Abstract
Aims
To test the in vivo activity of Cytochrome P450 (CYP) 2E1 in obese children vs. nonobese children, aged 11–18 years. Secondly, whether the activity of CYP2E1 in these patients is associated with NALFD, diabetes or hyperlipidaemia.
Methods
Seventy children were divided into groups by body mass index (BMI) standard deviation score (SDS). All children received 250 mg oral chlorzoxazone (CLZ) as probe for CYP2E1 activity. Thirteen blood samples and 20‐h urine samples were collected per participant.
Results
Obese children had an increased oral clearance and distribution of CLZ, indicating increased CYP2E1 activity, similar to obese adults. The mean AUC0–∞ value of CLZ was decreased by 46% in obese children compared to nonobese children. The F was was increased twofold in obese children compared to nonobese children, P < 0.0001. Diabetic biomarkers were significantly increased in obese children, while fasting blood glucose and Hba1c levels were nonsignificant between groups. Liver fat content was not associated with CLZ Cl.
Conclusion
Oral clearance of CLZ was increased two‐fold in obese children vs. nonobese children aged 11–18 years. This indicates an increased CYP2E1 activity of clinical importance, and dose adjustment should be considered for CLZ.
Keywords: drug metabolism, cytochrome P450, children, obesity, liver
What is Already Known About this Subject
Physiological changes accompanying obesity may result in altered drug pharmacokinetics, herein an altered hepatic clearance of some drugs.
Increased cytochrome P450 (CYP) 2E1 activity is well documented in obese adults.
CYP2E1 activity has never been investigated in obese children and adolescents.
What this Study Adds
This study provides new information about CYP 2E1 activity in obese children.
Oral clearance of clorzoxazone was found increased approximately twofold in obese children, aged 11–18 years, as compared to nonobese children. This is most likely to be due to an increased CYP2E1 activity.
The findings in this study may be of clinical importance in regards of chlorzoxazonel dosing in obese children.
Introduction
There is a lack of consensus on dose adjustments for specific drugs in obese paediatric patients. One of the main reasons is absence of clinical trials that can support evidence‐based dosing strategies 1, 2, 3.
The deficiency of trials has led to both over and under dosing of these patients 3, as obesity is more than a biometric measure. Due to changes in anatomy and physiology, obese children may respond differently to pharmacological exposures 3. Although total body weight is one of the most commonly used dosing metrics in paediatrics, it fails to account for changes in body composition and change in expression and activity of the cytochrome P450 enzymes 4, 5.
http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=262#1330 is a key player in the metabolism of endogenous substrates, including acetone and fatty acids and exogenous compounds, such as anaesthetics, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2299, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2322, tetrachloride, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239 (USAN: acetaminophen) 6. According to pain guidelines in children, the latter, is a commonly recommended as first line treatment for mild‐to‐moderate pain 7, and is the most used drug in paediatric general medical wards 8.
A recent study by Rongen et al. 4 investigated the pharmacokinetics of paracetamol in morbidly obese adult patients, with a specific emphasis on CYP2E1 mediated clearance (Cl). Paracetamol plasma concentrations were significantly lower in these patients. Although an increased dose of paracetamol may be anticipated to achieve a better pharmacodynamic response in obese patients, the authors warn against this, as the induced CYP2E1‐activity may also worsening the safety profile of paracetamol due to higher concentrations of the toxic metabolite http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6299 4.
In contrast, the CYP2E1‐mediated clearance of volatile anaesthetics, including http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7175 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7296 may not be induced by obesity 9. However, one study of obese Japanese adults, did find a higher clearance in mild obesity 10.
CYP2E1 activity can be determined noninvasively by oral administration of chlorzoxazone (CLZ), a centrally‐acting agent for treatment of muscle spasm 11, 12. In humans, CLZ is selectively hydroxylated by CYP2E1 to 6‐hydroxychlorzoxazone (6‐OH‐CLZ). The fraction of the administered dose recovered as 6‐OH‐CLZ times the total CLZ clearance can be used as an index of the CYP2E1 activity. Increased CYP2E1 activity in obese adult patients is by now well‐documented by use of this method 4, 13, 14, 15.
CLZ clearance was recently investigated in obese adults with nonalcoholic fatty liver diseases (NAFLD) 16. Therein, there were no difference in CYP2E1 activity between the two subgroups nonalcoholic fatty liver and nonalcoholic steatohepatitis. However, the degree of infiltration was lower, patients with nonalcoholic steatohepatitis were significantly older compared with patients with simple steatosis 10 and none of the results were compared with nonobese patients 16. Further, diabetes, increased serum triglyceride and serum cholesterol have been associated with increased CYP2E1 activity in adults 14, 17.
During recent decades, obesity has increased alarmingly in children, along with an increased incidence of NAFLD 18. In some studies, NAFDL has been recorded in up to 80% of obese children 19, while the prevalence was estimated to be 68% in a Danish study 20. CYP2E1 mediated clearance has never been investigated in obese children 21. Since children have been obese for a shorter period of time extrapolation of results from obese adults may not be reliable 4, as many of these children have not developed severe degrees of obesity‐related conditions, such as NAFLD, hypercholesterolaemia and diabetes.
The aims of the present study were to test whether the in vivo activity of CYP2E1 is altered in children with obesity as compared with nonobese children, aged 11–18 years, and whether the activity of CYP2E1 in these patients is associated with NALFD, diabetes or hyperlipidaemia.
