Abstract
Aims
We explored the patterns of antidepressant use during pregnancy.
Methods
A cohort of women who started a pregnancy in 2014 was identified using data from the French reimbursement healthcare system (covering approximately 99% of the population). Antidepressant usage (initiated before or during pregnancy) was assessed. Explored changes in antidepressant treatment were: associations, switches, discontinuation and resumption of antidepressants during pregnancy.
Results
The cohort included 766 508 pregnancies (755 519 women). Antidepressant use during pregnancy was 25.7 per 1000 [95% CI: 25.3–26.0]. New use concerned 3.9 per 1000 [95% CI: 3.7–4.0]; the most initiated class during pregnancy was selective serotonin reuptake inhibitors (SSRIs), while the most prescribed individual drug in second and third trimesters was amitriptyline, a tricyclic. Most changes were observed before pregnancy and during the first trimester: 63% of ongoing treatments in the year before pregnancy were discontinued before conception; 68% of treatments maintained after conception were discontinued during the first trimester; switches or antidepressant associations mostly occurred during the periconceptional period or during the first trimester. Regardless of initial antidepressant, switches to sertraline were the most frequent. Associations mainly consisted of a prescription of tri‐/tetracyclic or mirtazapine/mianserin in addition to an SSRI. Discontinuation during pregnancy led to treatment resumption in 22% of pregnancies.
Conclusions
These results suggest that pregnancy was planned or the treatment especially adapted in accordance with existing recommendations in a large proportion of women under antidepressants or in whom such treatments have been initiated after starting a pregnancy.
Keywords: antidepressive agents, drug utilization, insurance health reimbursement, pharmacoepidemiology, pregnancy
What is Already Known about this Subject
The decision whether to use antidepressants during pregnancy is complex and requires weighing up both maternal and child risks associated with treatment against those associated with depression not treated pharmacologically.
How antidepressant treatments are handled in this difficult context remains insufficiently explored, especially in terms of treatment discontinuation and drug switches.
What this Study Adds
Results suggest that antidepressant treatment adaptations might have been anticipated with regard to pregnancy (or pregnancy planned according to treatment course) in a large proportion of women.
The switches and product associations mostly appeared to comply with existing recommendations, as did the product choices made for treatments initiated during pregnancies.
Introduction
The prevalence of depression during pregnancy is high, ranging from 7% to 20% 1, 2, 3. Given that antenatal depression is considered a major risk factor for non‐optimal fetal development as well as for postnatal depression, a condition also linked to developmental problems in the child 4, 5, effective treatment of depression during pregnancy is needed. Non‐pharmacological interventions are preferable for mild‐to‐moderate depression, while antidepressants are indicated for more severe forms or when other treatment options are inaccessible or ineffective 6. Deciding whether to discontinue, change or initiate antidepressant usage during pregnancy requires weighing up both maternal and child risks associated with treatment against those associated with untreated depression. Selective serotonin reuptake inhibitors (SSRIs) are considered a first‐choice therapy during pregnancy owing to abundant and mostly reassuring data 6, 7; indeed, they constitute by far the most used antidepressants during pregnancy 8, 9, 10. However, uncertainties persist regarding a higher risk of heart defects with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4790 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=203 2, 11, as also suggested for http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2398 12, and of pulmonary arterial hypertension in newborns with any SSRIs used after the 20th week of gestation 13, 14. Furthermore, long‐term cognitive consequences related to antidepressant prenatal exposure is a matter of concern 15, and recent epidemiological evidence suggests an increased risk of autism spectrum disorder in children exposed in utero to SSRIs, and to serotonin and norepinephrine reuptake inhibitors (SNRIs) 16, 17, whereas other studies suggest no or a modest association 18, 19. Recommendations therefore advocate pursuing an effective antidepressant treatment during pregnancy without any change, owing to the risk of relapse.
How antidepressant treatments are handled in this difficult context remains insufficiently explored, especially in terms of treatment discontinuation and drug switches. Consequently, this study aimed to explore the patterns of antidepressant use during pregnancy in real clinical practice.
Methods
Design and data source
This was a historical cohort study using data from the French national healthcare insurance database (SNIIRAM) linked with the national hospital discharge database (PMSI). These two databases are linked by a unique personal identification number, anonymized using two successive hash‐scrambling operations.
In France, the national healthcare insurance system covers 99% of the whole population (more than 66 million individuals) irrespective of socioeconomic status. For people on welfare benefit because of very low income, all expenses are totally reimbursed. The SNIIRAM database consists of the anonymous and exhaustive recording of all reimbursements of patients' health expenditure, including drugs, physician visits, lab tests or imaging investigations. The base also contains data concerning the presence of certain long‐term diseases eligible for full reimbursement of health care for a given condition (e.g. mental and behavioural disorders). Full reimbursement of health care for long‐term diseases in France is subject to prior national health insurance approval, after examination of an official form signed by a physician certifying that the patient's condition requires full coverage, which strengthens the validity of diagnoses recorded. The latter are encoded according to the International Classification of Diseases, 10th Revision (ICD‐10). Other diagnoses, indications for prescribing, doses prescribed, or the duration of treatment are not collected in the SNIIRAM database. The PMSI database provides medical information on treatments given during any private and public hospital stays in France, including hospitalization dates and diagnoses coded according to ICD‐10, as well as medical and biological procedures. It covers hospital admissions in medical, surgical and obstetric wards, as well as in psychiatric hospitals.
In accordance with French regulations, ethics committee approval was not required for this observational study conducted on anonymous medico‐administrative data.
