) Vancomycin‐induced acute kidney injury in elderly Chinese patients: a single‐centre cross‐sectional study. Br J Clin Pharmacol, 84: 1706–1718. 10.1111/bcp.13594.
Contributor Information
Xiao‐yu Li, Email: li.xiaoyu@zs-hospital.sh.cn.
Qian‐zhou Lv, Email: lv.qianzhou@zs-hospital.sh.cn.
References
- 1. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–292. [DOI] [PubMed] [Google Scholar]
- 2. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health‐System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009; 49: 325–327. [DOI] [PubMed] [Google Scholar]
- 3. Jeffres MN, Isakow W, Doherty JA, Micek ST, Kollef MH. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care‐associated methicillin‐resistant Staphylococcus aureus pneumonia. Clin Ther 2007; 29: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 4. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017; 102: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costa e Silva VT, Marcal LJ, Burdmann EA. Risk factors for vancomycin nephrotoxicity: still a matter of debate. Crit Care Med 2014; 42: 2635–2636. [DOI] [PubMed] [Google Scholar]
- 6. Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, et al Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother 2011; 55: 5475–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kane‐Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis 65: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Yin Y, Liu XZ, Yao HJ, Li LX, Chen JH, et al Retrospective analysis of vancomycin nephrotoxicity in elderly Chinese patients. Pharmacology 2015; 95: 279–284. [DOI] [PubMed] [Google Scholar]
- 9. Wang ZB, Sun TS, Li GP. Regional differences and evolution of population aging in China in recent 20 years. Population Research 2013; 37: 66–77. [Google Scholar]
- 10. Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, et al Acute kidney injury in China: a cross‐sectional survey. Lancet 2015; 386: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 11. Kidney Disease . Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int (Suppl.) 2012; 2: 1–138. [Google Scholar]
- 12. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66: 82–98. [DOI] [PubMed] [Google Scholar]
- 14. Chen BY, Guan XD, He LX, Hu J, Huang ZY, Li GH, et al Vancomycin clinical application of Chinese experts consensus (2011 edition). Chin J New Drugs Clin Rem 2011; 30: 561–573. [Google Scholar]
- 15. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta‐analysis of vancomycin‐induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2012; 57: 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanrahan TP, Harlow G, Hutchinson J, Dulhunty JM, Lipman J, Whitehouse T, et al Vancomycin‐associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med 2014; 42: 2527–2536. [DOI] [PubMed] [Google Scholar]
- 17. Moffett BS, Hilvers PS, Dinh K, Arikan AA, Checchia P, Bronicki R. Vancomycin‐associated acute kidney injury in pediatric cardiac intensive care patients. Congenit Heart Dis 2015; 10: E6–E10. [DOI] [PubMed] [Google Scholar]
- 18. Otto GP, Sossdorf M, Breuel H, Schlattmann P, Bayer O, Claus RA, et al Renal outcome after vancomycin treatment and renal replacement therapy in patients with severe sepsis and septic shock: a retrospective study. J Crit Care 2014; 29: 656–661. [DOI] [PubMed] [Google Scholar]
- 19. Panwar B, Johnson VA, Patel M, Balkovetz DF. Risk of vancomycin‐induced nephrotoxicity in the population with chronic kidney disease. Am J Med Sci 2013; 345: 396–399. [DOI] [PubMed] [Google Scholar]
- 20. Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta‐analysis. PLoS One 2013; 8: e77169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katikaneni M, Lwin L, Villanueva H, Yoo J. Acute kidney injury associated with vancomycin when laxity leads to injury and findings on kidney biopsy. Am J Ther 2016; 23: e1064–e1067. [DOI] [PubMed] [Google Scholar]
- 22. Smith AP, Millares‐Sipin CA, James M, Cohen H. Impact of a pharmacist‐initiated vancomycin monitoring program. Consult Pharm 2016; 31: 505–510. [DOI] [PubMed] [Google Scholar]
- 23. Pietka M, Watrobska‐Swietlikowska D, Szczepanek K, Szybinski P, Sznitowska M, Kłęk S. Nutritional support teams: the cooperation among physicians and pharmacists helps improve cost‐effectiveness of home parenteral nutrition (HPN). Nutr Hosp 2014; 31: 251–259. [DOI] [PubMed] [Google Scholar]
- 24. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N, Goedken AM, Chrischilles EA, Carter BL. Cost‐utility analysis of physician‐pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens 2017; 35: 178–187. [DOI] [PubMed] [Google Scholar]
- 25. Farley TM, Izakovic M. Physician‐pharmacist collaboration in a pay for performance healthcare environment. Bratislava Med J 2015; 116: 517–519. [DOI] [PubMed] [Google Scholar]
- 26. Hirsch JD, Bounthavong M, Arjmand A, Ha DR, Cadiz CL, Zimmerman A, et al Estimated cost‐effectiveness, cost benefit, and risk. J Manag Care Spec Pharm 2017; 23: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Zhang BL, Zhang X. Acute renal failure induced by primary hyperuricemia in children: a case report. Zhonghua Er Ke Za Zhi 2005; 43: 525. [PubMed] [Google Scholar]
- 28. Ejaz AA, Mu W, Kang DH, Roncal C, Sautin YY, Henderson G, et al Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol 2007; 2: 16–21. [DOI] [PubMed] [Google Scholar]
- 29. Ejaz AA, Dass B, Kambhampati G, Ejaz NI, Maroz N, Dhatt GS, et al Lowering serum uric acid to prevent acute kidney injury. Med Hypotheses 2012; 78: 796–799. [DOI] [PubMed] [Google Scholar]
- 30. Burgess LD, Drew RH. Comparison of the incidence of vancomycin‐induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin‐tazobactam. Pharmacotherapy 2014; 34: 670–676. [DOI] [PubMed] [Google Scholar]
- 31. Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta‐analysis of observational clinical studies. Intensive Care Med 2010; 36: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β‐lactams for the treatment of osteomyelitis in diabetics: piperacillin‐tazobactam as compared with cefepime. Clin Microbiol Infect 2014; 20: O384–O389. [DOI] [PubMed] [Google Scholar]
- 33. Pan K, Ma L, Xiang Q, Li X, Li H, Zhou Y, et al Vancomycin‐associated acute kidney injury: a cross‐sectional study from a single center in China. PLoS One 2017; 12: e0175688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Im DS, Shin HJ, Yang KJ, Jung SY, Song HY, Hwang HS, et al Cilastatin attenuates vancomycin‐induced nephrotoxicity via P‐glycoprotein. Toxicol Lett 2017; 277: 9–17. [DOI] [PubMed] [Google Scholar]
- 35. Silveira Santos CGD, Romani RF, Benvenutti R, Ribas Zahdi JO, Riella MC, Mazza do Nascimento M. Acute kidney injury in elderly population: a prospective observational study. Nephron 2018; 138: 104–112. [DOI] [PubMed] [Google Scholar]
- 36. Petronijevic Z, Selim G, Petkovska L, Georgievska‐Ismail L, Spasovski G, Tozija L. The effect of treatment on short‐term outcomes in elderly patients with acute kidney injury. Open Access Maced J Med Sci 2017; 5: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li P, Qu LP, Qi D, Shen B, Wang YM, Xu JR, Jiang WH, Zhang H, Ding XQ, Teng J High‐dose versus low‐dose haemofiltration for the treatment of critically ill patients with acute kidney injury: an updated systematic review and meta‐analysis. BMJ Open 2017; 7: e014171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Cause of shock
Table S2 The severity of vancomycin‐induced acute kidney injury