Methods
The CYTONOX‐study is an open‐label explorative pharmacokinetic trial 22 conducted at the Children's Obesity Clinic, Department of Pediatrics, Holbaek and at the Phase 1 Unit, Department of Clinical Pharmacology, Copenhagen Denmark. The children were recruited between April 2016 and January 2017. To assure homogeneity between the two investigational sites, the same principal investigator was in charge of both study sites. Furthermore, the equipment used was identical, and blood and urine samples were analysed in the same laboratory. The study was approved by The Danish Health Authorities (EudraCT: 2014‐004554‐34) and the local Ethical Committee of Region Zealand (SJ‐455), who also approved the informed consent, signed for each trial participant by both parents. The trial was monitored by the GCP‐Unit in Copenhagen.
Patient population
Obese children were primarily recruited from Department of Paediatrics, University Hospital, Holbaek, where they were consecutively enrolled in the chronic care multidisciplinary intervention program at the Children's Obesity Clinic. The children had been referred from their general practitioners, school‐ and community‐based doctors, or paediatricians. Nonobese children were primarily recruited from varies schools in Zealand, Denmark. Obese children participated in the trial prior to entering treatment programme at the obesity clinic. Nonobese children were matched by age and sex.
Trial participants were included using the following criteria: (i) children aged 11–18 years, both sexes, of which girls had had their first period (Tanner stage 4); (ii) body mass index (BMI) standard deviation score (SDS) ≥2.33 for obese children and BMI SDS ≤ 1.28 for nonobese children corresponding to the 99th and 90th percentile, respectively. This is in accordance with the Danish age and sex‐adjusted references 23; (iii) children not allergic to CLZ; (iv) children not on any medication that interferes with CYP2E1 drug metabolism; (v) children not having acute or chronic liver disease, kidney disease, dialysis or other chronic diseases – except for minor disorders e.g. allergy and rash; (vi) and pregnancy tests were performed in all females to exclude pregnancy.
Design and blood sampling
Prior to administration of CLZ, participants were asked to refrain from consuming any substances that would alter basal activity of CYP2E1 including alcohol for at least 72 h and smoking for at least 96 h 13, 24. Following oral administration of 250 mg CLZ (tablet Klorzoxazon, Takeda), 2.5 ml blood samples were collected at prespecified time points: 10, 20, 30, 40, 50, 60, 90, 120 min and hourly during the next 4 h. Heparinized tubes were used and samples were centrifuged for 10 min, at –4°C, 2400 g and stored at –80°C until analysis. Urine was collected for 20 h in a container, the total volume measured and 10 ml aliquots, stored at –80°C, until analysis. All subjects underwent a health status test included past medical history, medications, and physical examination. A baseline blood sample was obtained for diabetic biomarkers (fasting glucose, insulin, proinsulin C peptide, fasting lipid profile [total cholesterol, low‐density lipoprotein cholesterol (LDL), triglycerides, high‐density lipoprotein cholesterol (HDL)], and liver biomarkers (alanine aminotransferase and aspartate aminotransferase, bilirubin, albumin). The updated computer model of homeostasis model assessment was downloaded from the internet (https://www.dtu.ox.ac.uk/homacalculator) and used to calculate the proxies of insulin resistance, β‐cell function and insulin sensitivity from fasting plasma glucose, serum insulin, and c‐peptide 25. In addition, liver fat content (LFC) profile was determined (see below). All trial participants were asked about race, including origins of their parents and grandparents.
CLZ plasma and urine quantification
CLZ and 6‐OH‐CLZ plasma and urine concentrations were quantified according to a previously published method 26 with changes with regard to the expected therapeutic concentrations. Urine samples (10 μl) were diluted in blank plasma (90 μl) and processed analogue to the pure plasma samples. In brief, liquid–liquid extraction was applied for clean‐up of CLZ and 6‐OH‐CLZ in plasma. Samples (100 μl) were spiked with internal standards D3‐CLZ and 13C6–6‐OH‐CLZ (25 μl) and β‐glucuronidase (200 μl/40 units) and incubated at 37°C overnight for conjugate hydrolysis. Subsequently tert‐butylmethylether (2.5 ml) was added, shaken (10 min), and centrifuged (10 min, 3000 g). Supernatants were evaporated to dryness (40°C), reconstituted by adding liquid chromatography eluent, and injected (25 μl) into the liquid chromatography–tandem mass spectrometry (MS/MS) system, which consisted of a Surveyor autosampler, quaternary high‐pressure liquid chromatography pump, and a TSQ 7000 triple stage quadrupole mass spectrometer (Thermo Fisher Scientific, Darmstadt, Germany). For chromatographic separation a Synergi Polar RP column (Phenomenex, Aschaffenburg, Germany) was used. The eluent consisted of acidified ammonium acetate buffer (A) and acetonitrile (B). For separation, a gradient programme at 0.5 ml min–1 was applied. From 0 to 0.5 min 95% A/5% B was used. From 0.5 to 4 min the ratio was linearly changed to 5% A/95% B and hold until 6.5 min. The eluent was introduced directly into the electrospray ion source of the MS/MS. MS/MS transitions monitored in the negative ion mode were m/z 168 → m/z 132 at 35 V for CLZ, m/z 171 → m/z 134 at 35 V for D3‐CLZ, m/z 184 → m/z 120 at 35 V for 6‐OH‐CLZ and m/z 190 → m/z 125 at 35 V for 13C6–6‐OH‐CLZ. The assay was validated according to common Food and Drug Administration and European Medicines Agency validation guidelines 27. Lower limits of quantification of CLZ and 6‐OH‐CLZ were 10.0 ng ml–1. The calibrated ranges for both analytes were 10.0–10 000 ng ml–1 with correlation coefficients >0.99. The overall accuracy varied between +0.1% and + 11.3%, and an overall precision ranging from 1.2% to 13.4%.