Study population
The date of conception is not recorded in French medico‐administrative databases. However, the databases contain information that allows it to be estimated. Consequently, we developed an algorithm to identify women who became pregnant in 2014; all pregnancy outcomes were considered, except for those that could not be captured in medico‐administrative databases (illegal abortion, thought to be very marginal in France; spontaneous abortion not requiring medical care), in order to provide a representative description of antidepressant use both before and during pregnancy. We first identified women with a diagnosis or procedure code related to any type of pregnancy outcome during 2014 and 2015 in a hospital setting or carried out in private practice: deliveries (live birth or stillbirth), spontaneous abortions (managed in hospital setting), elective abortions (legal abortions on demand performed before the end of the 12th week of gestation), therapeutic abortions (legal abortions for medical reasons performed after 12th week of gestation), ectopic pregnancy, hydatidiform mole and other abnormal products of conception. The date of conception was estimated from the date of pregnancy outcome using the gestational age provided in the databases. Information on gestational age is sometimes missing from databases (0.03% of deliveries and 0.46% of other pregnancy outcomes in hospital settings). In such situations, the gestational age was imputed using the median value of all observed gestational ages in 2014–2015, according to the pregnancy outcome, after reclassification of cases with discrepancies between gestational age and label. Sensitivity imputations were performed using the 5% and 95% percentiles of the median value of gestational ages observed in 2014–2015. For elective abortions in outpatient settings, as gestational age is not recorded in the databases, the date of conception was estimated by using national statistics regarding the gestational age at elective abortion carried out in private practices in France in 2012: the median gestational age (time from first day of last menstrual period) was 6 weeks (5th–95th percentiles: 5–7 weeks) 20. Lastly, pregnancies started in 2015 were excluded; the complete cohort of pregnancies started in 2014 that was thus identified included 873 992 pregnancies. Among these, we retained those of women aged 12–55 years on 1 January 2014, whose pregnancy lasted at least 14 days, and who had available health data for at least 12 months both prior to the estimated date of conception and throughout the duration of pregnancy. If more than one pregnancy occurred in 2014 for a given women, all pregnancies meeting the eligibility criteria were considered for analysis.
Women were described in terms of age, welfare recipient status and pregnancy outcome. Psychiatric disorders and comorbidities (diabetes, hypertension) in the year before or during pregnancy, and obstetric complications (threatened preterm labour, premature rupture of membranes) were identified using data from diagnoses related to hospital stays or chronic diseases, and reimbursement data in the 12‐month period preceding the estimated date of conception. All codes used for the identification of the studied comorbidities and medications are listed in Table S1.
Exposure
Antidepressant use during pregnancy was defined as at least one reimbursement during pregnancy or one before the estimated date of conception that overlapped it. Antidepressant new‐use corresponded to treatments for which antidepressant reimbursement was identified during pregnancy but not in the prior 12‐month period, i.e. to treatments initiated during pregnancy. Finally, antidepressant use discontinued before pregnancy was defined as at least one reimbursement in the 12‐month period preceding the estimated date of conception with no ongoing treatment at conception.
The SNIIRAM database does not directly provide the total duration of treatments but antidepressant treatment is issued for a maximum of 30 days in France, and individuals have to renew their treatment every month. We consequently estimated antidepressant exposure by using dates of successive antidepressant reimbursements identified in the year before and during pregnancy, each allowing 30 days of treatment after each reimbursement. Discontinuation of antidepressant treatment was defined as an absence of treatment renewal 45 days after the last reimbursement (i.e. allowing a 15‐day grace period following the putative end of the last reimbursement). An antidepressant treatment episode was defined as a period without discontinuation.
Exposure to antidepressants was defined according to the period of use (12 months before pregnancy, including the peri‐conceptional period, i.e. the 90‐day period preceding the estimated date of conception; first trimester: first 13 weeks of gestation; second trimester: 14th to 26th weeks of gestation; third trimester: 27th week of gestation and later). When a reimbursement included the peri‐conceptional period and trimesters or several trimesters, pregnancies were defined as exposed in both time periods.
The following antidepressant classes were considered: SSRIs, SNRIs, tri‐/tetracyclics, monoamine oxidase inhibitors, and other antidepressants (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7241, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=135, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=198 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7558) (Table S2).
Antidepressant treatment changes investigated
We considered four types of treatment changes during pregnancy: (i) associations of two antidepressants, (ii) switch to another antidepressant, (iii) antidepressant discontinuation (as described above), and (iv) resumption of antidepressants. During a treatment episode with reimbursements for two different antidepressants, treatment was considered as ‘association’ when both antidepressants were used concomitantly and continued. It was classified as a ‘switch’ if the first antidepressant identified was stopped after the second was initiated. Resumption of antidepressants was assessed in women who discontinued treatment and had presented at least two treatment episodes. Resumption was qualified as ‘similar’ if the antidepressant identified for two consecutive treatments was the same.
Data analysis
Characteristics of women who became pregnant in 2014 were examined according to the antidepressant exposure status: no use, use discontinued before pregnancy, and use during pregnancy (whether initiated before or during pregnancy). Antidepressant use was defined as the number of exposed pregnancies per 1000 pregnancies in the study population. Antidepressant new‐use was defined as the number of pregnancies with antidepressant initiation per 1000 pregnancies in the study population. Use and new‐use were first calculated for antidepressants overall and then for each class and each individual molecule. All estimates were stratified according to the period of use as described above. Discontinuation rate during a given period (before pregnancy and first trimester) was defined as the proportion of pregnancies in women who discontinued treatment and never resumed it per 100 pregnancies exposed at least 1 day during the time window of interest. All analyses were performed using SAS® software (SAS Institute, version 9.4, Cary, NC, USA).
Nomenclature of ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 21, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.
Results
Characteristics of study population
The cohort included 766 508 pregnancies (755 519 women), including 19 663 exposed to antidepressants at any time during pregnancy (Figure S1). Table 1 shows the principal characteristics and comorbidities stratified by antidepressant exposure status. As expected, women who used antidepressants during pregnancy appeared to have a higher frequency of psychiatric disorders and comorbidities (chronic or gestational hypertension and diabetes) than those who did not. They also appeared older and more likely to have pregnancy terminations (therapeutic or elective abortions). In agreement with observations in the general population of antidepressant users in France 22, they were also more likely to use anxiolytics or hypnotics (44.9% of women with anxiolytic/hypnotic treatment continued to use it during pregnancy).
Table 1.
Characteristics of study population
| Characteristics | No. of antidepressants used | Antidepressant treatment | |
|---|---|---|---|
| Discontinued before pregnancy | During pregnancy | ||
| N = 719 010 | N = 27 835 | N = 19 663 | |
| Age on 1 January 2014 (years), mean ± SD | 30.0 ± 5.5 | 31.1 ± 5.7 | 32.5 ± 5.6 |
| <18 | 5913 (0.8) | 81 (0.3) | 52 (0.3) |
| 18–24 | 106 581 (14.8) | 3461 (12.4) | 1543 (7.9) |
| 25–34 | 457 596 (63.6) | 16 443 (59.1) | 10 899 (55.4) |
| ≥35 | 148 920 (20.7) | 7850 (28.2) | 7169 (36.5) |
| Welfare recipient | 141 795 (19.7) | 6869 (24.7) | 4614 (23.5) |
| Psychiatric disorders | |||
| Registered as long‐term disease | |||
| In year before conception | 991 (0.1) | 295 (1.1) | 687 (3.5) |
| Depression or bipolar disorder | 377 (0.1) | 135 (0.5) | 363 (1.9) |
| Others | 621 (0.1) | 163 (0.6) | 336 (1.7) |
| During pregnancy | 597 (0.1) | 170 (0.6) | 489 (2.5) |
| Depression or bipolar disorder | 214 (<0.1) | 69 (0.3) | 263 (1.3) |
| Others | 386 (0.1) | 102 (0.4) | 233 (1.2) |
| At least one visit to a private psychiatrist | |||
| In year before conception | 11 571 (1.6) | 4409 (15.8) | 5049 (25.7) |
| During pregnancy | 6382 (0.9) | 1520 (5.5) | 4145 (21.1) |
| Concurrent psychotropics other than antidepressants | |||
| Exposure in year before conception | 85 583 (11.9) | 19 059 (68.5) | 13 836 (70.4) |
| of which (o.w.) anxiolytics/hypnotics | 83 271 (11.6) | 18 721 (67.3) | 13 480 (68.6) |
| o.w. antipsychotics | 2762 (0.4) | 1700 (6.1) | 2441 (12.4) |
| o.w. mood stabilizers | 2287 (0.3) | 485 (1.7) | 715 (3.6) |
| Exposure during pregnancy | 32 672 (4.5) | 3744 (13.5) | 9334 (47.5) |
| o.w anxiolytics/hypnotics | 29 276 (4.1) | 3475 (12.5) | 8820 (44.9) |
| o.w antipsychotics | 2931 (0.4) | 390 (1.4) | 1628 (8.3) |
| o.w mood stabilizers | 1719 (0.2) | 154 (0.6) | 419 (2.1) |
| Hospitalization for mental and behavioural disorders | |||
| In year before conception | 1030 (0.1) | 378 (1.4) | 398 (2.0) |
| During pregnancy | 437 (0.1) | 85 (0.3) | 323 (1.6) |
| Comorbidities | |||
| In year before conception | |||
| Chronic hypertension | 20 866 (2.9) | 1889 (6.8) | 1640 (8.3) |
| Diabetes | 6190 (0.9) | 294 (1.1) | 308 (1.6) |
| During pregnancy | |||
| Chronic or gestational hypertension | 47 766 (6.6) | 2468 (8.9) | 2112 (10.7) |
| Chronic or gestational diabetes | 43 580 (6.1) | 2055 (7.4) | 1724 (8.8) |
| Threatened preterm labour, premature rupture of membranes | 53 934 (7.5) | 2298 (8.3) | 1619 (8.2) |
| Pregnancy outcome | |||
| Delivery (live born and stillborn infants) | 561 888 (78.1) | 20 848 (74.9) | 14 168 (72.1) |
| Terminations (therapeutic or elective abortions) | 144 722 (20.1) | 6342 (22.8) | 5048 (25.7) |
| Other outcomes | 12 400 (1.7) | 645 (2.3) | 447 (2.3) |
Data are presented as number (%) of pregnancies unless otherwise indicated.
SD, standard deviation
Antidepressant use during pregnancy
Antidepressant use during pregnancy was estimated to be 25.7 per 1000 (19 663/766 508); it decreased over pregnancy, from 23.9 per 1000 in the first trimester to 10.4 and 8.4 per 1000 pregnancies in the second and third trimesters, respectively (Table 2). Of exposed pregnancies, 69.9% were exposed to SSRIs, 17.2% to SNRIs, and 13.8% to tri‐/tetracyclic antidepressants. A total of 5554 pregnancies were exposed to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4798 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7177/http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7547 since the estimated date of conception, corresponding to 30.3% of all pregnancies exposed to antidepressants during the first trimester. Sertraline was the most frequently used antidepressant during the third trimester (22.3% of the exposed pregnancies). Eleven pregnancies were exposed to monoamine oxidase inhibitors and are not described here.
Table 2.