Assessment of hepatic fat
Magnetic resonance (MR) measurements were performed in 3T Achieva MR imaging (MRI) system (Philips Medical Systems, Best, the Netherlands) or in open 1T Panorama HFO MRI system (Philips Medical Systems, Best, the Netherlands) as described by Chabanova et al. 28.
Liver fat fraction was measured based on a single voxel point resolved spectroscopy sequence. The water and fat peaks of the acquired spectra were fitted to obtain their areas using a standard postprocessing protocol for fitting metabolite peak areas available at the MRI systems.
The LFC was calculated according to the equation:
Peak areas at TE = 0 ms were corrected for T2 relaxation effects for each peak using an exponential least‐square fitting algorithm to the peak areas with the series of TE as described earlier 29.
Pharmacokinetic analysis
The pharmacokinetic parameters were determined by standard noncompartmental methods, using R software (version 3.2.3); CRAN packet PKNCA (version 0.8.1) 30.
The oral area under the plasma concentration–time curves (AUC) for CLZ and its 6‐OH‐CLZ metabolite were determined using the trapezoidal rule and extrapolated to infinity by a log‐linear estimation of the terminal elimination rate constant (k) for each compound. An elimination half‐life was obtained from the ratio ln2/k. The total oral clearance (CL/F) of CLZ was determined from the ratio of the administered oral dose (D) to the total area under the plasma concentration–time profile (AUC0–∞). The fractional clearance (Clf) by CYP2E1 by 6‐hydroxylation, was estimated from the product of CL/F and the fraction (fe) of the administered dose recovered as 6‐OH‐CLZ in the 0–20‐h urine sample (AUC 0–20). Prior to the calculation of fe, the CLZ dose (mg) and 6‐OH‐CLZ (ng ml–1) were converted into in mmol l–1, based on the molecular weight of CLZ 31 and 6‐OH‐CLZ 32. An apparent volume of distribution (Vd) was determined from the ratio of oral clearance to the elimination rate constant.
Statistical analysis
Demographic data are presented as medians (ranges) or numbers (percentages) as applicable. Data management was conducted by using R (version 3.2.3) The BMI SDS was calculated by the least means square method by converting BMI into a normal distribution by sex and age using the median coefficient of variation and a measure of the skewness 33, based on the Box–Cox power plot based on Danish BMI charts23.
Pharmacokinetic parameters were log10‐transformed before inclusion in the analyses to approximate a normal distribution. The difference between the means of the groups was tested for significance using Student t test. The level of statistical significance was set at P < 0.05. The relationship between the CLZ CL and the independent variables BMI SDS, diabetic (DM) biomarkers, lipid profile (LP) and LFC were examined by multivariate linear regression analysis.
Sample size
The sample size calculation was based on results from independent control (nonobese adults) and experimental subjects (obese adults). The response within each subject group was previously found with a standard deviation of 1.17.
If the true difference of the CL/F the experimental and control means is 0.8 ml min–1 kg–1, this study should include 34 experimental and 34 control subjects to be able to find significant differences with probability (power) of 0.8. The type I error probability associated with this test of this null hypothesis is 0.05.
Missing data
The proportion of missing data is presented in the result section. Data were handled as a full set analysis. To minimize missing data and avoid withdrawal bias, participants were replaced as soon as possible.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked corresponding to corresponding entries in http//http://www.guidetopharmacology.org, the common portal for data from the IUPHA/BPS Guide to PHARMACOLOGY 34, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 35.
Results
Patients and baseline characteristics
In total, 72 children were included. Two children withdrew from the study, both before administration of CLZ. Characteristics of all 70 participants (34 obese and 36 nonobese children) are presented in Table 1. Thirty‐five females and 35 males were included in the study, of whom 20 females and 14 males were obese. Sixty‐seven children were of Caucasian origin. One nonobese child was of Asian origin, and two obese children had one parent of Asian and African origin, respectively.
Table 1.
Demographic and baseline characteristics
| Obese [Median] | Nonobese [Median] | P | ||
|---|---|---|---|---|
| Age, years (range) | 14.06 (11.04–16.68) | 14.40 (11.08–17.74) | 0.38 | |
| Weight, kg (range) | 78 (56.2–123,9) | 56 (33.2–75.8) | <0.0001 | |
| BMI z score (range) | 2.83 (1.82–4.24) | 0.33 (–2.04–1.83) | <0.0001 | |
| Females/males (%) | 20 (29)/14 (20) | 15 (21)/21 (30) | 0.47 |
| Obese [Mean] | Nonobese [Mean] | Reference | P | |
|---|---|---|---|---|
| Lipid profile | ||||
| LDL (mmol l –1 ) | 2.0 | 1.63 | 1.1–3.4 | 0.01 |
| HDL (mmol l –1 ) | 1.07 | 1.23 | 1.0–2.3 | 0.0002 |
| Total cholesterol (mmol l –1 ) | 3.57 | 3.24 | 2.7–5.5 | 0.13 |
| Total triglyceride (mmol l –1 ) | 1.02 | 0.72 | 0.48–2.69 | 0.004 |
| Diabetic biomarkers | ||||
| Hba1c (mmol l –1 ) | 33.19 | 32.85 | 5.4–7.4 | 0.67 |
| Blood glucose (mmol l –1 ) | 5.06 | 5.19 | <7 | 0.26 |
| Insulin (pmol l –1 ) | 106.17 | 64.11 | 17.8–173.0 | <0.0001 |
| Proinsulin C peptide (mmol l –1 ) | 0.82 | 0.62 | 0.37–1.47 | 0.0003 |
| HOMA IR (%) | 1.87 | 1.35 | ‐ | 0.0005 |
| HOMA B (%) | 146.0 | 114.2 | ‐ | <0.0001 |
| HOMA IS (%) | 53.6 | 74.3 | ‐ | 0.0006 |
| Liver function tests | ||||
| ALAT (U l –1 ) | 25.66 | 19.93 | <41 | 0.001 |
| ASAT (U l –1 ) | 21.45 | 19.55 | 15–45 | 0.25 |
| Alkaline phosphatase (U l –1 ) | 153.14 | 163.59 | 151–457 | 0.59 |
| Bilirubin (μmol l –1 ) | 8.22 | 7.87 | 3.8–30.0 | 0.75 |
| Albumin (g l –1 ) | 37.00 | 37.56 | 39–47 | 0.35 |
| Liver MR | ||||
| LFC (%) | 1 (0.5–17.4) | 0.5 (0.5–2) | <5 | ‐ |
| Steatosis (%) | 5 (17) | 25 (83) | ‐ | ‐ |
BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; Hba1c, haemoglobin 1 ac; HOMA IR, B and IS, homeostasis model assessment insulin resistance, β cell function and insulin sensitivity; ALAT, alanine transaminase; ASAT, aspartate transaminase; MR, magnetic resonance; LFC, liver fat content
The two groups revealed significant differences in mean value of LDL and total triglyceride, although within range of the reference value, while HDL was significantly decreased in obese children. Total cholesterol did not differ between the two groups. Diabetic biomarkers insulin and proinsulin C peptide were significantly increased in obese compared to nonobese children, while fasting blood glucose and Hba1c levels were non‐significant between groups. The two groups revealed no significant differences in liver blood tests (alanine aminotransferase, aspartate aminotransferase and bilirubin).