Antidepressant use during pregnancy in 2014, overall and by trimester. Figures are number (per 1000) of pregnancies
| Overall | 1st trimester | 2nd trimester | 3rd trimester | |
|---|---|---|---|---|
| N = 766 508 | N = 766 508 | N = 603 945 a | N = 595 011 a | |
| Exposure to antidepressants any time during pregnancy (initiated before or during pregnancy) | ||||
| Overall | 19 663 (25.7) | 18 324 (23.9) | 6303 (10.4) | 4972 (8.4) |
| SSRIs | 13 753 (17.9) | 12 821 (16.7) | 4260 (7.1) | 3502 (5.9) |
| Escitalopram | 5863 (7.7) | 5630 (7.3) | 1480 (2.5) | 1057 (1.8) |
| Paroxetine | 3344 (4.4) | 2998 (3.9) | 888 (1.5) | 817 (1.4) |
| Sertraline | 2365 (3.1) | 1795 (2.3) | 1128 (1.9) | 1108 (1.9) |
| Fluoxetine | 2145 (2.8) | 2022 (2.6) | 605 (1.0) | 452 (0.8) |
| Citalopram | 1013 (1.3) | 955 (1.3) | 320 (0.5) | 234 (0.4) |
| SNRIs | 3381 (4.4) | 3293 (4.3) | 952 (1.6) | 708 (1.2) |
| Venlafaxine | 2477 (3.2) | 2404 (3.1) | 809 (1.3) | 627 (1.1) |
| Duloxetine | 825 (1.1) | 807 (1.1) | 129 (0.2) | 74 (0.1) |
| Milnacipran | 112 (0.2) | 110 (0.1) | 17 (<0.1) | 9 (<0.1) |
| Tri‐/tetracyclics | 2721 (3.6) | 2166 (2.8) | 1115 (1.9) | 809 (1.4) |
| Amitriptyline | 2102 (2.7) | 1638 (2.1) | 800 (1.3) | 540 (0.9) |
| Clomipramine | 535 (0.7) | 445 (0.6) | 290 (0.5) | 246 (0.4) |
| Dosulepine | 46 (0.1) | 43 (0.1) | 13 (<0.1) | 8 (<0.1) |
| Other antidepressants | 1200 (1.6) | 1143 (1.5) | 217 (0.4) | 117 (0.2) |
| Mianserin | 501 (0.7) | 475 (0.6) | 84 (0.1) | 52 (0.1) |
| Mirtazapine | 386 (0.5) | 362 (0.5) | 89 (0.2) | 44 (0.1) |
| Agomelatine | 261 (0.3) | 255 (0.3) | 26 (<0.1) | 8 (<0.1) |
| Tianeptine | 60 (0.1) | 59 (0.1) | 18 (<0.1) | 13 (<0.1) |
| Antidepressant new‐use during pregnancy | ||||
|---|---|---|---|---|
| Overall | 2971 (3.9) | 2062 (2.7) | 624 (1.0) | 285 (0.5) |
| SSRIs | 1802 (2.4) | 1321 (1.7) | 326 (0.5) | 155 (0.3) |
| Escitalopram | 701 (0.9) | 601 (0.8) | 71 (0.1) | 29 (0.1) |
| Paroxetine | 395 (0.5) | 276 (0.4) | 80 (0.1) | 39 (0.1) |
| Sertraline | 361 (0.5) | 159 (0.2) | 131 (0.2) | 71 (0.1) |
| Fluoxetine | 229 (0.3) | 187 (0.2) | 33 (0.1) | 9 (<0.1) |
| Citalopram | 115 (0.2) | 97 (0.1) | 11 (<0.1) | 7 (<0.1) |
| SNRIs | 248 (0.3) | 209 (0.3) | 29 (0.1) | 10 (<0.1) |
| Venlafaxine | 168 (0.2) | 139 (0.2) | 21 (<0.1) | 8 (<0.1) |
| Duloxetine | 69 (0.1) | 61 (0.1) | 6 (<0.1) | 2 (<0.1) |
| Tri‐/tetracyclics | 791 (1.0) | 425 (0.6) | 251 (0.4) | 115 (0.2) |
| Amitriptyline | 692 (0.9) | 371 (0.5) | 220 (0.4) | 101 (0.2) |
| Clomipramine | 76 (0.1) | 38 (0.1) | 25 (<0.1) | 13 (<0.1) |
| Other antidepressants | 148 (0.2) | 122 (0.2) | 19 (<0.1) | 7 (<0.1) |
| Mianserin | 76 (0.1) | 61 (0.1) | 11 (<0.1) | 4 (<0.1) |
| Mirtazapine | 40 (0.1) | 34 (<0.1) | 4 (<0.1) | 2 (<0.1) |
Numbers do not sum to totals as exposure to several antidepressants, or treatment initiation with several antidepressants, is possible. Results for overall use <0.1 per 1000 pregnancies are not shown in this table.
Number of ongoing pregnancies on first day of trimester
SNRIs: serotonin and norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors.
Treatment patterns during pregnancy
Discontinuations and initiations
Definitive treatment discontinuation before conception was observed in 27 835 (62.5%) of the 44 527 pregnancies exposed to antidepressants at least once during the year preceding conception (Figure S1); treatment discontinuation most frequently occurred more than 3 months before conception (77.2%).
Among the 16 262 pregnancies with an antidepressant treatment initiated before conception and continued during the first trimester, the discontinuation rate during the first trimester was 68.2%. Treatments initiated more than 3 months before pregnancy presented lower discontinuation rates than those initiated during the peri‐conceptional period (70% vs. 93%). Regarding pregnancies with exposure to a single class, the discontinuation rate appeared higher for other antidepressants (mianserin, mirtazapine, agomelatine and tianeptine) during the first trimester (85% vs. 66% to 70% for the other classes).
Initiation of an antidepressant during pregnancy was observed in 2971 pregnancies (3.9 per 1000); it decreased throughout pregnancy (2.7, 1.0 and 0.5 per 1000 pregnancies in first, second and third trimesters, respectively) (Table 2). At treatment initiation, the most prescribed class was SSRIs, while the most prescribed molecule during the second and third trimesters was a tricyclic (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=200, representing 35% of incident exposures for these trimesters). Treatment initiation with fluoxetine dropped between the first and third trimester but remained stable for paroxetine (Figure 1). Nearly 76% of antidepressant treatments initiated during pregnancy lasted less than 3 months; tri‐/tetracyclics were more likely to be used for at least two trimesters (25.5%) than other antidepressants.
Figure 1.
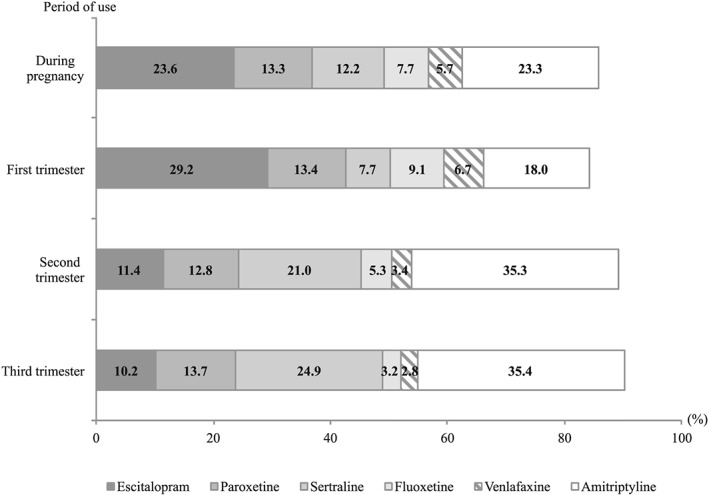
Distribution of antidepressants used during pregnancy, for pregnancies started in 2014 and during which antidepressant treatments were initiated. Number of pregnancies with incident exposure to a given antidepressant relative to the number of pregnancies with incident antidepressant exposure in 2014. Results for proportions < 5% not represented; several antidepressants could be used simultaneously at treatment initiation
Associations, switches and resumption of antidepressants
Overall, 12% of pregnancies were exposed to two antidepressants used either concomitantly (associations) or successively (switch). Associations and switches were frequently observed during the peri‐conceptional period and during the first trimester: this concerned 59.3% of associations and 62.9% of switches. The remaining associations/switches were observed constantly over the 9‐month follow‐up period preceding the peri‐conceptional period, and during the second and third trimesters.
Associations of two antidepressants were observed in 3.3% of exposed pregnancies; these mostly consisted of a tricyclic (amitriptyline or clomipramine) added to the initial antidepressant (most frequently an SSRI), but also of adding mirtazapine or mianserin to the ongoing treatment (Table 3). Switches to another antidepressant concerned 9.1% of exposed pregnancies. More than 50% cases of antidepressant replacements were within the same class, especially for SSRIs (67.9% of SSRI–SSRI switches). Regardless of the initial antidepressant, switches to sertraline were the most frequent, accounting for 21% (383/1823) within all switches performed, followed by es‐/citalopram (376/1823; 20.6%). One quarter was to paroxetine or fluoxetine (474/1823) (Table 3). An overlap of the two antidepressants for at least 3 months was observed in 3.1% of treatment episodes concerned by switches. Antidepressants were resumed in 21.6% of pregnancies in women who discontinued treatment after conception: in almost eight out of ten cases, the antidepressant resumed was similar to the previous one.
Table 3.
Description of associations of two antidepressants, or switching to another antidepressant, during pregnancy
|
Treatment episodes with associations of two ADs N = 733 |
||||
|---|---|---|---|---|
| Initial AD | ||||
| SSRIs | SNRIs | Tri‐/tetracyclics | Other ADs | |
| N = 494 | N = 153 | N = 50 | N = 36 | |
| Add of a tri‐/tetracyclic | 149 (30.2) | 59 (38.6) | 20 (40.0) | 2 (5.6) |
| of which | ||||
| amitriptyline | 112 (22.7) | 49 (32.0) | 4 (8.0) | 1 (2.8) |
| Add of an other AD | 148 (30.0) | 57 (37.3) | 9 (18.0) | 4 (11.1) |
| of which | ||||
| mirtazapine or mianserin | 133 (26.9) | 40 (26.1) | 6 (12.0) | 3 (8.3) |
| Add of a SSRI | 139 (28.1) | 32 (20.9) | 15 (30.0) | 23 (63.9) |
| of which | ||||
| paroxetine or fluoxetine | 48 (9.7) | 6 (3.9) | 8 (16.0) | 11 (30.6) |
| es‐/citalopram | 49 (9.9) | 12 (7.8) | 5 (10.0) | 8 (22.2) |
| sertraline | 42 (8.5) | 13 (8.5) | 2 (4.0) | 4 (11.1) |
| Add of a SNRI | 55 (11.1) | 5 (3.3) | 6 (12.0) | 7 (19.4) |
| of which | ||||
| venlafaxine | 38 (7.7) | 0 (0.0) | 3 (6.0) | 6 (16.7) |
|
Treatment episodes with switching to another AD N = 1823 |
||||
|---|---|---|---|---|
| Initial AD | ||||
| SSRIs | SNRIs | Tri‐/tetracyclics | Other ADs | |
| N = 1259 | N = 294 | N = 126 | N = 143 | |
|
Switch to a SSRI
of which |
855 (67.9) | 208 (70.7) | 80 (63.5) | 92 (64.3) |
| paroxetine or fluoxetine | 324 (25.7) | 79 (26.9) | 39 (31.0) | 32 (22.4) |
| es‐/citalopram | 232 (18.4) | 69 (23.5) | 32 (25.4) | 43 (30.1) |
| sertraline | 296 (23.5) | 60 (20.4) | 9 (7.1) | 17 (11.9) |
| Switch to a SNRI | 223 (17.7) | 28 (9.5) | 20 (15.6) | 35 (24.5) |
| of which | ||||
| venlafaxine | 173 (13.7) | 17 (5.8) | 9 (7.1) | 26 (18.2) |
| Switch to a tri‐/tetracyclic | 114 (9.1) | 42 (14.3) | 16 (12.7) | 11 (7.7) |
| of which | ||||
| amitriptyline | 69 (5.5) | 27 (9.2) | 8 (6.3) | 5 (3.5) |
| Switch to other AD | 67 (5.3) | 16 (5.4) | 10 (7.9) | 5 (3.5) |
| of which | ||||
| mirtazapine or mianserin | 45 (3.6) | 12 (4.1) | 9 (7.1) | 4 (2.8) |
Data are presented as number (%) of treatment episode with associations of two antidepressants or switching to another antidepressant. Results for switching from a monoamine oxidase inhibitor (n = 1) or adding of a monoamine oxidase inhibitor (n = 2) not shown in this table.