CLZ pharmacokinetics and in vivo CYP2E1 activity
In total, 894 blood samples and 63 urine samples were collected for pharmacokinetic analysis. CLZ pharmacokinetic data are summarized in Table 2. The mean AUC0–∞ value of CLZ was decreased by 46% in obese children compared to nonobese children, Figure 1. The mean CLZ CL/F was increased two‐fold in obese children compared to nonobese children, P < 0.0001 estimated in absolute values, and still significant different when adjusted for weight P < 0.0001, Table 2 and Figure 2.
Table 2.
Chlorzoxazone pharmacokinetics in obese vs. nonobese children, aged 11–18 years
| Obese (Mean) | Nonobese (Mean) | P‐value | Log10 95% CI on the difference (Obese vs. Nonobese) | |
|---|---|---|---|---|
| AUC (mg min ml –1 ) | 0.609 | 1.130 | <0.0001a | ‐ 0.34‐ ‐0.204 |
| AUC 6–OHCZX (mg min ml –1 ) | 0.293 | 0.315 | 0.45 | –0.0367‐00.81b |
| CL/F (ml min –1 ) | 407.38 | 218.78 | <0.0001a | 0.204–0.34 |
| Weight‐normalized Cl (ml min –1 kg –1 ) | 5.13 | 3.98 | <0.0001a | 0.052‐0.16 |
| 6‐OHCZX recov (%) | 62.74 | 57.45 | 0.36 | –0.045‐0.121 |
| Cl f (ml min –1 ) | 235.55 | 127.85 | <0.0001a | 0.158‐0.373 |
| CL f (ml min –1 kg –1 ) | 3.05 | 2.30 | 0.02 | 0.022–0.222 |
| C max (mg ml –1 ) | 0.005 | 0.009 | <0.0001a | 0.006–0.003 |
| T max (min) | 85.11 | 100 | 0.12 | –0.156‐ 0.018 |
| Vd (l) | 39.57 | 15.67 | <0.0001a | 0.31‐0.494 |
| Weight adjusted Vd (l kg –1 ) | 0.49 | 0.29 | <0.0001a | 0.309‐0.544 |
| T1/2 (h) | 1.12 | 0.83 | <0.0001a | 0.08 to 0.18 |
Highly significant,
has not been log10 transformed. CI, confidence interval; AUC, areal under the curve; CL/F, oral clearance; Clf, fractional clearance; Cmax, max concentration; Tmax, time taken to reach Cmax; Vd, volume of distribution; T1/2, half‐life
Figure 1.
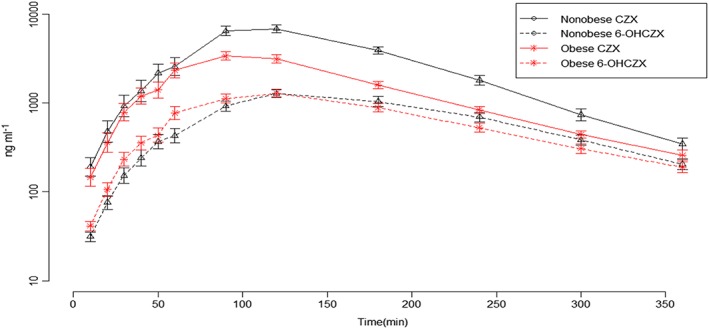
Chlorzoxazone (CZX) and 6‐OHCZX concentration–time profile in obese and nonobese children, aged 11–18 years, after a single oral dose
Figure 2.