ADs, antidepressants; SNRIs, serotonin and norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors.
Other ADs: mirtazapine, mianserin, agomelatine, tianeptine
Discussion
In this large population‐based study covering nearly all women who started a pregnancy in 2014 in France, antidepressant use during pregnancy was estimated to be 25.7 per 1000, in line with the estimated 23–36 per 1000 in Europe 9, 23, 24 and lower than estimates from North America (45–133 per 1000) 25, 26.
The term ‘antidepressant’ is no longer in accordance with the clinical indications of this pharmacological class, since most of these drugs are now approved for indications other than depression, e.g. anxiety disorders. In Canada, between 2006 and 2015, only 55% of antidepressant prescriptions were given for depression while 19% were for anxiety disorders 27. In any case, the prevalence of antidepressant use during pregnancy in France appeared much lower than the estimated prevalence of depression during pregnancy, ranging from 7% to 20% 1, 2, 3. This raises the question of the extent of the potential undertreatment during pregnancy, while optimal management should be promoted owing to the risk of untreated depression, both for mother and child 1. In the absence of information regarding the prevalence of severe depression during pregnancy (which constitutes the proper indication for antidepressants), it is difficult to estimate to what extent pregnant severely depressed women are undertreated.
The proportion of women who discontinue treatment before pregnancy was high, which could mean that they waited for the depression/the indication of antidepressants to be cured before planning a pregnancy, or that they planned a pregnancy and its anticipation motivated the treatment discontinuation. Also, within women who were using an antidepressant during pregnancy and did not discontinue it before conception, a large proportion stopped their treatment in the first months of pregnancy. All these situations could reflect the fact that pregnancy was anticipated or considered early on so that the treatment course could be adapted. While the latter situation can be considered positive as it is in line with international recommendations that emphasize the desire for pregnancy or the discovery of a pregnancy as situations requiring re‐evaluation of the relevance of antidepressant therapy, it does raise the question of the necessity of these treatments. Either they could be stopped with few risks related to the disease, meaning they were not fully medically justified in the first place, or they were stopped despite a potentially important risk related to the disease, which would lead to considering discontinuation as being potentially harmful. Also, we cannot identify the part of these treatments that could have been self‐stopped by patients in the absence of any medical recommendation. The interpretation of these results is indeed limited by the lack of information regarding treatment discontinuation, as in most medico‐administrative databases. In addition, most treatments initiated during pregnancy lasted less than 3 months. This duration does not comply with any recommendation and, at least for the main indications of antidepressants, cannot lead to any benefit for the mother, while the potential risks for the child are real.
Finally, although tricyclics are no longer recommended as a first line for the treatment of depression owing to their adverse effects and the life‐threatening danger of overdosing, amitriptyline was the most frequently used drug at treatment initiation during the second and third trimesters. This probably reflects prescriptions based on what was recommended for years in France in pregnant women, i.e. tricyclics rather than SSRIs, especially amitriptyline, whose sedative properties were also used in sleep disorders, which are rather prevalent after the end of the second trimester. Unfortunately, the extent of amitriptyline prescribed for a psychiatric indication, neuropathic pain or sleep disorders could not be explored in the database we used.
Some of the results are nonetheless encouraging. Consistent with previous British and American reports 23, 28, SSRIs accounted for approximately 70% of antidepressants prescribed during pregnancy, as recommended by international guidelines 6, except for fluoxetine and paroxetine owing to an incertitude regarding a higher risk of fetal cardiac abnormalities associated with exposure to these drugs 2, 11. The decrease in use of fluoxetine throughout pregnancy, especially for incident exposures, fits with public health warnings 29, and reflects the adherence to this recommendation by prescribers. This trend was not observed for paroxetine yet it carries the same suspicion of cardiac risk for the fetus. About 30% of pregnancies were exposed to sertraline or es‐/citalopram from the beginning, the drugs of choice in pregnancy. However, while guidelines are clear regarding the treatments to be considered as first choices for treating depression during pregnancy, they also discourage switching as an effective treatment, even with fluoxetine or paroxetine, during that period, as there is no evidence that the difference in safety profile across drugs outweighs the hazard of increasing the risk of relapse by switching from an effective drug 4.
In the present study, switches to another antidepressant and associations of antidepressants were essentially observed between the three‐month period before conception and the end of the first trimester of pregnancy. This is in contrast with guidelines that suggest that all treatment changes should be made before pregnancy starts whenever possible in order to minimize the risk of disease relapse and to limit the overall burden of fetal exposure to drugs. However, current knowledge does not clearly allow to determine the actual value of adding a second antidepressant to the ongoing treatment or switching to another 30. Compared with antidepressant associations, switching to another antidepressant has the potential advantage of limiting the risk of drug–drug interactions and adverse reactions, and the overall burden of fetal exposure to drugs. Nevertheless, some results from this study regarding these associations and switches were positive. Switching to another antidepressant was more frequently observed than associations of antidepressants. In such cases, sertraline or es‐/citalopram was the preferred drug, which is in line with clinical guidelines recommending these antidepressants as first‐choice drugs when starting treatment in pregnant women, especially if breastfeeding is planned 5. Coprescribing mirtazapine/mianserin with an SSRI now belongs to the best evidence‐based associations 31, 32, and this association was one of the most frequently found in the present study. Other observations were less favourable: one‐quarter of switches to paroxetine or fluoxetine and adding a tricyclic (especially amitriptyline) to an SSRI were also frequent. However, even this can be considered questionable owing to the increased risk of drug–drug interactions with a delayed elimination of the tricyclic antidepressant leading to increased plasma levels 6.