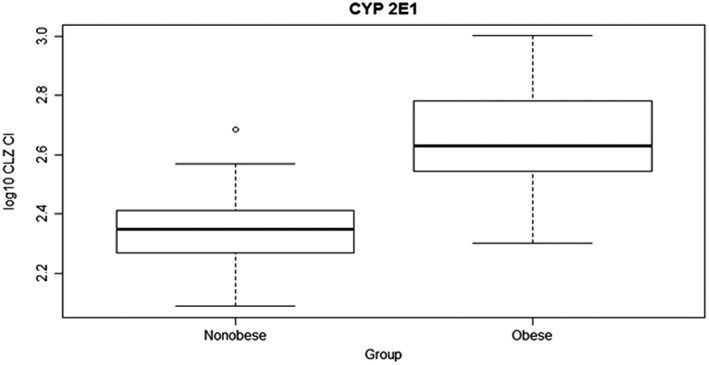
Chlorzoxazone clearance (CLZ Cl; log10 transformed) in obese and nonobese children (mean 2.61 vs. 2.34 ml min–1, 95% confidence interval 0.20–0.34, P < 0.0001)
There was no difference in mean AUC0–∞ of the main metabolite 6‐OH‐CLZ between the groups, P = 0.45. Further, the amount of 6‐OH‐CLZ recovered in the urine was not statistical different between the two groups P = 0.36. The fractional clearance by 6‐hydroxylation (CYP2E1 activity) was increased 46% in obese children compared to nonobese P = 0.0001, Figure 3, and 25% when adjusted for weight P = 0.02, Table 2. The apparent volume of distribution (Vd) of CLZ was also about 41% larger in obese children, Figure 4. Accordingly, the mean maximum plasma concentration (Cmax) was decreased in obese children P < 0.0001, but the time to reach the maximum plasma concentration (Tmax) of CLZ was not statistically significant between the two groups. Also, the mean CLZ t1/2 value was increased in children with obese as compared to normal weight children, P < 0.001.
Figure 3.
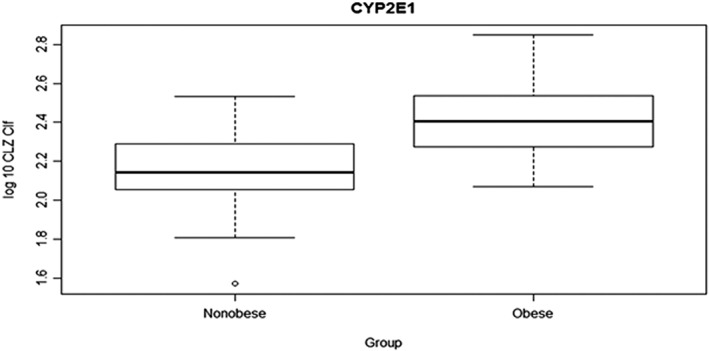
Chlorzoxazone fractional clearance (CLZ Clf; log10 transformed) in obese and nonobese children (mean 2.41 vs. 2.15 ml min–1, 95% confidence interval 0.16–0.37, P < 0.0001)
Figure 4.
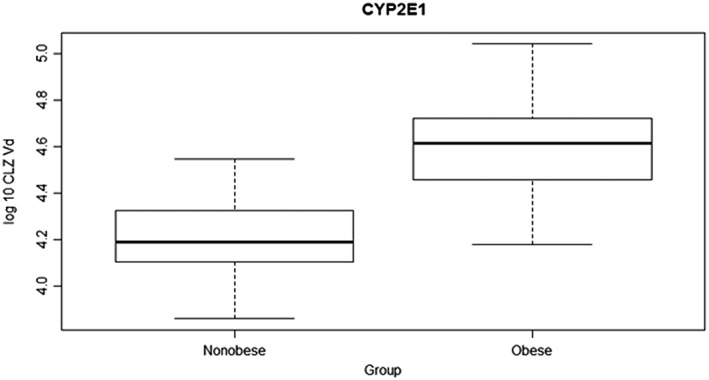
Chlorzoxazone volume of distribution (CLZ Vd; log10 transformed) in obese and nonobese children (mean 4.597 vs. 4.195, 95% confidence interval 0.31–0.49, P < 0.0001)
LFC
In total 30 children (18 obese) were MR scanned for liver fat content. Median value of liver fat content was twice as high in obese children, 1% (range 0.5–17.4%) vs. 0.5% (range 0.5–2.0%) in nonobese children. Hepatic steatosis was only found in five obese children (17%; 4.13–15), with mean BMI SDS 3.56 (range 2.73–4.24), four of whom were male.
Liver fat content was not associated with CLZ CL/F, see Figure 5.
Figure 5.
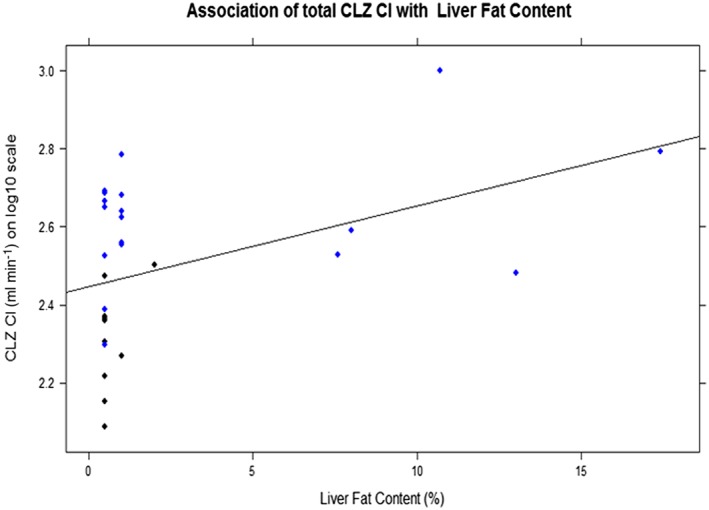
Association of chlorzoxazone clearance (CLZ Cl; log10 transformed) with liver fat content, R 2 0.15, P = 0.02
The effects of covariates (BMI SDS, DM biomarkers, LP and LFC) on CLZ CL/F
The relationships between the CLZ CL/F and the independent variables BMI SDS, DM biomarkers (serum glucose, glycosylated haemoglobin A1c, insulin, homeostasis model assessment insulin resistance), LP (LDL, HDL, total cholesterol, triglyceride), and LFC were examined by multivariate linear regression analysis. A statistically significant correlation was found between CLZ CL/F and BMI SDS [F(1, 0.1285) = 86.13, P < 0.0001], with an R2, of 0.563, Figure 6. Participant's predicted CLZ CL/F is equal to 102.30734 + 0.10129*BMI SDS ml min–1. Participant's CL/F increased 26% for each SDS unit. No statistically significant correlations were noted between any of the other covariates tested.