The present findings differ somewhat from those reported to date. Switches were mostly observed to fluoxetine and citalopram in the study by Jimenez‐Solem et al. performed in Denmark 33, and to paroxetine and venlafaxine in the study by Ramos et al. conducted in Quebec 34. Two reasons could explain these inconsistencies apart from potential national specificities: first, the methods used differed in terms of definitions, and second and more importantly, they were conducted using data from pregnant women exposed to antidepressants almost 20 years ago, in times when antidepressant use and recommendations were different. On the other hand, the results are consistent with those reported in a study conducted in pregnant women treated with SSRIs or SNRIs in four Nordic countries between 2008 and 2012 24. In that study, most switches were to the recommended sertraline.
The last result concerns women who resumed treatment during pregnancy. About 22% of women who discontinued treatment resumed it, frequently with the same antidepressant. This could be due to lack of compliance, relapse or to the clinician's difficulty in assessing the respective hazards of treatment and disease in the context of pregnancy 6, 35, 36, 37.
The study has several limitations. First, misclassifications, if any, concerning the estimated date of conception are likely modest. Imputation of missing data showed similar results when we used the 5% and 95% percentiles of the median value of gestational ages observed in 2014–2015 rather than the median value itself. This concordance was mainly due to the very low amount of missing data (less than 0.5%). Indications having justified the prescription were not directly provided in the databases and the relative proportions of treatments prescribed for depressive symptoms or anxiety were unknown, as was the severity of the disease that led to the treatment. Non‐pharmacological treatments such as behavioural therapy, which are the first‐line approach for mild‐to‐moderate forms of depression, could not be explored through the medico‐administrative databases we used because they are not reimbursed by the French healthcare insurance system and are therefore not recorded in its databases. As we used reimbursement data, we cannot be sure that women to whom antidepressants were reimbursed actually used them and in totality during the period following the date of reimbursement. The actual use of drugs and patterns of use can be obtained only directly from patients, but surveys based on collected data are limited in terms of sample size and recall bias. Furthermore, no information was available on drugs dispensed during hospital stays. This may have resulted in misclassifications regarding treatment discontinuation. However, considering a 45‐day window between two consecutive reimbursements for the definition of these discontinuations made it unlikely that hospitalizations resulted in a significant number of such errors, owing to the shortness of hospital stays in most cases. On the other hand, a strength is the nationwide representativeness of the databases used and the exhaustive recording of out‐of‐hospital drug reimbursements, suggesting that all community antidepressant exposures were captured.
In summary, the prevalence of antidepressant use during pregnancy in France was estimated to be 25.7 per 1000, in line with other European estimates. While this cannot be ascertained from such data, these results suggest that pregnancy was planned or the treatment especially adapted in a large proportion of women under antidepressants or in whom such treatments were initiated after pregnancy start. Most of these prescriptions appeared in accordance with existing recommendations, even if the lack of information concerning depression severity precludes any clear‐cut conclusion on this point. Remaining issues include the potential undertreatment of depression during pregnancy and the proportion of resumption of treatment for disease relapse.
Competing Interests
There are no competing interests to declare.
The present study is part of the Drugs Systematized Assessment in real‐liFe Environment (DRUGS‐SAFE) research program funded by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, ANSM). This program aims at providing an integrated system allowing the concomitant monitoring of drug use and safety in France. The potential impact of drugs, frailty of populations and seriousness of risks drive the research program.
This publication represents the views of the authors and does not necessarily represent the opinion of the French Medicines Agency.
Contributors
A.B.L., A.L.S.D., S.G., C.H.D., C.D.M., I.L., B.B. and A.P. conceptualized and designed the work. E.P. collected the data and carried out the analysis. A.B.L., A.L.S.D., S.G., C.H.D., C.D.M., I.L., B.B. and A.P. interpreted the data. A.B.L. wrote the first draft, and all the authors critically revised and approved the final manuscript.
Supporting information
Table S1 List of comorbidities, concurrent drugs and identification codes
Table S2 Antidepressant classes, individual drugs and identification codes
Figure S1 Flowchart of the cohort study. AD, antidepressant
Bénard‐Laribière, A. , Pambrun, E. , Sutter‐Dallay, A.‐L. , Gautier, S. , Hurault‐Delarue, C. , Damase‐Michel, C. , Lacroix, I. , Bégaud, B. , and Pariente, A. (2018) Patterns of antidepressant use during pregnancy: a nationwide population‐based cohort study. Br J Clin Pharmacol, 84: 1764–1775. 10.1111/bcp.13608.
References
- 1. Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol 2004; 103: 698–709. [DOI] [PubMed] [Google Scholar]
- 2. Berard A, Iessa N, Chaabane S, Muanda FT, Boukhris T, Zhao JP. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta‐analysis. Br J Clin Pharmacol 2016; 81: 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibanez G, Charles MA, Forhan A, Magnin G, Thiebaugeorges O, Kaminski M, et al Depression and anxiety in women during pregnancy and neonatal outcome: data from the EDEN mother‐child cohort. Early Hum Dev 2012; 88: 643–649. [DOI] [PubMed] [Google Scholar]
- 4. Vigod SN, Wilson CA, Howard LM. Depression in pregnancy. BMJ 2016; 352: i1547. [DOI] [PubMed] [Google Scholar]
- 5. Weisskopf E, Fischer CJ, Bickle Graz M, Morisod Harari M, Tolsa JF, Claris O, et al Risk–benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf 2015; 14: 413–427. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence . Antenatal and postnatal mental health: clinical management and service guidance (update). (Clinical Guideline 192.) 2014. Available at: http://guidance.nice.org.uk/CG192. Retrieved 29 June 2017.