Figure 6.
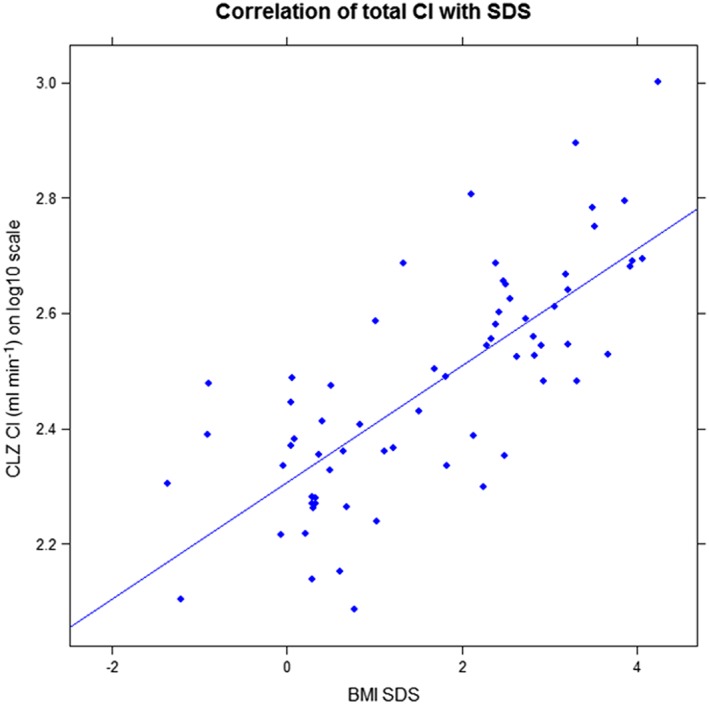
Association of chlorzoxazone clearance (CLZ Cl; log10 transformed) with body mass index standard deviation score (BMI SDS), R 2 0.563, P < 0.0001
Discussion
The importance of induced CYP2E1 activity has been highlighted in adult obese patients in several studies 4, 13, 14, 15.
In the present study, obese children have an increased oral clearance and distribution of chlorzoxazone, indicating induction of CYP2E1 activity similar to the findings in obese adults. O'Shea et al. 13 found a 50% increase in weight normalized obese adults, which was slightly lower in obese children (30%). Correspondingly, Emery et al. found a 46% decline in CLZ Cl in morbidly obese patients, 1 year after gastric bypass surgery 15. Moreover, the fractional clearance as a measure of CYP2E1 activity was also increased by >30% in the obese children. This induction was even more marked in obese adults approaching 60%, when using the same dosages of CLZ 13. The mean BMI in the latter study was 44 kg m–2, corresponding to extreme obesity 36. Since a positive correlation between BMI and CYP2E1 activity exist 16, this may explain some of the differences found between the two studies. Similar to Chtioui et al. 16, we observed a positive correlation between CYP2E1 activity and BMI SDS in obese children. These results are also in line with Rongen et al., who found that lean bodyweight was a significant parameter for CYP2E1‐mediated clearance of paracetamol in obese adults, by the use of population pharmacokinetic modelling 4. The same authors found that obesity in adults leads to lower paracetamol concentrations and an accelerated formations of the NAPQI‐conjugated metabolites cysteine and mercapturate metabolites 4. It is currently unknown whether earlier and greater formation of the CYP2E1mediated metabolites may contribute to paracetamol hepatotoxicity 4, or whether these findings will be similar in children. The latter is of clinical interest as paracetamol is often used as a painkiller in this group of patients.
In accordance with Rongen et al. 4 and O'Shea et al. 13, we found that the Cmax values were substantially lower in obese children. An incomplete CLZ absorption would result in a decreased F, which could also explain the increase in CLZ CL/F found in the present study. However, the fraction of the dose absorbed did not appear to be reduced in obese children, as the fraction of the dose recovered into the urine was not significantly different between the two groups. Further, if the absorption of CLZ was delayed, a lower ka would have produced a decreased Cmax and an extended Tmax. However, Tmax was not statistically significantly different in obese children compared with nonobese children. Hence, the current findings may be explained by altered disposition kinetics rather than altered CLZ absorption. This was also supported by the fact that the apparent volume of distribution was increased 69% in obese children compared with nonobese children, which is similar to the findings in obese adults by O'Shea et al. 13. Presumably, this reflects the increased ratio of body fat to lean tissue in obese children, consistent with CLZ being a highly lipid soluble drug. Furthermore, a trend toward a longer t1/2, has previously been found, and this was confirmed in our study. A significantly extended t1/2 may reflect a relatively higher concomitant increase in Vd (ratio 2.5) rather than total CL/F of CLZ (ratio 1.8), as increased CL/F otherwise would reduce the t1/2 value. In the study by Rongen et al., paracetamol t1/2 was equal between obese and nonobese patients 4, and this was explained by both Vd and CL being higher in these patients. Hence, the relative ratio may be drug dependent.
A reduced protein binding would increase the unbound CLZ fraction resulting in an increased CLZ intrinsic CL, and an increased CLZ Vd. Since, CLZ is a weak base and the concentration of α1‐acid glycoprotein is double in obesity 37 an altered CLZ protein binding would have the opposite effect. This is supported by a study by Wang et al. 17, who found CLZ Cl increased in obese adults with Type II diabetes as compared with healthy subjects, but no difference in the CLZ protein binding. Thus, the findings of the present study indicate that changes in CLZ CL/F reflect enhanced hepatic CYP2E1 activity.
The above findings may have clinical implications regarding drug overdose with paracetamol. First, as many treatment algorithms are based on an initial plasma sample of paracetamol 38, 39, 40, which might not be very accurate in very obese children. Secondly, the upregulation of CYP2E1 may also be linked to increased paracetamol‐induced hepatotoxicity 3. Third, antidote dosing based on total body weight, such as in N‐acetylcysteine administration, results in higher doses in obese children. A few case stories have been published, regarding fatality following administration of intravenous N‐acetylcysteine administration in obese adults 3. This is of concern, since obese adolescents represent a special population in medical toxicology, as they also have mental health risk factors (such as mood disorder) that increase their risk of drug overdose, as well as affecting their prognosis following overdose 3.
Additionally, CYP2E1 is known to play a key role in the hepatotoxicity of ethanol and of other xenobiotics through generation of reactive oxygen metabolites 41. In our study, 30% of the obese children, who had been MR scanned performed (in total 18 children), had steatosis. However, none of them exceeded 20%, and we did not find any association between CLZ CL/F and LFC, Figure 5. This is in contrast to other studies that found that NAFDL were associated to the hepatic expression and activity of CYP2E1 15, 42. However, steatosis was much more pronounced in the obese adults involving more than 50% of the hepatocytes 11.
Another cause of increased expression of CYP2E1 activity has been related to diabetes. Lucas et al. found increased CYP2E1 activity in both type I and II diabetic patients 14, although an association was only confirmed in patients with a body mass ≥ 30 kg m–2 17. In the present study, we found higher insulin and a higher proinsulin C peptide level in obese children compared with nonobese children, but glucose was still within the same range between the two groups. In contrast, glucose levels were also increased in adult obese patients 14, 15, 17. Interestingly, O'Shea 13 and Emery 15 argued that obesity may be a confounding factor when measuring the CYP2E1 activity in diabetes patients 13, 15. Additionally, Lucas et al. found that CYP2E1 activity is positive correlated to BMI, serum triglycerides and serum cholesterols 14. However, Wang et al. 17 did not find CLZ Cl to be significantly influenced by any of their examined covariates (weight, height, BMI, serum glucose, serum cholesterol and glycosylated haemoglobinA1c) 14, 17.
Although there are many similarities between obese adult and obese children in the CYP2E1 induced activity, pathophysiological changes related to obesity are not as pronounced in school‐age children and adolescents as in adults. Data from adult data should therefore be extrapolated with caution.
Limitations
One limiting factor of this study is that we did not investigate the influence of growth and development in infants and young obese children to in relation to the other changes of pathophysiological kinetics. Furthermore, only a relative small proportion of the obese children were MR scanned. Thus, the number was too small to draw any conclusion about CLZ CL/F and LFC. The strength on the study is on the other hand, is the large number of participants, the high number of samples, and the homogeneity of ethnical background. Last, it should be noted that the definition of childhood obesity varies between countries, which should be considered when comparing studies.
Conclusion
Oral clearance of CLZ was increased approximately twofold in obese children, aged 11–18 years, as compared to nonobese children. This is most likely to be due to a clinical important increased CYP2E1 activity, and this needs to be accounted for in the treatment strategies for obese children.
Competing Interests
There are no competing interests to declare.
The study was funded by the Danish Regions' “Medicinpuljen”. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Thank you to Jens Peter Konnerup Kampmann for contributing to the research.
Gade, C. , Dalhoff, K. , Petersen, T. S. , Riis, T. , Schmeltz, C. , Chabanova, E. , Christensen, H. R. , Mikus, G. , Burhenne, J. , Holm, J. C. , and Holst, H. (2018) Higher chlorzoxazone clearance in obese children compared with nonobese peers. Br J Clin Pharmacol, 84: 1738–1747. 10.1111/bcp.13602.