- 7. Chisolm MS, Payne JL. Management of psychotropic drugs during pregnancy. BMJ 2016; 532: h5918. [DOI] [PubMed] [Google Scholar]
- 8. Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population‐based cohort study from the Netherlands. Br J Clin Pharmacol 2008; 65: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlton RA, Jordan S, Pierini A, Garne E, Neville AJ, Hansen AV, et al Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population‐based study in six European regions. BJOG 2015; 122: 1010–1020. [DOI] [PubMed] [Google Scholar]
- 10. Leong C, Raymond C, Chateau D, Dahl M, Alessi‐Severini S, Falk J, et al Psychotropic drug use before, during, and after pregnancy: a population‐based study in a Canadian cohort (2001–2013). Can J Psychiatry 2017; 62: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet Gynecol 2011; 118: 111–120. [DOI] [PubMed] [Google Scholar]
- 12. Kallen B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel) 2013; 6: 1221–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huybrechts KF, Bateman BT, Palmsten K, Desai RJ, Patorno E, Gopalakrishnan C, et al Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA 2015; 313: 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M, et al Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ 2012; 344: d8012. [DOI] [PubMed] [Google Scholar]
- 15. Wibroe MA, Mathiasen R, Pagsberg AK, Uldall P. Risk of impaired cognition after prenatal exposure to psychotropic drugs. Acta Psychiatr Scand 2017; 136: 177–187. [DOI] [PubMed] [Google Scholar]
- 16. Boukhris T, Sheehy O, Mottron L, Berard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr 2016; 170: 117–124. [DOI] [PubMed] [Google Scholar]
- 17. Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369: 2406–2415. [DOI] [PubMed] [Google Scholar]
- 18. Sujan AC, Rickert ME, Oberg AS, Quinn PD, Hernandez‐Diaz S, Almqvist C, et al Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention‐deficit/hyperactivity disorder in offspring. JAMA 2017; 317: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Agerbo E, Ingstrup KG, Musliner K, Meltzer‐Brody S, Bergink V, et al Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ 2017; 358: j3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Institut National d'Etudes Démographiques . Statistiques d'IVG. Available at http://ivg_statistiques.site.ined.fr/ (last accessed 29 June 2017).
- 21. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Etchepare F, Sanglier T, Andre M, Verdoux H, Tournier M. Antidepressant treatment patterns in younger and older adults from the general population in a real‐life setting. Int J Geriatr Psychiatry 2014; 29: 928–935. [DOI] [PubMed] [Google Scholar]
- 23. Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from the Health Improvement Network. J Clin Psychiatry 2011; 72: 979–985. [DOI] [PubMed] [Google Scholar]
- 24. Zoega H, Kieler H, Norgaard M, Furu K, Valdimarsdottir U, Brandt L, et al Use of SSRI and SNRI antidepressants during pregnancy: a population‐based study from Denmark, Iceland, Norway and Sweden. PLoS One 2015; 10: e0144474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berard A, Sheehy O. The Quebec pregnancy cohort – prevalence of medication use during gestation and pregnancy outcomes. PLoS One 2014; 9: e93870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 2007; 196: e1–e5. [DOI] [PubMed] [Google Scholar]
- 27. Wong J, Motulsky A, Eguale T, Buckeridge DL, Abrahamowicz M, Tamblyn R. Treatment indications for antidepressants prescribed in primary care in Quebec, Canada, 2006–2015. JAMA 2016; 315: 2230–2232. [DOI] [PubMed] [Google Scholar]
- 28. Huybrechts KF, Palmsten K, Mogun H, Kowal M, Avorn J, Setoguchi‐Iwata S, et al National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry 2013; 35: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fluoxetine . Summary of product characteristics (SPC). Date of revision of text on the SPC: 04‐May‐2017N. Available at https://www.medicines.org.uk/emc/medicine/27769 (last accessed 29 June 2017).
- 30. Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al Evidence‐based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 2015; 29: 459–525. [DOI] [PubMed] [Google Scholar]
- 31. Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Moller HJ, et al World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 2013; 14: 334–385. [DOI] [PubMed] [Google Scholar]
- 32. Charpeaud T, Moliere F, Bubrovszky M, Haesebaert F, Allaili N, Bation R, et al [Switching and combining strategies of antidepressant medications]. Presse Med 2016; 45: 329–337. [DOI] [PubMed] [Google Scholar]
- 33. Jimenez‐Solem E, Andersen JT, Petersen M, Broedbaek K, Andersen NL, Torp‐Pedersen C, et al Prevalence of antidepressant use during pregnancy in Denmark, a nation‐wide cohort study. PLoS One 2013; 8: e63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramos E, Oraichi D, Rey E, Blais L, Berard A. Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG 2007; 114: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295: 499–507. [DOI] [PubMed] [Google Scholar]
- 36. Molenaar NM, Brouwer ME, Bockting CL, Bonsel GJ, van der Veere CN, Torij HW, et al Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non‐inferiority randomized controlled trial. BMC Psychiatry 2016; 16: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Swanson SA, Hernandez‐Diaz S, Palmsten K, Mogun H, Olfson M, Huybrechts KF. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol Drug Saf 2015; 24: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of comorbidities, concurrent drugs and identification codes
Table S2 Antidepressant classes, individual drugs and identification codes
Figure S1 Flowchart of the cohort study. AD, antidepressant