The study was conducted at the Phase One Clinic, Department of Clinical Pharmacology, Copenhagen University Hospital, Bispebjerg and Frederiksberg and at Department of Pediatrics, Zealand University Hospital, Holbaek
References
- 1. Han PY, Duffull SB, Kirkpatrick CMJ, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 2007; 82: 505–508. [DOI] [PubMed] [Google Scholar]
- 2. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010; 49: 71–87. [DOI] [PubMed] [Google Scholar]
- 3. Zuckerman M, Greller HA, Babu KM. A review of the toxicologic implications of obesity. J Med Toxicol 2015; 11: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Rongen A, Välitalo PAJ, Peeters MYM, Boerma D, Huisman FW, van Ramshorst B, et al Morbidly obese patients exhibit increased CYP2E1‐mediated oxidation of acetaminophen. Clin Pharmacokinet 2016; 55: 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cella M, Knibbe C, Danhof M, Della Pasqua O. What is the right dose for children? Br J Clin Pharmacol 2010; 70: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. García‐Suástegui WA, Ramos‐Chávez LA, Rubio‐Osornio M, Calvillo‐Velasco M, Atzin‐Méndez JA, Guevara J, et al The role of CYP2E1 in the drug metabolism or bioactivation in the brain. Oxid Med Cell Longev 2017; 4680732. 10.1155/2017/4680732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis T. NICE guideline: feverish illness in children—assessment and initial management in children younger than 5 years. Arch Dis Child Educ Pract 2013; 98: 232–235. [DOI] [PubMed] [Google Scholar]
- 8. Rashed AN, Wong IC, Wilton L, Tomlin S, Neubert A. Drug utilisation patterns in children admitted to a paediatric general medical ward in five countries. Drugs Real World Outcomes 2015; 2: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Hert S, Moerman A. Sevoflurane. F1000Research 2015; 4 (F1000 Faculty Rev): 626. [DOI] [PMC free article] [PubMed]
- 10. Higuchi H, Satoh T, Arimura S, Kanno M, Endoh R. Serum inorganic fluoride levels in mildly obese patients during and after sevoflurane anesthesia. Anesth Analg 1993; 77: 1018. [DOI] [PubMed] [Google Scholar]
- 11. Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, et al Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003; 37: 544–550. [DOI] [PubMed] [Google Scholar]
- 12. Lucas D, Ferrara R, Gonzalez E, Bodenez P, Albores A, Manno M, et al Chlorzoxazone, a selective probe for phenotyping CYP2E1 in humans. Pharmacogenetics 1999; 9: 377–388. [DOI] [PubMed] [Google Scholar]
- 13. O'Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6‐hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther 1994; 56: 359–367. [DOI] [PubMed] [Google Scholar]
- 14. Lucas D, Farez C, Bardou L, Vaisse J, Attali J, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam Clin Pharmacol 1998; 12: 553–558. [DOI] [PubMed] [Google Scholar]
- 15. Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, et al CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology 2003; 38: 428–435. [DOI] [PubMed] [Google Scholar]
- 16. Chtioui H, Semela D, Ledermann M, Zimmermann A, Dufour J‐F. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int 2007; 27: 764–771. [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol 2003; 55: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singer C, Stancu P, Coşoveanu S, Botu A. Non‐alcoholic fatty liver disease in children. Curr Health Sci J 2014; 40: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J, et al Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology 2008; 135: 1961–71.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fonvig CE, Chabanova E, Andersson EA, Ohrt JD, Pedersen O, Hansen T, et al 1H‐MRS measured ectopic fat in liver and muscle in Danish lean and obese children and adolescents. PLoS One 2015; 10: e0135018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe PCAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 2012; 51: 277–304. [DOI] [PubMed] [Google Scholar]
- 22. Gade C, Mikus G, Christensen HR, Dalhoff KP, Holm JC, Holst H. The CYTONOX trial. Dan Med J 2016; 63: A5226. [PubMed] [Google Scholar]
- 23. Nysom K, Mølgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45‐y‐old Danes: reference values and comparison with published European reference values. Int J Obes (Lond) 2001; 25: 177–184. [DOI] [PubMed] [Google Scholar]
- 24. Chiney MS, Schwarzenberg SJ, Johnson LA. Altered xanthine oxidase and N‐acetyltransferase activity in obese children. Br J Clin Pharmacol 2011; 72: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21: 2191–2192. [DOI] [PubMed] [Google Scholar]
- 26. Witt L, Suzuki Y, Hohmann N, Mikus G, Haefeli WE, Burhenne J. Ultrasensitive quantification of the CYP2E1 probe chlorzoxazone and its main metabolite 6‐hydroxychlorzoxazone in human plasma using ultra performance liquid chromatography coupled to tandem mass spectrometry after chlorzoxazone microdosing. J Chromatogr B 2016; 1027 (Suppl C): 207–213. [DOI] [PubMed] [Google Scholar]
- 27. 4252fnl.PDF ‐ ucm070107.PDF [internet]. Available at https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf,2001 (last accessed 25 October 2017).
- 28. Chabanova E, Bille DS, Thisted E, Holm J‐C, Thomsen HS. (1)H MRS assessment of hepatic steatosis in overweight children and adolescents: comparison between 3T and open 1T MR‐systems. Abdom Imaging 2013; 38: 315–319. [DOI] [PubMed] [Google Scholar]
- 29. Chabanova E, Bille DS, Thisted E, Holm J‐C, Thomsen HS. MR spectroscopy of liver in overweight children and adolescents: investigation of 1H T₂ relaxation times at 3T. Eur J Radiol 2012; 81: 811–814. [DOI] [PubMed] [Google Scholar]
- 30. Denney B. CRAN task view: analysis of pharmacokinetic data. 4 April 2017. Available at https://cran.r-project.org/view=Pharmacokinetics (last accessed 14 August 2017).
- 31. Pubchem . Chlorzoxazone [Internet]. Available at https://pubchem.ncbi.nlm.nih.gov/compound/2733.
- 32. Pubchem . 6‐Hydroxychlorzoxazone [Internet]. Available at https://pubchem.ncbi.nlm.nih.gov/compound/2734.
- 33. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992; 11: 1305–1319. [DOI] [PubMed] [Google Scholar]
- 34. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitahara CM, Flint AJ, de Gonzalez AB, Bernstein L, Brotzman M, MacInnis RJ, et al Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014; 11: e1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased alpha 1‐acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol 1984; 18: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N‐acetylcysteine. Lancet Lond Engl 1977; 2: 432–434. [DOI] [PubMed] [Google Scholar]
- 39. Chomchai S, Chomchai C. Predicting acute acetaminophen hepatotoxicity with acetaminophen‐aminotransferase multiplication product and the Psi parameter. Clin Toxicol Phila Pa 2014; 52: 506–511. [DOI] [PubMed] [Google Scholar]
- 40. Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol Phila Pa 2017; 55: 879–892. [DOI] [PubMed] [Google Scholar]
- 41. Lieber CS. Cytochrome P‐4502E1: its physiological and pathological role. Physiol Rev 1997; 77: 517–544. [DOI] [PubMed] [Google Scholar]
- 42. Weltman MD, Farrell GC, Hall P, Ingelman‐Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998; 27: 128–133. [DOI] [PubMed] [Google Scholar]


